Abstract
ABO blood groups is a cheap and affordable test that can be immediately retrieved from COVID-19 patients at the diagnosis. There is increasing evidence that non-O blood groups have both higher susceptibility and higher severity of COVID-19 infections. The reason behind such relationship seems elusive. Regarding susceptibility, Non-O individuals have Anti-A antibodies which can prevent viral entry across ACE-2 receptors, moreover, Non-O individuals are at higher risk of autoimmunity, hypercoagulable state, and dysbiosis resulting in an augmented tendency for vascular inflammatory sequelae of COVID-19. We can conclude, on the diagnostic level, that ABO blood groups can be potentially used for risk stratification of affected COVID-19 patients, to anticipate the deterioration of patients at higher risk for complications. On a therapeutic level, plasma from normal O blood group individuals might potentially replace the use of convalescent serum for the treatment of COVID-19.
Keywords: ABO blood group, Obesity, COVID-19, Immune dysregulation, Microbiota, Hypercoagulability
Abbreviations
- ACE2 receptor
Angiotensin Converting Enzyme 2 receptor
- AF
Atrial Fibrillation
- CD
Crohn’s Disease
- CRP
C-reactive protein
- CK-MB
Creatine Kinase–Myocardial band
DVT
- FMF
Familial Mediterranean Fever
- FUT2
Fucosyltransferase 2
- IFX
Infliximab
- IHD
Ischemic heart disease
- INR
International normalized ratio
- 2-MeSAMP
2 Methyl adenosine 5′monophosphate
- MS
Multiple Sclerosis
- PCI
Percutaneous Coronary Intervention
- PE
Pulmonary embolism
- vWF
Von Willibrand factor
- RA
Rheumatoid Arthritis
- SARS-CoV-2
Severe acute respiratory distress syndrome- Corona virus2
- SLE
Systemic Lupus Erythematosus
- SjS
Sjögren’s syndrome (SjS)
- SSc
Systemic Sclerosis
- T1DM
Type 1 Diabetes Mellitus
- WHO
World Health Organization
Background
Coronavirus Disease 2019 (COVID-19), is currently one of the worst pandemics reported after the Spanish flu of 1918, infecting over 31 million people and nearly one million dead as of September the 22nd, 2020. Severe Acute Respiratory syndrome Coronaviridae 2 (SARS-CoV-2), the culprit of COVID-19, is highly virulent and transmitted via droplet infection, resulting in an acute form of respiratory distress. It was first discovered in the city of Wuhan, China back in December 2019, and due to its rapid transmission, it spread to many countries, and by March, 11 2020, the World Health Organization (WHO) declared it as a global pandemic [1].
Patients with SARS-CoV-2 infection present with a wide range of symptoms. Most patients seem to have a mild form of the disease, and about 20% progress to severe disease, including pneumonia, respiratory failure, and even death [2].
This wide range of manifestations, and the unpredictable course of the illness has put significant pressure on healthcare systems worldwide. At these times of fear and uncertainty, members of the public have sought medical care with even mild symptoms for fear of developing the lethal forms of the disease.
Hypothesis
The use of affordable and rapid screening tests to help predict the severity of the disease and the occurrence of complications prior to their development, can be an efficient strategy to reduce the pressure on healthcare facilities and personnel worldwide. To date, many studies have been launched to evaluate the value of various serum markers in the prediction of COVID-19 severity. Such studies ranged from the use of simple affordable tests such as C-reactive protein (CRP) [3] Ferritin [4], and D-Dimer [5] to more expensive biomarkers, such as Interleukin 6 and single cell sequencing.
We hypothesize that ABO blood grouping system seems to be an interesting target to this strategy, being a very rapid and cheap test, and in some countries, it is readily available in the identification documents of each person [6].
Evaluation of the hypothesis
The idea of using blood groups to measure hosts susceptibility to infectious diseases is not a novel strategy, and has been previously proposed by Cooling [7].
There is conflicting data regarding COVID-19. To date, there are three published studies discussing the association between blood groups and disease severity/positivity including Zhao et al. [8], who suggested that blood type A is associated with the worst outcome, while blood type O is associated with mild symptoms. In his cohort, 75% of the deceased patients were non-O individuals while only 25% belonged to group O.
Latz and colleagues have shown in their series a link between ABO blood groups and host susceptibility to COVID-19 rather than a real effect of blood grouping on disease severity [9], [10].
