Summary
Acinetobacter baumannii is a Gram-negative bacterium primarily associated with hospital-acquired, often multidrug-resistant (MDR) infections. The ribosome-targeting antibiotics amikacin and tigecycline are among the limited arsenal of drugs available for treatment of such infections. We present high-resolution structures of the 70S ribosome from A. baumannii in complex with these antibiotics, as determined by cryoelectron microscopy. Comparison with the ribosomes of other bacteria reveals several unique structural features at functionally important sites, including around the exit of the polypeptide tunnel and the periphery of the subunit interface. The structures also reveal the mode and site of interaction of these drugs with the ribosome. This work paves the way for the design of new inhibitors of translation to address infections caused by MDR A. baumannii.
Keywords: ribosome, antibiotics, cryoelectron microscopy, antimicrobial resistance, structural biology
Graphical Abstract
Highlights
-
•
Cryo-EM structures of the ribosome from the pathogenic bacteria A. baumannii
-
•
Unique structural features compared with other bacterial ribosomes
-
•
The site and mode of binding of amikacin and tigecycline to this ribosome
-
•
A putative alternative tigecycline-binding site at the 50S central protuberance
Nicholson et al. present high-resolution cryo-EM structures of the ribosome from the pathogenic bacteria Acinetobacter baumannii in complex with the clinically relevant antibiotics amikacin and tigecycline, revealing species-specific features and modes of drug interactions. These structures pave the way for the design of new inhibitors to address multidrug-resistant A. baumannii infections.
Introduction
Acinetobacter baumannii is a formidable opportunistic pathogen and an important cause of hospital-acquired infection. This bacterium predominantly affects immunocompromised patients who require prolonged hospital stays, in whom it can cause pneumonia, meningitis, and infections of the blood, urinary tract, and skin and soft tissue (Dijkshoorn et al., 2007; Howard et al., 2012; Montefour et al., 2008; Peleg et al., 2008). As a Gram-negative bacterium equipped with an outer membrane and possessing an array of efflux transporters, A. baumannii is intrinsically well defended against antibacterial drugs. However, it also readily acquires novel resistance determinants through horizontal gene transfer, which has led to the rapid emergence of multidrug-resistant strains. These include strains resistant to most or all classes of antibiotics (Falagas and Bliziotis, 2007; Fournier et al., 2006; Perez et al., 2008; Potron et al., 2015). Accordingly, it has been classified as one of the ESKAPE pathogens, a designation reserved for those bacteria most commonly associated with multidrug resistance (Boucher et al., 2009).
The antibiotics available for treatment of A. baumannii infections in the clinic include beta-lactams, polymyxins, and ribosome-targeting antibiotics such as the aminoglycosides (e.g., amikacin) and tigecycline (a third-generation tetracycline derivative) (Fishbain and Peleg, 2010). However, the effectiveness of even these agents is diminishing, and there are few recently approved drugs or candidates in late-stage development to replace them (Asif et al., 2018; Baron et al., 2016; Perez et al., 2007; Poirel and Nordmann, 2006; Wright et al., 2017). Consequently, the World Health Organization has placed carbapenem-resistant A. baumannii in the top tier of their priority pathogens list for research and development of new antibiotics (Tacconelli et al., 2018). A more detailed molecular understanding of how current antibiotics bind their targets and exert their inhibitory effects on this pathogen may aid the design and development of such drugs.
The bacterial ribosome is one such antibiotic target (Wilson, 2014). Although structures of ribosomes and antibiotic-ribosome complexes from a range of bacterial species have been determined (Ban et al., 2000; Brodersen et al., 2000; Carter et al., 2000; Schluenzen et al., 2000; Schlunzen et al., 2001; Wimberly et al., 2000), only three species of pathogenic bacteria have known structures of drug-bound ribosomes, namely E. coli (Borovinskaya et al., 2007; Schuwirth et al., 2006), Staphylococcus aureus (Eyal et al., 2015; Halfon et al., 2019b), and Mycobacterium tuberculosis (Yang et al., 2017). Recently, the structures of the ribosomes from Pseudomonas aeruginosa (Halfon et al., 2019a) and A. baumannii (Morgan et al., 2020) were solved, but these did not include bound antibiotics. Expanding our repertoire of bacterial ribosome and antibiotic-ribosome complex structures will improve our understanding of species-specific translation and translation inhibition mechanisms, and inform the development of new antibiotics with improved activity against specific pathogens (Khusainov et al., 2016; Wilson, 2014). The variation in ribosomes between species can be substantial (e.g., in the form of rRNA expansion segments [Yang et al., 2017], unique ribosomal proteins [Hentschel et al., 2017; Yang et al., 2017], and protein paralogs [Khusainov et al., 2016]). However, variation can also be more subtle (e.g., different protein and rRNA folds, insertions, and deletions [Eyal et al., 2015] and chemical modifications [Byrgazov et al., 2013; Polikanov et al., 2015]). Specific examples include differences in helix h26 of the 16S rRNA, a region that interacts with the Shine-Dalgarno sequence and that varies slightly in length between ribosomes from Thermus thermophilus, E. coli, Bacillus subtilis, and S. aureus (Khusainov et al., 2016), and protein uL22, which lines the polypeptide exit tunnel and contains a single residue difference between B. subtilis and E. coli that leads to ribosome stalling during the translation of the MifM leader peptide in B. subtilis specifically (Sohmen et al., 2015). Even variations between ribosomes from different strains of the same species can have a significant functional impact, as seen for a strain of P. aeruginosa with a mutation in ribosomal protein uL6 that results in aminoglycoside resistance and ribosome instability (Halfon et al., 2019a).
In this study we present cryoelectron microscopy (cryo-EM) structures of the ribosome from A. baumannii in complex with the clinically relevant antibiotics amikacin and tigecycline, solved to high resolutions of 2.8 and 2.6 Å, respectively, allowing comparison with previous apo structures of the A. baumannii ribosome (Morgan et al., 2020). Structural comparison with ribosomes from other bacteria identifies several unique structural features, including differences around the exit of the polypeptide tunnel and at the subunit interface. These structures also reveal the molecular detail of interactions of amikacin and tigecycline with the A. baumannii ribosome and suggest the existence of an alternative tigecycline-binding site within the 50S subunit. Collectively, these structures contribute toward a greater understanding of species-specific translation mechanisms and provide a platform for the design and development of novel antibiotics needed to treat increasingly drug-resistant A. baumannii infections.
Results and Discussion
Structure Determination of the 70S Ribosome from A. baumannii
Ribosomes were extracted from the A. baumannii type strain ATCC 19606 and then purified by sucrose cushion and sucrose gradient centrifugation. Fractions corresponding to the dominant peak in the sucrose gradient sedimentation profile were collected (Figure S1A). The purified ribosomes were incubated with either amikacin or tigecycline, and visually inspected by cryo-EM to confirm the presence of intact 70S ribosomes (Figure S1B). Single-particle cryo-EM analysis was performed to reconstruct structures of the amikacin-ribosome and tigecycline-ribosome complexes to resolutions of 2.8 and 2.6 Å, respectively (Table S1 and Figure S1). The local resolution of the reconstructions ranges from ~2.3 Å in the core of the 50S subunit to >6 Å in the flexible peripheral regions of the ribosome. Image alignment was dominated by the larger 50S subunit, leaving the smaller 30S subunit, particularly its head, poorly resolved, due to movements in the ribosome necessary to facilitate the translocation of the tRNA-mRNA complex. These include intersubunit rotation between the 50S and the 30S subunits (Cornish et al., 2008) and 30S head swiveling (Ratje et al., 2010). Therefore, for each structure, the 50S subunit, the body of the 30S subunit, and the head of the 30S subunit were treated as three independent rigid bodies and their reconstructions refined to nominal resolutions of 2.7, 2.9, and 3.0 Å for the amikacin-ribosome complex and 2.5, 2.7, and 3.0 Å for the tigecycline-ribosome complex, respectively (Figure S2). This multibody refinement procedure vastly improved the density of the 30S head in the amikacin-ribosome structure, making it amenable to model building (Figures S1C and S2B), but was less successful for the tigecycline-ribosome structure (Figures S1E and S2C). Note that padding in Fourier space was not performed in order to save computer memory, resulting in artifacts around the edge of the box in Figure S2C. This noise was masked out before undertaking model building and refinement. The 30S head of the tigecycline-ribosome structure contained significantly more density outside the expected region compared with the 30S head of the amikacin-ribosome structure, suggesting that the particle subtraction and focused refinement procedures within multibody refinement were less effective (Figures S3A and S3B). Furthermore, despite similar Fourier shell correlation-derived resolution estimates for the 30S head in the two structures, the masked and sharpened 30S head map of the tigecycline-ribosome structure was visually poorer than the corresponding map from the amikacin-ribosome complex, with protein side-chain and RNA base density less consistently resolvable across the whole map (Figures S3C and S3D), no matter the sharpening B factor used. This comparatively small improvement in the tigecycline-ribosome reconstruction could not be rectified, despite trying a number of different masks to define the rigid-body boundaries. The reason for the larger improvement in the 30S head in the amikacin-ribosome reconstruction compared with the 30S head in the tigecycline-ribosome reconstruction is unclear. One possible reason is that tigecycline locks the 30S head to the 30S body in a way similar to that of spectinomycin (Mohan et al., 2014), reducing 30S head rotation, and thus the improvement through multibody refinement would be expected to be less significant than if the 30S head were rotating more freely. Indeed, the 30S head density is more complete in the tigecycline-ribosome pre-multibody refinement reconstruction than in the corresponding amikacin-ribosome reconstruction (Figure S1), a phenomenon that could be explained by reduced 30S head swivel when tigecycline is bound. Furthermore, principal component analysis reveals that the largest variation in the data for the tigecycline-bound ribosome is due to 50S-30S intersubunit rotation, compared with a combination of 30S head rotation and intersubunit rotation for the amikacin-bound ribosome (Figure S4) Tigecycline-induced locking of the 30S head is plausible, considering that the primary tigecycline binding site is at the interface of the 30S head and body (Jenner et al., 2013).
