Abstract
Background
: Topoisomerase II alpha (TOP2A) has been reported to play a crucial role in the tumorigenesis of various cancer types. However, the biological role of TOP2A in gallbladder cancer (GBC) remains unknown. The current study aimed to explore the function and potential mechanism of TOP2A in GBC.
Methods
: Based on Gene Expression Profiling Interactive Analysis data, we found TOP2A was significantly up-regulated in GBC tissues and resulting in shorter overall survival. Quantitative real-time polymerase chain reaction and immunohistochemistry were conducted to detect the expression of TOP2A in 45 pairs of GBC tissues and adjacent non-tumor tissues. In vitro, cell proliferation, migration, and invasion ability were examined by cell counting kit-8 and transwell assay, respectively. Epithelial-mesenchymal transition (EMT) related and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway-related markers were measured by Western blotting. Xenograft model assay was performed to evaluate the effect of TOP2A in vivo.
Results
: TOP2A was found up-regulated in GBC (tumor vs. normal, 12.62 vs. 0.34) and correlated with the late tumor node metastasis stage (P = 0.0032), present of lymph node metastasis (P = 0.0273), and poor prognosis in GBC patients (log-rank P = 0.028). In vitro and in vivo assays showed that knockdown of TOP2A notably inhibited cell proliferation, migration, invasion, EMT process, and tumor growth in GBC. In addition, TOP2A down-regulation significantly decreased the protein levels of phosphor (p)-PI3K, p-Akt, and p-mTOR.
Conclusion
: Our study demonstrates that TOP2A was overexpressed in GBC and associated with poor prognosis in GBC patients. TOP2A promotes GBC cell proliferation, migration, invasion, EMT process, and tumor growth through activating PI3K/Akt/mTOR signaling pathway, and may serve as a novel prognostic biomarker and therapeutic target for GBC.
Keywords: Topoisomerase II alpha, Gallbladder cancer, Proliferation, Metastasis, Epithelial-mesenchymal transition, Phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathway
Introduction
There is wide geographic variability in the frequency of gallbladder cancer (GBC), which is due to the interaction between genetic factors and their alteration.[1] In general, GBC is an aggressive and often lethal malignancy with a poor prognosis, characterized by a lack of earlier symptoms and high propensity to develop and metastasize.[2] So far, surgical resection is the only potentially curative therapy for GBC; however, most patients are diagnosed in advanced stages and 40% to 75% with metastatic disease, thus missing the optimal timing of treatment.[3,4] Moreover, GBC is highly resistant to currently known chemotherapy and radiotherapy.[5] Therefore, an in-depth understanding of the molecular mechanism of GBC and finding novel diagnostic and therapeutic targets are of great importance.
DNA topoisomerase II alpha (TOP2A) gene locates on the locus q21 of chromosome 17, which codes the key nuclear enzyme TOP2A.[6] TOP2A plays an important role in the processes of DNA replication and transcription by altering the topological states of DNA.[7] It acts as a catalyst to break double-strand DNA and promotes gene transcription during mitosis.[8,9] Many studies have reported that TOP2A is involved in the tumorigenesis of several cancers. For example, in colon cancer, TOP2A was found up-regulated and served as an oncogene, promoting proliferation and invasion of colon cancer cells.[10] In pancreatic cancer, TOP2A was shown as a direct target of miR-139 and induced the malignant progression of pancreatic cancer via β-catenin signaling pathway.[11] However, there is no study so far of the biological role of TOP2A in GBC.
In our present study, we found that the expression level of TOP2A was up-regulated in GBC and was associated with poor prognosis. The role of TOP2A was studied both in vitro and in vivo, the results demonstrated that TOP2A promotes the proliferation and metastasis of GBC via phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway.
Methods
Ethical approval
We obtained informed consent from all patients and the study was approved by the Research Ethics Committee of Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University (No. XHEC-H-2020-014). The animal study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (No. XHEC-A-2020-023).
Bioinformatics analysis
TOP2A expression in GBC samples and paired normal tissues, and the overall survival plot based on TOP2A expression was obtained from Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn).
Patients and specimens
Human GBC and adjacent non-tumor tissues were obtained from 45 GBC patients after cholecystectomy, between 2014 and 2018, who had not received prior radiotherapy or chemotherapy at the Department of General Surgery, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University, China.
Immunohistochemistry (IHC)
IHC was carried out following the standard immunoperoxidase staining procedure.[12] The staining intensity was classified as follows: negative (0%–5%), weak (6%–25%), moderate (26%–50%), and strong (>51%).
