The methods of fecal microbiota transplantation (FMT) and the related results vary a lot among centers worldwide. Therefore, methodologic standardization can improve the clinical practice and trials about FMT. A panel of 28 experts from 22 hospitals or institutes in 15 cities has contributed to the present consensus on washed microbiota transplantation (WMT).[1] This consensus provides guidance on the methodology of WMT, which is different from that of the manually FMT in recent experts’ consensus or recommendations.[2,3] All experts were assigned, according to their expertise, to the following five groups: Group 1, donor screening; Group 2, washing microbiota protocol, storing, and transport; Group 3, patient preparation; Group 4, delivery decision; Group 5, safety and management. Each statement and the following comment have passed a Delphi process and a face-to-face plenary meeting. The best available evidence was assessed according to the Grading of Recommendations Assessment, Development and Evaluation system.
Consensus was reached for all 31 statements after three rounds of anonymous voting and editing. Respectively, 100% of statements passed the 80% agreement threshold after the first round, 100% after the second round, and 100% after the third round. In the final round, all statements were presented to 28 of 28 (100%) panel members on December 12, 2019 in Nanjing, China, for a face-to-face meeting. The consensus report is shown in Table 1.
Table 1.
Statements released by Nanjing consensus on the methodology of washed microbiota transplantation.
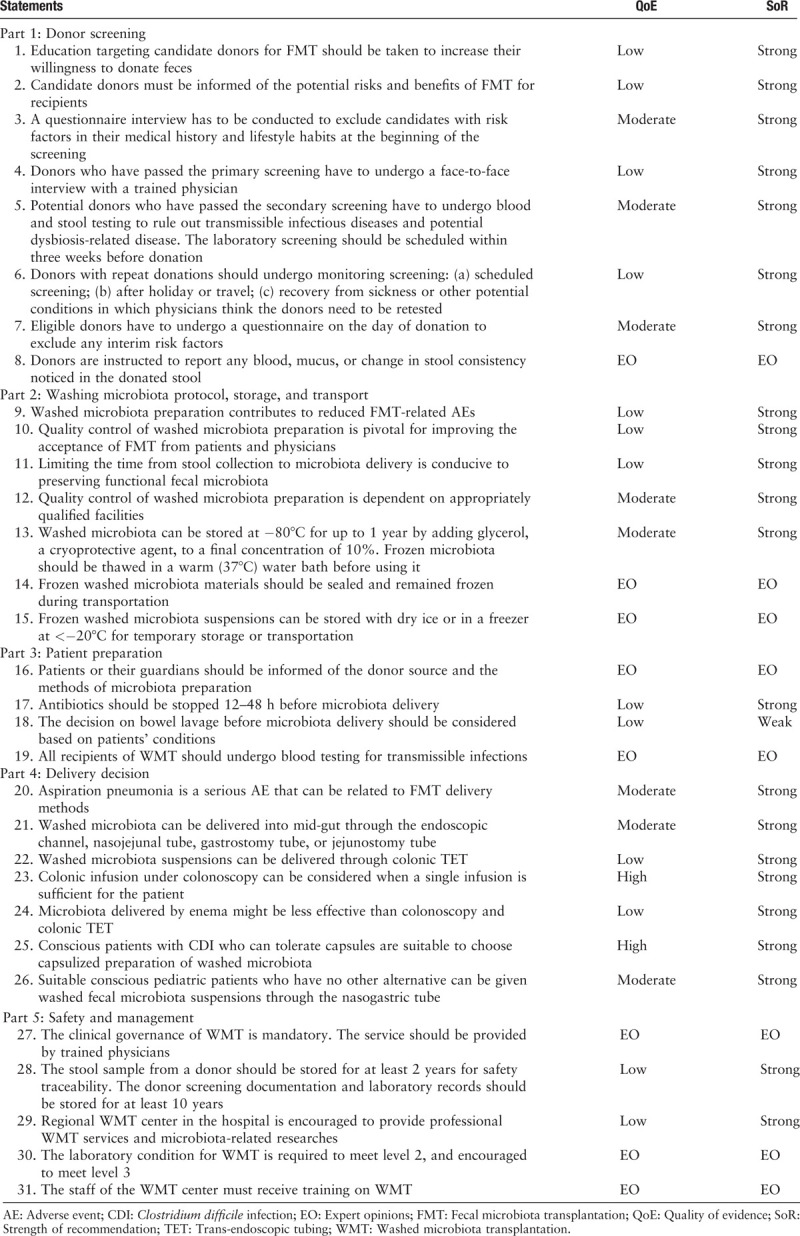
The methods of the consensus development process, each statement and the related quality of evidence, strength of recommendation/expert opinions, and comments with evidence are shown in Supplementary File 1. The brief laboratory methods for the preparation of washed microbiota suspensions are shown in Table 2.