They studied 7648 COVID-19 positive patients, among which only 40% belonged to type O blood group compared to 60% of non-O individuals. Their multivariate analysis revealed a statistically significant negative correlation between O blood group and testing positive for COVID-19 patients with an adjusted odds ratio of 0.84 [9].
Another interesting finding converging with our findings is the proportion of O blood group individuals to non-O blood group individuals across countries. A simple visual observation, shows that countries heavily struck by COVD-19 deaths such as Italy, United States of America, Brazil, and Spain all shared a percentage of group O individuals lower than 40% of the population. While countries showing relatively less COVID-19 mortality such as Saudi Arabia, Egypt, and Singapore all had a percentage of O blood group individuals greater than 40% [11], [12].
The aim of this article is to provide insights about the possible link between ABO blood groups and COVID-19 susceptibility, and the development of severe complications.
I-Host susceptibility
As mentioned earlier, Latz and colleagues recently demonstrated that type O individuals have a reduced likelihood of testing positive for COVID-19 infection. In this section we are trying to elucidate the protective mechanisms by which type O individuals are shielded against COVID-19.
Anti-A antibodies can neutralize SARS-CoV
Cheng et al. [13] was the first to describe an interesting negative relationship between blood type O and a reduced likelihood of SARS-1 in 2005. Guillon et al. [14] proved in his study two interesting findings; (1) virion particles replicating in epithelial cells of the respiratory tract in blood type A or B individuals are covered with A or B antigens, making the shed viral particles easily recognized by type O individuals harboring both anti-A and anti B antibodies in their sera, and (2) the similar configuration found between the A antigen and parts of the Angiotensin Converting enzyme 2 (ACE2) receptor, which is the primary site of entry for the virus into the body, and how this similarity might render Anti-A antibodies circulating in type O individuals able to prevent the binding and subsequent cellular entry of the virion into the cells.
A similar hypothesis was suggested by Silva-Filho and colleagues, they suggested that A antigen promotes the secretion of sialosides that facilitates the interaction between SARS-CoV-2 and body cells, while the lack of A and B antigens prevents the development of sialosides [15].
Furin and ABO blood groups
Furin is a cellular enzyme pertaining to the category of proprotein convertases. Furin is required for the cleavage-activation of many microbial agents. Specifically, some viruses harboring multi-basic cleavage sites in their protein sequence can be activated canonically with Furin. AbdelMassih and colleagues underlined the importance of Furin as a determinant of endothelial pathogenicity of SARS-CoV-2 [16]. Furin has gained interest in the past years as an important core player of metabolic syndrome, and little if no studies have explored its levels according to ABO blood groups. However, Li et al described a negative relationship between blood type O and the level of Furin-related proprotein convertases. This might signify that, Furin levels may follow a similar pattern, being reduced in blood type O individuals; leading to marked reduction of viral load and pathogenicity of type O individuals [17].
II-Increased manifestations of COVID-19 in non-O individuals
Despite the apparent contradiction between Zhao et al series and Fan and colleagues [18], there might be a reasonable merge between their findings. Fan and colleagues all concluded that patients presenting to hospitals were more likely to test positive if non-O blood group, while Zhao claimed that the disease severity in itself is reduced in type O individuals. People presenting to hospitals are usually driven by symptoms. So, confirming that symptomatic individuals that are mainly testing are non-O blood group goes in agreement with Zhao et al who stated that O type individuals are less likely to develop severe manifestations [9], [10].
AbdelMassih and colleagues underlined the importance of immune dysregulation, vascular inflammation, and thrombosis in the pathogenesis of COVID-19. Upregulated inflammation and prothrombotic tendency are an important predisposing factor for development of COVID-19 complications.
In this section, we are trying to determine if ABO blood grouping system is linked to upregulated inflammation or autoimmunity, and if a certain blood group is associated with increased prothrombotic tendency. Finally, yet importantly we are trying to elucidate if a variation of intestinal microbiota exists in different ABO blood groups, and if this variation increases the likelihood for augmented inflammation.