Cell culture
Human GBC cell lines (NOZ, SGC-996, GBC-SD, and OCUG) and 293T cells were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in medium (Williams’ medium for NOZ, Roswell Park Memorial Institute 1640 medium for SGC-996, Dulbecco modified Eagle medium for GBC-SD, OCUG, and 293T) containing 10% fetal bovine serum (Gibco, Sydney, Australia). Cells were incubated at 37°C in a 5% CO2 humidified incubator.
Total RNA extraction and quantitative real-time PCR (qRT-PCR)
TRIzol reagent (Invitrogen, CA, USA) was used to extract the total RNA from the tissue samples and cells. PrimeScript RT reagent kit (Takara, Shanghai, China) and SYBR Green (Takara) were applied for complementary DNA synthesis and qRT-PCR, respectively. Target genes were detected on an Applied Biosystems StepOnePlus real-time thermocycler (Thermofisher, NY, USA). The primer sequences are as follows: TOP2A: 5′-ACCATTGCAGCCTGTAAATGA-3′ (forward) and 5′-GGGCGGAGCAAAATATGTTCC-3′ (reverse); phosphatidylinositol 3-kinase: 5′-CCACGACCATCATCAGGTGAA-3′ (forward) and 5′-CCTCACGGAGGCATTCTAAAGT-3′ (reverse); protein kinase B: 5′-AGCGACGTGGCTATTGTGAAG-3′ (forward) and 5′-GCCATCATTCTTGAGGAGGAAGT-3′ (reverse); mammalian target of rapamycin: 5′-ATGCTTGGAACCGGACCTG-3′ (forward) and 5′-TCTTGACTCATCTCTCGGAGTT-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase: 5′-CAACAGCCTCAAGATCATCAGC-3′ (forward) and 5′-TTCTAGACGGCAGGTCAGGTC-3′ (reverse). Relative mRNA expression levels were quantified by the 2-ΔΔCT method.
Cell transfection
TOP2A was knockdown by small interfering RNA (siRNA) using Rfect transfection reagent according to the manufacturer's protocol. TOP2A and negative control (NC) siRNA were designed and synthesized by Genomeditech (Shanghai, China). TOP2A-siRNA1 (sense: GGUCAGAUUUGUUAUUAAAUU; antisense: UUUAAUAACAAAUCUGACCUG), TOP2A-siRNA2 (sense: GCUCAGUGCUAGCACAAUAUA; antisense: UAUUGUGCUAGCACUGAGCUA). The TOP2A full-length overexpression plasmids were synthesized and purchased from Genomeditech and used ViaFect reagent for transfection following the manufacturer's instructions. Lentivirus LV-shTOP2A and LV-NC using puromycin-resistant lentivirus PGMLV-SC5 vectors were constructed by Genomeditech. Lentiviral infection was performed according to the manufacturer's instructions. Puromycin was used to construct stable-transfected cells, and the transfection efficiency was confirmed by qRT-PCR and Western blot analysis.
Western blot analysis
Protein was extracted using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) with protease and phosphatase inhibitor cocktail. Equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, MA, USA). The membranes were incubated in 5% skim milk for 1 h at room temperature, and then with primary antibodies at 4°C overnight. Primary antibodies were all purchased from Cell Signaling Technology, NY, USA. After washing, the membranes were incubated with secondary antibody for 1 h at room temperature and finally detected by a Gel Doc 2000 (Bio-Rad, NY, USA).
Cell counting kit-8 (CCK-8) assay
The level of cell proliferation was determined by CCK-8 assay. GBC cells in 100 μL complete medium were plated into a 96-well plate at the density of 1500 cells per well. Each well was replaced with 10 μL CCK-8 reagent and 90 μL complete medium, and incubated at 37°C for 2 h away from light. The optical density at 450 nm was measured with a SpectraMax 190 Microplate Reader (Molecular Devices, NY, USA).
Migration and invasion assay
Transwell Chamber (Corning, New York, USA) was used for the migration assay and BioCoat Matrigel Invasion Chamber (Corning) for invasion assay. The lower chamber was put into a 24-well plate filled with 700 μL complete medium, 3 × 104 GBC cells in 200 μL serum-free medium were added into the upper chamber and incubated in a 37°C, 5% CO2 humidified incubator for 24 h. Next, cells were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet, and counted in five random fields under a microscope.
