Abstract
Rationale & Objective
Kidney biopsy data inform us about pathologic processes associated with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We conducted a multicenter evaluation of kidney biopsy findings in living patients to identify various kidney disease pathology findings in patients with coronavirus disease 2019 (COVID-19) and their association with SARS-CoV-2 infection.
Study Design
Case series.
Setting & Participants
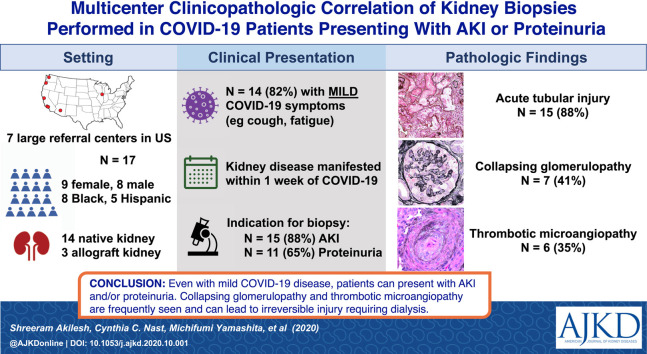
We identified 14 native and 3 transplant kidney biopsies performed for cause in patients with documented recent or concurrent SARS-CoV-2 infection treated at 7 large hospital systems in the United States.
Observations
Men and women were equally represented in this case series, with a higher proportion of Black (n = 8) and Hispanic (n = 5) patients. All 17 patients had SARS-CoV-2 infection confirmed by reverse transcriptase–polymerase chain reaction, but only 3 presented with severe COVID-19 symptoms. Acute kidney injury (n = 15) and proteinuria (n = 11) were the most common indications for biopsy and these symptoms developed concurrently or within 1 week of COVID-19 symptoms in all patients. Acute tubular injury (n = 14), collapsing glomerulopathy (n = 7), and endothelial injury/thrombotic microangiopathy (n = 6) were the most common histologic findings. 2 of the 3 transplant recipients developed active antibody-mediated rejection weeks after COVID-19. 8 patients required dialysis, but others improved with conservative management.
Limitations
Small study size and short clinical follow-up.
Conclusions
Cases of even symptomatically mild COVID-19 were accompanied by acute kidney injury and/or heavy proteinuria that prompted a diagnostic kidney biopsy. Although acute tubular injury was seen among most of them, uncommon pathology such as collapsing glomerulopathy and acute endothelial injury were detected, and most of these patients progressed to irreversible kidney injury and dialysis.
Index Words: Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), acute kidney injury (AKI), renal complications of COVID-19, collapsing glomerulopathy, thrombotic microangiopathy, kidney biopsy, allograft biopsy, antibody-mediated rejection (AMR), renal pathology, case series
Graphical abstract
Plain-Language Summary.
Acute kidney injury (AKI) is common in patients with coronavirus disease 2019 (COVID-19). We undertook a multicenter study to evaluate kidney biopsy findings in living patients to identify different kidney disease pathology in patients with COVID-19. Most patients in this case series developed AKI concurrent with mild COVID-19 symptoms. AKI and proteinuria were the most common indications for biopsy. Both common and rare pathologic processes such as acute tubular injury, collapsing glomerulopathy, and endothelial injury/thrombotic microangiopathy were the most common histologic findings. Two of the 3 transplant recipients developed active antibody-mediated rejection weeks after COVID-19. These data suggest that even symptomatically mild COVID-19 can be associated with AKI and/or heavy proteinuria and may warrant diagnostic kidney biopsy.
Since its identification, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its resulting disease, coronavirus disease 2019 (COVID-19), has grown into a worldwide pandemic with devastating medical and financial impacts. Though the primary targets of injury are the lungs and airways, heart and kidney involvement are common and portend a worse prognosis.1, 2, 3, 4 Pre-existing kidney disease or diseases known to contribute to decreased kidney function such as hypertension and diabetes are risk factors for an aggressive clinical course.2 , 5, 6, 7, 8 De novo kidney manifestations in SARS-CoV-2–infected patients include acute kidney injury (AKI), proteinuria, and hematuria.3 , 9 Various mechanisms have been proposed for kidney injury, including ischemia related to severe pulmonary injury, high levels of circulating proinflammatory cytokines (cytokine storm) and bradykinin, and possible direct infection of the renal parenchyma by SARS-CoV-2. However, very few patients with COVID-19 undergo kidney biopsy to identify the pathologic processes affecting the kidney because management of pulmonary disease and infection precautions often take precedence. Therefore, we aggregated kidney biopsies performed on patients with documented COVID-19 from multiple large medical centers in the United States to identify clinicopathologic correlations of decreased kidney function.
Methods
Identification of Kidney Biopsies of Patients With COVID-19
A request for collaboration and case contribution was sent to renal pathologists at large medical centers. When cases were identified at each institution, biopsy and electronic health record review were performed locally as human subjects exempt research with the approval of waiver of consent by local institutional review boards (IRBs): University of Washington (6 cases; STUDY00010368), Oregon Health Sciences University (2 cases; STUDY00017467), Cedars-Sinai Medical Center (3 cases; STUDY00000903); University of Chicago (1 case; IRB20-1260), University of Arizona (2 cases; protocol 2005664752), Stanford University (1 case; IRB 43782), and University of California Los Angeles (2 cases; IRB 20-001635).
APOL1 Genotyping
For case 1, genetic testing was performed at GeneDx Laboratories (Gaithersburg, MD). For case 6, genomic DNA was isolated from ethylenediaminetetraacetic acid–anticoagulated blood with the PureLink Genomic DNA kit (ThermoFisher Scientific). The region of the apolipoprotein L1 gene (APOL1) containing the G1 and G2 risk alleles was amplified by polymerase chain reaction (PCR) and the product was sequenced by automated sequencing in 2 reactions. Sequence data were analyzed by the most recent version of the Mutation Surveyor software (SoftGenetics).
Immunohistochemistry and In Situ Hybridization for SARS-CoV-2
For SARS-CoV-2 nucleocapsid protein, formalin-fixed paraffin-embedded tissue sections were immunohistochemically stained with an antibody to SARS-CoV nucleocapsid protein (catalogue #40143-T62; Sinobiological) at 1:2,000 dilution on Leica Bond III instruments after 20 minutes of antigen retrieval with epitope retrieval 2, followed by Leica Bond Polymer Refine detection chemistry. For RNA in situ hybridization, formalin-fixed paraffin-embedded tissue was stained with a SARS-CoV-2 probe (ACD Bio RNAscope Probe - V-nCoV2019-S; catalogue #848568) on Leica platform with 3,3′-diaminobenzidine, protease retrieval, and hybridization per manufacturer protocols.
