Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has led to surges of patients presenting to emergency departments (EDs) and potentially overwhelming health systems.
Objective
We sought to assess the predictive accuracy of host biomarkers at clinical presentation to the ED for adverse outcome.
Methods
Prospective observational study of PCR-confirmed COVID-19 patients in the ED of a Swiss hospital. Concentrations of inflammatory and endothelial dysfunction biomarkers were determined at clinical presentation. We evaluated the accuracy of clinical signs and these biomarkers in predicting 30-day intubation/mortality, and oxygen requirement by calculating the area under the receiver-operating characteristic curve and by classification and regression tree analysis.
Results
Of 76 included patients with COVID-19, 24 were outpatients or hospitalized without oxygen requirement, 35 hospitalized with oxygen requirement, and 17 intubated/died. We found that soluble triggering receptor expressed on myeloid cells had the best prognostic accuracy for 30-day intubation/mortality (area under the receiver-operating characteristic curve, 0.86; 95% CI, 0.77-0.95) and IL-6 measured at presentation to the ED had the best accuracy for 30-day oxygen requirement (area under the receiver-operating characteristic curve, 0.84; 95% CI, 0.74-0.94). An algorithm based on respiratory rate and sTREM-1 predicted 30-day intubation/mortality with 94% sensitivity and 0.1 negative likelihood ratio. An IL-6–based algorithm had 98% sensitivity and 0.04 negative likelihood ratio for 30-day oxygen requirement.
Conclusions
sTREM-1 and IL-6 concentrations in COVID-19 in the ED have good predictive accuracy for intubation/mortality and oxygen requirement. sTREM-1– and IL-6–based algorithms are highly sensitive to identify patients with adverse outcome and could serve as early triage tools.
Key words: Endothelial dysfunction, immune activation, biomarkers, COVID-19
Abbreviations used: Ang-2, Angiopoietin-2; AUROC, Area under the receiver-operating characteristic curve; COVID-19, Coronavirus disease; CRP, C-reactive protein; CRT, Classification and regression tree; ED, Emergency department; qSOFA, Quick Sepsis-related Organ Failure Assessment; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; sTREM-1, Soluble triggering receptor expressed on myeloid cells; TREM-1, Triggering receptor expressed on myeloid cells
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to surges of patients that can overwhelm health systems.1, 2, 3, 4 Medical resource constraints and scarcity are a new reality including lack of hospital beds, oxygen concentrators, and ventilators in both high- and low-income countries.5 , 6 There is an urgent need to have guidance allowing the rational allocation of scarce medical equipment and resources. In this context, it is critical to have triage tools in place permitting the early recognition of patients at risk of adverse outcomes, and allow for optimal resource allocation.7, 8, 9 Current prediction models to support clinical decision making for patients with coronavirus disease 2019 (COVID-19) were developed on the basis of demographic characteristics, clinical signs and symptoms, imaging techniques, biomarkers, or a combination of these variables; however, most are poorly validated and at risk of bias.10
Cytokine dysregulation during COVID-19 is suspected to contribute to disease severity, and multiple clinical trials are underway to test the efficacy of immunomodulatory therapies.11, 12, 13 A recent study identified different inflammatory cytokine profiles according to the stage of the disease, suggesting their usefulness for risk stratification in patients with COVID-19.13 IL-6, IL-8, and C-reactive protein (CRP) have been proposed for monitoring and prognosis purpose in the context of COVID-19.14, 15, 16 Triggering receptor expressed on myeloid cells (TREM-1) is a receptor expressed both on the surface of blood neutrophils and on mature monocytes/macrophages, which have been implicated in severe COVID-19.17, 18, 19 Soluble TREM-1 (sTREM-1) is its cleaved soluble circulating counterpart. As previously shown, sTREM-1 has a prognostic value in septic shock and other infectious states but has never been studied in the context of COVID-19.20, 21, 22 Furthermore, endothelial cell infection and dysfunction might explain systemic impaired microcirculatory and multiorgan dysfunction with onset of endotheliitis and host inflammatory response.23 , 24 In support of this contention, several studies have reported an association between markers of a procoagulable state (eg, D-dimers and fibrinogen) and patients outcome.25, 26, 27 In a French study, level of angiopoietin-2 (Ang-2) as marker of endothelial activation predicted with a medium accuracy the intensive care unit admission for COVID-19.28
Here, we investigate the predictive accuracy of a panel of host markers of inflammatory and endothelial dysfunction in patients with COVID-19 at presentation to the emergency department (ED), for 30-day intubation/mortality, as well as for 7-day intubation/mortality and 7- and 30-day oxygen requirement. We also evaluate the predictive value of biomarkers in combination with clinical signs and validated clinical severity scores.
Methods
Study design and participants
This prospective observational cohort study of patients with COVID-19 was conducted in the ED of Lausanne University Hospital (CHUV), a tertiary care center in Switzerland. We prospectively screened all patients (age ≥ 18 years) upon arrival at the ED with symptoms of an acute lower respiratory tract infection (cough, sputum, dyspnea, or chest pain for <21 days) between February 6, 2020, and April 3, 2020.29 Patients were included in this study if COVID-19 was confirmed by reverse transcription PCR for SARS-CoV-2 from a nasopharyngeal swab.
Patients’ demographic characteristics, comorbidities, symptoms, vital signs, and laboratory test results performed during routine care were recorded in the ED using a standardized electronic case report form in Research Electronic Data Capture. Easy-to-measure bedside clinical scores to identify patients at risk of poor outcome were calculated at inclusion: (1) quick Sequential Organ Failure Assessment (qSOFA): 1 point each for systolic hypotension (≤100 mm Hg), tachypnea (≥22/min), or altered mentation (Glasgow Coma Scale score ≤14),30 (2) CRB-65: 1 point each for confusion (Glasgow Coma Scale score ≤14), elevated respiratory rate (≥30/min), low blood pressure (systolic <90 mm Hg or diastolic ≤60 mm Hg), age 65 years or more.31 Clinical outcomes on days 7 and 30 were assessed by checking the electronic health record and by calling patients.