ACE-ABO-COVID-19 axis and increased cardiovascular risk
Dai was the first to try to elucidate why blood group O carriers tend to have better outcome when infected with COVID-19. His hypothesis relied mainlyon the relationship of O blood group to ACE2 tissue expression. Hypertension is the leading cause of mortality and represents the most important risk factor for the development of cardiovascular diseases. Hypertensive patients typically have over-elevated ACE (Angiotensin Converting Enzyme)/ANGII (Angiotensin II) axis, in which ACE positively regulates the level of angiotensin II (ANGII) in the renin–angiotensin–aldosterone system (RAS). ACE 2 is the entry receptor of SARS-CoV-2 into the cells and is one of the key regulators of tissue tropism of COVID-19. Higher levels of ACE2 were positively associated with heavier intracellular load of SARS-CoV-2, and linked to worst outcomes including ventilation, myocarditis, and endotheliitis. It was reported that the ABO blood group is associated with ACE activity and ACE inhibitor-induced cough among Chinese patients with essential hypertension. That is, the GATC haplotype of the four polymorphisms of the ABO gene (rs8176746, rs8176740, rs495828, rs12683493), which is prevalent among non-O blood type patients, is positively associated with ACE activity. Thereby, O blood type carriers should have lower ACE2 levels, which can offer an additional explanation to the milder manifestations of COVID-19 in blood group O carriers [19].
Autoimmunity and ABO system
The occurrence of autoimmune diseases is theorized to be due to the interplay between various genetic and environmental risk factors. Within the literature, there appears to be a relationship between the ABO blood groups and several autoimmune diseases. This association, examined through frequencies, disease severity, risk factor, is significant enough to consider the ABO blood groups a clinical parameter for performing genetic testing for these diseases. Moreover, an association exists between ABO groups and inflammatory processes evidenced by interactions unique to blood group antigens, which affect host-pathogen interactions, as well as disease susceptibility between individuals. This is evidenced by studies that have revealed that circulating glycoprotein levels, that are critical in endothelial function and inflammation, are majorly determined by ABO blood type [20]. Evidence suggests the possibility that ABO blood groups may influence the prognosis of some diseases by affecting the inflammatory status of its patients. A predilection of blood type A to more inflammatory end points appears to be common.
Interestingly some autoimmune diseases show a pattern of ABO association similar to that found in COVID patients.
Type 1 diabetes mellitus
In a cohort prospective study performed by Parente et al, examining ischemic heart disease events in 4531 Type 1 diabetes mellitus (DM) patients with diabetic nephropathy status (microalbuminuria) also implicated Type A blood. In their study, Type A blood was associated with the highest risk of Ischemic Heart disease (IHD) events (Hazard Ratio (HR) 1.94, Confidence Interval (CI) 95% 1.41–2.67, p < 000.1) compared to type O. Levels of high sensitivity C-reactive protein and the number of antibiotic purchases per year were significantly higher in the type A group. This suggests some sort of inflammatory/infectious mediator which links the type A blood association to certain outcomes [21]. Additionally, in a study by Oner et al, Type A blood was the most dominant genotype in all diabetic groups in both genders, while type AB was significantly more frequent among T1DM [22].
Rheumatic diseases
Within the range of rheumatic diseases, a retrospective study conducted by Çildağ et al showed that 42.5% of patients belonged to group A type, whereas 33.2% belonged to group O type. Blood type A patients were more likely to suffer from spondylo-arthropathy, undifferentiated connective tissue disease, Bechet’s disease, vasculitis, and Rheumatoid Arthritis while, familial Mediterranean fever (FMF), SLE, systemic sclerosis (SSc), and Sjögren’s syndrome (SjS) were more prevalent in blood type O. RA and Ankylosing Spondylitis with erosive arthritis are the most common rheumatic disorders, and were highest in the type A group [23]. A prospective study by Tamega et al, also showed that disseminated clinical forms of discoid lupus erythematous were more common in Type A blood (p < 0.05) [24].
Crohn's disease
The Fructosyltransferases 2 (FUT2) gene has been linked to the ABO antigens, as well as being a known locus for Crohn's Disease (CD). A study conducted on CD patients revealed that those who lacked the FUT2 gene and were non-O blood group were less protected than carriers of the secretor Type O blood group [25].
Likewise, another study showed an Odds Ratio >1 for all non-type O blood types indicating a predisposition for CD. Non type O non-secretor status was associated with a higher risk of complications including penetrating disease, and structures [26].
Most interestingly, is the finding from a cohort study which demonstrated ABO variations in CD patients and their response to Infliximab (IFX) treatment. Of the 197 patients who received Infliximab (IFX) treatment for one year, a significant correlation was found where blood type A had the highest risk of losing response to therapy [27].
Psoriasis
A link was found between blood groups and different triggers of Psoriasis activity. Of 683 patients a significant difference in the initiating trigger for psoriasis exists between the blood groups, where Type A group was triggered by infection (49.0%), while stress factor was triggering for O blood [28].