Results
Clinical Presentation
We identified 17 patients (8 men and 9 women) with SARS-CoV-2 infection confirmed by reverse transcriptase (RT)-PCR. Clinical characteristics of patients in this case series are summarized in Table 1 . Additional information for baseline laboratory and clinical parameters, medication use, and pathologic findings are detailed in Table S1. Median age of our patients was 54 (range, 34-77) years. The case series predominantly consisted of Black (n = 8; 47%) and Hispanic (n = 5; 29%) patients. Most patients had only mild COVID-19–related symptoms such as upper respiratory tract infection, cough, fatigue, or low-grade fevers, though at least 3 required hospitalization for hypoxic pulmonary failure. The patients presented with markers of decreased kidney function or kidney damage affecting either their native (n = 14) or in allograft (n = 3) kidneys. AKI (n = 15; median serum creatinine, 5.5 [range, 0.7-12] mg/dL) and heavy proteinuria (n = 11) were the most common indications for biopsy. Hypertension was almost uniformly present in this case series, while diabetes and obesity were less frequent.
Table 1.
Clinical Characteristics of Patients With COVID-19 and Kidney Biopsies
| Case | Demographics | COVID-19 Symptoms | HTN & DM Status | Kidney Markers | Additional Pertinent Labs | Reason for Kidney Bx | Bx Diagnosis |
|---|---|---|---|---|---|---|---|
| Podocytopathy | |||||||
| 1 | 46-yo Black man | Acute respiratory failure | HTNa | Scr, 8.7 mg/dL; UPCR, 13.7 g/g; no hematuria | APOL1 G1/G1 | Nephrotic syndrome, AKI | Collapsing FSGS, ATN |
| 2 | 60-yo Black woman | None | HTN | Scr, 5.7 mg/dL; UPCR, 21 g/g; no hematuria | NA | Nephrotic-range proteinuria, AKI | Collapsing FSGS, ATN |
| 3 | 58-yo Black woman | None | HTN | Scr, 10.2 mg/dL; UPCR, 20 g/g; no hematuria | NA | Nephrotic syndrome, AKI | Collapsing FSGS, ATN, AIN |
| 4 | 59-yo Black man | Abdominal pain, weakness, fever | Both | Scr, 11.9 mg/dL; proteinuria, >12 g/d; no data on hematuria | NA | Nephrotic-range proteinuria, AKI | FSGS, ATN, AIN |
| 5 | 52-yo White woman | Loss of exercise tolerance, splenomegaly | Neither | Scr, 0.7 mg/dL; proteinuria, 20 g/d; no hematuria | Leukopenia | Nephrotic syndrome | Minimal change disease |
| Podocytopathy + Renal TMA | |||||||
| 6 | 44-yo Hispanic man | Mild upper respiratory symptoms | Neither | Scr, 12 mg/dL; UPCR, 11.4 g/g; no hematuria | APOL1 G2/G2 | Nephrotic-range proteinuria, AKI | Collapsing FSGS, EM evidence of endothelial injury, ATN, TIN |
| 7 | 58-yo Black man | Cough, fever, hypoxia | Neither | Scr, 11.3 mg/dL; proteinuria, 4 g/d; hematuria | NA | Nephrotic-range proteinuria, AKI | Collapsing FSGS, TMA, ATN |
| Clinically Overt TMAPodocyte Injury | |||||||
| 8 | 47-yo Black man | None | HTN | Scr, 6.6 mg/dL (on dialysis); UPCR, 7.6 g/g; hematuria | Hb, 7.6 g/dL; Plt, 226 × 103/μL | Concern for TMA, AKI | Arteriolar-prominent TMA, collapsing FSGS, ATN |
| 9 | 63-yo Black woman | Fatigue | HTN | Scr, 6 mg/dL; UPCR, 20 g/g; hematuria | Anemia; thrombocytopenia; negative ANA; normal C3 & C4; normal ADAMTS13 | Concern for TMA; oliguric AKI and nephrotic- range proteinuria | Arteriolar and glomerular-prominent TMA, collapsing FSGS, ATN |
| 10 | 77-yo Hispanic woman | None | Both | Scr, 3.99 mg/dL; UPCR, 13.41 g/g; no hematuria | C3, 51 μg/mL; normal C4; HP, <9 mg/dL; elevated D-dimer; LDH, 365 U/L; BNP, 397 pg/mL; ADAMTS13, 45%; Hb, 10.0 g/dL; Plt, 32 × 103/μL | Concern for TMA, AKI, nephrotic syndrome | Arteriolar-prominent TMA, FSGS, mesangial immune complex deposition, ATN |
| 11 | 34-yo Hispanic man | Fatigue, weakness, shortness of breath | HTN | Scr, 11.4 mg/dL; UPCR, 2.1 g/g; no data on hematuria | Hb, 8.6 g/dL; thrombocytopenia; schistocytes; elevated D-dimer | Concern for TMA | Arteriolar and glomerular-prominent TMA |
| AKI + Underlying Disease | |||||||
| 12 | 69-yo White woman | Upper respiratory symptoms | Both | Scr, 4.0 mg/dL; UPCR, 5.7 g/g; no data on hematuria | Urinary tract infection; Escherichia coli | Nephrotic-range proteinuria, AKI | Postinfectious GN, advanced diabetic nephropathy, ATN |
| 13 | 34-yo White woman | Cough and runny nose; no fever | Both | Scr, 1.26 mg/dL; UPCR, 7 g/g; proteinuria, 2.5 g/d; no hematuria | NA | Nephrotic syndrome, AKI | Advanced diabetic nephropathy with FSGS, ATN |
| 14 | 67-yo Hispanic woman | Hypoxia, fever to 100.4 °F | HTN | Scr, 1.42 mg/dL; UA, 1+ proteinuria; hematuria | LDH, 451 U/L; Elevated D-dimer | AKI | ATN with κ light chain staining bias |
| Allograft AKI | |||||||
| 15 | 47-yo Black woman | Sore throat, nasal congestion, anosmia, cough, malaise, pleuritic chest pain, fever | HTN | Scr, 1.63 mg/dL; UA, 2+ proteinuria; no hematuria | Newly elevated donor-specific antibodies against HLA-DR53, DP, DQ4 and B44 | AKI in transplant recipient | Active AMR |
| 16 | 54-yo Asian man | Acute respiratory failure, fever to 104 °F, nausea/vomiting | Both | Scr, 1.9→5.2 mg/dL; UPCR, 3 g/g; no hematuria | Hb, 9.7 g/dL; Plt, 109 × 103/μL; aPL negative | AKI, proteinuria, edema in a transplant recipient | Chronic active AMR, IgAN, FSGS, possible TMA |
| 17 | 42-yo Hispanic man | Cough, sore throat, anosmia, headache, arthralgia, fever to 100.5 °F | HTN | Scr, 1.27→ 1.43 mg/dL; UPCR, 0.15 g/g; no hematuria | No DSAs | AKI in a transplant kidney | ATN |
Note: Reference ranges for clinical values: LDH, 100-200 U/L; BNP, <167 pg/mL; HP, 26-185 mg/dL; Hb, 14-17.5 g/dL; Plt, 150-350 × 103/μL.