Patients were classified into 2 groups according to COVID-19 severity: (1) outpatients and/or admission without oxygen requirement within 30 days of presentation, (2) hospital admission and oxygen requirement without intubation and/or death, and (2) intubation and/or death.
Host biomarkers of endothelial and immune dysfunction
A plasma sample was collected in the ED at enrollment and stored at −80°C without freeze-thaw until analysis. Plasma concentrations of endothelial and immune mediators (Ang-2, IL-6, IL-8, and sTREM-1) were analyzed head-to-head on a multianalyte Ella platform with custom-developed reagents from Protein Simple (San Jose, Calif) as described.32 CRP was quantified by ELISAs (R&D DuoSet, Minneapolis, Minn).
Statistical analyses
Differences between the 3 groups were evaluated by 1-way ANOVA, Kruskal-Wallis, or chi-square test, as appropriate. A bilateral P value of less than .05 was considered indicative of statistical significance.
The primary outcome was 30-day intubation/mortality. Secondary outcomes were 7-day intubation/mortality and 7- and 30-day oxygen requirement (all patients hospitalized with oxygen requirement, including those who were intubated or died, as they all received oxygen).
These outcomes are important for pragmatic clinical decision making in the ED. They are objective and therefore more reproducible in other settings than clinician decision to admit a patient to hospital or to the intensive care unit. We hypothesized first, that patients intubated or fatal within 30 days of the ED consultation absolutely require hospital admission and close monitoring and, second, that patients requiring oxygen supplementation within 30 days need close medical follow-up as outpatient or in-patient depending on health care resources.5 The diagnostic accuracy of clinical signs, clinical severity scores, and host biomarkers to predict intubation/death and oxygen requirement was assessed by calculating the area under the receiver-operating characteristic curve (AUROC). Biomarkers with excellent discrimination value (AUROC ≥ 0.80) as well as vital signs and clinical severity scores were selected for the multivariate analysis.33 The linearity of the continuous variables with respect to the logit of the dependent variable was assessed via the Box-Tidwell procedure and by inspecting the partial residuals. Nonlinear variables were transformed for multivariate logistic regression. The predictive validity of a combinatorial model adding top biomarkers to vital signs or clinical severity scores was measured using logistic regression, and the predicted probabilities were used to generate AUROC. The combinatorial models were compared using the DeLong method.
After having assessed the magnitude of the association (logistic regression) and the discrimination value (AUROC) of the aforementioned parameters, we performed another statistical analysis to increase the robustness of our findings. Classification and regression tree (CRT) analysis is a tree-building technique used to classify patients into various risk categories, and it is ideal to generate clinical decision rules.34 High sensitivity is an important feature for a triage tool to ensure safety. We therefore performed the analysis assigning the cost of misclassifying intubation or death as 10 times greater than the cost of misclassifying other patients as described.20 The CRT analysis was performed with all vital signs, clinical severity scores, and biomarkers as continuous variables to let the software decide on the optimal variables combination and best cutoffs. We decided on the following settings: minimum of 10 cases for parent node and 5 for child node, pruning to reduce overfitting, and maximum levels of 2 for tree depth. All analyses were performed with STATA (version 15.0, Stata Corp, College Station, Tex), R Core Team (2019), and IBM SPSS version 26 (IBM Corporation, Armonk, NY).
Consent for publication
All included patients signed an informed consent form.
Ethics approval
The Swiss Ethics Committee of the canton of Vaud (CER-VD 2019-02283) and the University Health Network – Toronto General Hospital (20-5442) gave ethical approval.
Results
Demographics and clinical and laboratory findings associated with 30-day COVID-19 severity
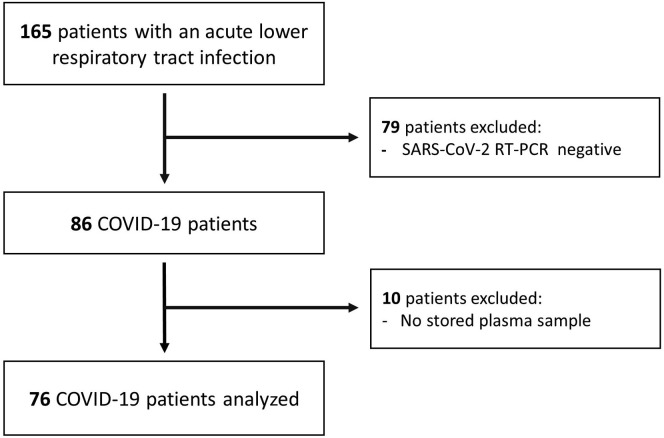
Among 165 patients prospectively included in the lower respiratory tract infection cohort, 76 patients were real-time PCR–confirmed for COVID-19 and had an available plasma sample for analysis (see Fig E1 in this article’s Online Repository at www.jacionline.org). Patients’ 30-day outcomes were as follow: 24 (32%) outpatients or patients hospitalized without oxygen requirement (17 outpatients, 7 hospitalized without oxygen requirement), 35 (46%) hospitalized with oxygen requirement, 17 (22%) intubated and/or dead (5 intubated, 7 deaths without previous intubation, 5 intubated who died). Seven-day intubation/death outcome differed by 2 patients compared with 30-day outcome. These 2 patients died 8 and 9 days after inclusion. The same number of patients required oxygen 7 and 30 days after inclusion; therefore, we present the 30-day oxygen requirement data.
Fig E1.
Flowchart of study participants.
After their initial consultation in ED, 20 patients were discharged home. Three of these subsequently required a secondary hospitalization for oxygen supplementation after a median of 2.6 days (interquartile range, 2.4-3.0). Among intubated patients, 1 was intubated shortly after enrollment and 9 were intubated later after a median of 2.0 days (interquartile range, 2.0-3.0). Twelve patients died after a median of 8.0 days (interquartile range, 2.8-10.3).