Multiple sclerosis
Multiple Sclerosis (MS) and ABO blood group association is proposed by several studies. Most studies point to the protective factor of Type O, and risk factor for non-Type O. A study in Basque showed a protective value of blood group O in MS compared to the presence of A, B, or Rh+ groups, which seemed to be a risk factor in developing MS [29].
Increased prothrombotic tendency in non-O individuals
Recent literature has linked the severity of COVID-19 to a state of hypercoagulability supported by the increased risk of thromboembolic events in severely ill patients. In this section, we will be presenting the available data regarding the association between blood type A, and a state of hypercoagulability, there by linking blood group A to the severity of COVID-19.
Ionesco et al. was the first to describe the relationship between blood groups and strokes, stating that in thrombotic cases there was a prevalence of blood-groups A and AB, and a deficiency of O and B, suggesting a correlation between the A1 allele and thrombophilia [30]. Later on, it was proved by a considerable number of studies that there is a higher incidence of both venous and arterial thromboembolic events in subjects with non-O blood groups compared to those with blood group O in the form of venous thromboembolism, deep vein thrombosis (DVT), and pulmonary embolism (PE) [30], [31], [32], [33], [34], [35]. Interestingly, a specific study showed that for atrial fibrillation (AF) and Deep Venous Thrombosis (DVT) patients, a higher warfarin dose was required (target INR 2–3) for patients in the A, B and AB blood groups than those in the O blood group (2.74 mg versus 3.19 mg, 3.32 mg and 3.14 mg, respectively, P < 0.05). However, there was no significant difference in requirements among the A, B, and AB blood groups [36].
A study done by Timur et al., showed a robust correlation between blood group A and the risk of myocardial infarction [Odds ratio (OR) = 2.50, 95% CI = 1.37–4.58, P = 0.003], higher baseline troponin T and Creatinine Kinase (CK-MB) index, post-Percutaneous Coronary Intervention (PCI) CK-MB index, platelet reactivity index (PRI), and being a poor responder against 2 Methyl adenosine 5′monophosphate (2-MeSAMP) (OR = 5.75, 95% CI = 1.51–21.90, P = 0.010) [37]. Moreover, another study showed that a significantly higher degree of atherosclerosis, calculated using the Gensini score, is associated with blood group A. The same study used multivariate linear regression to show an association between blood type A and the severity of coronary artery disease (β = 3.298, 95% CI: 0.91–6.505, P = 0.044). Diabetic subjects with blood group A were also associated with increased Gensini score (P = 0.02) [38].
The association between the different blood groups, and a state of hypercoagulability could be attributed to the higher plasma level of factor VIII and von Willebrand factor (vWF), and lower levels of vWF-cleaving protease (ADAMST13) in subjects with non-O blood group compared to those with blood type O. Also the proteolysis of VWF by ADAMTS13 seems to be faster in subjects of the O blood groups than in subjects with non-O blood group [34], [39]. Last but not least, few studies argued that the association is between the A allele of the genotype, rather than the A phenotype of the blood group and thrombophilia [30], [34].
In view of the above, we suggest that blood groups may act as a risk factor for developing thromboembolic events, and thus could be used for risk assessment in COVID-19
Intestinal microbiota and blood groups
The human intestinal tract possesses a complex and divergent microbial community which plays a pivotal role in human health, namely gut microbiota [40]. More than 90% of the gut microbiota belong to Firmicutes (e.g. Clostridium, Lactobacillus, and Bacillus), Bacteroidetes (e.g. Bacteroides), Proteobacteria (e.g. Escherichia), and Actinobacteria (e.g. Bifidobacterium). Gut microbiota is engaged in basic human biological processes including regulation of innate immunity and epithelial development [41]. Diversity in the gut microbiota decreases during aging and microbiome composition is altered to an imbalance state, so called dysbiosis, which leads to immune dysfunction and generalized inflammation [42]. Dysbiosis has been linked to various chronic conditions such as asthma, arthritis, type 2 diabetes, obesity, inflammatory bowel disease, atherosclerosis, and alcoholic liver disease [43].