Xenograft model assay
Female nude mice (4 weeks old, 18–22 g) were purchased from Shanghai SLRC Laboratory Animal Center and housed under appropriate conditions. Mice were randomly divided into two groups, five mice in each group, and 2 × 106 NOZ cells in 200 μL phosphate buffer saline (stably transfected with LV-NC or LV-shTOP2A) were injected into the left axilla of each mouse. Tumor volume was measured with calipers every week and estimated as: π/6 × length × width2. The mice were sacrificed after 4 weeks and the tumors were harvested for weighing and IHC assays.
Statistical analysis
All data were expressed as mean ± standard deviation of three independent experiments. Prism 7 (GraphPad Software, CA, USA) was used for statistical analysis. Paired t-test was used to analyze the TOP2A expression between GBC and adjacent non-tumor tissue. Chi-square test was performed to analyze the difference between TOP2A and clinicopathological variables. Overall survival was analyzed by Kaplan-Meier method and using log-rank test to determine the significance. Other data were analyzed through two-tailed t-test when necessary. P < 0.05 was considered statistically significant.
Results
TOP2A is up-regulated and correlated with poor prognosis in GBC
First, we performed bioinformatics analysis to tentatively determine the relationship between TOP2A and GBC. Based on the GEPIA database, we found that TOP2A expression is notably increased in GBC tissues compared with paired normal tissues [Figure 1A]. More importantly, high TOP2A expression seems related to poor prognosis [Figure 1B]. To further confirm the expression pattern of TOP2A in GBC, we conducted qRT-PCR and IHC staining to examine the expression level of TOP2A in 45 paired GBC samples, and investigated the correlation between TOP2A levels and clinicopathologic characteristics. Consistent with the prior results, we found that TOP2A was remarkably up-regulated in GBC tissues [Figure 1C and 1D], and high expression of TOP2A is correlated significantly with the late tumor node metastasis (TNM) stage, present of lymph node metastasis and poor prognosis [Table 1 and Figure 1E and 1F].
Figure 1.
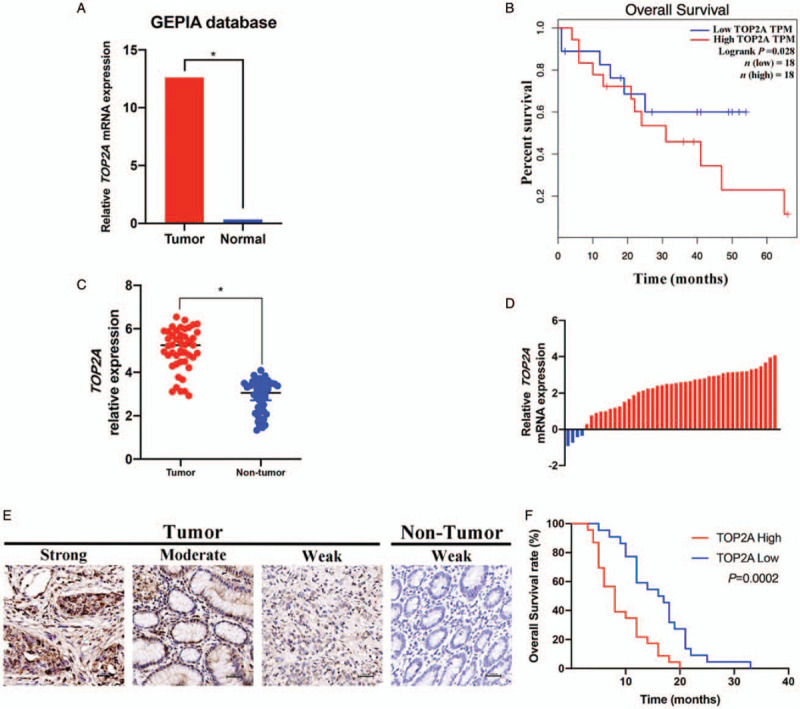
TOP2A is up-regulated and correlated with poor prognosis in gallbladder cancer. (A) Based on the data from GEPIA (http://gepia.cancer-pku.cn), GBC is up-regulated in GBC tissue compared with normal tissue. (B) High expression of TOP2A leads to poor prognosis in GBC patients. (C) The TOP2A expression in 45 paired GBC tissues and adjacent non-tumor tissues. (D) The comparison of TOP2A expression between GBC tissues and paired non-tumor tissues. (E) Representative images of immunohistochemistry staining for TOP2A expression in GBC tissues and non-tumor tissues. (F) The association between TOP2A expression and overall survival rate in GBC patients. ∗P < 0.05. GBC: Gallbladder cancer; TOP2A: Topoisomerase II alpha.