Table I presents patient demographics, clinical characteristics, and laboratory results. Although sex distribution, prevalence of comorbidities, and duration of symptoms were similar between the 3 severity groups, patients in the intubation/mortality group were significantly older than the other participants. Respiratory rate, oxygen saturation, qSOFA, and CRB-65 were associated with 30-day intubation/mortality. Leukocyte count and creatinine were significantly lower in patients without oxygen requirement.
Table I.
Characteristics of study participants at inclusion in the ED according to 30-day clinical outcomes
| Characteristic | All (n = 76) | No oxygen requirement (n = 24) | Oxygen requirement (n = 52) |
P value∗ | |
|---|---|---|---|---|---|
| No intubation/death (n = 35) | Intubation/death (n = 17) | ||||
| Demographic | |||||
| Sex: female, n (%) | 43 (57) | 12 (50) | 22 (62) | 9 (52) | .584 |
| Age (y), mean ± SD | 62 ± 17 | 54 ± 19 | 62 ± 15 | 72 ± 12 | .002 |
| Residence in nursing home, n (%) | 8 (10) | 0 (0) | 4 (11) | 4 (23) | .057 |
| Current smoker, n (%) | 1 (1.3) | 1 (4.0) | 0 (0) | 0 (0) | .334 |
| Comorbidities, n (%) | |||||
| Any | 55 (72) | 18 (75) | 26 (74) | 11 (64) | .724 |
| Hypertension | 36 (47) | 9 (37) | 19 (54) | 8 (47) | .447 |
| Asthma | 18 (23) | 9 (37) | 6 (17) | 3 (17) | .157 |
| Diabetes | 17 (22) | 4 (16) | 10 (28) | 3 (17) | .486 |
| Obesity† | 19 (27) | 6 (28) | 8 (25) | 5 (31) | .894 |
| Cardiovascular disease‡ | 10 (13) | 2 (8.0) | 3 (8.6) | 5 (29) | .080 |
| Chronic obstructive pulmonary disease | 3 (3.9) | 1 (4.0) | 0 (0) | 2 (11) | .124 |
| Neurological disorder§ | 11 (14) | 2 (8.0) | 3 (8.6) | 6 (35) | .022 |
| Active cancer | 3 (3.9) | 0 (0) | 2 (5.7) | 1 (5.9) | .486 |
| Hepatitis or liver cirrhosis | 1 (1.3) | 0 (0) | 1 (2.9) | 0 (0) | .552 |
| Chronic renal failure‖ | 3 (3.9) | 0 (0) | 2 (5.7) | 1 (5.9) | .486 |
| Chronic inflammatory diseases¶ | 4 (5.3) | 4 (16.7) | 0 (0) | 0 (0) | .010 |
| Symptoms | |||||
| Duration (d), median (IQR) | 7 (5.7-11) | 7 (5-11) | 7 (7-11) | 8 (4-10) | .905 |
| History of fever, n (%) | 62 (82) | 18 (75) | 28 (80) | 16 (100) | .105 |
| Cough, n (%) | 68 (90) | 21 (87) | 33 (94) | 14 (87) | .602 |
| Dyspnea, n (%) | 58 (76) | 15 (62) | 30 (86) | 13 (76) | .120 |
| Vital signs at inclusion in ED | |||||
| Glasgow Coma Scale score <15, n (%) | 2 (2.7) | 0 (0) | 0 (0) | 2 (12) | .032 |
| Temperature (°C), median (IQR) | 37.0 (36.7-38.2) | 37.1 (37.0-37.0) | 37.6 (37.0-38.0) | 38.0 (37.0-38.0) | .102 |
| Systolic BP (mm Hg), median (IQR) | 133 (122-142) | 133 (119-141) | 133 (125-143) | 135 (118-142) | .889 |
| Heart rate (bpm), median (IQR) | 85 (77-95) | 80 (75-85) | 87 (80-100) | 85 (82-98) | .018 |
| Respiratory rate (vpm), median (IQR) | 23 (18-28) | 20 (17-23) | 23 (18-28) | 26 (25-34) | .001 |
| Oxygen saturation, median (IQR) | 96 (93-97) | 96 (96-97) | 95 (94-97) | 93 (91-95) | .001 |
| Clinical scores at inclusion in ED | |||||
| qSOFA ≥ 2, n (%) | 2 (2.7) | 0 (0.0) | 0 (0.0) | 2 (12) | .034 |
| CRB-65 ≥ 2, n (%) | 9 (13) | 0 (0.0) | 3 (8.6) | 6 (35) | .003 |
| Laboratory findings at inclusion in ED | |||||
| Leukocyte count (G/L), median (IQR) | 6.2 (4.8-8.0) | 5.1 (4.4-6.0) | 6.4 (5.4-7.5) | 8.7 (5.7-10) | .002 |
| Hemoglobin (g/L), median (IQR) | 139 (129-149) | 145 (139-154) | 136 (127-147) | 135 (131-145) | .053 |
| Platelet count (G/L), median (IQR) | 212 (160-288) | 226 (163-274) | 209 (158-293) | 186 (158-275) | .720 |
| Creatinine (μmol/L), median (IQR) | 91 (79-109) | 91 (70-98) | 89 (77-113) | 105 (88-126) | .034 |
| Radiology | |||||
| Infiltrate on chest radiograph, n (%) | 54 (76) | 10 (53) | 29 (83) | 15 (88) | .018 |
| Clinical outcome, n (%) | |||||
| 7-d intubation/death | 15 (20) | ||||
| 30-d intubation/death | 17 (22) | ||||
| 7-d oxygen requirement | 52 (68) | ||||
| 30-d oxygen requirement | 52 (68) | ||||
BMI, Body mass index; BP, blood pressure; bpm, beats per minute; CKD, chronic kidney disease; FiO2, fraction of inspired oxygen; IQR, interquartile range; vpm, ventilations per minute.
Missing values: obesity 7, duration of symptoms 8, fever 1, cough 1, vital signs 5, blood cell count 1, chest radiograph 6, lung ultrasound 5, CRB-65 4, qSOFA 3.