Abbreviations: AIN, acute interstitial nephritis; AKI, acute kidney injury; AMR, antibody-mediated rejection; ANA, antinuclear antibody; aPL, antiphospholipid antibody; APOL1, apolipoprotein L1 gene; ATN, acute tubular necrosis; BNP, brain natriuretic peptide; Bx, biopsy; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; DSAs, donor-specific antibodies; EM, electron microscopy; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; Hb, hemoglobin; HP, haptoglobin; HTN, hypertension; IgAN, immunoglobulin A nephropathy; Labs, laboratory test results; LDH, lactate dehydrogenase; NA, not available; Plt, platelet count; Scr, serum creatinine; TIN, tubulointerstitial nephritis; TMA, thrombotic microangiopathy; UA, urinalysis; UPCR, urinary protein-creatinine ratio; yo, year-old.
DM status not available.
The most severe AKI presentations were in the 6 patients who presented with severe hypertension (often >150/100 mm Hg; cases 6-11). Four of these (cases 8-11) had characteristic laboratory features of thrombotic microangiopathy (TMA), including anemia, thrombocytopenia, elevated serum lactate dehydrogenase level, and low haptoglobin level with normal ADAMTS13 (von Willebrand factor protease) activity. Three of 4 had additional conditions that may have contributed to TMA, including cytotoxic and/or illicit drug use and poorly controlled hypertension.
The first of these patients (case 8) presented with mental status changes and intracranial hemorrhage and ultrasound imaging revealed atrophic-appearing kidneys, suggesting long-standing poorly controlled blood pressure. The second patient (case 9) had mental status changes, normal complement levels, and had previously been treated with gemcitabine for adenocarcinoma. She presented with oliguric AKI and nephrotic-range proteinuria. The third patient (case 10) had evidence of activation of the alternative complement pathway (low serum C3 with normal C4 levels), which has been reported in patients with COVID-19.10 The fourth patient initially presented only with fatigue (case 11) and 2 days later COVID-19 was diagnosed. At that time, his serum creatinine level was 1.62 mg/dL, and 23 days later, his pulmonary symptoms had worsened and he was admitted for pulmonary edema and high blood pressure (165/87 mm Hg). His social history was significant for active cocaine use and he was being treated with gemcitabine, vinorelbine, and doxorubicin for primary refractory classic Hodgkin lymphoma (first diagnosed in 2017, followed by multiple cycles of chemotherapy with failure and progression). His serum creatinine level was 3.9 mg/dL on the day of admission to the hospital and he had evidence of hemolytic anemia (hemoglobin, 8.6 g/dL; hematocrit, 27%; schistocytes on blood smear (2+); activated partial thromboplastin time, 22.7 seconds; fibrinogen activity, 180 mg/dL; and D-dimer level, 7,546 ng/mL).
For the kidney transplant recipients, the original cause of kidney failure for case 15 was focal segmental glomerulosclerosis (FSGS) in the setting of HIV infection. She received a deceased donor allograft in 2015. Her course was complicated by acute vascular rejection approximately 10 months after transplantation. The patient was out of state when she was hospitalized with a 4-day history of COVID-19 symptoms and elevated serum creatinine level (baseline, 1.1 mg/dL). She eventually recovered without requiring intubation. Upon return to her home state and follow-up with her transplant nephrologist, her serum creatinine level was found to be persistently elevated with the appearance of several new high-titer donor-specific antibodies. This prompted a kidney biopsy and initiation of treatment for antibody-mediated rejection after the report was returned.
The original cause of kidney failure for case 16 was unknown, presumed due to undiagnosed hypertension or calculi. He received a deceased donor allograft in 2000 and his subsequent course was relatively uncomplicated without biopsy-diagnosed episodes of rejection. In 2019, he presented with elevated serum creatinine level increasing from his baseline of 1.5 to 2.2 mg/dL and increasing proteinuria (urinary protein-creatinine ratio [UPCR], 3 g/g). At that time, chronic transplant glomerulopathy with negative C4d and focal glomerulitis (g2) in the setting of a low positive HLA-DQ titer was biopsy diagnosed. In spring 2020, the patient presented with progressive hypoxemia requiring up to 4 L/min of oxygen by nasal canula (he never required intubation) and AKI. At this time, tacrolimus and mycophenolate mofetil treatments were discontinued and he was maintained on 10 mg/d of prednisone until he was stable enough to undergo biopsy approximately 6 weeks after COVID-19 symptom onset.
Patient 17 had kidney failure due to FSGS, and he received a living related kidney transplant 19 months before the diagnosis of COVID-19. His posttransplantation course had been uncomplicated. He developed AKI in the setting of mild COVID-19 symptoms.
There was often a delay between the onset of kidney symptoms or COVID-19 diagnosis and performance of the kidney biopsy. Reasons for this delay were varied but included an abundance of caution with infectious precautions at the onset of the pandemic (cases 5, 9, 16, and 17), hemodynamic instability (cases 1 and 8), observation during initial improvement that was followed by further decline (cases 2, 3, and 11), and scheduling hurdles (cases 12 and 15). These delays were not unique to a given institution and are likely comparable to biopsy-scheduling practices at large referral centers nationwide.
Pathologic Findings
Native Biopsies
The findings on kidney biopsy are summarized in Table 2 , with clinical follow-up summarized in Table 3 . Clinically, 15 of 17 patients presented with an acute increase in serum creatinine level, and 13 of these patients demonstrated histologic evidence of acute tubular injury in their biopsy specimens (Fig 1 A). Eleven patients demonstrated FSGS, which in 7 patients assumed the form of collapsing glomerulopathy (Fig 1B-D). One of these patients (case 6) had collapsing glomerulopathy diagnosed 3 years prior and had been clinically stable with a baseline serum creatinine level of 2 mg/dL and UPCR of 2 g/g. During his COVID-19 course, his serum creatinine increased to 12 mg/dL with a UPCR of 11.4 g/g. All 11 patients with FSGS, as well as the patient with minimal change disease diagnosed (case 5), presented with UPCR > 3 g/g, which correlated with diffuse podocyte foot-process effacement by electron microscopy. Two patients were tested and found to have high-risk APOL1 genotypes (cases 1 and 6).