Interestingly, a correlation between the composition of the intestinal mucosal microbiota and the ABO blood type of the host was demonstrated by Mäkivuokko et al., who concluded that the ABO blood grouping is associated with the variations in relative proportion and general profiles of intestinal microbiota [44]. In this study, blood group A individuals exhibited a higher %G (Guanosine) + C (Cytosine) microbes, dominated by Actinobacteria when compared to other blood groups. Actinobacteria are a group of Gram-positive bacteria with high guanine and cytosine content in their Deoxyribonucleic acid (DNA) [45]. Actinobacteria are reported to be increased in chronic inflammatory conditions as, in Crohn’s disease and ulcerative colitis [46].
Notably, another study showed that Blautia was lower in the group A-secretors compared with the non-A-secretors, (O and B) and the highest abundance in O-secretors [47]. Blautia is a species of anaerobic, gram-positive bacteria found in the gut. They showed a beneficial anti-inflammatory role, along with other relevant Taxa, in the recovery process from Vibrio cholerae infection and microbiota maturation in children [48]. In addition, the Blautia genus was decreased in irritable bowel syndrome, nonalcoholic fatty liver diseases, Crohn's disease, and diabetes. This might relate to the favorable prognosis in COVID-19 among type O individuals and the poor prognosis in Blood type A.
The gut microbiota influenced the course of COVID-19 owing to its bidirectional association with the lung and immune system. Angiotensin Converting Enzyme 2 (ACE2) receptors are highly expressed in the gastrointestinal epithelium [48]. SARS-CoV-2 may interfere with nutrient absorption by binding to ACE2 receptors, causing gastroenteritis-like symptoms and disrupting intestinal homeostasis. Dysbiosis in gut microbiota results in gut leakage leading to secondary infection and systemic inflammation. Interchangeably, dysbiosis disrupts the gut barrier integrity, which may accelerate viral dissemination of SARS-CoV-2 from the lung into the intestinal lumen via the circulatory and lymphatic system [49].
In a recent study on alteration of gut microbiome, COVID-19 patients had; a significantly reduced bacterial diversity, a significantly higher relative abundance of opportunistic pathogens, such as Streptococcus, Rothia, Veillonella, and Actinomyces, and a lower relative abundance of beneficial symbionts. The gut microbial signature of patients with COVID-19 was different from that of healthy controls. Moreover, CRP and D-dimer levels showed direct correlation with COVID-19 enriched bacteria (Streptococcus, Rothia, Veillonella, and Actinomyces) [50].
These evidences indicate a possible relationship between blood group antigens, dysbiosis in the GIT microbiome, and COVID-19.
III-ABO grouping system and affected subgroups in COVID-19
It is currently established that certain subgroups of individuals are at a higher risk of developing COVID-19, especially those suffering from metabolic syndrome. Several theories have been suggested to explain the relative higher risk of COVID-19 complications in such subgroups, however, the theory of upregulated inflammation in both clusters is the most widely accepted to date. In this section we present an overview of the distribution of ABO blood groups in such subgroup.
Data regarding the association between ABO blood groups and metabolic syndrome are conflicting. A study done be Ahmed and colleagues [51] reported an increased prevalence of obesity in non-O individuals, while Smith et al (Smith et al., 2018) proved the exact opposite in his series. Awasaidi and colleagues reported no association between body mass index and the ABO system [52]. Despite these conflicting data, vascular inflammatory complications of obesity are definitely more abundant in non-O individuals. As mentioned earlier, Hong et al. [38] reported a higher incidence of coronary artery diseases in non-O subjects. Nevertheless, Type 2 diabetes, is linked to blood group A, with markedly reduced likelihood in blood type O individuals [53].
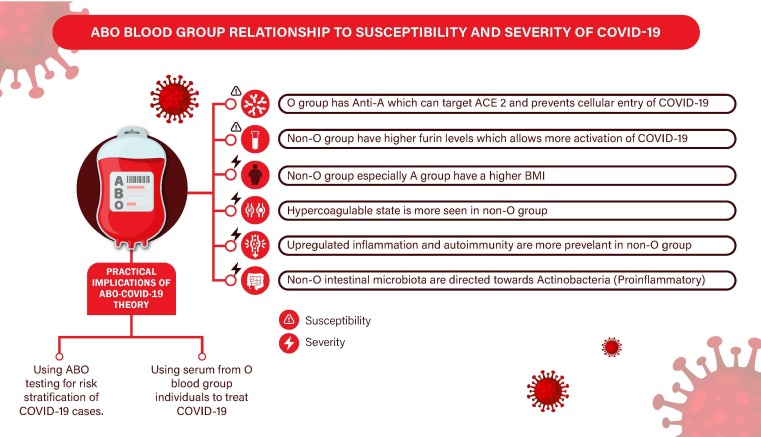
Fig. 1 summarizes the above mentioned mechanisms linking Non-O blood type o increased susceptibility and severity of COVID-19.
