Abstract
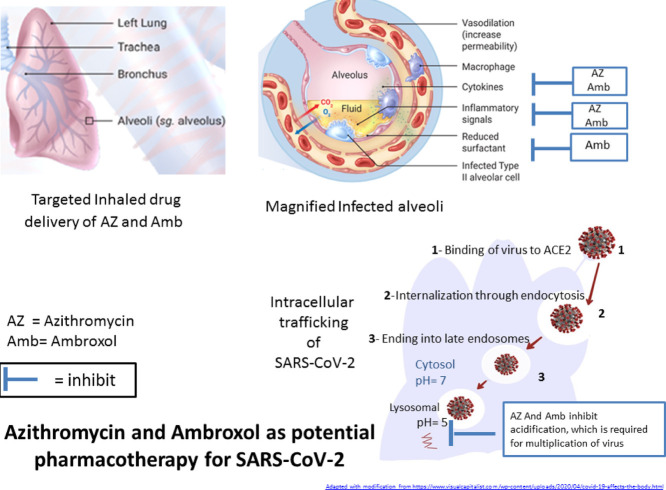
Knowing the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to bind to the angiotensin-converting enzyme 2 (ACE2) receptor and to enter cells via endocytosis paved the way for repositioning of old drugs as potential treatment of COVID-19, the disease caused by SARS-CoV-2 infection. This paper highlights the potential of azithromycin and ambroxol to treat COVID-19. Azithromycin and ambroxol share lysosomotropic characteristics, i.e. they penetrate and accumulate inside the late endosomes and lysosomes and may possibly interfere with multiplication of the virus inside cells. In addition, both of these drugs have anti-inflammatory effects. Ambroxol has a proven antiviral effect and a unique stimulatory action on the secretion of surfactant by alveolar type II cells, the main target of SARS-CoV-2. Surfactant may form a fundamental defence mechanism against the virus. Involvement of nasal epithelial cells in SARS-CoV-2 entry suggested advantageous use of inhaled drug delivery of these two drugs over the use of systemic administration. Inhaled drug delivery could aid in targeting the drug to the exact site of action with little or no side effects. To conclude, administration of these two drugs using a special drug delivery system provides two kinds of drug targeting: (i) tissue targeting through using an inhaled drug delivery system to achieve high drug concentrations at the respiratory epithelial tissue that overexpress the ACE2 receptor for virus binding; and (ii) cellular targeting of the virus in the acidic vesicles (late endosomes and lysosomes), which represent the fate of endocytic viruses.
Keywords: SARS-CoV-2, COVID-19, Azithromycin, Ambroxol, Lysosomotropic agent
Graphical abstract
1. Introduction
Coronaviruses (CoVs) belong to the family Coronaviridae that consists of four genera, α-CoVs, β-CoVs, γ-CoVs and δ-CoVs. The viruses responsible for the outbreak in China in 2003, known as severe acute respiratory syndrome coronavirus (SARS-CoV), as well as the second outbreak caused by Middle East respiratory syndrome coronavirus (MERS-CoV) that caused severe acute respiratory disease in the Middle East in 2012 are β-CoVs [1].
In the last week of 2019, a novel coronavirus, now know as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), caused an outbreak in Wuhan, China. It is believed that it started in a seafood market and then spread very rapidly through Wuhan City and China. The disease caused by this virus has been named coronavirus disease 2019 (COVID-19). SARS-CoV-2 spread very quickly in China and then to many other countries, causing a life-threatening global pandemic.
Clinical features of COVID-19 include fever, cough and pneumonia leading to death in certain cases [2]. Reported deaths in Europe have increased in a way that every effort should be made to work together to fight the disease. On 12 March 2020, the World Health Organization (WHO) announced COVID-19 as a worldwide pandemic. To date, millions of people worldwide have been infected with SARS-CoV-2 and hundreds of thousands have died due to COVID-19.
Many pharmacological and non-pharmacological treatments have been tried and a total of 238 registered clinical trials were conducted before the end of February 2020, some of them including repositioning of old drugs (https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/). Several reports suggested lysosomotropic agents such as chloroquine and hydroxychloroquine as potential pharmacological treatments. Indeed, hydroxychloroquine has been used in China and other countries and it has been shown that hydroxychloroquine is effective against SARS-CoV-2 in vitro [3].
