Abstract
Background
Coronavirus Disease 2019 (COVID-19) has become a worldwide pandemic and affected more than 227 countries or territories, resulting in more than 25 million cases with over 0•85 million deaths, as of September 2, 2020. Taiwan has been successful in countering the COVID-19 outbreak, however, the potential risk for asymptomatic infections and the prevalence rates remain unknown. We aimed to estimate the seroprevalence of COVID-19 in Taiwan via serologically testing hospital patients with neither symptoms indicative of nor positive nucleic acid test for SARS-CoV-2 infection.
Methods
Residual specimens from laboratory blood tests for outpatient and emergency department patients visiting a medical centre in Taipei, Taiwan, within one week in May and another in July, 2020, were collected. We used Elecsys Anti-SARS-CoV-2 Assay to screen and further validated cases with high cutoff index by a confirmatory ELISA assay. We also analysed antibody responses against SARS-CoV-2 along disease progression in four nucleic acid test confirmed COVID-19 patients.
Findings
Blood samples from a total of 14,765 patients were tested. The unweighted seroprevalence of anti-SARS-CoV-2 antibodies was 0•07% [95% CI, 0•04%-0•13%]; after weighting with the population demographics of Taiwan, the estimated overall seroprevalence was 0•05% [95% CI, 0•02%-0•10%]. Furthermore, based on data of the four COVID-19 cases, the seroconversion dates for IgM were as early as 9 days and that for IgG 11 days after symptoms onset.
Interpretation
We screened the anti-SARS-CoV-2 antibodies in a small-scale population-based study and observed an approximately 0•05% seroprevalence of COVID-19, indicating that the current containment protocols emphasising mask wearing, hand washing, social distancing and mandatory quarantine for all incomers are effective in Taiwan.
Funding
Taipei Veterans General Hospital, Taipei, Taiwan.
Keywords: anti-SARS-CoV-2 serological antibodies, COVID-19, Seroprevalence, Pandemic;Taiwan
Research in context.
Evidence before this study
Taiwan has been efficacious in countering the current global COVID-19 epidemics, with only 489 confirmed cases and 7 deaths, accounting for 2•07 and 0•03 per 100,000 of the general population, respectively, as of September 2, 2020. However, the potential risk for asymptomatic infections and the prevalence rates of COVID-19 remain unknown. We searched PubMed for peer-reviewed articles and preprint reports on “seroprevalence”, “SARS-CoV-2 antibody”, “anti-SARS-CoV-2”, and similar terms, up to August 31, 2020. There were only a few and most serological studies focused on specific subpopulation in “hotspot” regions of the world. Additionally, in most serological studies only one single type of laboratory test was performed, which might generate more false positive results and over-estimate the prevalence of anti-SARS-CoV-2 antibodies.
Added value of this study
This is the first study that establishes an algorithm for COVID-19 serology test composed of two integrated platforms and screens the presence of anti-SARS-CoV-2 antibodies in a significant number of individuals to accurately estimate the COVID-19 seroprevalence in Taiwan. Additionally, our testing dates were grouped into two separate weekly periods, one in May and one in July, to investigate the temporal variability of COVID-19 seroprevalence; no increase of seroprevalence rates was observed in the second weekly period.
Implications of all the available evidence
Our findings reveal a 0•05% seroprevalence of COVID-19 in Taiwan, which is a relatively low seroprevalence observed compared to other countries worldwide. Also, there is no increase of anti-SARS-CoV-2 detection rate 7 to 10 days after a long weekend holiday full of indoor and outdoor social activities all around the island, indicating that epidemic prevention measures in Taiwan currently are appropriate and effective. The low seroprevalence of COVID-19 in Taiwan, found in our study, helps evaluate the population proportion of the suspected asymptomatic infection and provide information for making public policies to manage the possible next wave of COVID-19 pandemics.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19), the infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified on December 12, 2019 in Wuhan, China and has spread rapidly around the world [1], [2]. The COVID-19 has affected more than 227 countries or territories and led to more than 25 million confirmed cases, with over 0•85 million deaths globally, as of September 2, 2020 [3].