Table 1.
TOP2A expression and clinicopathologic parameters in gallbladder cancer (GBC) patients, n.

TOP2A expression is overexpressed and promotes cell proliferation in GBC cell lines
The results of qRT-PCR and Western blotting revealed that TOP2A expression was also enhanced in GBC cell lines [Figure 2A and 2B]. To investigate the effects of TOP2A on GBC cell proliferation, we used siRNA transfection to knockdown the expression of TOP2A in NOZ and SGC-996 cell lines and used TOP2A plasmid to increase the expression of TOP2A in OCUG and GBC-SD cell lines, qRT-PCR and Western blotting were carried out to detect the knockdown and overexpression efficiency [Figure 2B and 2C]. Then CCK-8 assays were performed and the results showed that TOP2A knockdown significantly inhibited the proliferation of GBC cells, while TOP2A overexpression enhanced the proliferation of GBC cells [Figure 2D].
Figure 2.
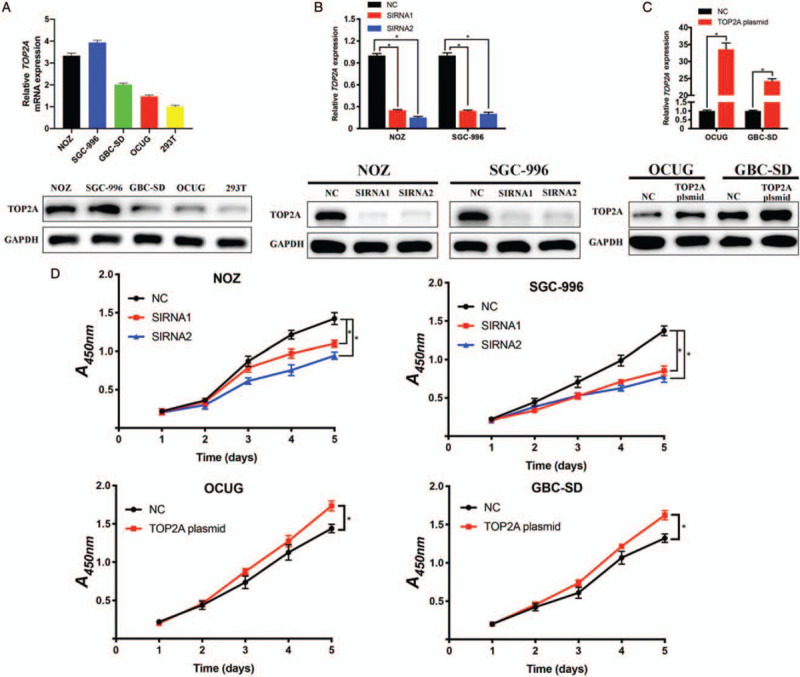
TOP2A is overexpressed and promotes cell proliferation in gallbladder cancer cell lines. (A) Relative mRNA and protein expression of TOP2A in GBC cell lines (NOZ, SGC-996, GBC-SD, and OCUG) and human renal epithelial cell line 293T. (B and C). Knockdown and overexpression efficiency of TOP2A in GBC cell lines were measured by quantitative reverse transcription polymerase chain reaction and Western blotting, respectively. (D) Cell proliferation of GBC cell lines was detected by cell counting kit-8 assay. ∗P < 0.05. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; NC: Negative control; SIRNA: Small interfering RNA; TOP2A: Topoisomerase II alpha.
TOP2A knockdown suppresses migration, invasion, and epithelial-mesenchymal transition (EMT) process of GBC cell
Exploring whether TOP2A affects GBC metastasis, we applied transwell migration and invasion assays. As shown in Figure 3A, TOP2A silencing inhibited the capacity of migration and invasion of NOZ and SGC-996 cells. Moreover, TOP2A overexpression significantly increased the migration and invasion of GBC-SD cells [Figure 3B]. EMT is a process that highly associated with tumor initiation, migration, and invasion,[13] and based on the results of our prior studies we reasonably assumed that TOP2A was involved in the EMT process of GBC cells. To prove this hypothesis, we performed Western blotting to detect the expression of key EMT-related markers and found that TOP2A knockdown up-regulated the expression of tight junction protein 1 (ZO-1), E-cadherin, whereas decreased the expression of N-cadherin, vimentin, and snail [Figure 3C].