Homology models based on experimental structures of E. coli ribosomal proteins and rRNA were fitted and refined into the A. baumannii antibiotic-ribosome cryo-EM multibody reconstructions (Table 1). The overall structure comprises a large 50S subunit, composed of 23S rRNA, 5S rRNA, and 28 ribosomal proteins, and a small 30S subunit composed of 16S rRNA and 20 ribosomal proteins. These constituent parts form the recognizable structural elements of the ribosome, including the central protuberance, L1 stalk, and L12 stalk of the 50S subunit, and the head, body, platform, shoulder, and spur of the 30S subunit (Figure 1). At this resolution, most rRNA nucleobases (Figures 1A and 1D) and protein side chains (Figures 1B and 1C) can be distinguished in both subunits. Most rRNA residues were modeled, with missing regions mostly belonging to the flexible stalks of the 50S subunit, which had poorly resolved electron microscopy (EM) density, as has been seen previously in other ribosome structures. Of the 54 known core ribosomal proteins, 41 were modeled with side chains included, 7 had ambiguous or poor side-chain density and were modeled, at least in part, without side chains, and 6 were not modeled (Table S2). These unmodeled proteins are known to be located in flexible parts of the ribosome (uL1, bL9, uL10, uL11, and bL12), or to be loosely associated (bS1), and all had weak or non-existent density. Density corresponding to protein bL31, which bridges the two subunits, was resolved only in the tigecycline-ribosome reconstruction. Weak density was seen in the E site for mRNA (modeled as polyuridine) and tRNA (modeled as “E-site tRNA,” derived from the E-site fMet-tRNA of PDB: 5AFI) (Figure S5). These densities likely correspond to a mixture of different tRNAs and mRNAs that remain associated with a subpopulation of ribosomes through the purification procedure.
Table 1.
Model Refinement and Validation Statisticsa
| Amikacin-Ribosome 50S | Amikacin-Ribosome 30S Body | Amikacin-Ribosome 30S Head | Tigecycline-Ribosome 50S | Tigecycline-Ribosome 30S Body | Tigecycline-Ribosome 30S Head | |
|---|---|---|---|---|---|---|
| Map resolution (Å) (FSC = 0.143) | 2.7 | 2.9 | 3.0 | 2.5 | 2.7 | 3.0 |
| Map sharpening B factor (Å2) | −47.3 | −55.7 | −51.6 | −82.5 | −99.3 | −131.4 |
| Model Composition | ||||||
| Non-hydrogen atoms | 83,514 | 33,322 | 16,864 | 83,855 | 33,279 | 16,906 |
| Protein residues | 3,069 | 1,496 | 852 | 3,112 | 1,496 | 852 |
| Nucleic acid residues | 2,800 | 1,040 | 477 | 2,800 | 1,040 | 477 |
| Metal ions | 161 | 59 | 24 | 163 | 56 | 24 |
| Ligand | none | amikacin | none | tigecycline ×3 | none | tigecycline |
| General Validation | ||||||
| CCb (model to map fit) | 0.86 | 0.83 | 0.86 | 0.86 | 0.84 | 0.70 |
| Clashscore | 4.84 | 7.09 | 5.25 | 5.86 | 9.42 | 15.11 |
| Root-mean-square Deviations | ||||||
| Bond lengths (Å) | 0.007 | 0.012 | 0.007 | 0.008 | 0.010 | 0.007 |
| Bond angles (°) | 0.775 | 0.974 | 0.702 | 0.730 | 0.794 | 0.853 |
| Protein Geometry Validation | ||||||
| Rotamer outliers (%) | 7.08 | 11.02 | 7.15 | 5.70 | 8.22 | 11.24 |
| Ramachandran outliers (%) | 0.03 | 0.20 | 0.00 | 0.07 | 0.00 | 0.24 |
| Ramachandran favored (%) | 94.46 | 90.54 | 92.58 | 95.42 | 91.90 | 91.03 |
| RNA Geometry Validationc | ||||||
| Sugar pucker outliers (%) | 0.64 | 0.38 | 0.21 | 0.57 | 0.38 | 0.42 |
| Backbone conformation outliers (%) | 17.14 | 20.48 | 17.19 | 15.89 | 19.71 | 22.85 |
Obtained from Phenix refinement log unless otherwise stated.
CC, correlation coefficient.
Obtained from MolProbity Web server.
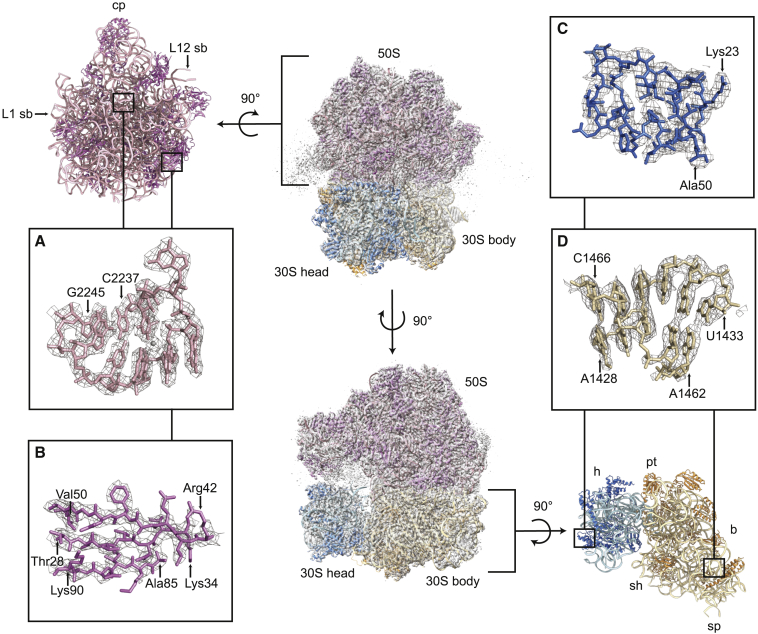
Figure 1.
Structure of the 70S Ribosome from A. baumannii
Center: two views of the A. baumannii ribosome-amikacin complex with atomic models of the 50S (rRNAs light pink, proteins dark pink), 30S body (rRNA light brown, proteins dark brown) and 30S head (rRNA light blue, proteins dark blue), and corresponding EM densities (gray volume). These three models were refined separately in the three multibody reconstructions (after sharpening and masking), and the models and maps are superimposed. Top left: atomic model of the 50S subunit showing key structural elements (cp, central protuberance; L1 sb, L1 stalk base; L12 sb, L12 stalk base). Bottom right: atomic model of the 30S subunit showing key structural elements (h, head; pt, platform; b, body; sp, spur; sh, shoulder).
(A) EM density (gray mesh) and atomic model of the 23S rRNA P loop C2237–G2245.
(B) EM density (gray mesh) and atomic model of a β sheet in protein bL19: Thr28–Lys34, Arg42–Val50, Ala85–Lys90.
(C) EM density (gray mesh) and atomic model of a helix-turn-helix in protein uS14: Lys23–Ala50.
(D) EM density (gray mesh) and atomic model of part of the 16S rRNA helix h44: A1428–U1433, A1462–C1466.
See also Table S2.
The A. baumannii Ribosome Has Unique Structural Features, Including Around the Exit of the Polypeptide Tunnel and the Subunit Interface
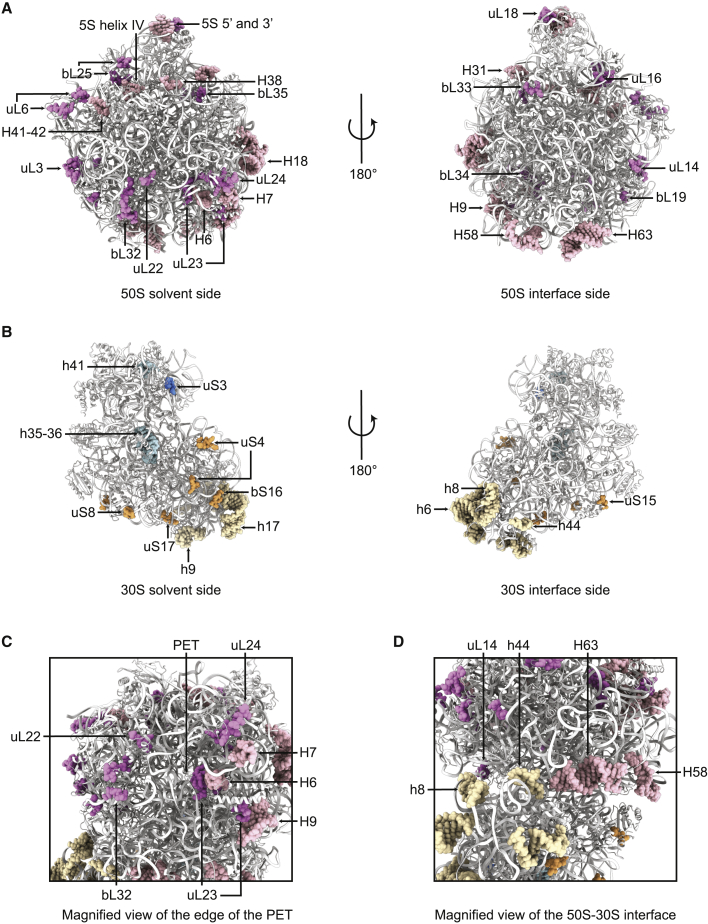
Structural differences in bacterial ribosomes may be exploited to design drugs that have improved activity against pathogenic species or that avoid harmful side effects resulting from disturbance of the gut microbiota (Dethlefsen et al., 2008). To identify unique structural features in the A. baumannii ribosome, the structure was compared with that of ribosomes from other bacteria, including E. coli (PDB: 4YBB and PDB: 5MDZ) (James et al., 2016; Noeske et al., 2015), S. aureus (PDB: 5LI0) (Khusainov et al., 2016), and T. thermophilus (PDB: 5E81) (Rozov et al., 2016). Insertions, deletions, and differences in the fold of all modeled ribosomal proteins and rRNA in the A. baumannii ribosome compared with E. coli were identified (Table S3). The overall architecture of this ribosome is broadly similar to that of other bacterial ribosomes; in particular, the regions around the catalytic peptidyl transferase center and decoding center are structurally conserved. Differences were mostly located on the solvent-facing portions of the subunits, as well as around the periphery of the subunit interface (Figures 2A and 2B).