Common initial symptoms of COVID-19 include fever, dry cough and fatigue, which resemble respiratory illness caused by other viruses or bacteria [4]. Diagnosis of COVID-19 becomes problematic due to overlapping clinical presentations, especially during epidemics of seasonal flu. Thus, confirmation of COVID-19 depends on the laboratory diagnostic tests. The methods of COVID-19 confirmatory tests used in the laboratory are of two major categories, one is molecular assays for detection of SARS-CoV-2 viral RNA based on polymerase chain reaction (PCR), and the other is a serological assay which detects anti-SARS-CoV-2 antibodies produced by patients against viral antigenic proteins [5].
Currently, the viral nucleic acid test, which targets different SARS-CoV-2 genomic regions such as the ORF1b and nucleocapsid (N), spike (S), RNA-dependent RNA polymerase (RdRP), and envelope (E) genes, is the gold standard practice for clinical diagnosis of COVID-19 [6], [7]. Although it is the most widely used methodology for detecting SARS-CoV-2 infections, reverse transcription quantitative polymerase chain reaction (RT-qPCR) also has disadvantages such as high false negative incidences [4], time-consuming and requiring expensive laboratory instrumentation operated by highly skilled laboratory personnel. These problems cause a noteworthy delay of early diagnosis and timely quarantine to minimize the spread of COVID-19.
Furthermore, chasing after individuals having contact with confirmed COVID-19 cases based on clinical symptoms is not an ideal way for estimating the proportion of the population infected; thus an effective screening method is needed with respect to epidemiology. Serological antibody testing is a more suitable assay for screening and estimating the prevalence of the disease, and helping make public policies for the infection control [8], [9]. Antibodies to SARS-CoV-2 can be successfully detected from patients approximately one week after the infection [10]. Serologically detecting anti-SARS-CoV-2 antibodies provides evidence of a previously infected population as well as information regarding how many asymptomatic cases may have occurred [11], [12]. Compared to RT-qPCR assays, the antibody detection assays are more advantageous with faster turn-around time, higher throughput and less workload.
Taiwan has been rather successful in countering the current COVID-19 outbreak, with only 489 confirmed cases (2.07 per 100,000) and 7 deaths (0•03 per 100,000), respectively, accounting for 0•0019% and 0•0008% of the global total as of September 2, 2020. With experiences from SARS epidemics, Taiwan was able to quickly and efficiently carry out strategies fighting against COVID-19. In January, before the outbreak of COVID-19, Taiwan had already commenced airport and onboard quarantine measures for passengers who arrived from Wuhan or transited through China, Hong Kong, and Macau. Hospitals activated negative pressure isolation wards and public places were requested to provide body temperature monitoring and hand sanitizers. In March, the Taiwan government implemented a series of strict epidemic prevention policies, including international travel restriction, wearing surgical masks and maintaining social distance. Effective policies and decent public health education protected Taiwan from community transmission [13]. However, the potential risk for asymptomatic infections and the prevalence rates of COVID-19, which are critical for Taiwan's epidemic prevention actions, remain largely unknown.
In this study, we performed the Elecsys Anti-SARS-CoV-2 serology test to screen approximately 15,000 blood samples collected from a medical center in Taiwan and correlated with a confirmatory COVID-19 ELISA test developed by Academia Sinica to validate the accuracy of the testing platforms. While a diagnostic algorithm for anti-SARS-CoV-2 serology testing was established, the seroprevalence of anti-SARS-CoV-2 antibodies in Taiwan was also estimated. Serological antibody responses against SARS-CoV-2 along disease progression in four COVID-19 patients were also examined to further evaluate the clinical utility of the anti-SARS-CoV-2 antibodies tests.