2. Lysosomotropic agents
Lysosomotropic agents are cationic (basic) drugs that are protonated and become trapped inside the late endosomes or lysosomes. This process is known as lysosomal trapping where any lipophilic (logP > 1) or amphiphilic drug with ionisable amines (pKa > 6) can accumulate in lysosomes. This process contributes to pre-systemic extraction of drugs by lysosome-rich organs such as the liver and lungs [4].
The pH gradient between the neutral cytosol (pH 7.0–7.2) and the acidic lysosome (pH 4.5–5) is the major driving force delivering lysosomotropic drugs into the lysosomes via passive diffusion [5]. This is followed by a protonation step of basic groups in the acidic environment of lysosomes or any acidic counterparts such as late endosomes, which converts the neutral molecule into a protonated molecule that cannot diffuse out of the lipid bilayer. Lysosomal trapping during cellular drug uptake leads to extremely high drug concentrations within the lysosome in comparison with the cytosol [6].
2.1. Endocytosis as a mode of cellular uptake of coronaviruses
Briefly, endocytosis acts as the main mechanism for CoV uptake into various cell types. However, of the different endocytosis routes, clathrin-mediated endocytosis and cathepsin-mediated S protein cleavage are the two critical endocytic pathways for viral entrance and infection of SARS-CoV-2 [7].
Following endocytosis, internalised cargoes enter the early endosome where they are either recovered for recycling to the plasma membrane or are delivered to the late endosome that fuses with the lysosome for subsequent degradation [8]. The main functions of the endocytic pathways are for retrieval and recycling of internalised cargo proteins. These processes play key roles in the pathophysiology of some human diseases such as viral infections and neurodegenerative diseases, e.g. Parkinson's disease [9,10].
Possible involvement of the endocytic pathways as a cellular uptake mechanism of the newly emerging SARS-CoV-2 has been reported [11]. It is now known that SARS-CoV-2 can enter host cells utilising the same receptor as SARS-CoV, namely angiotensin-converting enzyme 2 (ACE2) [12]. ACE2 receptors are present in the epithelium of the human lungs and small intestine [13]. However, very recently, ACE2 receptors were also identified in the epithelial cells of the nose and upper respiratory tract [14]. Another recent study showed that although SARS-CoV-2 binds to the same ACE2 receptor as SARS-CoV, binding in the case of SARS-CoV-2 is with higher affinity than for SARS-CoV [15]. Knowing that SARS-CoV-2 can enter cells through endocytosis may explain the effectiveness of some lysosomotropic agents such as chloroquine and hydroxychloroquine [16,17].
2.2. The importance of autophagy
Autophagy is a highly conserved process in which intracellular components such as damaged organelles and protein aggregates are sequestered into a double-membrane vesicle called the autophagosome. The autophagosome then fuses with the lysosome to form the autolysosome for degradation and ultimate recycling of the resulting macromolecules [18,19]. Autophagy plays a vital role as a host defence mechanism against viral infections [20]. In the case of MERS-CoV, some viral proteins lead to the induction of incomplete autophagy, however further work is needed to confirm the role of autophagy in the antiviral immune response [21,22].
3. Repositioning or repurposing of two old drugs
Owing to the high risk and rapid spread of COVID-19, there is an urgent need for an approved drug in order to save the lives of millions of people. Repositioning of old drugs is a good approach to introduce drugs in a short time without waiting for lengthy pre-clinical and clinical studies. This article suggests the repositioning of two drugs, namely azithromycin and ambroxol.
3.1. Azithromycin
Azithromycin, which is a member of the macrolide class of antibiotics used for infectious diseases, was approved by the US Food and Drug Administration (FDA) in 2002. Two characteristics of azithromycin proposed it as a potential therapy for SARS-CoV-2 infection.
First, azithromycin has favourable physicochemical properties. It is a dicationic drug with high lipid solubility (logP = 3.03) that can accumulate in the acidic cellular compartment, meaning it can be considered a lysosomotropic agent. Indeed, azithromycin has distinctive effects on endocytosis; it not only interferes with the acidification process but also helps in dissecting various steps of the endocytic apparatus, including fusogenicity with lysosomes [23].
Second, azithromycin has an antiproliferative and autophagic effect on airway smooth muscle [24]. In addition, azithromycin inhibits arachidonic acid release and prostaglandin E2 synthesis, with consequent impairment of lysosome function that may lead to an anti-inflammatory effect [25].