Table 2.
Kidney Biopsy Findings in Patients With SARS-CoV-2 Infection
| Case | Diagnosis | ATN | TMA Features | Chronic Vascular Disease | LM Findings | IF Findings | EM Findings |
|---|---|---|---|---|---|---|---|
| Podocytopathy | |||||||
| 1 | Collapsing FSGS, ATNa | Present | None | Mild AS | 9 glomeruli: 55% global GS; 10% FSGS (1 collapsing); 75% IFTA | Not performed | Diffuse FPE; no immune deposits; no TRIs; no viral particles |
| 2 | Collapsing FSGS, ATN | Severe | None | Mod AS | 4 glomeruli: 25% global GS; 50% FSGS (2 collapsing); 20% IFTA | Negative | Segmental FPE; no TRIs; no viral particles |
| 3 | Collapsing FSGS, ATN, AINa | Severe | None | Severe AS | 25 glomeruli: 50% global GS; 24% FSGS (6 collapsing); 10% IFTA | Negative | 75% FPE; no TRIs; no viral particles |
| 4 | FSGS, ATN, AIN | Severe | None | Mod AS, severe arteriolosclerosis | 19 glomeruli: 42% global GS; 5% FSGS; 25% IFTA | Negative | Diffuse FPE; TRIs present; no viral particles |
| 5 | Minimal change disease | No | None | Minimal AS | 23 glomeruli: 17% global GS; 0% FSGS; 5% IFTA | Negative | Diffuse FPE; no TRIs; probable clathrin-coated vesicles in endothelial cells and podocytes |
| Podocytopathy + Renal TMA | |||||||
| 6 | Collapsing FSGS, EM evidence of endothelial injury, ATN, TIN | Present | EM evidence of endothelial injury | None | 4 glomeruli: 25% global GS; 25% FSGS (1 collapsing); mod IFTA | Negative | Diffuse FPE; mild subendothelial space expansion; no TRIs; no viral particles |
| 7 | Collapsing FSGS, TMA, ATNa | Severe | Thrombi in 1 glomerulus and arteriole | Mild AS | 21 glomeruli: 10% global GS; 15% FSGS (3 collapsing); 0% IFTA | Negative | Diffuse FPE; TRIs present; no viral particles |
| Clinically Overt TMAPodocyte Injury | |||||||
| 8 | Arteriolar-prominent TMA, collapsing FSGS, ATN | Present | Arterial fibrin thrombi, swollen endothelium | Mod AS | 14 glomeruli: 25% global GS; 7% FSGS (1 collapsing); 30% IFTA | Medulla only | Extensive FPE; variable subendothelial space expansion; no immune deposits; no TRIs; no viral particles |
| 9 | Arteriolar and glomerular-prominent TMA, FSGS, ATNa | Severe | Arterial and glomerular fibrin thrombi, segmental GBM duplication | Severe AS | 36 glomeruli: 0% global GS; 3% FSGS (1 collapsing); 10% IFTA | Negative | Diffuse FPE; endothelial swelling, ischemic wrinkling and occasional duplication of GBMs |
| 10 | Arteriolar-prominent TMA, FSGS, mesangial immune complex deposition, ATN | Severe | Arteriolar TMA | Severe AS | 10 glomeruli: 0% global GS; 50% FSGS; 30% IFTA | Segmental granular mesangial & capillary wall staining for polyclonal IgG (2+), IgM (1+), C1q (1+) | Diffuse FPE; mesangial immune deposits; no subendothelial expansion; TRIs present; probable multivesicular bodies in podocytes, no viral particles |
| 11 | Arteriolar and glomerular-prominent TMA | Mild | Arterial and glomerular fibrin thrombi, endothelial swelling, mesangiolysis, GBM duplication | None | 11 glomeruli: 0% global GS; 0% FSGS; 0% IFTA | Negative | Segmental FPE; marked subendothelial space expansion and accumulation of flocculent material; segmental duplication of GBMs |
| AKI + Underlying Disease | |||||||
| 12 | Postinfectious GN, advanced diabetic nephropathy, ATN | Severe | None | Mod AS, severe AH | 15 glomeruli: 28% global GS; 7% FSGS; 40% IFTA | Irregular coarse granular glomerular C3 (3+) | Subepithelial, paramesangial deposits; frequent FPE; GBM thickening and mesangial sclerosis; no TRIs; no viral particles |
| 13 | Advanced diabetic nephropathy, ATN | Severe | None | Severe AH | 20 glomeruli: 15% global GS; 15% FSGS; mild to mod IFTA | Negative | GBM thickening and mesangial sclerosis; no TRIs; no viral particles |
| 14 | ATNa | Present | None | Mild AS | 14 glomeruli: 14% global GS; 0% FSGS; 10% IFTA | κ light chain staining bias in tubular droplets and casts | Segmental FPE; no deposits or crystals; no TRIs; no viral particles |
| Allograft AKI | |||||||
| 15 | Active AMR | No | None | Mod AS, AH | 25 glomeruli: 10% global GS; 0% FSGS; 50% IFTA | C4d diffusely positive | Segmental FPE; no TRIs; no viral particles |
| 16 | Chronic active AMR, IgAN, FSGS, TMA | No | Single arterial thrombus | Severe AH | 4 glomeruli: 25% global GS; 50% FSGS; 50% IFTA | IgA (1-2+); C4d (positive in 10% of PTCs) | Subendothelial space expansion; segmental FPE; rare mesangial deposits; no TRIs; no viral particles |
| 17 | ATN | Mild | None | None | 19 glomeruli: 0% global GS; 0% FSGS; 0% IFTA | C4d negative | Not performed |
Abbreviations: AH, arteriolar hyalinosis; AIN, acute interstitial nephritis; AMR, antibody-mediated rejection; AS, arteriosclerosis; ATN, acute tubular necrosis/acute tubular injury; EM, electron microscopy; FPE, podocyte foot-process effacement; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; GS, glomerulosclerosis; IF, immunofluorescence; IFTA, interstitial fibrosis and tubular atrophy; IgAN, immunoglobulin A nephropathy; LM, light microscopy; Mod, moderate; PTC, peritubular capillary; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TIN, tubulointerstitial nephritis; TMA, thrombotic microangiopathy; TRIs, tubuloreticular inclusions.