Figure 2.
Unique Structural Features of the A. baumannii Ribosome
Insertions, deletions, and differences in the fold of loops and secondary structures of the ribosomal proteins and rRNA in the A. baumannii ribosome compared with E. coli (PDB: 5MDZ and 4YBB).
(A) Two views of the atomic model of the 50S subunit (white cartoon), with unique features highlighted (rRNA light pink spheres, protein dark pink spheres).
(B) Two views of the atomic model of the 30S subunit (white cartoon), with unique features highlighted (30S body rRNA light brown spheres, protein dark brown spheres; 30S head rRNA light blue spheres, protein dark blue spheres).
(C) Magnified view of unique features around the edge of the PET (polypeptide exit tunnel).
(D) Magnified view of unique features around the subunit interface.
The structure of the A. baumannii ribosome-amikacin complex is used, but all highlighted differences hold true for the A. baumannii ribosome-tigecycline complex.
See also Table S3.
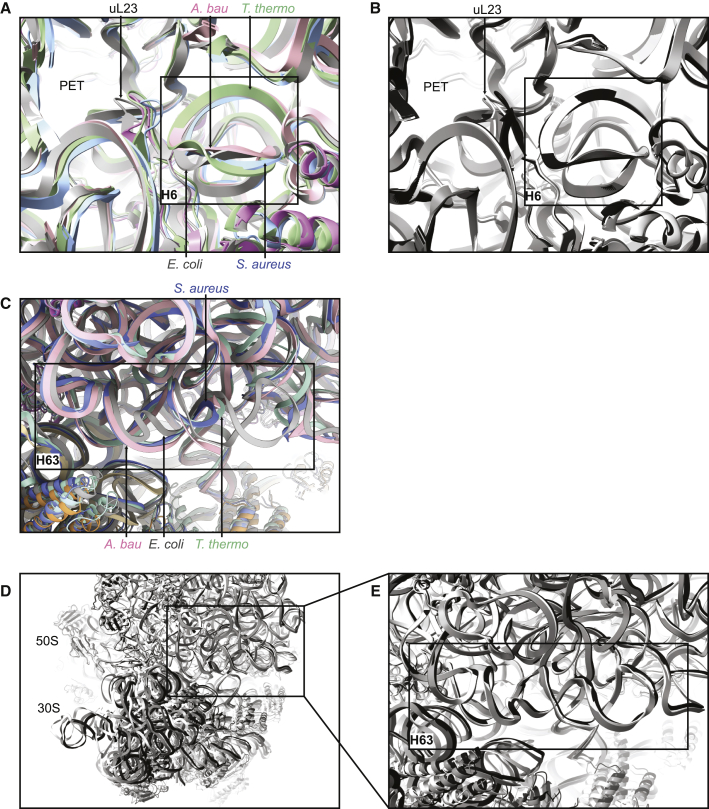
Hotspots of structural differences were identified around the exit of the polypeptide tunnel on the solvent-exposed face of the 50S subunit, specifically in proteins uL22, uL23, uL24, and bL32, and in the 23S rRNA helices H6, H7, and H9 (Figure 2C). This site is where ribosome-associated factors dock, such as the molecular chaperone trigger factor (Deuerling et al., 2019), the signal recognition particle, and the Sec translocon (Jomaa et al., 2016). For example, the 23S rRNA helix H6, situated near uL23 at the edge of the polypeptide tunnel exit, takes up a different fold in A. baumannii compared with E. coli, S. aureus, and T. thermophilus (Figure 3A). The fold of H6 is consistent across multiple E. coli structures, no matter the structural technique or ribosome buffer used, so this difference in fold is attributed to species specificity (Figure 3B). Note that the fold of the nearby β-hairpin loop of uL23, although clearly different between the different species (Figure 3A), does not always maintain the same fold in different E. coli structures (Figure 3B). This makes it more difficult to assign as a species-specific difference with as high a confidence as H6, as different buffer conditions or crystal contacts could be playing a role. Clusters of differences were also identified around the periphery of the subunit interface, specifically in the 23S rRNA helices H58 and H63, the 16S rRNA helices h8 and h44, and protein uL14 (Figure 2D). The consensus reconstruction (i.e., the EM map determined without using multibody refinement) was used to check the validity of the multibody models in these regions, because models fitted to multibody maps can be difficult to interpret at the body interfaces (Nakane et al., 2018). H63 is shorter in S. aureus and T. thermophilus than in E. coli or A. baumannii, but takes up a slightly different conformation in E. coli compared with A. baumannii (Figure 3C). Contacts at the interface keep the ribosome intact and are important for the dynamic processes involved in translocation (Liu and Fredrick, 2016) and some antibiotics, such as neomycin and thermorubin, inhibit translation by perturbing these intersubunit bridges (Bulkley et al., 2012; Wang et al., 2012). To confirm the validity of the unique structural features in H63 of A. baumannii, as well as the other identified features near the subunit interface, we compared these helices and proteins with those in E. coli ribosome structures exhibiting a range of 50S-30S rotation states: the two ribosome conformations in PDB: 4YBB and the ribosome from PDB: 5MDZ (Figure 3D). The conformations of these helices and proteins remained consistent across these three E. coli ribosome structures, and hence were all different from the corresponding conformations in the A. baumannii ribosome (Figure 3E shows this for H63). This strongly suggests that the unique structural features identified near the subunit interface truly are species-specific differences, rather than differences arising from different ribosome rotation states.
Figure 3.
Unique Structural Features in the 23S rRNA Helices H6 and H63
(A) H6 of A. baumannii (pink) takes a different conformation compared with H6 of E. coli (gray, PDB: 4YBB), S. aureus (blue, PDB: 5LI0), and T. thermophilus (green, PDB: 5E81).
(B) H6 maintains the same conformation across various E. coli ribosome structures, as shown here in a crystal structure (light gray, PDB: 4YBB ribosome I; mid-gray, PDB: 4YBB ribosome II) and an EM structure (dark gray, PDB: 5MDZ), unlike the nearby β-hairpin loop of uL23, which shows some variation.
(C) H63 of A. baumannii (pink) takes a different conformation than H63 of E. coli (gray, PDB: 4YBB) and is longer than in S. aureus (blue, PDB: 5LI0) and T. thermophilus (green, PDB: 5E81).
(D) E. coli ribosome models aligned on the 50S ribosomal subunit, representing a range of rotation states of the 30S ribosomal subunit. Empty ribosome in an intermediate rotated state (light gray, PDB: 4YBB ribosome I), empty ribosome in non-rotated state (mid-gray, PDB: 4YBB ribosome II), and ribosome with A-site and P-site tRNA (dark gray, PDB: 5MDZ).
(E) H63 in these three E. coli ribosome models. Despite the difference in intersubunit rotation states, the conformation of H63 remains similar across the three models.
The structure of the A. baumannii ribosome-amikacin complex is used, but the structures of all highlighted regions hold true for the A. baumannii ribosome-tigecycline complex. PET, polypeptide exit tunnel.
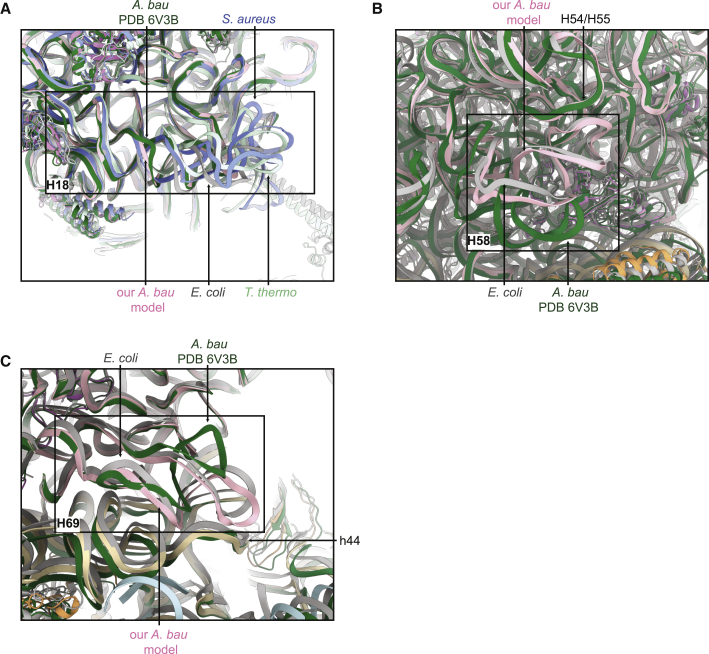
The structure of the ribosome from A. baumannii was very recently solved in empty and tRNA-bound states, and the authors identified three unique structural features of this ribosome (Morgan et al., 2020). First, H18 was significantly shorter than in other bacterial ribosomes, which is confirmed in our structure (Figure 4A). The authors also identified a conformational difference in H58, which forms a straight helix, without bending to contact H54/H55 as it does in other ribosomes. However, in our structure, although H58 is longer and has a slightly different conformation in A. baumannii compared with E. coli, the helix still follows an overall similar path, forming the contact with H54/H55 (Figure 4B). Finally, the authors found that H69 bends toward the 50S subunit instead of forming intersubunit bridge B2a/d with h44 of the 30S subunit, much the same as seen in the aminoglycoside-resistant P. aeruginosa ribosome structure (Halfon et al., 2019a), and they suggested this could contribute toward low aminoglycoside susceptibility. However, the strain of A. baumannii used in their study (AB0057) is reported to be susceptible to aminoglycosides amikacin and tobramycin (Adams et al., 2008), and in our structure, H69 forms contacts with h44 as seen in other bacterial ribosomes (Figure 4C), suggesting a more complex and subtle relationship between the conformation of H69 and aminoglycoside resistance. The cause of the differences in H58 and H69 in these two structures is unclear. Indeed, there are no sequence differences in the stem-loops or in the proteins in the immediate vicinity that might explain any change in conformation. However, similar purification procedures and final ribosome buffers were used when preparing the two samples, so it is unlikely that these differences are artifactual. Note that these differences are not due to modeling errors, as the density is quite clear for both helices in both structures (Figure S6). Discrepancies between these two structures imply that even ribosomes from different strains of the same bacterial species may have unique structural features, and further study could help us to understand divergent antibiotic susceptibilities in different strains.