Fig. 1.
ABO blood group relationship to susceptibility and severity of COVID-19. Abbreviations: ACE2: Angiotensin Converting Enzyme 2, BMI: Body Mass Index, COVID-19 (Coronavirus disease 2019).
Practical implications
Main practical implication: using ABO blood group for risk stratification of COVID-19 cases
ABO blood grouping is a cheap and affordable test. Clustered data from the current outbreak and from former outbreaks by coronaviridae affirms a positive correlation between Non-O blood group particularly blood type A and both infection susceptibility and severity of complications. Such findings go in agreement with many pathogenic mechanisms linked to non-O blood groups such as immune dysregulation, symbiosis, and increased prothrombotic state. The same prevalence is shared by metabolic syndrome, related complications, and elderly individuals. Using ABO blood grouping for risk stratification and determination of patients in need for close follow up should be combined with other previously mentioned stratification markers, whether clinical such as the age, diabetes and obesity, or laboratory such as CRP, ferritin, and IL-6. Also the role of ABO blood group as an initial stratification criterion should be first confirmed by cohort multicenter studies to confirm and refute its usefulness.
Secondary practical implication: Using plasma from O individuals to treat COVID-19
Passive antibody therapy involves the administration of antibodies against a given agent to a susceptible individual for the purpose of preventing or treating an infectious disease due to that agent. The use of human convalescent sera for blocking SARS-CoV-2 entry into the human cells has been proposed and applied in many countries as a treatment for COVID-19. However, one of the most important barriers of such strategy was the availability of donors who have recovered and developed a good titer of Anti-SARS-CoV-2 antibodies [54].
As mentioned earlier, Guillon and colleagues proved in 2008 that anti-A antibodies in the sera of normal blood group O individuals can block ACE-2 receptors. This effect would fulfill the same biologic effect of human convalescent serum, preventing cellular entry of SARS-CoV-2 [14].
Raising this therapeutic hypothesis, is being confronted by the fact that if serum of Type O individuals is therapeutically effective, why Group O individuals still contract the disease? Two major factors are to be considered to be able to answer this question:
-
–
Is the level of anti-A antibodies uniform in all Type O individuals? Intriguingly, the level of anti-A antibodies is not constant. De França and colleagues described the presence of multiple environmental predictors of anti-A antibodies according to age, sex, racial background [55]. Saphire et al demonstrated a decline in the level of Anti-A antibodies with aging [56]. De França et al demonstrated low titer of Anti-A antibodies in sera of males above 50 compared to females. The latter two findings add additional explanation to the already present body of evidences about the susceptibility of males and old aged individuals to COVID-19 [56].
-
–
Is the type of Anti-A antibodies uniform across all individuals and in the serum of B type individuals vs. O type individuals? Gerard et al outlined in a short communication, the protective effect O blood group compared to B blood group against COVID-19. They found out that there is statistically significant difference between B and O individuals in the infection rates of COVID-19. This difference was attributed not only to the higher level of Anti-A antibodies in the serum of O group individuals, but also to a the phenotypic difference between Anti-A antibodies in type O individuals compared to Type B persons. Anti-A antibodies in group O individuals is mainly of the Ig G type, while in type B persons it is mainly Ig M. Ig M is mainly confined to the blood due its large molecular weight, however Ig G can easily operate in different body fluids and occasionally on epithelial surfaces. The epithelial surface being the most important checkpoint for viral entry in the upper respiratory tract [57].
Therefore applying our therapeutic hypothesis has some limitations, serum can solely be used in Type O individuals, and only after ensuring enough of a titer of Anti-Antibodies that can convey protection to recipients. This hypothesis should finally be taken into consideration carefully, and should be confirmed or refuted through robust clinical trials before applying it.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I want to thank the founders and advisors of Research Accessibilility Team, a program that we started in September 2019 to allow students to learn scientific writing and Research. I wanted also to thank the families of my co-authors, who have beautifully raised them. We also want to thank all the medical staff all around the world, whose sacrifices have saved many lives to their dear ones.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110343.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Mahase E. Covid-19: WHO declares pandemic because of ‘alarming levels’ of spread, severity, and inaction. BMJ. 2020;368 doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 2.Kim S.Y., Kim D.W. Does the clinical spectrum of coronavirus disease 2019 (COVID-19) show regional differences? Clin Exp Otorhinolaryngol. 2020;13:83–84. doi: 10.21053/ceo.2020.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L. C-reactive protein levels in the early stage of COVID-19. Médecine Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas-Vargas M., Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Pública. 2020;44:1. doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long H. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed Res Int. 2020;2020 doi: 10.1155/2020/6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentas F.J., Clark P.A. Blood type discrepancies on military identification cards and tags: a readiness concern in the U.S. Army. Mil Med. 1999;164:785–787. [PubMed] [Google Scholar]
- 7.Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. 1–18; 2020. doi:10.1101/2020.03.11.20031096.