2. Materials and methods
2.1. Study design and participants
The residual specimens obtained from routine laboratory blood tests for outpatient and emergency department patients visiting Taipei Veterans General Hospital, Taipei, Taiwan from 25 May to 30 May (weekly period 1) and from 6 July to 8 July (weekly period 2), 2020 were enrolled. This two-weekly-period strategy for sample collection is planned to investigate the variability of seroprevalence, if present, during different time periods. It is noteworthy to mention that there was a 4-day long holiday (Dragon Boat Festival, June 25 to 28) one week before the weekly period 2 in July, which had lots of public activities, both outdoors and indoors, in Taiwan. The specimens were firstly screened for anti-SARS-CoV-2 antibodies using Elecsys Anti-SARS-CoV-2 Assay (Roche Molecular System Inc., CA, USA) and all the cases with a cutoff index (COI) ≥ 1•00, together with an equivalent number of randomly selected negative cases, were further evaluated by Academia Sinica ELISA assay, which is an Enzyme-linked Immunosorbent Assay (ELISA) developed by the Laboratory of Professor Shie-Liang Hsieh in Academia Sinica, Taiwan. In addition, longitudinal series of blood samples collected from our only four COVID-19 patients admitted in March to May were also included in this study to use as control samples as well as to assess the immune reaction in response to SARS-CoV-2 infection. Data of the viral nucleic acid tests were obtained from chart review. This study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital (ID No. 2020-06-002CC and 2020-06-011B). The informed consent requirement was waived.
2.2. Laboratory analysis
The Elecsys Anti-SARS-CoV-2 Assay (Roche Molecular System Inc., CA, USA) is an immunoassay which uses a recombinant protein representing the nucleocapsid (N) antigen for in vitro qualitative detection of antibodies (including IgM and IgG) against SARS-CoV-2 in human serum and plasma. This assay is run on the Roche cobas e801 automatic system according to the manufacturer's instruction. The readout of a sample is given as a cutoff index (COI) which compares the mean chemiluminescent signal of a sample to the calibrator. Samples with a reported COI greater than 1•00 are considered positivity. The sensitivity of the Elecsys Anti-SARS-CoV-2 Assay, according to the manufacturer, is 88•1% [95% CI, 77•1%-95•1%] (N=59) 7-13 days after nucleic acid PCR confirmation and 100•0% [95% CI, 88•1%-100•0%] (N=29) beyond 14 days after confirmation; the specificity of the Assay is 99•80% [95% CI, 99•58%-99•92%] (N=5,272).
The anti-SARS-CoV-2 Academia Sinica ELISA assay is designed to differentiate IgG and IgM antibodies detected. Briefly, the serum samples are applied into ELISA plates which were pre-coated with the recombinant protein representing SARS-CoV-2 spike antigen provided by Professor Shie-Liang Hsieh. After incubation and then washing, the wells are subsequently hybridized with either HRP-conjugated donkey anti-human IgG (H+L) or HRP-conjugated goat anti-human IgM antibodies. Finally, the enzymatic activity is generated with the addition of the substrate tetramethylbenzidine (TMB) and the optical density (OD) is measured at 450 nm wavelength using a microplate reader (SunriseTM, TECAN, Switzerland). Each sample is run in triplicate. Samples with a mean OD more than the cut-off values (IgG: 0•134; IgM: 0•168) were considered positivity. Given that the COVID-19 cases in Taiwan are relatively rare, only few confirmed COVID-19 patients are reported to have received serology test. Thus, we performed the comparison between Academia Sinica ELISA assay and Elecsys Anti-SARS-CoV-2 Assay by measuring 34 samples (beyond 14 days after PCR confirmation) from our confirmed COVID-19 patients and 20 samples from healthy donors. The results showed 100% both sensitivity and specificity of Academia Sinica ELISA assay.
2.3. Statistics analysis
Seroprevalence is determined as the proportion of study individuals who are tested positive in both Elecsys Anti-SARS-CoV-2 Assay and Academia Sinica ELISA assay. The sex and age specific relative risks are calculated to assess the strength of an association by considering the incidence of an event in the corresponding group and comparing that with the incidence in a reference group. A relative risk of 1 indicates no association; a relative risk other than 1 indicates an association. 95% confidence intervals (CI) are calculated using exact binomial models. For all analyses, a p value of < 0•05 indicates statistical significance. We calculated age-standardized seroprevalence using weights derived from the population demographics of Taiwan in 2019 Department of Household Registration, Ministry of the Interior [14]. Confidence intervals for weighted estimates are calculated using the Korn-Graubard method. All statistical analyses are performed using SAS Statistics v•6•1 (SAS, Cary, NC, USA) and GraphPad Prism v•8•0 (GraphPad Software, San Diego, CA, USA).
