Abstract
Background
The epidemiology of psychiatric symptoms among patients with coronavirus disease 2019 is poorly characterized.
Objective
This article sought to identify the prevalence of anxiety, depression, and acute stress disorder among hospitalized patients with coronavirus disease 2019.
Methods
Adult patients recently admitted to nonintensive care unit medical ward settings with coronavirus disease 2019 were eligible for enrollment. Enrolled patients were screened for depression, anxiety, and delirium. Subsequently, patients were followed up by phone after 2 weeks and rescreened for depression, anxiety, and acute stress disorder symptoms. Subjects' medical records were abstracted for clinical data.
Results
A total of 58 subjects were enrolled; of whom, 44 completed the study. Initially, 36% of subjects had elevated anxiety symptoms and 29% had elevated depression symptoms. At 2-week follow-up, 9% had elevated anxiety symptoms, 20% had elevated depression symptoms, and 25% had mild-to-moderate acute stress disorder symptoms. Discharge to home was not associated with improvement in psychiatric symptoms.
Conclusions
A significant number of patients hospitalized with coronavirus disease 2019 experienced symptoms of depression and anxiety. While anxiety improved after index admission, depression remained fairly stable. Furthermore, a significant minority of patients experienced acute stress disorder symptoms, though these were largely mild to moderate.
Key words: anxiety, depression, epidemiology, mental health, post traumatic stress
Background
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in China in late 2019 and was declared a pandemic by the World Health Organization on March 11, 2020.1 Since its emergence, COVID-19 has infected more than 21 million people and caused more than 750,000 deaths.2 As of late September 2020, it continues to spread unabated throughout the world.
Viral epidemics and pandemics including H1N1 influenza, Ebola, and Zika were associated with neuropsychiatric disorders owing to both direct effects of infection and psycho-social-economic factors associated with epidemics.3 , 4 More specifically, severe epidemic coronaviruses such as severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome were associated with a high prevalence of psychiatric morbidity including mood, anxiety, and posttraumatic symptoms.5 , 6 Although COVID-19 differs from previous coronaviruses of the 21st century in incidence and distribution of disease, it has been hypothesized that its impact on mental health will be substantial.7 , 8 This impact is not limited to those directly infected by the virus; psychosocial stressors associated with the pandemic may exacerbate or precipitate psychiatric illness in uninfected individuals. Health care workers at the frontline are particularly vulnerable to a range of indirect psychiatric complications including posttraumatic stress disorder, anxiety, and depression.9 , 10
The psychosocial impact on the general population and health care workers notwithstanding, there are reasons to believe that those directly infected with SARS-COV-2 are at particular risk of psychiatric complications during both the acute and recovery phases of illness. A number of putative mechanisms exist by which COVID-19 may induce psychiatric symptoms including (1) the virus's direct effect on the central nervous system, (2) the neuropsychiatric effects of systemic and central nervous system inflammation, (3) the psychologic impact of contact isolation and the stigma of infectious disease, and (4) social role disruption and impairment in function associated with serious illness.11
While early research examined the respiratory complications and constitutional symptoms of COVID-19, emerging evidence suggests that neuropsychiatric complications are prevalent in a large number of patients with COVID-19, particularly those with severe disease.12 , 13 A collaborative case series from the United Kingdom documenting neurological and neuropsychiatric symptoms in 153 patients indicated that altered mental status was the most common neuropsychiatric symptom in the study population but did not focus on primary psychiatric symptoms such as depression and anxiety.14 Several surveys have sought to quantify mood symptoms in health care workers and patients with existing primary affective disorders; notably, these have been milieu studies investigating the impact of pandemic on individuals at large—not in those infected with SARS-2-CoV specifically.15 , 16 However, data from 1 cohort of patients with COVID-19 suggest high rates of anxiety, depression, and other psychiatric symptoms.17 Patients admitted to the hospital for COVID-19 may be a population at especially increased risk of developing psychiatric symptoms given the severity of their disease, the unique stress of hospitalization under contact isolation, and the possibility of iatrogenic psychiatric effects of medications and other interventions.