- 9.Latz C.A. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020 doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zietz M., Tatonetti N. Testing the association between blood type and COVID-19 infection, intubation, and death. medRxiv. 2020 doi: 10.1101/2020.04.08.20058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garratty G., Glynn S.A., McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ ethnic groups in the United States. Transfusion. 2004;44:703–706. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal A. ABO and Rh (D) group distribution and gene frequency; the first multicentric study in India. Asian J Transfus Sci. 2014;8:121. doi: 10.4103/0973-6247.137452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1447. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 14.Guillon P. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva-Filho J.C., de Melo C.G.F., de Oliveira J.L. The influence of ABO blood groups on COVID-19 susceptibility and severity: a molecular hypothesis based on carbohydrate-carbohydrate interactions. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AbdelMassih A.F. A multicenter consensus: a role of furin in the endothelial tropism in obese patients with COVID-19 infection. Obes Med. 2020 doi: 10.1016/j.obmed.2020.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S. ABO blood group in relation to plasma lipids and proprotein convertase subtilisin/kexin type 9. Nutr Metab Cardiovasc Dis. 2015;25:411–417. doi: 10.1016/j.numecd.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Fan Q. Association between ABO blood group system and COVID-19 susceptibility in Wuhan. Front Cell Infect Microbiol. 2020;10:1–7. doi: 10.3389/fcimb.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol. 2020;27:1436–1437. doi: 10.1177/2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M. ABO blood type is associated with renal outcomes in patients with IgA nephropathy. Oncotarget. 2017;8(43) doi: 10.18632/oncotarget.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parente E.B. Relationship between ABO blood groups and cardiovascular disease in type 1 diabetes according to diabetic nephropathy status. Cardiovasc Diabetol. 2020;19:68. doi: 10.1186/s12933-020-01038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oner C., Dogan B., Telatar B., Celik Yagan C.F., Oguz A. Frequency of ABO/rhesus blood groups in patients with diabetes mellitus. J Coll Physicians Surg Pak. 2016;26:74–75. [PubMed] [Google Scholar]
- 23.Cildag S., Kara Y., Senturk T. ABO blood groups and rheumatic diseases. Eur J Rheumatol. 2017;4:250–253. doi: 10.5152/eurjrheum.2017.17044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de A. Tamega A., da S.P. Bezerra L.V.G., de P. Pereira F., Miot H.A. Blood groups and discoid lupus erythematosus. An Bras Dermatol. 2009;84:477–481. doi: 10.1590/s0365-05962009000500005. [DOI] [PubMed] [Google Scholar]
- 25.Ye B.D. Association of FUT2 and ABO with Crohn’s disease in Koreans. J Gastroenterol Hepatol. 2020;35:104–109. doi: 10.1111/jgh.14766. [DOI] [PubMed] [Google Scholar]
- 26.Forni D. ABO histo-blood group might modulate predisposition to Crohn’s disease and affect disease behavior. J Crohns Colitis. 2014;8:489–494. doi: 10.1016/j.crohns.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Yu Q. The role of ABO blood groups in Crohn’s disease and in monitoring response to infliximab treatment. Blood Transfus. 2016;14:460–464. doi: 10.2450/2016.0199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirant M. Therapeutic and etiologic considerations related to blood group and triggers in psoriasis-A retrospective study. Dermatol Ther. 2020;33 doi: 10.1111/dth.13401. [DOI] [PubMed] [Google Scholar]
- 29.Lopetegi I, et al. ABO blood group distributions in multiple sclerosis patients from Basque Country; O(-) as a protective factor. Mult Scler J 5; 2019, 2055217319888957–2055217319888957. [DOI] [PMC free article] [PubMed]
- 30.Ionescu D.A., Bicescu E., Marcu I. Cerebral thrombosis, cerebral hæmorrhage and abo blood-groups. Lancet. 1976;307:278–280. doi: 10.1016/s0140-6736(76)91405-7. [DOI] [PubMed] [Google Scholar]
- 31.Sun X., Feng J., Wu W., Peng M., Shi J. ABO blood types associated with the risk of venous thromboembolism in Han Chinese people: a hospital-based study of 200,000 patients. Sci Rep. 2017;7:42925. doi: 10.1038/srep42925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasan S.K. ABO blood group and risk of thromboembolic and arterial disease. Circulation. 2016;133:1449–1457. doi: 10.1161/CIRCULATIONAHA.115.017563. [DOI] [PubMed] [Google Scholar]