2.4. Role of the funding source
The funder of the study had no role in study design, the collection, analyses, and interpretation of data, or the writing and submission of the manuscript. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
A total of 14,765 blood samples obtained from 9,777 patients from 25 May to 30 May, 2020 (weekly period 1) and 4,988 patients from 6 July to 8 July, 2020 (weekly period 2) were tested for the presence of anti-SARS-CoV-2 antibodies. Overall, there were 7,290 (49•37%) male and 7,475 (50•63%) female; 13,721 (92•9%) were from the Outpatient Department and 1,044 (7•07%) were from the Emergency Department. The age distribution were 617 (4•18%) aged 20-29 years, 1,070 (7•25%) aged 30-39 years, 1,536 (10•40%) aged 40-49 years, 2,762 (18•71%) aged 50-59 years, 3,847 (26•05%) aged 60-69 years, 2,823 (19•12%) aged 70-79 years, 1,546 (10•47%) aged 80-89 years, and 564 (3•82%) aged over 90 years. (Table 1 and Supplement Table 1).
Table 1.
Summary of prevalence of serological anti-SARS-CoV-2 antibodies evaluated by Elecsys Anti-SARS-CoV-2 Assay and Academia Sinica ELISA assay categorized by age and sex
|
Number of samples |
Proportion of sample, % |
Elecsys screening Positive |
ELISA confirmatory Positive (IgG/IgM) |
Proportion of ELISA Positive, % (95% CI) |
Relative risk (95% CI) |
p value | |
|---|---|---|---|---|---|---|---|
| Total | 14765 | 100 | 25 | 11 (7 / 4) | 0•07 (0•04-0•13) | •• | •• |
| Age, years | |||||||
| 20-29 | 617 | 4•18 | 0 | 0 (0 / 0) | 0•00 (0•00-0•60) | 1•52 (0•06-37•35) | 0•796 |
| 30-39 | 1070 | 7•25 | 1 | 1 (0 / 1) | 0•09 (0•00-0•52) | 2•64 (0•17-42•14) | 0•493 |
| 40-49 | 1536 | 10•40 | 3 | 1 (0 / 1) | 0•07 (0•00-0•36) | 1•84 (0•12-29•36) | 0•667 |
| 50-59 | 2762 | 18•71 | 5 | 1 (1 / 0) | 0•04 (0•00-0•20) | 1•02 (0•06-16•33) | 0•988 |
| 60-69 | 3847 | 26•05 | 7 | 4 (3 / 1) | 0•10 (0•03-0•27) | 3•24 (0•36-28•96) | 0•293 |
| 70-79 | 2823 | 19•12 | 4 | 1 (1 / 0) | 0•04 (0•00-0•20) | 1 (ref) | •• |
| 80-89 | 1546 | 10•47 | 3 | 1 (0 / 1) | 0•06 (0•00-0•36) | 1•83 (0•11-29•17) | 0•670 |
| ≥90 | 564 | 3•82 | 2 | 2 (2 / 0) | 0•35 (0•04-1•27) | 10•01 (0•91-110•22) | 0•060 |
| Sex | |||||||
| Male | 7290 | 49•37 | 15 | 7 (5 / 2) | 0•10 (0•04-0•20) | 1•79 (0•53-6•13) | 0•351 |
| Female | 7475 | 50•63 | 10 | 4 (2 / 2) | 0•05 (0•01-0•14) | 1 (ref) | •• |
Female and age 70-79 years groups are used as the reference for relative risk calculation, respectively, to which other groups are compared.
95% confidence intervals are estimated using exact binomial models.