Figure 4.
Structural Comparison of the Ribosome from Two Different Strains of A. baumannii
(A) H18 takes up different conformations in A. baumannii (pink, our model; dark green, PDB: 6V3B), E. coli (gray, PDB: 4YBB), S. aureus (blue, PDB: 5LI0), and T. thermophilus (light green, PDB: 5E81).
(B) H58 bends to interact with H54/H55 in our model of the A. baumannii ribosome (pink, strain ATCC 19606) and the E. coli ribosome (gray, PDB: 4YBB), but not in a different A. baumannii ribosome (dark green, strain AB0057, PDB: 6V3B).
(C) H69 reaches toward the 30S subunit to interact with h44 in our model of the A. baumannii ribosome (pink, strain ATCC 19606) and the E. coli ribosome (gray, PDB: 4YBB), but instead bends back toward the 50S subunit in a different A. baumannii ribosome (dark green, strain AB0057, PDB: 6V3B).
The structure of the A. baumannii ribosome-amikacin complex is used to describe the ATCC 19606 A. baumannii ribosome, but the structures of all highlighted regions hold true for the A. baumannii ribosome-tigecycline complex.
See also Figure S6.
The Interaction of Antibiotics Amikacin and Tigecycline with the A. baumannii Ribosome
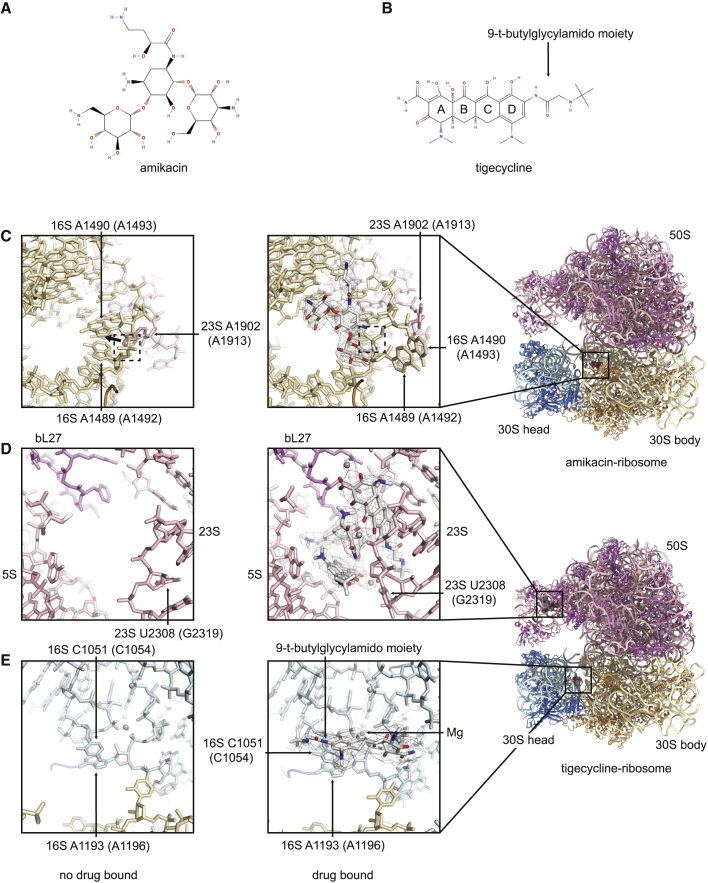
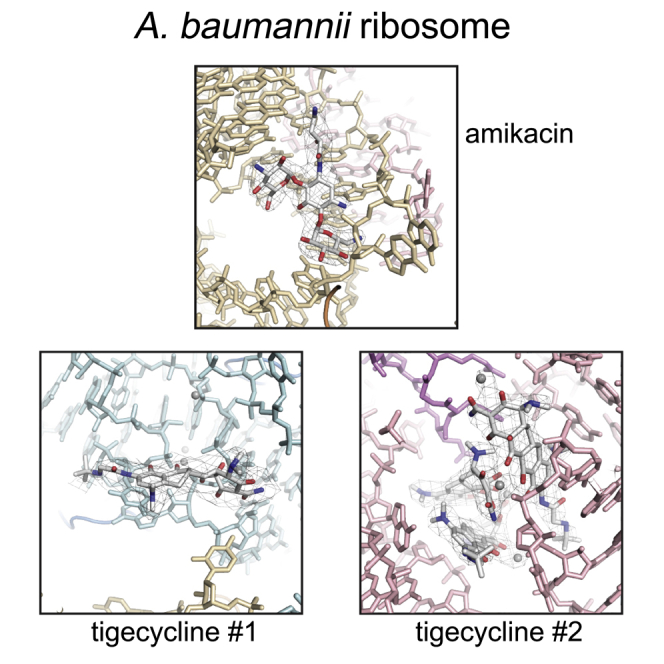
Additional cryo-EM density is present for amikacin and tigecycline in the amikacin-ribosome and tigecycline-ribosome cryo-EM reconstructions, respectively (Figure 5). In this section, A. baumannii rRNA nucleotide numbering will be followed by the identity of the corresponding E. coli nucleotide in parentheses. Aminoglycosides are known to impede the translocation of the mRNA-tRNA complex through the ribosome (Tsai et al., 2013), inhibit ribosome recycling (Borovinskaya et al., 2007), and promote translational misreading (Wilson, 2009). Like other aminoglycosides (Carter et al., 2000; Ogle et al., 2001), amikacin binds within an internal loop in h44 of the 16S rRNA at the A site and sterically overlaps with nucleotides A1489 (A1492) and A1490 (A1493), promoting an alternate conformation where these nucleotides are flipped out into the decoding center (Figure 5C). These nucleotides usually probe the minor groove of the codon-anticodon duplex in the A site, and hence distinguish cognate from non-cognate tRNAs. The stabilization of this flipped-out conformation even in the presence of non-cognate tRNA is a plausible mechanism for aminoglycoside-induced misreading (Magnet and Blanchard, 2005). Nearby, A1902 (A1913) of the 23S rRNA H69 moves toward the tRNA binding site, and the phosphate of A1490 (A1493) moves in toward to center of h44, away from the decoding center (Figure 5C). These movements fit with an alternative model proposing that aminoglycoside binding promotes misreading by inducing local changes in h44 and H69, which relax the constraints of the decoding pocket and otherwise compensate for energetically unfavorable non-cognate tRNA-mRNA interactions (Demeshkina et al., 2012). It may be that the concerted effect of both these mechanisms ultimately drives misreading.
Figure 5.
Interactions of Amikacin and Tigecycline with the A. baumannii Ribosome
(A) Structural formula of amikacin.
(B) Structural formula of tigecycline. Both drawn in MolView.
(C) The aminoglycoside binding site with amikacin bound (right), drug shown as an atomic model (white) with carved EM density (gray mesh). The tigecycline-ribosome structure, left, shows this site with no amikacin bound. Nucleotides A1489 and A1490 of the 16S rRNA and A1902 of the 23S rRNA, and the phosphate bridging A1489 and A1490, which change conformation upon drug binding, are highlighted.
(D) A secondary tigecycline binding site with tigecycline bound (right), drug shown as an atomic model (white) with carved EM density (gray mesh). The amikacin-ribosome structure, left, shows the site with no tigecycline bound. Nucleotide U2308 of the 23S rRNA, which changes conformation upon drug binding, is highlighted.
(E) The primary tigecycline binding site with tigecycline bound (right), drug shown as an atomic model (white) with carved EM density (gray mesh). The amikacin-ribosome structure, left, shows the site with no tigecycline bound. Nucleotides C1051 and A1193 of the 16S rRNA, along with a magnesium ion, which all interact with the drug, are highlighted.