Figure 3.
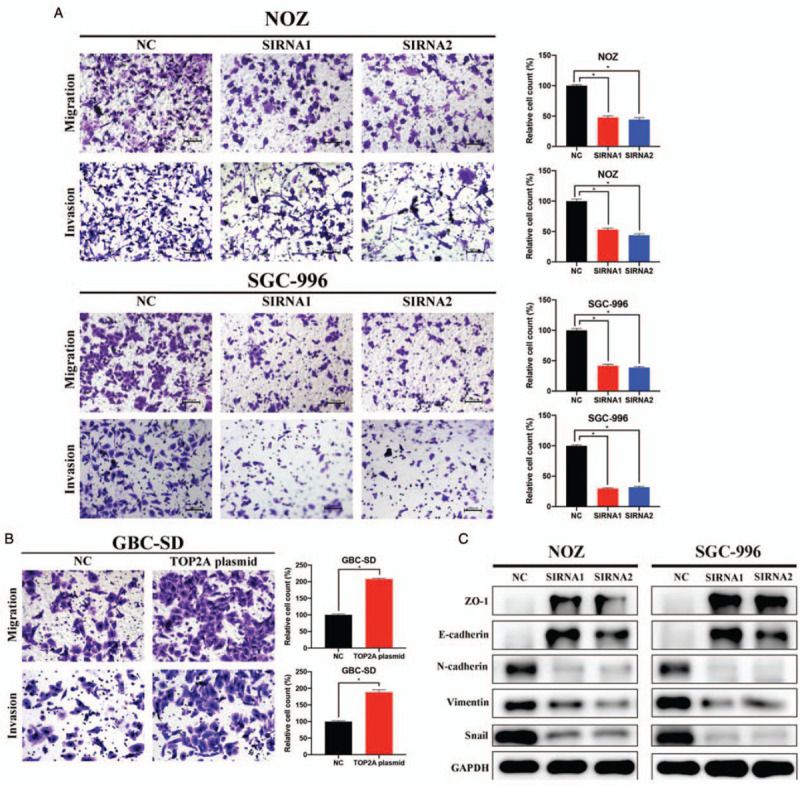
TOP2A knockdown suppresses migration, invasion, and EMT process of gallbladder cancer cells. (A and B). Transwell assays demonstrated that the down-regulation of TOP2A inhibited the migration and invasion of NOZ and SGC-996 cells. Original magnification, 200×. (C) EMT-related markers were detected by Western blotting. ∗P < 0.05. EMT: Epithelial-mesenchymal transition; NC: Negative control; SIRNA: Small interfering RNA; TOP2A: Topoisomerase II alpha.
TOP2A promotes GBC progression via PI3K/Akt/mTOR pathway
PI3K/Akt/mTOR signaling pathway is one of the most frequently activated pathways in a majority of human cancer types, and plays a vital role in tumor initiation, proliferation, and metastasis.[14,15] Several previous studies have demonstrated that PI3K/Akt/mTOR pathway is involved in the progression of GBC,[16–18] therefore, we explored whether TOP2A plays its role in GBC via PI3K/Akt/mTOR pathway. As shown in Figure 4A and 4B, the silencing of TOP2A showed no effect on the expression of total PI3K, Akt, and mTOR, but dramatically down-regulated the phosphorylation levels of these regulators. Then we conducted rescue experiments to further confirm that TOP2A works by activating PI3K/Akt/mTOR pathway. As shown in Figure 4C, TOP2A overexpression enhanced the proliferation of NOZ cells, and LY294002 (PI3K inhibitor) could reverse this enhancement induced by TOP2A. The above results demonstrated that TOP2A promoted GBC progression through activating PI3K/Akt/mTOR pathway.
Figure 4.