Table 2.
General steps for the preparation of washed microbiota suspensions.

Contributors: Fa-Ming Zhang conceived, organized, and developed the project. Siew Chien Ng, Xing-Xiang He, Deng-Chyang Wu, Kai-Chun Wu, and Cheng-Tang Chiu chaired the working group 1, group 2, group 3, group 4, and group 5, respectively. All panel members joined the consensus development and manuscript revision, and approved the final manuscript.
Experts of FMT-standardization Study Group: Qing Cao, Shanghai Children's Medical Center; Paul Kay Sheung Chan, Chinese University of Hong Kong; Cheng-Hsun Chiu, Chang Gung Memorial Hospital; Cheng-Tang Chiu, Chang Gung Memorial Hospital; Bo-Ta Cui, Second Affiliated Hospital of Nanjing Medical University; Ming-Ming Deng, Affiliated Hospital of Southwest Medical University; Ying Fang, Children's Hospital of Xi’an City; Bai-Sui Feng, Second Affiliated Hospital of Zhengzhou University; Xing-Xiang He, First Affiliated Hospital of Guangdong Pharmaceutical University; Wen-Hung Hsu, Kaohsiung Medical University Hospital; Yun-Lian Hu, Hubei Hospital of Traditional Chinese Medicine; Jing-Nan Li, Peking Union Medical College Hospital; Yi Li, Sir Run Run Hospital of Nanjing Medical University; Yu Liu, Sir Run Run Hospital of Nanjing Medical University; Ying-Lei Miao, First Affiliated Hospital of Kunming Medical University; Siew Chien Ng, Chinese University of Hong Kong; Yong-Zhan Nie, Xijing Hospital of Digestive Diseases; Jun Wang, Chinese Academy of Sciences; Jun-Ping Wang, Shanxi Provincial People's Hospital; Deng-Chyang Wu, Kaohsiung Medical University; Kai-Chun Wu, Xijing Hospital of Digestive Diseases; Fang Xiao, Tongji Hospital; Shao-Qi Yang, General Hospital of Ningxia Medical University; Yong Yu, Fifth Affiliated Hospital of Zhengzhou University; Fa-Ming Zhang, Second Affiliated Hospital of Nanjing Medical University; Xiao-Yin Zhang, Third People's Hospital of Shenzhen; Yong-Jian Zhou, Guangzhou First People's Hospital; Yin Zhu, First Affiliated Hospital of Nanchang University.
Funding
The work was supported by grants from the Jiangsu Province Creation Team and Leading Talents Project (No. 2017-1-57) and the National Clinical Research Center for Digestive Diseases, Xi’an, China (No. 2015BAI13B07).
Conflicts of interest
Fa-Ming Zhang conceived the concept of GenFMTer and transendoscopic enteral tubing and devices related to them. The other authors declare no conflict of interest.
Supplementary Material
Footnotes
How to cite this article: Fecal Microbiota Transplantation-standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J 2020;133:2330–2332. doi: 10.1097/CM9.0000000000000954
References
- 1.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019; 68:2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SC, Kamm MA, Yeoh YK, Chan PKS, Zuo T, Tang W, et al. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2020; 69:83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 2020; 11:251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