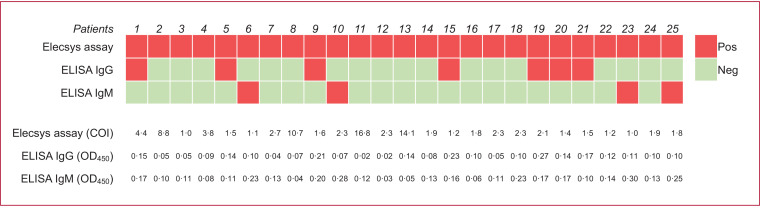
To estimate the seroprevalence, we implemented an anti-SARS-CoV-2 antibodies serology test algorithm (Supplement Fig. 1). Twenty-five out of 14,765 specimens were tested positive for anti-SARS-CoV-2 antibodies using Elecsys Anti‑SARS‑CoV‑2 Assay, which were furthered tested using Academia Sinica ELISA assay (Fig. 1). Samples positive for both assays were considered as true positivity. In the first weekly period in May, 9,777 patients were screened and 7 were tested positive; the unweighted seroprevalence was 0•07% [95% CI, 0•03%-0•15%]. In the second weekly period in July, one week after the Dragon Boat Festival holiday, 4,988 patients were screened and 4 were tested positive; the unweighted seroprevalence was 0•08% [95% CI, 0•02%-0•20%] (Table 2). No significant difference in seroprevalence was observed between these two weekly periods. Taken together, the overall unweighted prevalence of anti‑SARS‑CoV‑2 antibodies was 0•07% [95% CI, 0•04%-0•13%]. Approximately 78•2% of patients enrolled in this study were over 50 years old, which is noticeably skewed from the age distribution of Taiwanese population. To more precisely represent the actual seroprevalence of anti-SARS-CoV-2 antibodies in Taiwan, we weighted the prevalence by age. The overall weighted seroprevalence was 0•05% [95% CI, 0•02%-0•10%], with 0•06% [95% CI, 0•02%-0•12%] in the first weekly period and 0•03% [95% CI, 0•00%-0•11%] in the second weekly period. The reason that there is more difference between unweighted and weighted seroprevalences in the second weekly period (0•08% versus 0•03%) compared to that in the first weekly period (0•07% versus 0•06%) appeared to be that all the anti-SARS-CoV-2 antibody-positive cases in the second weekly period are in the old age group (≥ 60 years), as shown in Table 2. Moreover, the risk of seropositivity showed no statistically significant difference among different subgroups in sex, age, types of appointments and medical specialties (Table 1 and Supplement Table 1).
Fig. 1.
Comparison of the results of the Elecsys Anti‑SARS‑CoV‑2 Assay and Academia Sinica ELISA assay in 25 Elecsys Assay positive cases. Among 25 individuals tested with COI ≥ 1•00 by Elecsys Assay, seven were tested positive for SARS-CoV-2 anti-spike RBD IgG antibodies (IgG+ / IgM-), four were tested positive for SARS-CoV-2 anti-spike RBD IgM antibodies (IgG- / IgM+), and the other 14 were tested double negative (IgG- / IgM-) by ELISA assay. Results of the qualitative Elecsys Assay and ELISA assay are amended as positive (red) or negative (green).
Table 2.
Seroprevalence of anti-SARS-CoV-2 antibodies, unweighted and weighted with age, in weekly periods 1 and 2
|
Weekly Period 1 (2020.05.25 - 2020.05.30) |
Weekly Period 2 (2020.07.06 - 2020.07.08) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Total case number |
Screening Positive No.* |
Confirmatory Positive no.† |
Unweighted prevalence |
Weighted prevalence |
Total case number |
Screening Positive No.* |
Confirmatory Positive no.† |
Unweighted prevalence |
Weighted prevalence |
|
| Age, years | ||||||||||
| 20-29 | 411 | 0 | 0 | 0•00% | 0•000% | 206 | 0 | 0 | 0•00% | 0•000% |
| 30-39 | 727 | 1 | 1 | 0•01% | 0•021% | 343 | 0 | 0 | 0•00% | 0•000% |
| 40-49 | 1049 | 3 | 1 | 0•01% | 0•015% | 487 | 0 | 0 | 0•00% | 0•000% |
| 50-59 | 1830 | 5 | 1 | 0•01% | 0•008% | 932 | 0 | 0 | 0•00% | 0•000% |
| Young age group (< 60) | 4017 | 9 | 3 | 0•03% | 0•044% | 1968 | 0 | 0 | 0•00% | 0•000% |
| 60-69 | 2551 | 3 | 2 | 0•02% | 0•010% | 1296 | 4 | 2 | 0•04% | 0•020% |
| 70-79 | 1852 | 4 | 1 | 0•01% | 0•003% | 971 | 0 | 0 | 0•00% | 0•000% |
| 80-89 | 1001 | 2 | 0 | 0•00% | 0•000% | 545 | 1 | 1 | 0•02% | 0•005% |
| ≥ 90 | 356 | 1 | 1 | 0•01% | 0•002% | 208 | 1 | 1 | 0•02% | 0•003% |
| Old age group (≥ 60) | 5760 | 10 | 4 | 0•04% | 0•015% | 3020 | 6 | 4 | 0•08% | 0•028% |
| All ages‡ | 9777 | 19 | 7 | 0•07% (0•03-0•15) | 0•06% (0•02-0•12) | 4988 | 6 | 4 | 0•08% (0•02-0•20) | 0•03% (0•00-0•11) |
Cases positive for Elecsys Anti-SARS-CoV-2 Assay.