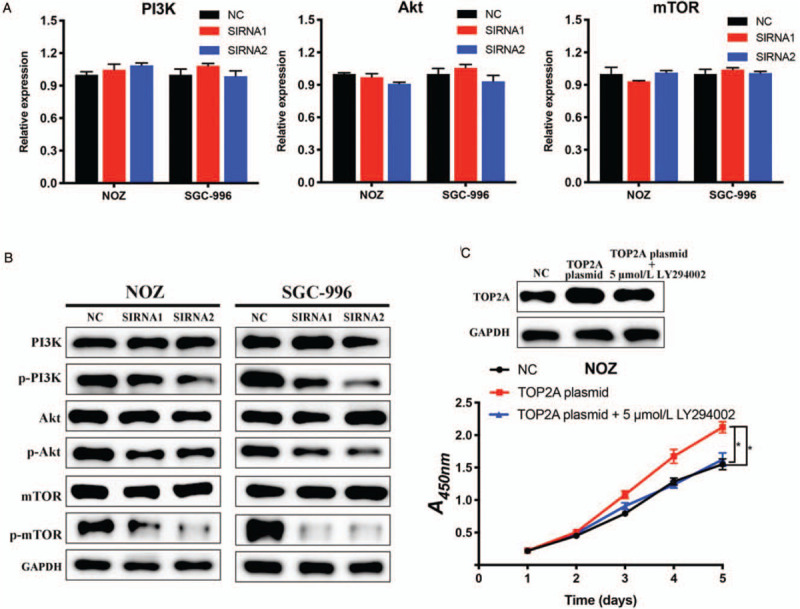
TOP2A promotes gallbladder cancer progression via PI3K/Akt/mTOR signaling pathway. (A) The mRNA expression levels of PI3K, Akt, and mTOR were measured by quantitative reverse transcription polymerase chain reaction. (B) Western blotting results showed that the silencing of TOP2A decreased the phosphorylation levels of phosphatidylinositol 3-kinase, protein kinase B, mammalian target of rapamycin. (C) 5 μmol/L LY294002 (PI3K inhibitor) reversed the proliferation accelerated by TOP2A overexpression. ∗P < 0.05. PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B; mTOR: Mammalian target of rapamycin; NC: Negative control; SIRNA: Small interfering RNA; TOP2A: Topoisomerase II alpha.
Down-regulation of TOP2A suppresses GBC tumor growth in vivo
To investigate the function of TOP2A on GBC growth in vivo, we applied xenograft model assay. First, we used LV-NC, LV-shTOP2A, and puromycin to construct stably transfected NOZ cells, the knockdown efficiency of LV-shTOP2A was verified by qRT-PCR and Western blotting [Figure 5A]. Then we injected the NOZ cells into nude mice to construct xenograft models. As shown in Figure 5B–5D, the tumor volume and weight of the LV-shTOP2A group is significantly lower than that of the LV-NC group. What's more, IHC analysis demonstrated that TOP2A silencing notably decreased the expression level of TOP2A, Ki-67, proliferating cell nuclear antigen, N-cadherin, vimentin, phosphor (p)-Akt, and p-mTOR. These in vivo results are consistent with the in vitro results, suggesting TOP2A acts as a tumor activator in GBC via PI3K/Akt/mTOR pathway.
Figure 5.
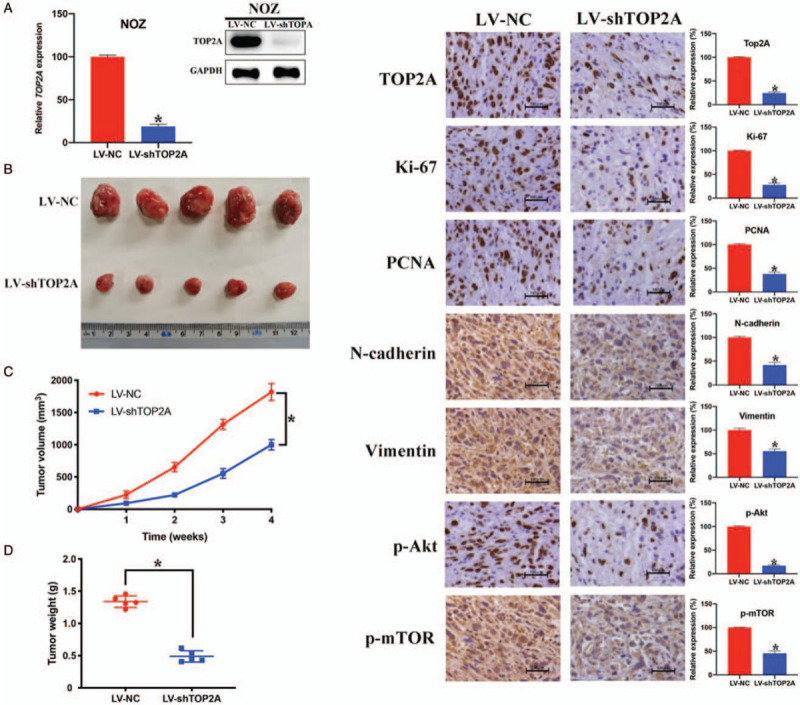
Down-regulation of TOP2A suppresses gallbladder cancer tumor growth in vivo. (A) The knockdown efficiency of LV-shTOP2A was confirmed by quantitative reverse transcription polymerase chain reaction and Western blotting. (B) Xenograft model assay showed TOP2A down-regulation inhibited GBC tumor growth in nude mice. (C and D). Tumor volume and weight in LV-NC group and LV-shTOP2A group. (E) Immunohistochemistry results of TOP2A, Ki-67, proliferating cell nuclear antigen, N-cadherin, vimentin, phosphorylation-protein kinase B, and phosphorylation-mammalian target of rapamycin. ∗P < 0.05. Scale bar = 100 μm. Akt: Protein kinase B; LV: Lentivirus; mTOR: Mammalian target of rapamycin; NC: Negative control; PCNA: Proliferating cell nuclear antigen; TOP2A: Topoisomerase II alpha.