In this prospective longitudinal cohort study, our aim was to assess the point prevalence of neuropsychiatric symptoms in hospitalized patients with COVID-19 using existing diagnostic screening tools administered remotely and to identify associations between illness severity in COVID-19 admissions and psychiatric symptoms. While there is a growing body of literature regarding the effect of viral epidemic illnesses on mental health, these studies have been cross-sectional or retrospective in nature. To our knowledge, this study is the first to examine the prevalence of mood symptoms in patients hospitalized with COVID-19 in a prospective manner.
Methods
Study Overview
Eligible patients were enrolled during a 1-month period at 2 sites at a large academic medical center—an urban tertiary care center and a community hospital—in New York City. After subject consent and enrollment, investigators administered a study entry survey and symptom assessment and contacted the participant's nurse to assess for delirium. Screening at study entry was performed 0–96 hours after an enrolled patient was admitted from the intensive care unit (ICU) or emergency department to an inpatient (non-ICU) hospital service. Fourteen to 17 days after enrollment, investigators contacted participants for follow-up assessment. Consent and assessments were administered by telephone. Additional data were retrieved from the medical record (Figure 1 ).
Figure 1.
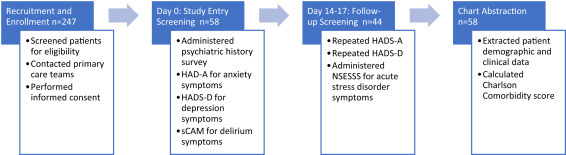
Study Overview.
HADS = Hospital Anxiety and Depression Scale; NSESSS = National Stressful Events Survey Acute Stress Disorder Short Scale.
For participants whose responses indicated a clinically significant level of symptoms, the investigator informed the patient's primary team, so that the patient could be further assessed and offered psychiatric intervention when warranted. If the participant had been discharged to home or a rehabilitation facility, the investigator referred the participant to a complimentary mental health referral and resource hotline in New York City.
The Columbia University Irving Medical Center Institutional Review Board approved the study protocol; verbal informed consent was obtained from all participants before administration of survey and screening assessments.
Eligibility and Recruitment
Patients were eligible for enrollment if they met the following criteria: (1) positive test for COVID-19 by nasopharyngeal SARS-2-CoV RT-PCR or admission diagnosis of coronavirus 2019 disease based on high clinical suspicion; (2) admitted to an inpatient non-ICU medical or surgical service from a system emergency department or ICU less than 96 hours prior; (3) primarily English-speaking adults; and (4) had capacity to provide verbal consent.
Investigators identified eligible patients using the hospital network's system-wide electronic medical record infection-control list, which enumerates all patients on isolation at a given time. If a patient appeared to meet criteria for enrollment, an investigator contacted the patient's primary care team using secure messaging to request they approach the patient for permission for the study team to contact them. If granted, a patient was called for informed consent and enrollment.
Study Survey and Screening Assessments
At time of study entry, investigators administered a survey to obtain a brief psychiatric history that queried existing diagnoses, treatment modalities, and history of prior psychiatric hospitalizations. The Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire used in adult patients with physical illness, was administered to determine symptoms of depression and anxiety.18 In addition, investigators asked the patient's primary nurse to complete the short form of the Confusion Assessment Method, a 5-item validated tool used to screen for delirium in hospitalized adults.19
At 2-week-follow up, participants were asked to repeat the HADS and to complete the National Stressful Events Survey Acute Stress Disorder Short Scale.20 The National Stressful Events Survey Acute Stress Disorder Short Scale is a validated 7-item questionnaire used to measure the severity of symptoms consistent with acute stress disorder in adults; if symptoms of acute stress disorder persist for greater than 1 month, participants may develop posttraumatic stress disorder. Because each item on the measure is rated on a 5-point scale, a raw composite score is divided by 7 to calculate an average total score; if the result is a fraction, it is rounded to the nearest whole number. A score of 0 points suggests no acute stress disorder; 1 point corresponds to mild, 2 points to moderate, 3 points to severe, and 4 points to extreme symptoms.