Cases positive for both Elecsys Anti-SARS-CoV-2 Assay and Academia Sinica ELISA assay.
Prevalence of all ages is shown as percentage with 95% CI; for weekly periods 1 & 2 combined, the total case number is 14,765 with 11 confirmed seropositive cases, and the unweighted prevalence is 0•07% (0•04-0•13) and the weighted prevalence is 0•05% (0•02-0•10).
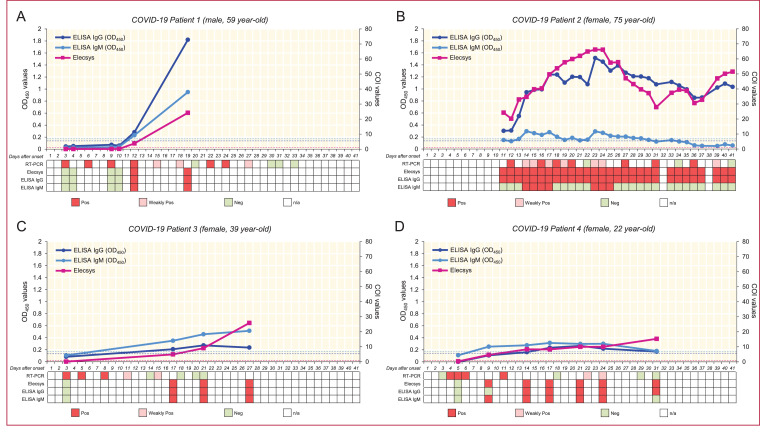
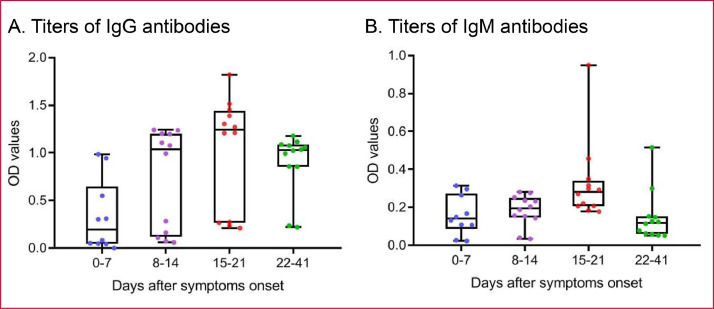
The longitudinal blood samples obtained from four COVID-19 patients were evaluated for the presence of anti-SARS-CoV-2 antibodies, and the results were compared with the presence of viral RNA detected using RT-PCR assays on nasal and pharyngeal swab specimens. As shown in Fig. 2, the levels of viral RNA fluctuated during clinical courses, however, the anti-SARS-CoV-2 antibodies showed persistent positivity after the seroconversion dates. For our first patient, the results of two antibody test platforms showed seroconversion at day 12 after symptoms onset. The second patient, who was confirmed at other hospital and transferred to our hospital 10 days after symptoms onset, was tested positive for Elecsys assay and Academia Sinica ELISA IgG assay at day 11, and the titers of IgM were within gray zones after day 11. The third patient, who missed the day 4 to day 16 blood samples, showed seroconversion in both platforms at day 17 after symptoms onset. The last patient showed a seroconversion for Elecsys assay and Academia Sinica ELISA IgM assay at day 9, and positive for IgG assay at day 14. Based on the observation on our four confirmed patients, the seroconversion dates for IgM were approximately beyond 9 days and that for IgG were approximately beyond 11 days after symptoms onset. We also compared the titers of IgG and IgM antibodies against SARS-CoV-2 in four patients at different days after symptoms onset (Fig. 3). The trends of antibody production and the plateau of IgG and IgM titers varied widely across patients. After symptoms onset, the IgG and IgM antibody titers gradually increased up to the third week and decreased after the third week and, as expected, the titers of IgM showed more dramatic decrease compared to that of IgG.