An inhaled formulation of azithromycin may be advantageous. It can be prepared as a dry powder for inhalation using spray-drying technology with the aid of a suitable diluent. A dry powder formulation of azithromycin was successfully achieved with good inhaler characteristics suitable for inhalation delivery [26].
Very recently, supportive evidence came from French studies about the potential benefit of azithromycin in treating COVID-19. These studies indicated that using azithromycin in combination with hydroxychloroquine resulted in a beneficial effect in the treatment of COVID-19 at an early stage and might reduce the contagiousness of the disease [27,28]. However, these studies were observational studies conducted on small numbers of patients and there are arguments about the safety of such a combination. Nevertheless, there is a need for well-designed controlled studies to confirm the assumed role of azithromycin.
3.2. Ambroxol
Ambroxol is a mucolytic agent used in the treatment of respiratory diseases. Ambroxol is a basic (pKa = 9.01) cationic drug with lipophilic properties (logP = 2.9), enabling it to act as a lysosomotropic agent. In addition, ambroxol exhibits a novel mechanism by accumulating in lamellar bodies and acting as a lysosomal secretagogue. This may also play a role in its use for lysosomal storage diseases [29]. Moreover, ambroxol can induce autophagy, which may have a role in fighting SARS-CoV-2 [30].
The main effect of ambroxol is on mucus regulation, however a wide range of pharmacological effects of ambroxol have been confirmed, including anti-inflammatory, reduction of arachidonic acid metabolites and pro-inflammatory cytokines, and antioxidant properties. In addition, ambroxol aids in the enhancement of local defence molecules involved in respiratory viral replication [31].
It has been reported that SARS-CoV-2 binds to alveolar type 2 (AT2) epithelial cells where ACE2 receptors are highly expressed [32]. AT2 cells are responsible for the production of surfactant. One mechanism of action of ambroxol is to act specifically on AT2 cells and to stimulate the release of surfactant, which aids in preventing influenza virus multiplication and in maintaining alveolar function and preventing alveolar collapse [33].
The antiviral effect of ambroxol may be due to upregulation of natural inhibitors of proteases that represents a potential therapeutic approach to suppress viral airway replication. A study conducted on rats showed that treatment of animals with ambroxol prior to infection with influenza A virus led to a substantial suppression of virus multiplication as well as improved survival [33].
Despite the presence of registered clinical trials on the prodrug bromhexine, the precursor of ambroxol (https://clinicaltrials.gov/ct2/show/NCT04355026), there is no registered clinical trial on ambroxol in COVID-19. Interestingly, one Chinese study mentioned the use of ambroxol as symptomatic treatment of cough in children with COVID-19 [34]. In addition, there are three case reports mentioning the use of ambroxol as symptomatic treatment for relief of phlegm [35,36].
As mentioned previously, there is evidence that SARS-CoV-2 may invade host cells through binding to ACE2 receptors present in the nasal cavity. Accordingly, inhaler administration of ambroxol may represent an efficient delivery method of ambroxol to the target site with fewer systemic side effects. Notably, ambroxol inhalation has been approved as an efficient and convenient therapy for other respiratory diseases [37] and is available in some countries as an ampule for inhalation.
4. Conclusion
Both azithromycin and ambroxol have lysosomotropic properties, allowing these two drugs to block virion infectivity. In addition, they have anti-inflammatory effects, while ambroxol has an additional proven antiviral effect and a surfactant stimulatory effect that may contribute in the innate immune mechanism against the virus.
The point highlighted here is the necessity of exploiting recent knowledge about the involvement of nasal epithelial cells in SARS-CoV-2 entry as well as knowledge of the involvement of endocytosis as a cellular uptake mechanism for SARS-CoV-2. This work suggests the repositioning of two old drugs, namely azithromycin and ambroxol, with the advantageous use of inhaler drug delivery over the use of systemic administration. Further investigations are required to confirm the effectiveness of these two drugs. It is worth mentioning here that both of these candidates (azithromycin and ambroxol) lack the serious side effects encountered with other lysosomotropic agents such as chloroquine and hydroxychloroquine.