- 33.Tirado I. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93:468–474. doi: 10.1160/TH04-04-0251. [DOI] [PubMed] [Google Scholar]
- 34.Schleef M. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128:100–107. doi: 10.1111/j.1365-2141.2004.05249.x. [DOI] [PubMed] [Google Scholar]
- 35.Shusterman M., Golub E., Mowrey W.B., Broder A. The association between ABO blood types and venous thromboembolism in individuals with a positive antiphospholipid profile is varied by sex. Lupus. 2018;27:319–326. doi: 10.1177/0961203317721352. [DOI] [PubMed] [Google Scholar]
- 36.Zou S. Effect of ABO blood groups on the response to warfarin. Am J Med Sci. 2020;360:50–54. doi: 10.1016/j.amjms.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Timur A.A. The relation between ABO blood types and clinical and platelet function parameters in patients who underwent percutaneous coronary intervention. Coron Artery Dis. 2019;30:51–58. doi: 10.1097/MCA.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 38.Hong X.L. Association of ABO blood groups with the severity of coronary artery disease: a cross-sectional study. J Geriatr Cardiol. 2019 doi: 10.11909/j.issn.1671-5411.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rios D.R.A. Relationship between ABO blood groups and von Willebrand factor, ADAMTS13 and factor VIII in patients undergoing hemodialysis. J Thromb Thrombolysis. 2012;33:416–421. doi: 10.1007/s11239-012-0719-5. [DOI] [PubMed] [Google Scholar]
- 40.Guinane C.M., Cotter P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinninella E. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aleman F.D.D., Valenzano D.R. Microbiome evolution during host aging. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb Ecol Heal Dis. 2015;26 doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mäkivuokko H. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012;12:94. doi: 10.1186/1471-2180-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barka E.A. Taxonomy, physiology, and natural products of actinobacteria. Microbiol Mol Biol Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D., Zeng M.Y., Núñez G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med. 2017;49 doi: 10.1038/emm.2017.24. e339–e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gampa A., Engen P.A., Shobar R., Mutlu E.A. Relationships between gastrointestinal microbiota and blood group antigens. Physiol Genomics. 2017;49:473–483. doi: 10.1152/physiolgenomics.00043.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang L. Analysis of microbiota in elderly patients with Acute Cerebral Infarction. PeerJ. 2019;7 doi: 10.7717/peerj.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aktas B., Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol. 2020;44:265–272. doi: 10.3906/biy-2005-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu S. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed H.O. Association of ABO group types to overweight and obesity: Based on six years of experience in two centers in Sulaimani governorate, Kurdistan Region/Iraq. Obes Med. 2019;13:21–25. [Google Scholar]
- 52.Alwasaidi T.A. Relation between ABO blood groups and obesity in a Saudi Arabian population. J Taibah Univ Med Sci. 2017;12:407–411. doi: 10.1016/j.jtumed.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meo S.A., Rouq F.A., Suraya F., Zaidi S.Z. Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2016;20:237–242. [PubMed] [Google Scholar]
- 54.Rajendran K. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020;0–3 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de França N.D.G., Poli M.C.C., de A. Ramos P.G., da Borsoi C.S., Colella R. Titers of ABO antibodies in group O blood donors. Rev Bras Hematol Hemoter. 2011;33:259–262. doi: 10.5581/1516-8484.20110073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saphire D.G., Rudolph N.S., Hackleman S.M., Stone W.H. The effect of age on the level of human ABO blood group antibodies. Aging Clin Exp Res. 1993;5:177–184. doi: 10.1007/BF03324152. [DOI] [PubMed] [Google Scholar]
- 57.Gérard C., Maggipinto G., Minon J. COVID-19 and ABO blood group: another viewpoint. Br J Haematol. 2020;190 doi: 10.1111/bjh.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.