Differences between the 3 groups evaluated by 1-way ANOVA, Kruskal-Wallis, or χ2, as appropriate.
BMI > 30 kg/m2.
Heart failure, coronary disease.
Stroke, dementia, Parkinson.
Stage III-V according to CKD classification.
Autoimmune or chronic inflammatory disease.
Immune and endothelial activation mediators are associated with 30-day COVID-19 severity
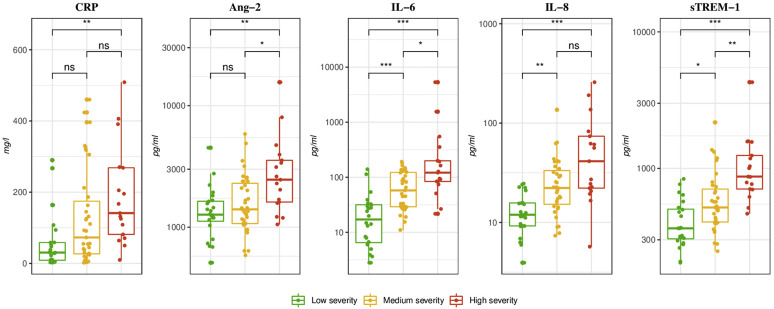
After correction for multiple comparisons, plasma concentrations of the immune activation markers, IL-6 and sTREM-1, were significantly higher between groups of increasing severity (Fig 1 ). CRP was significantly different between the 2 extreme patient groups, that is, those without oxygen requirement and those who were intubated/died. The concentration of the endothelial activation marker Ang-2 was significantly higher in ED patients at presentation who subsequently were intubated/died within 30 days of enrollment. IL-8 was significantly lower in patients who did not require oxygen.
Fig 1.
Plasma concentration of immune and endothelial dysfunction markers at inclusion in the ED according to 30-day COVID-19 outcome. ns, Nonsignificant. Boxplot with median and interquartile range. Concentrations reported in pg/mL except CRP in mg/L. P values were computed using the Wilcoxon-Mann Whitney test and were adjusted for multiple comparisons using Bonferroni method. ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001.
Vital signs, clinical severity scores, and biomarkers predict 30-day intubation/mortality and oxygen requirement
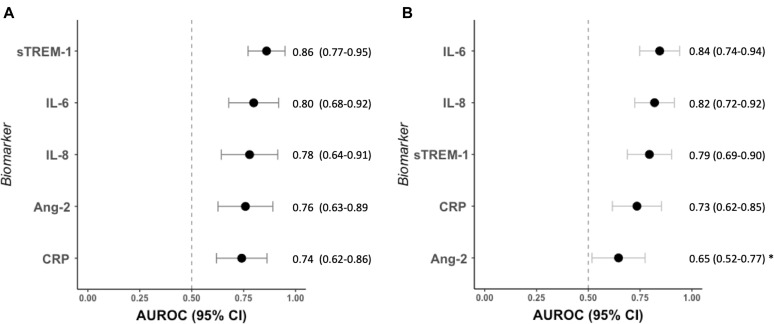
Of clinical signs, respiratory rate and oxygen saturation in the ED displayed the best predictive accuracy (AUROC, 0.77, 95% CI, 0.64-0.89, and AUROC, 0.78, 95% CI, 0.66-0.89, respectively) for 30-day intubation/mortality. Severity scores (qSOFA and CRB-65) did not perform better than respiratory rate or oxygen saturation alone (Table II ). sTREM-1 and IL-6 measured in the ED were the best predictive markers for 30-day intubation/mortality (AUROC, 0.86, 95% CI, 0.77-0.95, and AUROC, 0.80, 95% CI, 0.68-0.92, respectively); however, these were not significantly better than IL-8, Ang-2, or CRP, which all demonstrated an acceptable prognostic value (AUROC, >0.70) (Fig 2 , A).
Table II.
Prognostic accuracy of vital signs and clinical scoring systems alone and in combination with top predicting biomarkers for 30-day intubation/mortality in patients with COVID-19
| Model | AUROC (95% CI) |
||
|---|---|---|---|
| Clinical parameter | (+) sTREM-1 | (+) IL-6 | |
| Heart rate | 0.58 (0.43-0.74) | 0.85 (0.76-0.95)∗ | 0.81 (0.69-0.92)∗ |
| Respiratory rate | 0.77 (0.64-0.89) | 0.86 (0.59-0.89)∗ | 0.85 (0.74-0.96) |
| Oxygen saturation | 0.78 (0.66-0.89) | 0.86 (0.77-0.95)∗ | 0.86 (0.76-0.96) |
| qSOFA | 0.71 (0.60-0.82) | 0.85 (0.74-0.96)∗ | 0.85 (0.74-0.96) |
| CRB-65 | 0.75 (0.66-0.88) | 0.88 (0.79-0.98)∗ | 0.87 (0.77-0.97)∗ |
| sTREM-1 | 0.86 (0.77-0.95) | ||
| IL-6 | 0.80 (0.68-0.92) | ||
Missing values: CRB-65 4, qSOFA 3, Heart rate 1, Respiratory rate 1.
AUROCs were calculated from the predictive probabilities of logistic regression models to 30-d mortality/intubation. AUROC of clinical parameters alone and combined with sTREM-1 or IL-6 are presented. Differences in AUROCs were assessed using the DeLong method.
P < .05 comparing the clinical parameter AUROC vs the combined clinical parameter with sTREM-1 or IL-6 AUROC.
Fig 2.
Prognostic accuracy of host biomarkers measured at ED in patients with COVID-19 for (A) 30-day mortality and/or intubation and (B) 30-day oxygen requirement. Nonparametric ROC curves were generated and AUROCs were plotted to illustrate the ability of these markers to discriminate between patient groups. Each AUROC was compared with other using the DeLong method. AUROCs for the outcome of each biomarker are presented to the right of its respective forest plot, with 95% CIs in parentheses. ∗Ang-2 performed significantly worse than sTREM-1, IL-6, and IL-8 (P < .05) to predict 30-day oxygen requirement. No other comparison reached a statistically significant difference (P < .05).