SARS-CoV-2 positive by polymerase chain reaction at time of kidney biopsy.
Table 3.
Timing and Outcomes of Kidney Disease in Patients With COVID-19
| Case | Time From COVID-19 Presentation to: |
Kidney Biopsy Diagnosis | Treatment and/or Outcome at Time of Writing | |
|---|---|---|---|---|
| Kidney Manifestations | Kidney Biopsya | |||
| Podocytopathy | ||||
| 1 | Concurrent | 2 wkb | Collapsing FSGS, ATN | Dialysis |
| 2 | No COVID-19 symptoms | 4 wk | Collapsing FSGS, ATN | Unknown |
| 3 | No COVID-19 symptoms | 8 db | Collapsing FSGS, ATN, AIN | Dialysis, with improvement in pre-dialysis Scr |
| 4 | Concurrent | 11 d | FSGS, ATN, AIN | Unknown |
| 5 | Concurrent | 7 wk | Minimal change disease | Prednisone; full remission after 4 wk of treatment |
| Podocytopathy + Renal TMA | ||||
| 6 | 1 wk | 6 wk | Collapsing FSGS, EM evidence of endothelial injury, ATN, TIN | Dialysis |
| 7 | Concurrent | 4 db | Collapsing FSGS, TMA, ATN | Dialysis, no other specific treatment; Scr returned to 1.5 mg/dL |
| Clinically Overt TMAPodocyte Injury | ||||
| 8 | No COVID-19 symptoms | 25 d | Arteriolar-prominent TMA, Collapsing FSGS, ATN | Dialysis (symptomatically improved with BP control, but discharged to outpatient dialysis) |
| 9 | 3-5 d | 10-14 db | Arteriolar and glomerular-prominent TMA, collapsing FSGS, ATN | Dialysis |
| 10 | No COVID-19 symptoms | 3 d | Arteriolar-prominent TMA, FSGS, mesangial immune complex deposition, ATN | Dialysis |
| 11 | 2-3 d | 4-5 wk | Arteriolar and glomerular-prominent TMA | PLX, eculizumab, prednisone; remains on dialysis |
| AKI + Underlying Disease | ||||
| 12 | Unknown | 4 wk | Postinfectious GN, advanced diabetic nephropathy, ATN | Dialysis |
| 13 | Concurrent | 4 d | Advanced diabetic nephropathy, ATN | Stable and asymptomatic at the time of discharge with Scr of 1.1 mg/dL; volume status and rash had improved; never required dialysis |
| 14 | Concurrent | 5 db | ATN with κ light chain staining bias | Scr returned to 1 mg/dL; eventually diagnosed with Waldenström: FLC ratio of 15.1 (κ = 80.7 mg/dL [ref < 1.9 mg/L], λ = 5.35 mg/L [ref < 2.35 mg/L]) |
| Allograft AKI | ||||
| 15 | 6 wk | 6 wk | Active AMR | PLX + IVIG ×3, IV methylprednisolone followed by rituximab |
| 16 | Concurrent | 6 wk | Chronic active AMR, IgAN, FSGS, possible TMA | Held MMF, reduced tacrolimus and initiated low-dose prednisone (10 mg/d); Scr improved to 2.7 mg/dL |
| 17 | Concurrent | 7 wk | ATN | Scr returned to 1.3 mg/dL |
Abbreviations: AIN, acute interstitial nephritis; AKI, acute kidney injury; AMR, antibody-mediated rejection; ATN, acute tubular injury; BP, blood pressure; COVID-19, coronavirus disease 2019; EM, electron microscopy; FLC, free light chain; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; IgAN, immunoglobulin A nephropathy; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; PLX, plasma exchange; ref, reference range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Scr, serum creatinine; TIN, tubulointerstitial nephritis; TMA, thrombotic microangiopathy.
If no COVID-19 symptoms, the interval start is when SARS-CoV-2 positive by reverse transcriptase–polymerase chain reaction.
SARS-CoV-2 positive by polymerase chain reaction at time of kidney biopsy.
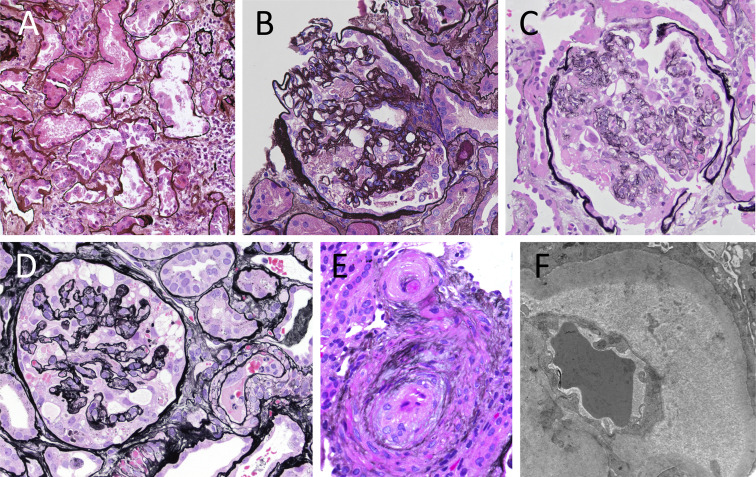
Figure 1.
Kidney biopsy findings from representative cases. (A) Case 7, acute tubular necrosis; (B) case 7, collapsing glomerulonephritis (GN); (C) case 6, collapsing GN; (D) case 9, collapsing GN and thrombotic microangiopathy (TMA); (E) case 8, TMA (A-E: Jones methenamine silver; original magnification, ×400); (F) case 8, TMA (electron microscopy; original magnification, ×20,900).
Six patients (cases 6-11) demonstrated evidence of acute endothelial cell injury by light and/or electron microscopy including all 4 clinically suspected of having acute TMA (cases 8-11). This ranged from mild injury with ultrastructural evidence of glomerular subendothelial space widening and/or loss of endothelial cell fenestrae to severe injury manifest by endothelial cell swelling and fibrin thrombi within glomerular hilar arterioles and small arteries evident by light microscopy (Fig 1D-F). Peritubular capillary thrombi were not seen in any cases in this series. Microangiopathic features were seen in the setting of collapsing glomerulopathy (cases 6-9) and/or with prominent arteriolar involvement in the background of severe hypertension (cases 8-11). Additional findings of acute interstitial nephritis, immunoglobulin A (IgA) nephropathy, mesangial immune complex deposition of uncertain significance, monoclonal gammopathy (later diagnosed as Waldenström macroglobulinemia), postinfectious glomerulonephritis, minimal change disease, and diabetic nephropathy were also identified (cases 3, 5, and 12-15).