E. coli numbering is shown in parentheses.
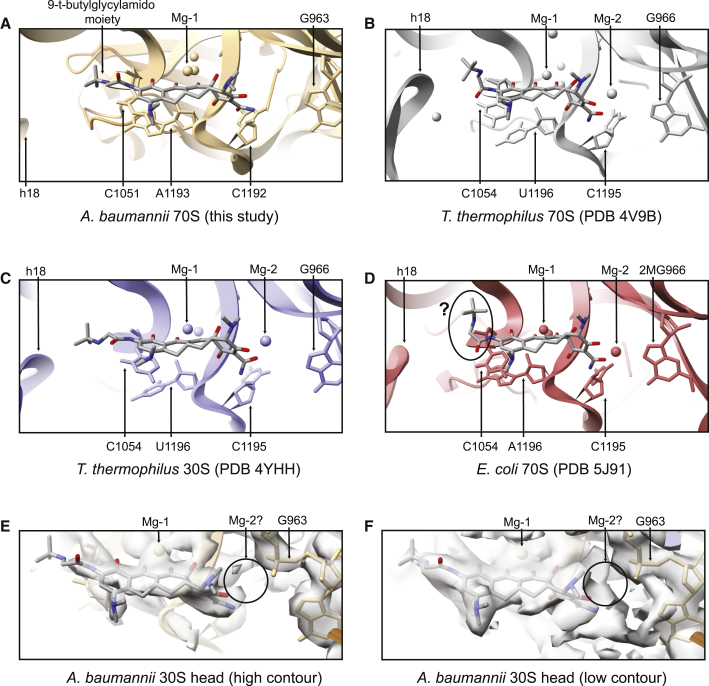
Tetracyclines inhibit translation elongation by binding to the 30S ribosomal subunit and interfering with the delivery of A-site tRNA (Brodersen et al., 2000; Pioletti et al., 2001). Tigecycline, a third-generation tetracycline derivative, targets the head of the 30S subunit to overlap with the A site in a way similar to that of tetracycline. It interacts with the phosphate backbone of h34 of the 16S rRNA through coordination between polar groups of rings B and C and a magnesium ion (Mg-1), and with the 16S rRNA nucleotides C1051 (C1054) and A1193 (A1196) through a stacking interaction of its 9-t-butylglycylamido moiety (Figure 5E), a group not present in tetracycline. Also, the sugar ring of C1192 (C1195) is in a position to form polar interactions with the amide of ring A of tigecycline. These interactions are similar to those seen in other structures (Figure 6) (Cocozaki et al., 2016; Jenner et al., 2013; Schedlbauer et al., 2015); however, there are a few noteworthy differences. First the nature of the stacking interaction of tigecycline with C1051 (C1054) varies slightly in the different structures. This interaction is thought to account for tigecycline's increased ability to interfere with A-site tRNA binding as well as its increased binding affinity compared with tetracycline (Jenner et al., 2013), and may also hinder access of the ribosomal protection protein TetM, likely explaining tigecycline's ability to evade TetM-mediated resistance (Jenner et al., 2013; Schedlbauer et al., 2015). In the A. baumannii 70S ribosome-tigecycline and the T. thermophilus 30S-tigecycline complexes, the base of C1051 (C1054) appears to form a pi-pi stacking interaction with ring D of tigecycline (Figures 6A and 6C), whereas it appears to stack with the amide of the 9-t-butylglycylamido moiety in the T. thermophilus 70S ribosome-tigecycline and E. coli 70S ribosome-tigecycline complexes (Figures 6B and 6D). Furthermore, although the density of the 9-t-butylglycylamido moiety is not strong in our data, it appears to adopt an extended conformation, similar to that seen when tigecycline is bound to the T. thermophilus 30S subunit, rather than a bent conformation as seen when bound to the whole T. thermophilus 70S ribosome. This bent conformation has previously been suggested to help accommodate a “closed” conformation of h18, which occurs when the 30S head and shoulder rotate inwards toward the decoding center (Schedlbauer et al., 2015). This is supported by a comparison of the structures of tigecycline bound to the T. thermophilus 70S ribosome and the T. thermophilus 30S ribosomal subunit, where the movement of h18 away from the tigecycline site in the latter correlates with an extended conformation of tigecycline's 9-t-butylglycylamido moiety (Figures 6B and 6C). In the A. baumannii ribosome-tigecycline structure presented here, h18 is even farther away from the tigecycline site, and the 9-t-butylglycylamido moiety adopts an extended conformation, following the expected trend (Figure 6A). (Note that the consensus map was used to confirm the relative proximity of these features, because the tigecycline site forms part of the 30S head, whereas h18 is part of the 30S body.) It should be noted that the 9-t-butylglycylamido moiety adopts a bent conformation in the E. coli 70S ribosome structure, despite h18 being far from the tigecycline binding site, which appears to contradict this trend (Figure 6D). However, the density corresponding to this moiety is not well defined, so it is not clear whether it in fact adopts a bent, rather than an extended, conformation (Cocozaki et al., 2016). In addition, all previous tigecycline-ribosome structures place a magnesium ion (Mg-2) that coordinates ring A of tigecycline to the phosphate of G963 (2N-methyl-G966). Through examination of the exact location of this ion, in the T. thermophilus 70S-tigecycline structure this coordination appears to occur primarily through ring A's hydroxyl oxygen, in the T. thermophilus 30S-tigecycline structure primarily through the ring's amide oxygen, and in the E. coli 70S-tigecycline structure there is an even mixture of the two (Figure 6). However, there is no density for a second magnesium ion in the A. baumannii ribosome-tigecycline map at high contour levels (Figure 6E). At lower contour levels there is some density present in this region, though at these levels, signal is difficult to discern from noise (Figure 6F). This is in contrast to the magnesium ion in site 1 (Mg-1), which has strong and clearly defined EM density.
Figure 6.
The Primary Tigecycline Binding Site in Ribosomes and Ribosome Subunits of Different Bacteria
(A–D) Atomic models of the primary tigecycline binding site in ribosomes and ribosome subunits of different bacteria. The nature of tigecycline binding is broadly similar across the structures, with some differences in the stacking interaction of tigecycline with C1051 (C1054) of 16S rRNA, the conformation of the 9-t-butylglycylamido moiety, and the coordination of a second magnesium ion. (A) Atomic model of tigecycline (gray) bound to the 70S of the A. baumannii ribosome (brown). The 30S head model and h18 from the 30S body model are shown. The consensus map was used to confirm the relative proximity of these features. (B) Atomic model of tigecycline (gray) bound to the 70S T. thermophilus ribosome (gray, PDB: 4V9B). (C) Atomic model of tigecycline (gray) bound to the 30S T. thermophilus ribosomal subunit (blue, PDB: 4YHH). (D) Atomic model of tigecycline (gray) bound to the 70S E. coli ribosome (red, PDB: 5J91). The density is not strong enough to support either an extended or a bent conformation of the 9-t-butylglycylamido moiety, as indicated by the question mark.
(E) EM density of the 30S head of the A. baumannii ribosome-tigecycline complex at the primary tigecycline site, high-contour level.
(F) EM density of the 30S head of the A. baumannii ribosome-tigecycline complex at the primary tigecycline site, low-contour level. It is difficult to discern possible magnesium ion density (Mg-2) from noise.
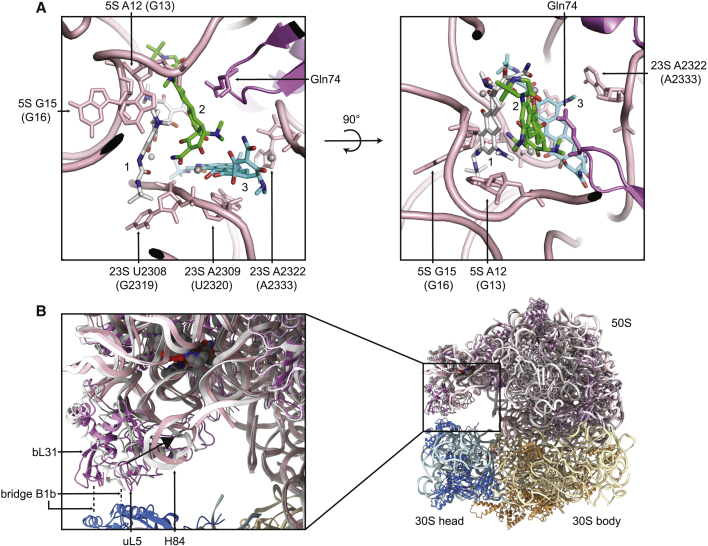
Additional density for tigecycline was also seen in the 50S at the central protuberance. Here, three tigecycline molecules bind in a cavity between the 23S rRNA, the 5S rRNA, and protein bL27. This is accommodated by a significant conformational change of the surrounding rRNA, with a particularly large movement by U2308 (G2319) of the 23S rRNA, which flips out to interact with the 9-t-butylglycylamido moiety of one of the molecules (Figure 5D). The three molecules interact with one another through stacking interactions and through bridging magnesium ions, as well as with the backbone and bases of the surrounding rRNA and protein bL27 (Figure 7A, the three molecules labeled 1, 2, and 3). Interactions between these ligands and the surrounding ribosome were calculated using Arpeggio (Jubb et al., 2017). From the 5S rRNA, the base of A12 (G13) forms a carbon-pi interaction with a methyl group of the amine of ring D of tigecycline 1, and the 2′-OH of G15 (G16) forms a donor-pi interaction with ring D of tigecycline 1. From the 23S rRNA, a carbonyl and a sugar ring oxygen of U2308 (G2319) form polar contacts with the 9-t-butylglycylamido moiety of tigecycline 1, the base of A2309 (U2320) forms a pi-pi stacking interaction with ring D of tigecycline 3, and the base of A2322 (A2333) forms a carbon-pi interaction with a methyl group of the amine of ring D of tigecycline 3. Finally, the main-chain carbonyl and Cγ of Gln74 of bL27 form van der Waals contacts with the amide of ring A and ring B of tigecycline 2. Additional interactions between the tigecycline molecules and the surrounding phosphate rRNA backbone are facilitated by coordination of magnesium ions, most clearly seen by the ion that bridges the phosphate of 23S A2309 (U2320) with oxygen atoms of ring A of tigecycline 2 and rings B and C of tigecycline 3. These interactions are summarized in 2D in Figures S7A–S7C. Overall, the presence of these tigecycline molecules promotes a series of interactions bridging the 23S and 5S rRNAs, with contributions from bL27.
Figure 7.
A Secondary Tigecycline Binding Site at the 50S Central Protuberance
(A) Two views of the secondary binding site in the A. baumannii ribosome-tigecycline structure. The three tigecycline molecules are labeled 1 (white), 2 (green), and 3 (cyan). The 23S rRNA nucleotides U2308, U2309, and A2322; the 5S rRNA nucleotides A12 and G15; and the bL27 residue Gln74, which interact with the drug molecules, are labeled. Magnesium ions are shown as gray spheres.
(B) Changes in the conformation of the central protuberance and intersubunit bridge B1b upon tigecycline binding at this secondary site. The atomic model of the tigecycline-bound ribosome (50S pink, 30S body brown, 30S head blue) is overlaid with the atomic model of the amikacin-bound 50S, which has no tigecycline bound (white). H84 and uL5 undergo a shift upon tigecycline binding, and bL31 becomes partially resolved in the density.
E. coli numbering is shown in parentheses.
See also Figure S7.
To investigate the possibility of tigecycline binding to this secondary site in other bacteria, the structure of this site in the A. baumannii ribosome-amikacin model was compared with that in ribosome structures from E. coli, S. aureus, and T. thermophilus, so that the site is compared in its empty state (Figure S7). The sites in A. baumannii and E. coli are very similar (Figure S7D), with the most noticeable differences found in U2308 (G2319) of the 23S rRNA, which takes up slightly different conformations in the two structures. However, given that this base dramatically changes conformation on tigecycline binding anyway, it is difficult to conclude whether this difference would have an impact on binding. Another obvious difference is the identity of residue 74 of bL27, which is glutamine in A. baumannii but proline in E. coli. However, in A. baumannii this residue appears only to form van der Waals contacts with tigecycline through its main chain and Cγ, and these contacts could probably still be made with a proline residue. The secondary tigecycline site differs much more greatly in S. aureus and T. thermophilus (Figure S7E). In these structures, the surrounding rRNA and the loop in bL27 take up different folds compared with their equivalents in A. baumannii. Overall, this crude analysis suggests that tigecycline might be able to bind to this site in E. coli, but is less likely to bind in S. aureus and T. thermophilus. However, experimental evidence is needed to confirm or refute this hypothesis.