No vital signs or clinical severity scores determined in the ED displayed good predictive accuracy for subsequent oxygen requirement (AUROC, <0.80) (Table II). The need for 30-day oxygen supplementation was best predicted by IL-6 (AUROC, 0.84; 95% CI, 0.75-0.94) and IL-8 (AUROC, 0.82; 95% CI, 0.72-0.92) (Fig 2, B). sTREM-1 and CRP showed an acceptable performance, which was not statistically lower than that of IL-6. Ang-2 performed significantly worse than IL-6, IL-8, and sTREM-1 (P < .005).
Biomarkers of immune and endothelial dysfunction improve the predictive accuracy of validated clinical scores and vital signs
Combinations of biomarkers from similar or different pathophysiological pathways associated with severe infections may further improve the prognostic accuracy. However, we tested all biomarker combinations and no combination improved predictive accuracy over single markers.
In previous reports, combination of clinical signs or validated clinical scores with biomarkers improved prognostic accuracy when compared with clinical features alone.20 , 35 Therefore, we combined clinical parameters with sTREM-1 and IL-6, the top predicting biomarkers (AUROC ≥ 0.80) of 30-day intubation/mortality (Table II). The combination of respiratory rate with sTREM-1 performed significantly better than the respiratory rate alone (P = .024), but not better than sTREM-1 alone. We found similar results when combining clinical scores and sTREM-1 (Table II).
Multivariate analysis of combination of biomarkers and vital signs to predict oxygen requirement are presented in Table E1 in this article’s Online Repository at www.jacionline.org. Combining respiratory rate with IL-6 or IL-8 significantly improved the predictive value of the model when compared with respiratory rate only. Similarly, the predictive value of CRB-65 and qSOFA for oxygen requirement was significantly improved by a combination with IL-6 or IL-8. No combination performed better than IL-6 or IL-8 alone.
To summarize, none of the tested combination improved significantly the prediction accuracy compared with the biomarkers alone.
sTREM-1–based algorithms predict 30-day intubation/mortality in patients with COVID-19
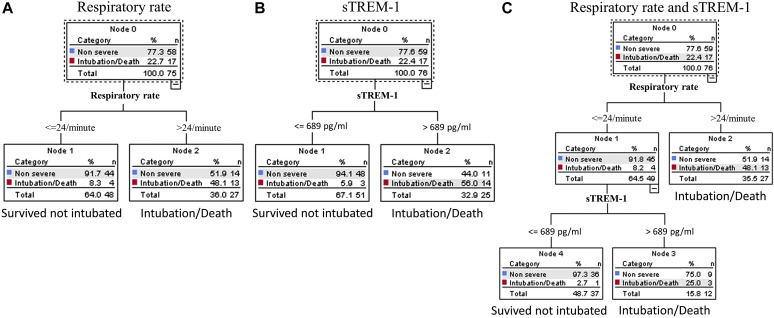
After having evaluated the magnitude of the association between clinical signs, scores, biomarkers, and adverse outcome, we performed a CRT analysis to find optimal variables and cutoff points suitable to generate simple algorithms. The resulting classification tree represents visual decision making.
CRT analysis including all biomarkers, vital signs, and clinical scores identified a model including sTREM-1 only (Fig 3 ). To allow for diagnostic stewardship, we performed the analysis with sTREM-1, forcing respiratory rate and oxygen saturation, the top predicting clinical signs, as the first variable in the algorithm. Although the algorithms including respiratory rate or sTREM-1 alone had a higher specificity, the algorithm including respiratory rate and sTREM-1 exhibited the highest sensitivity (94%) and the smallest negative likelihood ratio (0.10), allowing a large decrease in the posttest probability of day-30 intubation/death at first presentation to the ED (see Table E2 in this article’s Online Repository at www.jacionline.org). The specificity of this combined algorithm was 61% and its positive likelihood ratio 2.3. We also tried to force oxygen saturation in the algorithm but it was not possible without overfitting the model.
Fig 3.
CRT analysis algorithms to predict day-30 intubation/mortality in COVID-19 at ED. The algorithms were generated for (A) respiratory rate, (B) sTREM-1, and (C) respiratory rate and sTREM-1. CRT analysis including all biomarkers, vital signs, and clinical scores identified the model including sTREM-1 only (Fig 3, B). CRT was then performed with respiratory rate only (Fig 3, A) and with a combination of respiratory rate and sTREM-1 (Fig 3, C). For all algorithms, the cost of misclassifying a patient who was intubated or died was designated as 10 times the cost of misclassifying a patient who survived without intubation. Cutoff points selected by the CRT analysis are shown between the parent and child nodes. The outcome prediction of the model is indicated below each terminal node.
Because it is also important to identify patients who require immediate medical attention, we also assessed the performance of this algorithm to predict 7-day intubation/death. The sensitivity was maintained at 93%, the specificity at 59%, and the negative and positive likelihood ratio were 0.17 and 2.3, respectively.
Because we could use this triage tool to identify patients requiring oxygen, we tested this algorithm to predict 7- and 30-day oxygen requirement. Although it was highly specific (100%), its sensitivity was low (25%) in this setting.