Discussion
TOP2A has been proved to be associated with various cancers, such as colon cancer and pancreatic cancer.[10,11] But the role of TOP2A in GBC progression remains unknown. In our current study, we found that TOP2A expression was enhanced in GBC tissues and associated with TNM stage, lymph node metastasis, and poor prognosis. Based on our in vitro and in vivo studies, we demonstrated that TOP2A facilitates the proliferation and metastasis of GBC via PI3K/Akt/mTOR pathway.
GBC is the 5th most common digestive tract malignant tumor, and is characterized by rapid progressing and metastasis to the distant.[1,19] In this study, we explored the relationship between TOP2A and GBC by performing bioinformatics analysis and analyzing 45 paired samples of GBC. As expected, higher expression of TOP2A resulting in late TNM stage, presence of lymph node metastasis, and lower overall survival in GBC patients. Then we investigated the effects of TOP2A on GBC both in vitro and in vivo. According to the results, TOP2A plays a critical role in the progression of GBC, accelerating proliferation, migration, and invasion of the GBC cell.
EMT is a cellular process that highly associated with tumor progression, metastasis, and resistance to therapy.[20,21] Therefore, we investigated whether the EMT process is involved in the metastasis induced by TOP2A. We performed Western blotting to detect the expression levels of a series of markers of EMT. After knockdown of TOP2A, the expression of ZO-1 and E-cadherin was down-regulated while the expression of N-cadherin, vimentin, and snail was up-regulated, suggesting TOP2A facilitates the EMT process in GBC progression.
Hyperactivation of the PI3K/Akt/mTOR signaling pathway is one of the most crucial and ordinary alterations in human cancers.[22] PI3K and Akt are the main proteins involved in this signaling pathway, and this pathway is stimulated by assorted oncogenes and growth factor receptors.[23,24] PI3K/Akt/mTOR pathway has been reported to be associated with GBC progression,[16–18] however to the best of our knowledge, there is no study about the relationship between TOP2A and GBC or TOP2A and PI3K/Akt/mTOR pathway. Hence, in our study, we explored the association between GBC, TOP2A, and PI3K/Akt/mTOR pathway. Based on the Western blotting results, depletion of TOP2A significantly inhibited the expression of p-PI3K, p-Akt, and p-mTOR in the protein level. And the promoting effect induced by TOP2A overexpression could be depleted by LY294002 (PI3K inhibitor). What's more, IHC staining using xenograft tumors further confirmed that TOP2A silencing inactivates the Akt/mTOR pathway. Taken together, the above results demonstrate that TOP2A regulated GBC via activating PI3K/Akt/mTOR pathway.
In summary, our data indicate that the expression of TOP2A is significantly up-regulated in GBC tissue and highly relevant for poor prognosis in GBC patients. Moreover, TOP2A promotes GBC proliferation, migration, invasion, and EMT process through activating the PI3K/Akt/mTOR pathway. These findings suggest that TOP2A plays a vital role in GBC progression, and might be a novel diagnostic and therapeutic biomarker for GBC.
Conflicts of interest
None.