Allograft Biopsies
Two of the 3 transplant recipients (cases 15 and 16) demonstrated microvascular inflammation and positive C4d staining in peritubular capillaries in association with elevated levels of donor-specific antibodies, meeting Banff criteria for active antibody-mediated rejection. The discovery of IgA nephropathy in case 16 was considered incidental de novo disease because the patient’s original cause of kidney failure 20 years prior was considered to be hypertension/calculi. Glomeruli in this case showed mild mesangial expansion and focal mesangial hypercellularity, but these findings could also be attributed to chronic and active antibody-mediated rejection. A third allograft biopsy specimen (case 17) showed acute tubular injury, and the patient’s serum creatinine level returned to baseline shortly after the biopsy.
Lack of Evidence for Direct Infection of the Kidney by SARS-CoV-2
Using electron microscopy, earlier reports have suggested direct viral infection of the kidney.11 , 12 However, unambiguous identification of viral particles in autopsy tissue is difficult due to post mortem degradation artifacts. We therefore reasoned that 3 criteria were required to establish direct viral infection of tissues: (1) viral particles of expected size (80-140 nm) with the electron-dense dots of the nucleocapsid cores and, when visible, spikes facing the lumen of vacuoles13 , 14; (2) viral particles present in the appropriate subcellular compartment (eg, intracisternal spaces of the endoplasmic reticulum and Golgi, cytoplasmic vesicles, and extracellular spaces) compatible with known viral replication and trafficking pathways15; and (3) orthogonal validation by immunohistochemistry or RNA in situ hybridization (with appropriate clinical validation and controls). These criteria were not met in any of our cases and therefore we could not establish direct viral infection of the kidney in the patients in this case series. Viral particles were not identified by ultrastructural examination in any of our cases. Electron microscopy revealed intracellular vesicular structures with diameters of ~100 nm, which by consensus expert opinion represented normal cytoplasmic organelles (eg, clathrin-coated vesicles and multivesicular bodies) and not true viral particles. In addition, immunohistochemistry for SARS-CoV-2 nucleocapsid and RNA in situ hybridization for viral genomes were negative for all 4 patient samples on which it was performed (cases 1, 5, 10, and 12).
Clinical Outcome and Follow-up
There were often several weeks of delay between kidney manifestations and the biopsy procedure, and most patients were RT-PCR negative for SARS-CoV-2 at the time of biopsy (Table 3). Because most patients had only mild COVID-19–related symptoms, treatment focused on managing their kidney disease diagnosed on biopsy, usually with diuretics and blood pressure control. This resulted in improvement in 7 of 12 patients; at least 8 patients required dialysis, including 6 of 7 patients with collapsing glomerulopathy diagnosed, and all 6 presenting with suspected or clinically confirmed TMA. Seven patients remain on dialysis at the time of writing.
Discussion
In this series of patients with COVID-19, the most frequent indications for biopsy were an acute increase in serum creatinine level (15/17; 88%), new onset of proteinuria (11/17; 65%), and hematuria (4/17; 24%). All patients’ kidney manifestations presented concurrently with or within 1 week of RT-PCR–confirmed SARS-CoV-2 infection, and most had only mild COVID-19 symptoms. However, there was often several weeks’ delay between the onset of COVID-19 symptoms or confirmed positive RT-PCR test results and the kidney biopsy procedure (Table 3). Therefore, most biopsies were performed after individuals were RT-PCR negative and considered to be of low infection risk.
Histologically, acute tubular injury was seen in the majority (14/17; 82%) of this biopsy series. The most striking finding is the high incidence of collapsing glomerulopathy (7/17; 41%), which has been reported by multiple groups in association with SARS-CoV-2 infection.16, 17, 18, 19, 20 The mechanism driving this acute glomerular injury process is possibly related to interferon production, a known trigger of collapsing glomerulopathy.21, 22, 23 It is less likely that direct podocyte infection is an etiologic factor because 3 of the 4 patients who were negative for virus by immunohistochemistry and/or in situ hybridization had podocytopathies (1 each with FSGS, collapsing glomerulopathy, and minimal change disease).
We were able to perform APOL1 genotyping for 1 Black patient (case 1) who was found to carry the high-risk G1/G1 genotype. Although we did not genotype the other patients in this case series, 6 of 7 patients with collapsing glomerulopathy were of Black race, consistent with other reports demonstrating a strong association between collapsing glomerulopathy in the setting of SARS-CoV-2 infection with high-risk APOL1 genotypes,17 , 19 , 24 a susceptibility that parallels its incidence in HIV-infected patients.24 , 25 The last patient with collapsing glomerulopathy (case 6) was a Hispanic individual from Mexico who was also found to have a high-risk G2/G2 APOL1 genotype, although the prevalence of homozygous high-risk APOL1 genotypes in mainland Hispanics is 0.1%.26
Acute TMA and more subtle ultrastructural evidence of acute endothelial cell injury were also common findings in this case series (6/17; 35%). Four of 6 of these patients had underlying hypertension, and 2 had prior or ongoing treatment with gemcitabine (1 of whom also used cocaine) and thus had potential additional drivers for TMA besides SARS-CoV-2 infection. Four patients’ biopsy specimens with TMA/endothelial injury also demonstrated collapsing glomerulopathy, and 2 allograft biopsy specimens showed endothelial cell injury in the setting of antibody-mediated rejection.
Interestingly, TMA has been associated with collapsing glomerulopathy,27, 28, 29, 30 and endothelial injury is the most common manifestation of antibody-mediated rejection.31 , 32 Thus, the frequent finding of acute TMA and more subtle ultrastructural evidence of acute endothelial cell injury appears to be the manifestation of the underlying disease processes in the patients in this case series. Endothelial injury and dysfunction are emerging mechanisms in severe COVID-19, and other groups have suggested that endothelial injury may be a manifestation of SARS-CoV-2 infection and contribute to kidney injury.11 , 33 , 34 Our series demonstrates that a broad clinical and pathologic spectrum of acute endothelial injury may exist even in the absence of significant COVID-19–related respiratory symptoms, and some of these changes can be attributed to co-existing diseases. It is possible that SARS-CoV-2 infection may exacerbate clinical situations predisposing to endothelial injury such as hypertension, antibody-mediated rejection, prothrombotic states, and endothelial toxins. Additional data will be helpful to determine whether this is an important clinical consideration.