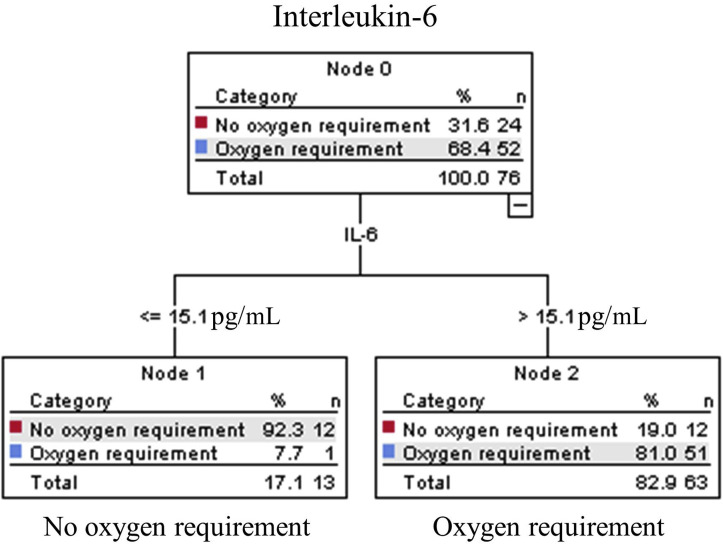
IL-6–based algorithms predict 30-day oxygen requirement in patients with COVID-19
The CRT analysis performed with all clinical signs, severity scores, and biomarkers to predict 30-day oxygen requirement selected IL-6 only (with a cutoff ≤15.1 pg/mL vs >15.1 pg/mL). This algorithm had a sensitivity of 98%, specificity of 50, a negative likelihood ratio at 0.04, and a positive likelihood ratio at 2.0 (see Fig E2 and Table E2 in this article’s Online Repository at www.jacionline.org). To allow for diagnostic stewardship, we performed the analysis with IL-6, forcing respiratory rate and oxygen saturation, as the first variable in the algorithm. However, it was not possible without overfitting the model.
Fig E2.
CRT analysis algorithms to predict day-30 oxygen requirement in COVID-19 at ED. CRT analysis including all biomarkers identified the model including IL-6 only. The cost of misclassifying a patient who required oxygen was designated as 10 times the cost of misclassifying a patient who did not require oxygen. Cutoff point selected by the CRT analysis is shown between the parent and child nodes. The outcome prediction of the model is indicated below each terminal node.
Because we could also use this triage tool to identify patients at high risk of poor outcomes, we tested this algorithm to predict 7- and 30-day intubation/death. It had an excellent sensitivity (100%) but a poor specificity (21%) for 7-day intubation/death and showed a similar performance for 30-day intubation/death (sensitivity at 100% and specificity at 22%).
Discussion
The development of an accurate triage tool to aid clinical decision making in the ED is critical for the early and rapid recognition of patients with COVID-19 at risk for deterioration and to optimize resource allocation in the context of a global pandemic.10 Immune and endothelial activation contribute to immunopathology, microvascular dysfunction, and a procoagulable state and have been proposed as pathophysiological pathways leading to adverse outcomes associated with COVID-19.23 , 25 , 36, 37, 38 In this prospective cohort of SARS-CoV-2–infected patients, we test the hypothesis that measuring markers of these pathways at first presentation to the ED may enable early triage and risk stratification of patients with COVID-19. We report that sTREM-1 and IL-6, when measured at presentation to the ED, have good predictive accuracy for 30-day clinical deterioration and 30-day oxygen requirement, respectively. No combination of biomarkers and clinical data performed better than single biomarkers.
Among the biomarkers of immune and endothelial dysfunction tested, sTREM-1 had the best prognostic accuracy for day-30 intubation/death. CRP elevation has been previously associated with severe COVID-19 and has been proposed as a biomarker to predict COVID-19 severity alone or combined with other variables.11 , 39, 40, 41, 42 In our study, the predictive accuracy of CRP was less than that of sTREM-1. Respiratory rate and oxygen saturation measured at the ED were the best predictive vital sign and performed as well as clinical scores. Adding sTREM-1 significantly improved the predictive accuracy of respiratory rate alone. We developed a simple algorithm based sequentially on respiratory rate (cutoff point at 24/min) and sTREM-1 (cutoff point at 689 pg/mL) with an excellent predictive accuracy for ruling out 7-day and 30-day risk of respiratory failure or death. Ultimately, the availability and use of sTREM-1 rapid tests such as the “near patient” 1 hour test used in this study,32 or point-of-care test versions suitable for low-income settings, might enable the early recognition of patients in EDs or outpatient clinics at risk of adverse outcomes with a high sensitivity. However, the performance of this algorithm to identify patients with a lower level of severity (those requiring oxygen) was not optimal with a low sensitivity. Our data do not support the use of a respiratory rate/sTREM-1 triage algorithm for oxygen requirement.
To our knowledge, this is the first study evaluating sTREM-1 in the context of COVID-19 risk stratification. Our results warrant further evaluation of sTREM-1 as an early COVID-19 triage tool in the ED. TREM-1 is a receptor from the immunoglobulin superfamily expressed both on blood neutrophils and on mature monocytes/macrophages. It participates in signaling pathways that amplify inflammatory responses inducing release of proinflammatory mediators such as TNF-α and IL-1β.18 , 21 , 43 Its soluble counterpart, sTREM-1, results from the proteolytic cleavage of membrane-anchored TREM-1 by metalloproteinases, after stimulation of TREM-1 by proinflammatory molecules, such as LPS and activation of Toll-like receptor 4.17 , 44 , 45 The mononuclear phagocyte system is likely implicated in severe COVID-19 via, among others, the Toll-like receptor 4-tumor necrosis factor receptor-associated factor 6 (TRAF6)-nuclear factor kappa B (NF-κB) pathway.19 Therefore, activation of the TREM-1 signaling pathway on monocytes/macrophages might contribute to the development of a cytokine storm in the context of COVID-19, and justify the use of the sTREM-1 concentration to predict adverse outcome. However, given our limited sample size, our findings warrant further validation studies. Previous reports showed that sTREM-1 has a high prognostic accuracy for adverse outcomes in febrile patients and in those presenting with septic shock, and is superior to widely used biomarkers including CRP and procalcitonin.21 An sTREM-1–based algorithm developed to predict all-cause mortality in febrile patients attending ED in an African low-resource setting showed a high prognostic performance and was superior to CRP and procalcitonin.20 These data suggest that sTREM-1 could be used as an early prognostic tool in the ED to support safe triage of patients with a broad spectrum of infections.