Acknowledgment
Special thanks to Dr Moustafa Alissa Alkhalaf for interesting scientific discussion.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Zheng Y, Shang J, Yang Y, Liu C, Wan Y, Geng Q, et al. Lysosomal proteases are a determinant of coronavirus tropism. J Virol. 2018;92:e01504–e01518. doi: 10.1128/JVI.01504-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazmi F, Hensley T, Pope C, Funk RS, Loewen GJ, Buckley DB, et al. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells) Drug Metab Dispos. 2013;41:897–905. doi: 10.1124/dmd.112.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mindell JA. Lysosomal acidification mechanisms. Ann Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt MV, Lienau P, Fricker G, Reichel A. Quantitation of lysosomal trapping of basic lipophilic compounds using in vitro assays and in silico predictions based on the determination of the full pH profile of the endo-/lysosomal system in rat hepatocytes. Drug Metab Dispos. 2019;47:49–57. doi: 10.1124/dmd.118.084541. [DOI] [PubMed] [Google Scholar]
- 7.Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen PJ, Steinberg F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol. 2018;19:679–696. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- 9.Schreij AM, Fon EA, McPherson PS. Endocytic membrane trafficking and neurodegenerative disease. Cell Mol Life Sci. 2016;73:1529–1545. doi: 10.1007/s00018-015-2105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nour AM, Modis Y. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol. 2014;24:449–454. doi: 10.1016/j.tcb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sungnak W, Huang N, Bécavin C, Berg M; HCA Lung Biological Network. SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. ArXiv March 2020 Mar 13;arXiv:2003.06122v1 [Preprint].
- 15.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney J. Chloroquine as a potential treatment and prevention measure for the 2019 novel coronavirus: a review. Preprints. 2020 doi: 10.20944/preprints202003.0275.v1. [DOI] [Google Scholar]
- 18.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3:588–596. doi: 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Wang K, Xing Y, Tu J, Yang X, Zhao Q, et al. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun. 2019;10:5770. doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP, et al. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281:86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 24.Stamatiou R, Paraskeva E, Boukas K, Gourgoulianis KI, Molyvdas P-A, Hatziefthimiou AA. Azithromycin has an antiproliferative and autophagic effect on airway smooth muscle cells. Eur Respir J. 2009;34:721–730. doi: 10.1183/09031936.00089407. [DOI] [PubMed] [Google Scholar]
- 25.Nujić K, Banjanac M, Munić V, Polančec D, Eraković Haber V. Impairment of lysosomal functions by azithromycin and chloroquine contributes to anti-inflammatory phenotype. Cell Immunol. 2012;279:78–86. doi: 10.1016/j.cellimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wang X, Lin X, Liu X, Tian B, Tang X. High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int J Pharm. 2010;395:205–214. doi: 10.1016/j.ijpharm.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Gautret P, Lagier JC, Parola P, Van Thuan Hoang Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fois G, Hobi N, Felder E, Ziegler A, Miklavc P, Walther P, et al. A new role for an old drug: ambroxol triggers lysosomal exocytosis via pH-dependent Ca2+ release from acidic Ca2+ stores. Cell Calcium. 2015;58:628–637. doi: 10.1016/j.ceca.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Choi SW, Gu Y, Peters RS, Salgame P, Ellner JJ, Timmins GS, et al. Ambroxol induces autophagy and potentiates rifampin antimycobacterial activity. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01019-18. e01019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beeh KM, Beier J, Esperester A, Paul LD. Antiinflammatory properties of ambroxol. Eur J Med Res. 2008;13:557–562. [PubMed] [Google Scholar]
- 32.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang B, Yao DF, Ohuchi M, Ide M, Yano M, Okumura Y, et al. Ambroxol suppresses influenza-virus proliferation in the mouse airway by increasing antiviral factor levels. Eur Respir J. 2002;19:952–958. doi: 10.1183/09031936.02.00253302. [DOI] [PubMed] [Google Scholar]
- 34.Shen K-L, Yang Y-H, Jiang R-M, Wang T-Y, Zhao D-C, Jiang Y, et al. Updated diagnosis, treatment and prevention of COVID-19 in children: experts’ consensus statement (condensed version of the second edition) World J Pediatr. 2020;16:232–239. doi: 10.1007/s12519-020-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92:461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X-X. Therapeutic effect of inhaling ambroxol hydrochloride in treatment of infants with bronchopneumonia. Journal of Xinxiang Medical College. 2007:5. http://en.cnki.com.cn/Article_en/CJFDTotal-XXYX200705027.htm [accessed 8 October 2020] [Google Scholar]