To our knowledge, this is the first report of infection-related glomerulonephritis (case 12) in the setting of COVID-19. Infection-related glomerulonephritis is most commonly associated with bacterial infections but has been seen in association with viral infections such as parvovirus B19 and influenza virus H1N1.35, 36, 37 Our patient manifested kidney symptoms 19 days after testing positive for SARS-CoV-2, indicating that the timing of glomerulonephritis fits with the pathogenesis of postinfectious glomerulonephritis. However, this patient also had nodular diabetic nephropathy and a concurrent urinary tract infection, and it is possible that the urinary tract infection was a cause of or contributor to the infection-related glomerulonephritis. We also report a case of minimal change disease in 1 patient (case 5). In contrast to the Black patient with a high-risk (G1/G1) APOL1 genotype who presented with minimal change disease in a recent report,20 our patient is of White race. However, it is well appreciated that minimal change disease may manifest after viral infections or interferon treatment,38, 39, 40, 41 therefore representing another pathway by which SARS-CoV-2 infection may trigger a podocytopathy.
Two of the 3 kidney transplant recipients reported here developed new donor-specific antibodies and antibody-mediated rejection weeks after SARS-CoV-2infection, which raises the possibility of immune stimulation of alloantibody production during viral infection.42 , 43 In 1 of these patients (case 16), immunosuppression was reduced during acute COVID-19 hypoxemia, which may have predisposed to the development of antibody-mediated rejection. However, in the other patient (case 15), treatment for antibody-mediated rejection was initiated only after the diagnostic kidney biopsy was completed and in the setting of new high-titer anti-HLA∗DR53 antibodies. Emergence of de novo donor-specific antibodies has also been recently been reported in a pediatric heart transplant recipient who was infected with SARS-CoV-2.44 Kidney allograft recipients may therefore be at increased risk for developing de novo donor-specific antibodies and active antibody-mediated rejection after SARS-CoV-2 infection.
Direct viral infection of the kidney by SARS-CoV-2 has been proposed as a possible mechanism of kidney injury and is an attractive concept because both podocytes and proximal tubular epithelial cells express high levels of the angiotensin-converting enzyme type 2 entry receptor.11 , 45, 46, 47 We could not establish direct kidney infection by SARS-CoV-2 in our case series, though a caveat is that there was often a several-week delay between diagnosis of COVID-19 and performing the kidney biopsy. We note that the temporal relationship between onset of kidney manifestations, COVID-19 symptoms, and diagnostic biopsy has not been clearly reported in the previous studies to date.10 , 16 , 17 , 20 , 48 Detectable SARS-CoV-2 may wane quickly with time after the onset of infection similar to SARS-CoV.49 , 50 Therefore, these delays meant that most patients in this series may have cleared virus from their kidneys and were therefore RT-PCR negative by nasopharyngeal swab at the time of biopsy. Even with this caveat, most recent biopsy and autopsy case series have concluded that there is no significant SARS-CoV-2 infection of the kidney,16 , 20 , 24 , 48 , 51, 52, 53 which is in contrast to the possibility of direct infection that was reported in 2 earlier studies.11 , 45 Therefore, our study adds to the accumulating evidence that direct and persistent infection of the kidney by SARS-CoV-2 does not appear to play a significant role in kidney disease in most patients with COVID-19.
Many studies have relied solely on electron microscopy to establish direct viral infection of tissues. However, without orthogonal validation and rigorous controls, this approach is problematic because there are many ultrastructural mimics of viral particles.51 , 54, 55, 56 Even in the lung, using validated immunohistochemistry and RNA in situ hybridization, very few cells appear to contain detectable virus.45 , 52 If direct viral infection of the kidney occurs, the lower viral load compared to lung tissue would make ultrastructural identification of exceedingly rare SARS-CoV-2 particles in the kidney improbable among the ubiquitous viral mimics.45 In contrast to the patients in 2 autopsy series or those in a recent biopsy series,11 , 20 , 48 , 53 most patients reported here had only mild COVID-19 symptoms while presenting with AKI, heavy proteinuria, and/or severe hypertension. Some diseases detected in this case series may represent diagnoses coincident with COVID-19. However, the high incidence of typically rare disease processes such as collapsing glomerulopathy and TMA raises the possibility that SARS-CoV-2 infection contributed to these injuries, even in the absence of direct infection of the renal parenchyma. Given that most SARS-CoV-2–infected patients exhibit only mild symptoms such as those in our case series, the incidence of AKI and proteinuria may increase as the pandemic progresses and disproportionately affect more vulnerable populations with pre-existing risk factors for kidney disease or high-risk APOL1 genotypes.
Article Information
Authors’ Full Names and Academic Degrees
Shreeram Akilesh, MD, PhD, Cynthia C. Nast, MD, Michifumi Yamashita, MD, PhD, Kammi Henriksen, MD, Vivek Charu, MD, PhD, Megan L. Troxell, MD, PhD, Neeraja Kambham, MD, Erika Bracamonte, MD, Donald Houghton, MD, Naila I. Ahmed, MD, Chyi Chyi Chong, MD, Bijin Thajudeen, MD, Shehzad Rehman, MD, Firas Khoury, MD, Jonathan E. Zuckerman, MD, PhD, Jeremy Gitomer, MD, Parthassarathy C. Raguram, MD, Shanza Mujeeb, MD, Ulrike Schwarze, MD, M. Brendan Shannon, MD, Iris De Castro, MD, Charles E. Alpers, MD, Behzad Najafian, MD, Roberto F. Nicosia, MD, PhD, Nicole K. Andeen, MD, and Kelly D. Smith, MD, PhD.
Authors’ Contributions
Research idea and study design: SA, NKA, KDS; data acquisition: all authors; data analysis/interpretation: all authors; study supervision: SA, NKA, KDS. NKA and KDS contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank Dr Kimberly Muczynski (University of Washington) for contributing clinical information and follow-up for this series.
Peer Review
Received July 21, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Pathology Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form October 4, 2020.
Footnotes
Complete author and article information provided before references.
Table S1: Additional information on cases.
Supplementary Material
Table S1: Additional information on cases.