Fig. 2.
Longitudinal assessment with viral RNA and serological antibodies testing for four COVID-19 patients. Shown is the variation of presence of viral RNA and antibodies against SARS-CoV-2 in four confirmed COVID-19 patients: male, 59 year-old (Patient 1; A), female, 75 year-old (Patient 2; B), female, 39 year-old (Patient 3; C), and female, 22 year-old (Patient 4; D). The results of Elecsys Assay are presented as COI values (purple), and the results of ELISA assay are presented as OD values (IgG: dark blue; IgM: light blue) measured by 450 nm. Results of the qualitative viral RNA testing, Elecsys Assay and ELISA assay are amended as positive (red), weakly positive (pink), negative (green), and n/a (white). The dash lines denote the cut-off value for ELISA IgG assay (dark blue, 0•134), ELISA IgM assay (light blue, 0•168), and Elecsys Assay (purple, 1•00).
Fig. 3.
Titers of antibodies against SARS-CoV-2 in four COVID-19 patients at different days after symptoms onset. Levels of IgG (A) and IgM (B) antibodies against SARS-CoV-2 at different time periods after symptoms onset in four COVID-19 patients are plotted. The IgG and IgM antibody titers gradually increased up to the third week and decreased thereafter with a more dramatic decrease back to the titers of the first week in the IgM antibodies. The boxplots show medians and first and third quartiles, and the whiskers show minimal to maximal range below and above the box.
4. Discussion
To study patients with COVID-19 solely depending on testing viral nucleic acids from the nasopharyngeal swabs of symptomatic individuals would fail to identify patients who have infected with no or mild symptoms or have recovered from the infection. The limited availability of the viral RNA tests has been another caveat in quarantine for COVID-19 pandemics. The results of serology test for anti-SARS-CoV-2 antibodies in our study provide important information to help assess the regional epidemiology of COVID-19. Currently, there were 489 confirmed COVID-19 cases (2•07 per 100,000) in Taiwan as of September 2, 2020. However, in our study, the estimated seroprevalence was 0•05%, implying that approximately 11,800 adults might have anti-SARS-CoV-2 antibodies, which is substantially 24-fold greater than the case number of confirmed COVID-19 in Taiwan. Given the above experimental data and calculative information, a large proportion of Taiwanese people might have been infected with SARS-CoV-2 virus, however, most of them were asymptomatic or mildly symptomatic and not qualified for a diagnostic viral nucleic acid test. The etiological mechanisms of this subclinical manifestation of COVID-19 remain enigmatic and await further investigation. Noteworthy, compared with reports in the literature [8], [9], [15], the seroprevalence of 0•05% in Taiwan is significantly lower than that in most regions of the world and there is no increase of detection rate 7 to 10 days after a long weekend holiday full of social activities all around the island, indicating that epidemic preventions nowadays in Taiwan are appropriate and effective.
The risk along with serological anti-SARS-CoV-2 assays for population screening is the possibility of false-positive results, which might lead to the erroneous assumption of past or present infection and subsequently putative immunity, which may place the individual into a hazardous situation of acquiring or transmitting COVID-19 [16]. Therefore, we conducted two serological assays with different capturing antigens and established the testing algorithm to minimize the possibility of producing false-positive results. In this study, out of 14,765 hospital patients screened by using Elecsys Anti-SARS-CoV-2 Assay we discovered 25 individuals with positive results and among them only 11 were tested positive based on forward confirmation using Academia Sinica ELISA assay. The possible reasons for discrepancy between these two assays include that Elecsys Anti-SARS-CoV-2 Assay had been demonstrated to have cross-reactivity with cytomegalovirus and Epstein-Barr virus IgM/IgG antibodies [17]. Of note, the observation that three patients with highest Elecsys Assay COI values (patients 8, 11, 13 in Fig. 1) are tested negative by using the confirmatory Academia Sinica ELISA assay is suggestive of the occurrence of cross-reactivity rather than sensitivity limitation.