Footnotes
How to cite this article: Lyu WJ, Shu YJ, Liu YB, Dong P. Topoisomerase II alpha promotes gallbladder cancer proliferation and metastasis through activating phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Chin Med J 2020;133:2321–2329. doi: 10.1097/CM9.0000000000001075
References
- 1.Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol 2015; 21:12211.doi: 10.3748/wjg.v21.i43.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi MY, Maithel SK. Updates on gallbladder cancer management. Curr Oncol Rep 2018; 20:21.doi: 10.1007/s11912-018-0664-3. [DOI] [PubMed] [Google Scholar]
- 3.Duffy A, Capanu M, Aboualfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) 2010; 98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 4.Hickman L, Contreras C. Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am 2019; 99:337–355. doi: 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Tewari M, Kumar S, Shukla S, Shukla H. Analysis of wedge resection of gallbladder bed and lymphadenectomy on adequate oncologic clearance for gallbladder cancer. Indian J Cancer 2016; 53:552–557. doi: 10.4103/ijc.IJC_88_17. [DOI] [PubMed] [Google Scholar]
- 6.Christoforos N, Ignacio B, Chaysavanh M, José GM, Roderic G, Pérez-Ortín JE, et al. Topoisomerase II regulates yeast genes with singular chromatin architectures. Nucleic Acids Res 2013; 41:9243–9256. doi:10.1093/nar/gkt707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunciacantarero M, Martinezcanales S, Andréspretel F, Santpere G, Ocaña A, Galanmoya EM. Functional transcriptomic annotation and protein-protein interaction network analysis identify NEK2, BIRC5, and TOP2A as potential targets in obese patients with luminal. Breast Cancer Res Treat 2018; 168:613–623. doi: 10.1007/s10549-017-4652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu KZ, Wang GN, Fitzgerald J, Quachthithu H, Rainey MD, Cattaneo A, et al. DDK dependent regulation of TOP2A at centromeres revealed by a chemical genetics approach. Nucleic Acids Res 2016; 44:8786–8798. doi: 10.1093/nar/gkw626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terashima M, Ichikawa W, Ochiai A, Kitada K, Kurahashi I, Sakuramoto S, et al. TOP2A, GGH, and PECAM1 are associated with hematogenous, lymph node, and peritoneal recurrence in stage II/III gastric cancer patients enrolled in the ACTS-GC study. Oncotarget 2017;8:57574-57582. doi: 10.18632/oncotarget.15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Xu J, Zhao J, Bai J. Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A. J Cell Biochem 2018; 119:7256–7263. doi: 10.1002/jcb.26916. [DOI] [PubMed] [Google Scholar]
- 11.Pei YF, Yin X-M, Liu X-Q. TOP2A induces malignant character of pancreatic cancer through activating β-catenin signaling pathway. Biochim Biophys Acta Mol Basis Dis 2018; 1864:197–207. doi: 10.1016/j.bbadis.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Meseure D, Vacher S, Drak Alsibai K, Nicolas A, Chemlali W, Caly M, et al. Expression of ANRIL - polycomb complexes - CDKN2A/B/ARF genes in breast tumors: identification of a two-gene (EZH2/CBX7) signature with independent prognostic value. Mol Cancer Res 2016; 14:623–633. doi: 10.1158/1541-7786.MCR-15-0418. [DOI] [PubMed] [Google Scholar]
- 13.Pastushenko I, Blanpain C. EMT Transition States During Tumor Progression and Metastasis. Trends Cell Biol 2019; 29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lien E, Dibble C, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol 2017; 45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 2002; 5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Yang M, Guo X, Yang Z, Liu S, Ji Y, et al. Estrogen-related receptor-α promotes gallbladder cancer development by enhancing the transcription of Nectin-4. Cancer Sci 2020; 111:1514–1527. doi: 10.1111/cas.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Bian R, Li Y, Gao Y, Liu Y, Xu Y, et al. Liensinine induces gallbladder cancer apoptosis and G2/M arrest by inhibiting ZFX-induced PI3K/AKT pathway. Acta Biochim Biophys Sin 2019; 51:607–614. doi: 10.1093/abbs/gmz041. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, et al. ERBB2Genomic/mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut 2019; 68:1024–1033. doi: 10.1136/gutjnl-2018-316039. [DOI] [PubMed] [Google Scholar]
- 19.Dutta U. Gallbladder cancer: can newer insights improve the outcome? J Gastroenterol Hepatol 2012; 27:642–653. doi: 10.1111/j.1440-1746.2011.07048.x. [DOI] [PubMed] [Google Scholar]
- 20.Nieto MA, Yun-Ju Huang R, Jackson RA, Thiery JP. EMT: 2016. Cell 2016; 166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 21.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 22.Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019; 698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 23.Brazil D, Hemmings B. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 2001; 26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 24.Mei XL, Zhong S. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin Med J 2019; 132:454–465. doi: 10.1097/CM9.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]