The elevation of inflammatory cytokines such as IL-6 has previously been reported in association with severe COVID-19.12 , 14 , 15 , 46 Here, we show that IL-6 was the best predictor of oxygen requirement in patients with COVID-19. The combination of respiratory rate with IL-6 performed significantly better than respiratory rate alone. An IL-6–based algorithm (IL-6 cutoff point of 15.1 pg/mL) had a high sensitivity and low negative likelihood ratio, allowing safe outpatient management in selected patients. This algorithm would have identified at their first ED visit the 3 patients who were secondary admitted for oxygen supplementation, as well as 4 of 7 (57%) patients who were admitted but never received oxygen supplementation. Measurement of IL-6 with a rapid point-of-care test at the ED might support clinicians in their decision of patients’ hospital admission.
Our study had limitations including a small number of cases, leading to a lack of power to show significant differences between the prognostic accuracy of tested biomarkers, as well as combinations of biomarkers and vital signs versus biomarkers alone. Another limitation is the lack of external validation of our algorithms in another cohort. We used a 30-day outcome to avoid missing late events. Day-30 outcomes might represent late complications unrelated to the viral infection. However, intubations or deaths all occurred within the first 9 days of inclusion and oxygen requirement during the first 7 days. In our cohort, we conclude that 30-day outcome represents early complications related to the viral disease. Indeed, most adverse outcomes occurred quickly after hospitalization as reported in previous studies.12 , 47
Nevertheless, our data support sTREM-1 and IL-6 as potential candidates for point-of-care test, which could be used as a triage tool at presentation to predict disease severity in COVID-19.
sTREM-1– or IL-6–based algorithms are complementary because they identify patients with a differing clinical COVID-19 severity. One outcome might be a preferred triage tool to decide on patient admission early in the outbreak, whereas the other might be more appropriate to prevent overwhelming health care systems. Indeed, in case of scarce resources, we prioritize admission of patients at a higher risk of severe outcome (intubation/death: RR/sTREM-1 algorithm), whereas less severe patients who will subsequently only require oxygen (IL-6 algorithm) could be managed as outpatients with oxygen supplementation to free up hospital beds. Medical resource allocation is a dynamic process affected by demands on health systems. Such increased demand lead to a shortage of medical supplies and induce the need of optimal resource allocation.5 However, larger studies are needed to differentiate which model is superior for ED triage.
In this study, both algorithms were very sensitive, with a low negative likelihood ratio ensuring safe management of patients. Ultimately biomarker-based algorithms such as these could enhance clinical decision making and resource allocation; however, this will require further prospective trials to confirm their risk stratification performance in actual practice.
Clinical implications.
Our findings suggest that sTREM-1 and IL-6 concentrations measured at presentation to the ED can be used as triage tools in patients with COVID-19 to decide on outpatient management or close monitoring.
Acknowledgments
We thank all the patients who accepted to participate and make this study possible. We thank Professor Carron, head of the emergency department, who supported the study. We thank all health care workers of the Emergency Department, Internal Medicine Ward, Infectious Disease Service, and Intensive Care Unit of the University Hospital of Lausanne, who managed patients with COVID-19. We thank Hélène Gerhard Donnet and Marie-Josée Brochu Vez for helping in sample collection at the emergency department. We are grateful to Martin Delaloye who helped us with sample storage and triage.
Footnotes
This work was supported in part by an academic award of the Leenaards Foundation (to N.B.-B.) and by the Foundation of Lausanne University Hospital (to N.B.-B.); Canadian Institutes of Health Research (Foundation grant no. FDN-148439 to K.C.K.); CIHR COVID-19 grant (grant nos. 447092 and VR3-172649 to K.C.K.); National Research Council of Canada Industrial Research Assistance Program (NRC-IRAP) (grant no. 947684 to K.C.K.); GeoSentinel Foundation ( to K.C.K.); FAST grants Thistledown Foundation (to K.C.K); Slaight Family Foundation (to K.C.K.); Tesari Foundation (to K.C.K); and the Canada Research Chair program (to K.C.K.). The funding bodies had no role in the design of the study and collection, analysis and interpretation of data, and writing the manuscript.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Prognostic accuracy of vital signs and clinical scoring systems alone and in combination with top predicting biomarkers for 30-day oxygen requirement in patients with COVID-19
| Model | AUROC (95% CI) |
||
|---|---|---|---|
| Clinical parameter | (+) IL-6 | (+) IL-8 | |
| Heart rate | 0.70 (0.59-0.83) | 0.87 (0.78-0.97)∗ | 0.86 (0.77-0.94)∗ |
| Respiratory rate | 0.72 (0.59-0.84) | 0.85 (0.76-0.95)∗ | 0.85 (0.76-0.94)∗ |
| qSOFA | 0.66 (0.54- 0.78) | 0.84 (0.74-0.94)∗ | 0.85 (0.75-0.94)∗ |
| CRB-65 | 0.67 (0.55-0.78) | 0.83 (0.72-0.93)∗ | 0.83 (0.73-0.92)∗ |
| IL-6 | 0.84 (0.75-0.94) | ||
| IL-8 | 0.82 (0.72-0.92) | ||
Missing values: CRB-65 4, qSOFA 3, Heart rate 1, Respiratory rate 1.
AUROCs were calculated from the predictive probabilities of logistic regression models to 30-d oxygen requirement. Clinical parameter AUROC alone and combined with IL-6 or IL-8 are presented. Differences in AUROCs were assessed using the DeLong method.
P < .05 comparing the clinical parameter AUROC vs the combined clinical parameter with IL-6 or IL-8 AUROC.
Table E2.