The manifestation of antibody responses against SARS-CoV-2 is still unclear. In studies of Xiang et al. and Lee et al., the seroconversion days for anti-SARS-CoV-2 IgG or IgM antibodies in confirmed COVID-19 patients were both within 7 days after symptoms onset [18], [19]. Long et al., by analyzing 63 confirmed COVID-19 patients, demonstrated that all patients achieved IgG or IgM seroconversion within 20 days after symptoms onset and the median day for both IgG and IgM was 13 days; they also observed three types of seroconversion: synchronous seroconversion of IgG and IgM, IgM seroconversion earlier than that of IgG, and IgM seroconversion later than that of IgG [10]. In our study, the seroconversion days for IgG were approximately beyond 11 days and that of IgM were approximately beyond 9 days after symptoms onset. Further investigation with larger number of patients is needed.
This study has certain limitations. The enrolled patients in our study are from outpatient and emergency departments of a medical center and individuals younger than 20 years are excluded due to the IRB regulations, which make this study population not fully randomly selected and represented. However, given the setting of a 2,600-bed hospital in the capital, the seroprevalence derived from this study would be expected higher or no less than that of the general population of Taiwan. Nevertheless, we have weighted age distribution of the general population to adjust the results and to reflect a more substantial “real world” seroprevalence.
To the best of our knowledge, this is the first small-scale population study in which a serology testing algorithm for anti-SARS-CoV-2 antibodies was established and a significant number of domestic residents were screened for the presence of antibodies to estimate the seroprevalence of COVID-19 in Taiwan. Since the outbreak of COVID-19, Taiwan government has implemented effective policies to protect Taiwan from community transmission. However, without widely screening for anti-SARS-CoV-2 antibodies, we are not able to assess the epidemiology of COVID-19 in Taiwan.
The results in the present study reveal a 0•05% seroprevalence of COVID-19 in Taiwan, which may help evaluate the prevalence of asymptomatic SARS-CoV-2 infection and provide valuable information for government's decision making to adapt plans for facing challenges from the possible next pandemic to come.
Declaration of Competing Interest
All authors of this manuscript have indicated that they have no conflicts of interest that relate to the content of this manuscript.
Acknowledgments
Data sharing statement
All deidentified data used within the study is available on request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100041.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. September 2, 2020.
- 4.Xie J, Ding C, Li J, Wang Y, Guo H, Lu Z. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020 doi: 10.1002/jmv.25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6(5):591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao ZC. Efficient management of novel coronavirus pneumonia by efficient prevention and control in scientific manner. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):163–166. doi: 10.3760/cma.issn.1001-0939.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Administration UFaD. Emergency use authorizations for coronavirus disease 2019 (COVID-19). https://www.fdagov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices#covid19ivd. 2020. [PubMed]
- 8.Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA. 2020 doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 11.Pollan M, Perez-Gomez B, Pastor-Barriuso R, Oteo J, Hernan MA, Perez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395(10230):1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu W-T, Laporte RP, Wu J. Determinants of Taiwan's early containment of COVID-19 incidence. American Public Health Association. 2020 [Google Scholar]
- 14.Dept. of Household Registration MotI. Population by sex and 5 year age group. https://www.risgovtw/app/portal. 2019.
- 15.Doi A, Iwata K, Kuroda H, Hasuike T, Nasu S, Kanda A. Estimation of seroprevalence of novel coronavirus disease (COVID-19) using preserved serum at an outpatient setting in Kobe, Japan: A cross-sectional study. medRxiv. 2020;2020 doi: 10.1016/j.cegh.2021.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farnsworth CW, Anderson NW. SARS-CoV-2 Serology: Much Hype. Little Data. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muench P, Jochum S, Wenderoth V, Ofenloch-Haehnle B, Hombach M, Strobl M. Development and validation of the Elecsys Anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. medRxiv. 2020;2020 doi: 10.1128/JCM.01694-20. 06.16.20132803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CY, Lin RTP, Renia L, Ng LFP. Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front Immunol. 2020;11:879. doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.