These interactions appear to have a long-range effect beyond the binding pocket, with H84 of the 23S rRNA pulled toward the binding site, along with uL5, which is pulled away from its interaction with the 30S where it normally forms part of bridge B1b (Figure 7B). Protein bL31, which also forms part of bridge B1b, is partly resolved in this tigecycline-bound structure but much less well resolved in the amikacin-bound structure, so it appears that these conformational shifts make bL31 more rigid.
It could be that this additional tigecycline binding at the central protuberance of the 50S is an artifact from incubation of the ribosome with excess drug. Indeed, the stacking of three separate drug molecules is unusual, and no unambiguous density for tigecycline in this site was seen in the other two tigecycline-70S ribosome structures (Cocozaki et al., 2016; Jenner et al., 2013), which are both X-ray crystal structures involving ribosomes from different species. However, it should be noted that none of the previously reported secondary binding sites for tetracycline within the 30S subunit were occupied in the EM density of our structure (Brodersen et al., 2000; Pioletti et al., 2001). In our density, the site at the central protuberance was the only place where density for tigecycline was seen, other than the reported primary site, suggesting that tigecycline binds at least moderately tightly to this secondary site, and it is possible that the rearrangement of bridge B1b caused by the binding of tigecycline here could affect the stability of the ribosome or the dynamics of translation. Whether tigecycline binding at this site contributes a secondary mode of action for the drug beyond inhibition of A-site tRNA delivery is currently unknown.
Accelerating Antibiotic Discovery Informed by Ribosome Structures
In summary, we have presented the structure of the A. baumannii ribosome in complex with clinically relevant antibiotics. Unique structural features were identified, for example, regions around the exit of the polypeptide tunnel, which could be targeted to interfere with ribosome-associated factor binding and hence nascent chain folding or targeting, and regions around the subunit interface, which could be targeted to destabilize ribosome integrity or dynamics. Furthermore, the interactions of the antibiotics amikacin and tigecycline with the A. baumannii ribosome were elucidated, revealing changes in ribosome conformations and, in the case of tigecycline, a putative additional binding site.
As in this study, previously determined structures of drug-ribosome complexes tend to involve empty ribosomes (Cocozaki et al., 2016), or sometimes ribosomes with tRNA and mRNA bound in vitro (Jenner et al., 2013). These structures provide an important starting point to understand the action of ribosome-targeting antibiotics in different bacteria and may be used to aid the design of new drugs. They also pave the way for structural studies on more complex systems in which ribosomes are stalled or inhibited mid-translation by the drug in question to gain snapshots of antibiotics “in action,” trapping the ribosome in particular conformational states. Some previous structures have already been determined using this approach, such as the erythromycin-bound ErmBL- and ErmCL-stalled bacterial ribosomes (Arenz et al., 2014a, 2014b) and the PF846-stalled human ribosome (Li et al., 2019). Building a repertoire of structures of drug-bound ribosomes that are empty, filled with tRNA and mRNA, or stalled in particular conformational states, and which are isolated from a variety of bacterial species and strains, will provide a strong platform for the design of new drugs with improved activity against specific species or strains of bacteria and which inhibit different stages of translation. Only by accelerating development of new antibiotics will we be able to successfully treat increasingly drug-resistant infections in the future, including those caused by A. baumannii.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Acinetobacter baumannii | ATCC | ATCC 19606 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| amikacin | Cayman chemical | Cat# 15405 |
| tigecycline | LKT Labs | Cat# T3324 |
| Deposited Data | ||
| Motion corrected micrographs of amikacin-ribosome | This study | EMPIAR-10406 |
| Motion corrected micrographs of tigecycline-ribosome | This study | EMPIAR-10407 |
| Density map of amikacin-ribosome 50S | This study | EMD-10809 |
| Density map of amikacin-ribosome 30S body | This study | EMD-10869 |
| Density map of amikacin-ribosome 30S head | This study | EMD-10892 |
| Density map of tigecycline-ribosome 50S | This study | EMD-10898 |
| Density map of tigecycline-ribosome 30S body | This study | EMD-10914 |
| Density map of tigecycline-ribosome 30S head | This study | EMD-10915 |
| Coordinates of amikacin-ribosome 50S | This study | PDB 6YHS |
| Coordinates of amikacin-ribosome 30S body | This study | PDB 6YPU |
| Coordinates of amikacin-ribosome 30S head | This study | PDB 6YS5 |
| Coordinates of tigecycline-ribosome 50S | This study | PDB 6YSI |
| Coordinates of tigecycline-ribosome 30S body | This study | PDB 6YT9 |
| Coordinates of tigecycline-ribosome 30S head | This study | PDB 6YTF |
| Homology model template for initial model building. E. coli 70S ribosome | (James et al., 2016) | Protein Data Bank 5MDZ |
| fMet-tRNA atomic model for modelling of E-site tRNA | (Fischer et al., 2015) | Protein Data Bank 5AFI |
| Software and Algorithms | ||
| RELION 3.0 | (Zivanov et al., 2018) | https://www3.mrc-lmb.cam.ac.uk/relion/index.php?title=Main_Page |
| MotionCor2 | (Zheng et al., 2017) | https://emcore.ucsf.edu/ucsf-motioncor2 |
| gCTF v1.18 | (Zhang, 2016) | https://www.mrc-lmb.cam.ac.uk/kzhang/Gctf/ |
| SWISS model | (Schwede et al., 2003) | https://swissmodel.expasy.org/ |
| Coot 0.8.9.2 | (Emsley and Cowtan, 2004) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Phenix v1.17.1-3660 | (Adams et al., 2010) | https://www.phenix-online.org/ |
| MolProbity | (Chen et al., 2010) | http://molprobity.biochem.duke.edu/ |
| UCSF ChimeraX-0.9 | (Goddard et al., 2018) | https://www.cgl.ucsf.edu/chimerax/ |
| ImageJ | (Schneider et al., 2012) | https://imagej.nih.gov/ij/ |
| Pymol 2.3.2 | https://pymol.org/2/ | |
| MolView | http://molview.org/ | |
| Arpeggio | (Jubb et al., 2017) | http://biosig.unimelb.edu.au/arpeggioweb/ |
| LigPlot+ v2.1 | (Laskowski and Swindells, 2011) | https://www.ebi.ac.uk/thornton-srv/software/LigPlus/ |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Neil Ranson (n.a.ranson@leeds.ac.uk).
Materials Availability
The study did not generate new unique reagents.
Data and Code Availability
CryoEM motion-corrected micrographs generated in this study are available in EMPIAR, along with extracted particle stacks (EMPIAR-10406 and 10407 for amikacin and tigecycline respectively). CryoEM multibody refinement maps are available in the EMDB for amikacin (EMD-10809 (50S), EMD-10869 (30S body) and EMD-10892 (30S head)) and for tigecycline (EMD-10898 (50S), EMD-10914 (30S body) and EMD-10915 (30S head)). Corresponding atomic models are available in the PDB for amikacin (6YHS (50S), 6YPU (30S body), and 6YS5 (30S head)) and for tigecycline (6YSI (50S), 6YT9 (30S body), and 6YTF (30S head)). Half-maps, masks used for multibody refinement and post-processing, and pre-multibody consensus reconstructions are all available as part of the EMDB entries. See Key Resources Table for more details.
Experimental Model and Subject Details
A. baumannii strain 19606 was used for these studies, and cells were grown in LB media in shaking incubators. Additional details are provided in the Method Details section.
Method Details
70S Ribosome Purification
Two litres of A. baumannii type strain ATCC 19606 were grown at 37ºC in LB media and harvested at early-mid log phase (OD600 of ~0.5). The cell pellet was washed with 10 mM Tris-HCl pH 7.5 and stored at -80°C. Each 1 g of cell pellet was resuspended in 2 mL lysis buffer (20 mM HEPES-KOH pH 7.5, 100 mM NH4Cl, 20 mM Mg(OAc)2, 0.5 mM EDTA, 1 mM DTT) supplemented with cOmplete protease inhibitor cocktail (Roche, one tablet per 10 mL) and RNase-free DNase (300 U). The resuspension was lysed using a cell disruptor (two passes at 25K psi) and cleared by centrifugation at 30,000 x g for 30 minutes. The top 80% of the supernatant was collected and recentrifuged at 30,000 x g for 30 minutes, and the resulting supernatant layered onto a sucrose cushion buffer (10 mM HEPES-KOH pH 7.5, 500 mM KCl, 25 mM Mg(OAc)2, 1.1 M sucrose (40% w/v), 0.5 mM EDTA, 1 mM DTT) and spun by ultracentifugation at 150,000 x g for 16 hours. The resulting pellet was gently resuspended in 200 μL of sucrose gradient buffer (10 mM HEPES-KOH pH 7.5, 100 mM KCl, 10 mM Mg(OAc)2, 0.5 mM EDTA, 1 mM DTT) and layered on top of a 10-40% w/v sucrose density gradient (made by dissolving different amounts of sucrose in sucrose gradient buffer). Ultracentrifugation was subsequently carried out at 50,000 x g for 16 hours, and the fractions corresponding to the largest A260 peak were collected and dialysed into storage buffer (10 mM HEPES-KOH pH 7.5, 50 mM KCl, 10 mM NH4Cl, 10 mM Mg(OAc)2, 1 mM DTT) using a 20K molecular weight cutoff Slide-A-Lyzer MINI Dialysis Device (Thermo Scientific), flash frozen in liquid nitrogen, and stored at −80°C.