Prognostic performance characteristics of CRT models for 7- and 30-day intubation/mortality and 30-day oxygen requirement outcomes in patients with COVID-19
| Performance | Prediction of 30-d intubation/mortality |
Prediction of 7-d intubation/mortality |
Prediction of 30-d oxygen requirement |
||
|---|---|---|---|---|---|
| sTREM-1 | RR | sTREM-1 + RR | sTREM-1 + RR | IL-6 | |
| Sensitivity | 83% | 77% | 94% | 93% | 98% |
| Specificity | 81% | 76% | 61% | 59% | 50% |
| Positive predictive value | 56% | 48% | 41% | 36% | 81% |
| Negative predictive value | 94% | 92% | 97% | 97% | 92% |
| LR+ | 4.4 | 3.2 | 2.4 | 2.3 | 2.0 |
| LR− | 0.21 | 0.30 | 0.10 | 0.17 | 0.04 |
LR+, Positive likelihood ratio; LR−, negative likelihood ratio; RR, respiratory rate.
References
- 1.Tanne J.H., Hayasaki E., Zastrow M., Pulla P., Smith P., Rada A.G. Covid-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ. 2020;368 doi: 10.1136/bmj.m1090. https://www.bmj.com/content/368/bmj.m1090 Available at: [DOI] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.COVID-19 in Europe: the Italian lesson. Lancet. 2020;395:1110–1111. doi: 10.1016/S0140-6736(20)30690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arango C. Lessons learned from the coronavirus health crisis in Madrid, Spain: how COVID-19 has changed our lives in the last 2 weeks. Biol Psychiatry. 2020;88:e33–e34. doi: 10.1016/j.biopsych.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 6.Vergano M., Bertolini G., Giannini A., Gristina G.R., Livigni S., Mistraletti G. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Crit Care. 2020;24:165. doi: 10.1186/s13054-020-02891-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson N., Laydon D., Nedjati Gilani G., Imai N., Ainslie K., Baguelin M. Report 9: impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. 2020. http://spiral.imperial.ac.uk/handle/10044/1/77482 Available at:
- 8.Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts, Interim guidance. Available at: https://apps.who.int/iris/bitstream/handle/10665/331473/WHO-nCov-IPC-HomeCare-2020.3-eng.pdf?sequence=1&isAllowed=y. Accessed May 26, 2020.
- 10.Wynants L., Calster B.V., Bonten M.M.J., Collins G.S., Debray T.P.A., Vos M.D. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Q, Li P, Ye X, Huang X, Mo X, Wang Q, et al. Longitudinal peripheral blood transcriptional analysis of COVID-19 patients captures disease progression and reveals potential biomarkers [published online ahead of print May 8, 2020]. medRxiv. 10.1101/2020.05.05.20091355. [DOI]
- 14.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., Bergwelt-Baildon M. von. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T., Zhang J., Yang Y., Ma H., Li Z., Zhang J. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Jiang L., Li X., Lin F., Wang Y., Li B. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Piña V., Soares-Schanoski A., Rodríguez-Rojas A., Fresno C del, García F., Vallejo-Cremades M.T. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 18.Bouchon A., Dietrich J., Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 19.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard-Greenblatt M., Boillat-Blanco N., Zhong K., Mbarack Z., Samaka J., Mlaganile T. Prognostic accuracy of soluble triggering receptor expressed on myeloid cells (sTREM-1)-based algorithms in febrile adults presenting to Tanzanian outpatient clinics. Clin Infect Dis. 2020;70:1304–1312. doi: 10.1093/cid/ciz419. [DOI] [PubMed] [Google Scholar]
- 21.Brenner T., Uhle F., Fleming T., Wieland M., Schmoch T., Schmitt F. Soluble TREM-1 as a diagnostic and prognostic biomarker in patients with septic shock: an observational clinical study. Biomarkers. 2017;22:63–69. doi: 10.1080/1354750X.2016.1204005. [DOI] [PubMed] [Google Scholar]
- 22.Wright S.W., Lovelace-Macon L., Hantrakun V., Rudd K.E., Teparrukkul P., Kosamo S. sTREM-1 predicts mortality in hospitalized patients with infection in a tropical, middle-income country. BMC Med. 2020;18:159. doi: 10.1186/s12916-020-01627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smadja D.M., Guerin C.L., Chocron R., Yatim N., Boussier J., Gendron N. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23:611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodhead M., Blasi F., Ewig S., Garau J., Huchon G., Ieven M. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011;17:E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer T.T., Ewig S., Marre R., Suttorp N., Welte T. CRB-65 predicts death from community-acquired pneumonia∗. J Intern Med. 2006;260:93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 32.Leligdowicz A., Conroy A.L., Hawkes M., Zhong K., Lebovic G., Matthay M.A. Validation of two multiplex platforms to quantify circulating markers of inflammation and endothelial injury in severe infection. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Applied logistic regression. Hoboken (NJ): John Wiley & Sons, Ltd; 2005. Assessing the fit of the model; pp. 143–202.https://onlinelibrary.wiley.com/doi/abs/10.1002/0471722146.ch5 Available at: [Google Scholar]
- 34.Lewis RJ. An introduction to Classification and Regression Tree (CART) analysis. Annual Meeting of the Society for Academic Emergency Medicine, 2000, San Francisco.
- 35.Erdman L.K., Dhabangi A., Musoke C., Conroy A.L., Hawkes M., Higgins S. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry B.M., Oliveira MHS de, Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med (CCLM) 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 40.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaquero LM, Barrado MES, Escobar D, Arribas P, Gonzalez JR, Bermejo JF, et al. C-Reactive protein and SOFA score as early predictors of critical care requirement in patients with COVID-19 pneumonia in Spain [published online ahead of May 24, 2020]. medRxiv. 10.1101/2020.05.22.20110429. [DOI]
- 42.Wang L. C-reactive protein levels in the early stage of COVID-19. Méd Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchon A., Facchetti F., Weigand M.A., Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 44.Carrasco K., Boufenzer A., Jolly L., Le Cordier H., Wang G., Heck A.J. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol Immunol. 2019;16:460–472. doi: 10.1038/s41423-018-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arts R.J.W., Joosten L.A.B. Meer JWM van der, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93:209–215. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 46.Wang C., Fei D., Li X., Zhao M., Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020:1–2. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]