References
- 1.Hou Y.J., Okuda K., Edwards C.E. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):16–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189(6):e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 11.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley B.T., Maioli H., Johnston R. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller S.E., Brealey J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98(1):231–232. doi: 10.1016/j.kint.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller S.E., Goldsmith C.S. Caution in identifying coronaviruses by electron microscopy. J Am Soc Nephrol. 2020;31(9):2223–2224. doi: 10.1681/ASN.2020050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith C.S., Tatti K.M., Ksiazek T.G. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10(2):320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaillard F., Ismael S., Sannier A. Tubuloreticular inclusions in COVID-19–related collapsing glomerulopathy. Kidney Int. 2020;98(1):241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma Y., Nasr S.H., Larsen C.P., Kemper A., Ormsby A.H., Williamson S.R. COVID-19–associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2(4):493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilk A.J., Rustagi A., Zhao N.Q. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanungo S., Tamirisa S., Gopalakrishnan R., Salinas-Madrigal L., Bastani B. Collapsing glomerulopathy as a complication of interferon therapy for hepatitis C infection. Int Urol Nephrol. 2010;42:219–222. doi: 10.1007/s11255-009-9594-1. [DOI] [PubMed] [Google Scholar]
- 23.Abid Q., Best Rocha A., Larsen C.P. APOL1-associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI) Am J Kidney Dis. 2020;75(2):287–290. doi: 10.1053/j.ajkd.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H., Larsen C.P., Hernandez-Arroyo C.F. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020;31(8):1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasembeli A.N., Duarte R., Ramsay M. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26(11):2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer H.J., Stilp A.M., Laurie C.C. African ancestry-specific alleles and kidney disease risk in Hispanics/Latinos. J Am Soc Nephrol. 2017;28(3):915–922. doi: 10.1681/ASN.2016030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal A.K., Nordquist J.A., Rodgers C.L. Glomerulopathy and arteriolopathy in congenital nephrotic syndrome: light, electron, and fluorescence microscopy studies. Hum Pathol. 1977;8(3):344–349. doi: 10.1016/s0046-8177(77)80032-4. [DOI] [PubMed] [Google Scholar]
- 28.Meehan S.M., Pascual M., Williams W.W. De novo collapsing glomerulopathy in renal allografts. Transplantation. 1998;65(9):1192–1197. doi: 10.1097/00007890-199805150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Stokes M.B., Davis C.L., Alpers C.E. Collapsing glomerulopathy in renal allografts: a morphological pattern with diverse clinicopathologic associations. Am J Kidney Dis. 1999;33(4):658–666. doi: 10.1016/s0272-6386(99)70216-7. [DOI] [PubMed] [Google Scholar]
- 30.Nadasdy T., Allen C., Zand M.S. Zonal distribution of glomerular collapse in renal allografts: possible role of vascular changes. Hum Pathol. 2002;33(4):437–441. doi: 10.1053/hupa.2002.124333. [DOI] [PubMed] [Google Scholar]
- 31.Fogo A.B. Talking back: the podocytes and endothelial cells duke it out. Kidney Int. 2016;90(6):1157–1159. doi: 10.1016/j.kint.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Halloran P.F., Venner J.M., Madill-Thomsen K.S. Review: the transcripts associated with organ allograft rejection. Am J Transplant. 2018;18:785–795. doi: 10.1111/ajt.14600. [DOI] [PubMed] [Google Scholar]
- 33.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhaveri K.D., Meir L.R., Flores Chang B.S. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara S., Hirata M., Ito K. Post-infectious acute glomerulonephritis with podocytopathy induced by parvovirus B19 infection. Pathol Int. 2018;68(3):190–195. doi: 10.1111/pin.12643. [DOI] [PubMed] [Google Scholar]
- 36.Marco H., Guermah I., Matas L. Postinfectious glomerulonephritis secondary to erythrovirus B19 (parvovirus B19): case report and review of the literature. Clin Nephrol. 2016;85:238–244. doi: 10.5414/CN108694. [DOI] [PubMed] [Google Scholar]
- 37.Jain T., Hemington L., Etuwewe B. A case of post-infectious glomerulonephritis following infection with influenza A subtype H1N1. Pediatr Nephrol. 2011;26:151–152. doi: 10.1007/s00467-010-1559-1. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.R., Lee S.B., Kim I.Y. Relapse of minimal change disease following infection with the 2009 pandemic influenza (H1N1) virus. Clin Exp Nephrol. 2012;16:329–332. doi: 10.1007/s10157-011-0562-6. [DOI] [PubMed] [Google Scholar]
- 39.Haruki A., Ishikawa E., Katayama K. Spontaneous remission of adult-onset minimal change nephrotic syndrome associated with influenza B infection: a case report. 2018;19:162. doi: 10.1186/s12882-018-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rettmar K., Kienast J., van de Loo J. Minimal change glomerulonephritis with reversible proteinuria during interferon α2a therapy for chronic myeloid leukemia. Am J Hematol. 1995;49(4):355–356. doi: 10.1002/ajh.2830490417. [DOI] [PubMed] [Google Scholar]
- 41.Markowitz G.S., Nasr S.H., Stokes M.B., D’Agati V.D. Treatment with IFN-α, -β, or -γ is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5(4):607–615. doi: 10.2215/CJN.07311009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Orsogna L., van den Heuvel H., van Kooten C., Heidt S., Claas F.H.J. Infectious pathogens may trigger specific allo-HLA reactivity via multiple mechanisms. Immunogenetics. 2017;69:631–641. doi: 10.1007/s00251-017-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amir A.L., D’Orsogna L.J.A., Roelen D.L. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115(15):3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 44.Russell M.R., Halnon N.J., Alejos J.C., Salem M.M., Reardon L.C. COVID-19 in a pediatric heart transplant recipient: emergence of donor-specific antibodies. J Heart Lung Transplant. 2020;39(7):732–733. doi: 10.1016/j.healun.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon R., Otto E.A., Sealfon R. SARS-CoV-2 receptor networks in diabetic kidney disease, BK-virus nephropathy and COVID-19 associated acute kidney injury [preprint posted online August 24, 2020]. medRxiv. https://doi.org/10.1101/2020.05.09.20096511
- 47.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P., Uppal N.N., Wanchoo R. COVID-19–associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauter J.L., Baine M.K., Butnor K.J. Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology. 2020;77(6):915–925. doi: 10.1111/his.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholls J.M., Butany J., Poon L.L.M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3(2):222–229. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cassol C.A., Gokden N., Larsen C.P., Bourne T.D. Appearances can be deceiving - viral-like inclusions in COVID-19 negative renal biopsies by electron microscopy. Kidney360. 2020;1(8):824–828. doi: 10.34067/KID.0002692020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Best Rocha A., Stroberg E., Barton L.M. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab Investig. 2020;100:1485–1489. doi: 10.1038/s41374-020-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golmai P., Larsen C.P., DeVita M.V. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roufosse C., Curtis E., Moran L. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98(2):505–506. doi: 10.1016/j.kint.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith K.D., Akilesh S., Alpers C.E., Nicosia R.F. Am I a coronavirus? Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldsmith C.S., Miller S.E., Martines R.B., Bullock H.A., Zaki S.R. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395(10238):e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Additional information on cases.