CryoEM
Purified 70S ribosomes (120 nM) were incubated with amikacin (100 μM) or purified 70S ribosomes (240 nM) were incubated with tigecycline (71.7 μM) at room temperature for 30 minutes. Quantifoil grids (R1.2/1.3, 400 mesh, with a 2 nm carbon layer) were glow discharged (10 mA, 30s, Quorum GloQube), 3 μL of the drug-ribosome reaction mixture applied, excess sample immediately blotted off and vitrification performed by plunging into liquid nitrogen-cooled liquid ethane at 100% humidity and 4°C using an FEI Vitrobot Mark IV (ThermoFisher). Data for both samples were collected on a ThermoFisher Titan Krios electron microscope (Astbury Biostructure Laboratory, University of Leeds) at 300 kV. Data collection was set up as described previously (Thompson et al., 2018). For the amikacin-ribosome sample, an electron dose of 58 e-/Å2 was applied, split into 1.16 e-/Å2 dose per frame across a 10 s exposure recorded by a Gatan K2 summit detector in counting mode with an object sampling of 1.07 Å/pixel. A magnification of 130,000 across a defocus range of -0.8 to -2.7 μm was used. For the tigecycline-ribosome sample, an electron dose of 62 e-/Å2 was applied, split into 1.44 e-/Å2 dose per frame across a 1.1 s exposure recorded by a ThermoFisher Falcon 3EC detector in integrating mode with an object sampling of 1.065 Å/pixel. A magnification of 75,000 across a defocus range of -0.8 to -2.6 μm was used. 2717 and 6228 micrograph movies of the amikacin- and tigecycline-ribosome samples were collected respectively, and following culling of micrographs with poor ice quality, 554 and 6228 micrograph movies remained respectively (Table S1).
Image Processing
Drift-corrected and dose-corrected averages of each movie were created using MOTIONCOR2 (Zheng et al., 2017) and the contrast transfer functions determined using Gctf (Zhang, 2016). All subsequent image processing steps were carried out using RELION 3.0 (Zivanov et al., 2018). Particles were picked using Laplacian-of-Gaussian autopicking and reference-free 2D classification and 3D classification performed on binned-by-4 particles to remove junk images. The remaining particles were re-extracted without binning and aligned and refined in 3D using a 60 Å low-passed filtered ab initio starting model made by a stochastic gradient descent procedure. The number of particles feeding into the final reconstructions were 51,958 and 231,159 for the amikacin-ribosome and tigecycline-ribosome samples respectively. Multibody refinement was performed using soft extended masks to define the 50S, 30S body and 30S head as rigid bodies (Figure S2). This procedure uses iteratively improved partial signal subtraction and focussed refinement to generate higher quality reconstructions for each body (Nakane et al., 2018). The resulting reconstructions were subjected to post-processing to mask out solvent and estimate and correct for the B-factors. The final resolutions were estimated using the gold-standard Fourier shell correlation (FSC = 0.143) criterion. Local resolution was estimated using RELION 3.0 (Figures S1 and S2).
Atomic Model Building and Refinement
The cryoEM structure of an E. coli ribosome (PDB 5MDZ) (James et al., 2016) was used as a starting reference for modelling the A. baumannii 23S, 16S and 5S rRNAs into the post-processed multibody reconstructions. Homology models were generated for the ribosomal proteins using the SWISS model server (Schwede et al., 2003) and rigid-body fit into the reconstructions in UCSF Chimera (Pettersen et al., 2004) using PDB 5MDZ to guide placement. The models were inspected using COOT (Emsley and Cowtan, 2004), and in all three amikacin-ribosome multibody reconstructions and the tigecycline-ribosome 50S and 30S body multibody reconstructions, regions of protein where side chains could not be resolved were modelled without side chains, and regions where the protein or rRNA backbone could not be traced were deleted. The tigecycline-ribosome 30S head reconstruction was of slightly poorer quality than the other maps and so such highly stringent trimming of the model was not carried out. Instead, the full amikacin-ribosome 30S head model was predicted to be a good approximation for the tigecycline-ribosome 30S head and hence was used as a starting model and retained with no further deletion of backbone or side chains. Density for tRNA was present in the ribosome E-site, likely corresponding to a mixture of different tRNAs, was modelled using fMet-tRNA from E. coli (PDB 5AFI) (Fischer et al., 2015) as a starting model. Only the regions near the 50S and 30S subunits which had resolved nucleotide density were retained. Density corresponding to a short mRNA at the E-site was also resolved, and this was modelled as a short polyuridine chain (Figure S5). COOT was used to manually adjust the models to improve map and rotamer fit and reduce Ramachandran outliers, before iterative rounds of model refinement and manual model editing were carried out using PHENIX real space refine (Adams et al., 2010) and COOT respectively. Models were validated using MolProbity (Chen et al., 2010) within PHENIX and PDB OneDep (Berman et al., 2003). Throughout the process, the models for the 50S, 30S body and 30S head were kept separate and refined independently into their corresponding maps, as this reflects the data from the multibody refinement procedure which generates independent reconstructions (Nakane et al., 2018). Details of the final model are found in Table S2. Model refinement and validation statistics are found in Table 1.
Figures and Model Analysis
Figures were made using UCSF ChimeraX (Goddard et al., 2018), ImageJ (Schneider et al., 2012), , LigPlot+ (Laskowski and Swindells, 2011), PyMol, and MolView. Interactions between the tigecycline molecules in the secondary binding site and the surrounding ribosome were calculated and visualised using Arpeggio (Jubb et al., 2017).
Quantification and Statistical Analysis
All cryoEM data sets were processed using RELION (Table S1). All resolutions reported are based on the “gold-standard” FSC 0.143 criterion (Figure S1 and S2). FSC curves were calculated using soft-edged masks. Refinement statistics of all atomic models are summarized in Table S1. These models were also evaluated based on MolProbity scores (Chen et al (2010)) and Ramachandran plots.
Acknowledgments
This work was supported by a Wellcome Trust 4-year PhD studentship to D.N. (203743/Z/16/Z) and BBSRC grant to AJO (BB/R004048/1). All cryo-EM was performed at the Astbury Biostructure Laboratory (ABSL), which was funded by the University of Leeds and the Wellcome Trust (108466/Z/15/Z). We thank ABSL for helping with cryo-EM data collection and Drs. Matthew Iadanza and Shaun Rawson for assistance with Python scripting.
Author Contributions
D.N. carried out experiments and analyzed the data. D.N., T.A.E., A.J.O., and N.A.R. conceived and directed the project. D.N., T.A.E., A.J.O., and N.A.R. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: August 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.str.2020.08.004.
Contributor Information
Thomas A. Edwards, Email: t.a.edwards@leeds.ac.uk.
Alex J. O'Neill, Email: a.j.oneill@leeds.ac.uk.
Neil A. Ranson, Email: n.a.ranson@leeds.ac.uk.
Supplemental Information
References
- Adams M.D., Goglin K., Molyneaux N., Hujer K.M., Lavender H., Jamison J.J., MacDonald I.J., Martin K.M., Russo T., Campagnari A.A. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 2008;190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P.D., Afonine P.V., Bunkoczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz S., Meydan S., Starosta A.L., Berninghausen O., Beckmann R., Vazquez-Laslop N., Wilson D.N. Drug sensing by the ribosome induces translational arrest via active site perturbation. Mol. Cell. 2014;56:446–452. doi: 10.1016/j.molcel.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz S., Ramu H., Gupta P., Berninghausen O., Beckmann R., Vazquez-Laslop N., Mankin A.S., Wilson D.N. Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide. Nat. Commun. 2014;5:3501. doi: 10.1038/ncomms4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif M., Alvi I.A., Rehman S.U. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018;11:1249–1260. doi: 10.2147/IDR.S166750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N., Nissen P., Hansen J., Moore P.B., Steitz T.A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- Baron S., Hadjadj L., Rolain J.M., Olaitan A.O. Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int. J. Antimicrob. Agents. 2016;48:583–591. doi: 10.1016/j.ijantimicag.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Berman H., Henrick K., Nakamura H. Announcing the worldwide protein data bank. Nat. Struct. Biol. 2003;10:980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- Borovinskaya M.A., Pai R.D., Zhang W., Schuwirth B.S., Holton J.M., Hirokawa G., Kaji H., Kaji A., Cate J.H. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat. Struct. Mol. Biol. 2007;14:727–732. doi: 10.1038/nsmb1271. [DOI] [PubMed] [Google Scholar]
- Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Brodersen D.E., Clemons W.M., Jr., Carter A.P., Morgan-Warren R.J., Wimberly B.T., Ramakrishnan V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Bulkley D., Johnson F., Steitz T.A. The antibiotic thermorubin inhibits protein synthesis by binding to inter-subunit bridge B2a of the ribosome. J. Mol. Biol. 2012;416:571–578. doi: 10.1016/j.jmb.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrgazov K., Vesper O., Moll I. Ribosome heterogeneity: another level of complexity in bacterial translation regulation. Curr. Opin. Microbiol. 2013;16:133–139. doi: 10.1016/j.mib.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.P., Clemons W.M., Brodersen D.E., Morgan-Warren R.J., Wimberly B.T., Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocozaki A.I., Altman R.B., Huang J., Buurman E.T., Kazmirski S.L., Doig P., Prince D.B., Blanchard S.C., Cate J.H., Ferguson A.D. Resistance mutations generate divergent antibiotic susceptibility profiles against translation inhibitors. Proc. Natl. Acad. Sci. U S A. 2016;113:8188–8193. doi: 10.1073/pnas.1605127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish P.V., Ermolenko D.N., Noller H.F., Ha T. Spontaneous intersubunit rotation in single ribosomes. Mol. Cell. 2008;30:578–588. doi: 10.1016/j.molcel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeshkina N., Jenner L., Westhof E., Yusupov M., Yusupova G. A new understanding of the decoding principle on the ribosome. Nature. 2012;484:256–259. doi: 10.1038/nature10913. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L., Huse S., Sogin M.L., Relman D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuerling E., Gamerdinger M., Kreft S.G. Chaperone interactions at the ribosome. Cold Spring Harb. Perspect. Biol. 2019;11:a033977. doi: 10.1101/cshperspect.a033977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Eyal Z., Matzov D., Krupkin M., Wekselman I., Paukner S., Zimmerman E., Rozenberg H., Bashan A., Yonath A. Structural insights into species-specific features of the ribosome from the pathogen Staphylococcus aureus. Proc. Natl. Acad. Sci. U S A. 2015;112:E5805–E5814. doi: 10.1073/pnas.1517952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M.E., Bliziotis I.A. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents. 2007;29:630–636. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Fischer N., Neumann P., Konevega A.L., Bock L.V., Ficner R., Rodnina M.V., Stark H. Structure of the E. coli ribosome-EF-Tu complex at <3 A resolution by Cs-corrected cryo-EM. Nature. 2015;520:567–570. doi: 10.1038/nature14275. [DOI] [PubMed] [Google Scholar]
- Fishbain J., Peleg A.Y. Treatment of acinetobacter infections. Clin. Infect. Dis. 2010;51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- Fournier P.E., Vallenet D., Barbe V., Audic S., Ogata H., Poirel L., Richet H., Robert C., Mangenot S., Abergel C. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard T.D., Huang C.C., Meng E.C., Pettersen E.F., Couch G.S., Morris J.H., Ferrin T.E. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon Y., Jimenez-Fernandez A., La Rosa R., Espinosa Portero R., Krogh Johansen H., Matzov D., Eyal Z., Bashan A., Zimmerman E., Belousoff M. Structure of Pseudomonas aeruginosa ribosomes from an aminoglycoside-resistant clinical isolate. Proc. Natl. Acad. Sci. U S A. 2019;116:22275–22281. doi: 10.1073/pnas.1909831116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon Y., Matzov D., Eyal Z., Bashan A., Zimmerman E., Kjeldgaard J., Ingmer H., Yonath A. Exit tunnel modulation as resistance mechanism of S. aureus erythromycin resistant mutant. Sci. Rep. 2019;9:11460. doi: 10.1038/s41598-019-48019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel J., Burnside C., Mignot I., Leibundgut M., Boehringer D., Ban N. The complete structure of the Mycobacterium smegmatis 70S ribosome. Cell Rep. 2017;20:149–160. doi: 10.1016/j.celrep.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Howard A., O'Donoghue M., Feeney A., Sleator R.D. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James N.R., Brown A., Gordiyenko Y., Ramakrishnan V. Translational termination without a stop codon. Science. 2016;354:1437–1440. doi: 10.1126/science.aai9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner L., Starosta A.L., Terry D.S., Mikolajka A., Filonava L., Yusupov M., Blanchard S.C., Wilson D.N., Yusupova G. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc. Natl. Acad. Sci. U S A. 2013;110:3812–3816. doi: 10.1073/pnas.1216691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa A., Boehringer D., Leibundgut M., Ban N. Structures of the E. coli translating ribosome with SRP and its receptor and with the translocon. Nat. Commun. 2016;7:10471. doi: 10.1038/ncomms10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb H.C., Higueruelo A.P., Ochoa-Montano B., Pitt W.R., Ascher D.B., Blundell T.L. Arpeggio: a web server for calculating and visualising interatomic interactions in protein structures. J. Mol. Biol. 2017;429:365–371. doi: 10.1016/j.jmb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusainov I., Vicens Q., Bochler A., Grosse F., Myasnikov A., Menetret J.F., Chicher J., Marzi S., Romby P., Yusupova G. Structure of the 70S ribosome from human pathogen Staphylococcus aureus. Nucleic Acids Res. 2016;44:10491–10504. doi: 10.1093/nar/gkw933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski R.A., Swindells M.B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Li W., Ward F.R., McClure K.F., Chang S.T., Montabana E., Liras S., Dullea R.G., Cate J.H.D. Structural basis for selective stalling of human ribosome nascent chain complexes by a drug-like molecule. Nat. Struct. Mol. Biol. 2019;26:501–509. doi: 10.1038/s41594-019-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Fredrick K. Intersubunit bridges of the bacterial ribosome. J. Mol. Biol. 2016;428:2146–2164. doi: 10.1016/j.jmb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Blanchard J.S. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 2005;105:477–498. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- Mohan S., Donohue J.P., Noller H.F. Molecular mechanics of 30S subunit head rotation. Proc. Natl. Acad. Sci. U S A. 2014;111:13325–13330. doi: 10.1073/pnas.1413731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefour K., Frieden J., Hurst S., Helmich C., Headley D., Martin M., Boyle D.A. Acinetobacter baumannii: an emerging multidrug-resistant pathogen in critical care. Crit. Care Nurse. 2008;28:15–25. quiz 26. [PubMed] [Google Scholar]
- Morgan C.E., Huang W., Rudin S.D., Taylor D.J., Kirby J.E., Bonomo R.A., Yu E.W. Cryo-electron microscopy structure of the acinetobacter baumannii 70S ribosome and implications for new antibiotic development. mBio. 2020;11 doi: 10.1128/mBio.03117-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane T., Kimanius D., Lindahl E., Scheres S.H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife. 2018;7:e36861. doi: 10.7554/eLife.36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noeske J., Wasserman M.R., Terry D.S., Altman R.B., Blanchard S.C., Cate J.H. High-resolution structure of the Escherichia coli ribosome. Nat. Struct. Mol. Biol. 2015;22:336–341. doi: 10.1038/nsmb.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J.M., Brodersen D.E., Clemons W.M., Jr., Tarry M.J., Carter A.P., Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F., Endimiani A., Bonomo R.A. Why are we afraid of Acinetobacter baumannii? Expert Rev. Anti. Infect. Ther. 2008;6:269–271. doi: 10.1586/14787210.6.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F., Hujer A.M., Hujer K.M., Decker B.K., Rather P.N., Bonomo R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pioletti M., Schlunzen F., Harms J., Zarivach R., Gluhmann M., Avila H., Bashan A., Bartels H., Auerbach T., Jacobi C. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 2001;20:1829–1839. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- Polikanov Y.S., Melnikov S.V., Soll D., Steitz T.A. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat. Struct. Mol. Biol. 2015;22:342–344. doi: 10.1038/nsmb.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potron A., Poirel L., Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int. J. Antimicrob. Agents. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Ratje A.H., Loerke J., Mikolajka A., Brunner M., Hildebrand P.W., Starosta A.L., Donhofer A., Connell S.R., Fucini P., Mielke T. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–716. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A., Demeshkina N., Khusainov I., Westhof E., Yusupov M., Yusupova G. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016;7:10457. doi: 10.1038/ncomms10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer A., Kaminishi T., Ochoa-Lizarralde B., Dhimole N., Zhou S., Lopez-Alonso J.P., Connell S.R., Fucini P. Structural characterization of an alternative mode of tigecycline binding to the bacterial ribosome. Antimicrob. Agents Chemother. 2015;59:2849–2854. doi: 10.1128/AAC.04895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluenzen F., Tocilj A., Zarivach R., Harms J., Gluehmann M., Janell D., Bashan A., Bartels H., Agmon I., Franceschi F. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Schlunzen F., Zarivach R., Harms J., Bashan A., Tocilj A., Albrecht R., Yonath A., Franceschi F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuwirth B.S., Day J.M., Hau C.W., Janssen G.R., Dahlberg A.E., Cate J.H., Vila-Sanjurjo A. Structural analysis of kasugamycin inhibition of translation. Nat. Struct. Mol. Biol. 2006;13:879–886. doi: 10.1038/nsmb1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T., Kopp J., Guex N., Peitsch M.C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohmen D., Chiba S., Shimokawa-Chiba N., Innis C.A., Berninghausen O., Beckmann R., Ito K., Wilson D.N. Structure of the Bacillus subtilis 70S ribosome reveals the basis for species-specific stalling. Nat. Commun. 2015;6:6941. doi: 10.1038/ncomms7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Thompson R.F., Iadanza M.G., Hesketh E.L., Rawson S., Ranson N.A. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nat. Protoc. 2018;14:100–118. doi: 10.1038/s41596-018-0084-8. [DOI] [PubMed] [Google Scholar]
- Tsai A., Uemura S., Johansson M., Puglisi E.V., Marshall R.A., Aitken C.E., Korlach J., Ehrenberg M., Puglisi J.D. The impact of aminoglycosides on the dynamics of translation elongation. Cell Rep. 2013;3:497–508. doi: 10.1016/j.celrep.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Pulk A., Wasserman M.R., Feldman M.B., Altman R.B., Cate J.H., Blanchard S.C. Allosteric control of the ribosome by small-molecule antibiotics. Nat. Struct. Mol. Biol. 2012;19:957–963. doi: 10.1038/nsmb.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.N. The A-Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 2009;44:393–433. doi: 10.3109/10409230903307311. [DOI] [PubMed] [Google Scholar]
- Wilson D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- Wimberly B.T., Brodersen D.E., Clemons W.M., Jr., Morgan-Warren R.J., Carter A.P., Vonrhein C., Hartsch T., Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- Wright H., Bonomo R.A., Paterson D.L. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin. Microbiol. Infect. 2017;23:704–712. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Yang K., Chang J.Y., Cui Z., Li X., Meng R., Duan L., Thongchol J., Jakana J., Huwe C.M., Sacchettini J.C. Structural insights into species-specific features of the ribosome from the human pathogen Mycobacterium tuberculosis. Nucleic Acids Res. 2017;45:10884–10894. doi: 10.1093/nar/gkx785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.Q., Palovcak E., Armache J.P., Verba K.A., Cheng Y., Agard D.A. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CryoEM motion-corrected micrographs generated in this study are available in EMPIAR, along with extracted particle stacks (EMPIAR-10406 and 10407 for amikacin and tigecycline respectively). CryoEM multibody refinement maps are available in the EMDB for amikacin (EMD-10809 (50S), EMD-10869 (30S body) and EMD-10892 (30S head)) and for tigecycline (EMD-10898 (50S), EMD-10914 (30S body) and EMD-10915 (30S head)). Corresponding atomic models are available in the PDB for amikacin (6YHS (50S), 6YPU (30S body), and 6YS5 (30S head)) and for tigecycline (6YSI (50S), 6YT9 (30S body), and 6YTF (30S head)). Half-maps, masks used for multibody refinement and post-processing, and pre-multibody consensus reconstructions are all available as part of the EMDB entries. See Key Resources Table for more details.