Once study entry and follow-up assessments were completed, investigators performed a review of each participant's electronic medical record to abstract demographic data, clinical data, and details about the index hospital admission. Chart diagnoses were used to calculate a patient's Charlson Comorbidity score, which quantifies an individual's morbidity and mortality risk.21
Outcomes
The primary goal of the study was to describe the point prevalence of delirium, depressed mood, and anxiety among surveyed patients acutely hospitalized with COVID-19 and the point prevalence of depressed mood, anxiety, and acute stress disorder at 2 weeks in this same population. A secondary aim was to determine if any risk factors predicted a higher prevalence of psychiatric symptoms in the study cohort. Specifically, we sought to test the hypotheses that (1) severity of illness as indexed by prior ICU stay and by inflammatory markers are associated with a more significant psychiatric symptom burden and (2) discharge to home, rather than to rehabilitation or skilled nursing care is associated with lower symptoms at 2-week follow-up, on the premise that intensive care unit admission, inflammatory markers, and disposition correlate with disease severity and functional status.
Statistical Analysis
We report characteristics of enrolled patients as counts and percentages. We report psychiatric screening scores stratified as per demographic and clinical characteristics. Continuous variables are expressed as means and interquartile ranges. We did not impute missing data. As screening score results were not normally distributed and asymmetric, we used nonparametric tests to evaluate associations and mean differences. Spearman's rank correlation was used to calculate associations; the Mann-Whitney U Test, the nonparametric equivalent of the unpaired t-test, was used to compare means between 2 groups; and the Kruskall-Wallis test, the nonparametric equivalent of a 1-way ANOVA, was used to compare means between multiple groups. Significance values were adjusted using the post hoc Bonferroni correction for multiple tests. We analyzed all data using SPSS Statistics, version 27.
Results
Recruitment
Daily searches of the system's electronic health record performed between April 29, 2020 and June 1, 2020 identified 247 English-speaking patients admitted to inpatient (non-ICU) services with COVID-19. Of these, 111 patients (45%) met eligibility criteria; of whom, 58 consented to participate. The most common reasons for exclusion were a history of neurocognitive disorder or chronic cognitive disorder, an admission diagnosis of delirium, or because a patient was nonverbal in the setting of tracheostomy for COVID-19 complicated by hypoxic respiratory failure. Of note, 80 of the 247 identified patients were excluded from recruitment because of cognitive impairment or delirium (Figure 2 ).
Figure 2.
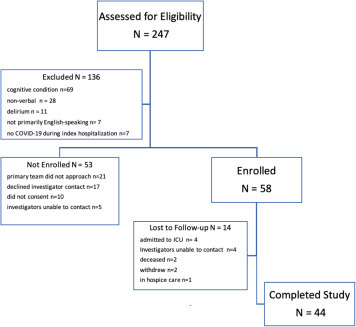
Patient Eligibility, Enrollment, and Study Completion.
Demographic and Clinical Characteristics
Of the 58 enrolled participants, 44 (76%) individuals completed both initial and follow-up assessments. Participants' mean age (range) was 59 (25–95) years, and 37 (65%) were men. Twenty-four patients (41%) were black or African American, 14 (24%) were white, 11 (19%) were of unknown race, and 9 (16%) of other races; 27 patients (47%) identified as non-Hispanic or Latino, 17 (29%) as Hispanic or Latino, and 14 (24%) were of unknown ethnicity; race and ethnicity were nonexclusive categories. Most patients were residents of the New York City boroughs of Manhattan (29 [50%]) and the Bronx (19 [33%]).
Fifteen (26%) participants had a past psychiatric history and 11 (19%) had a history of substance use disorder. The mean Charlson Index score of patients was 9.9 points, which correlates with an in-hospital mortality rate of greater than 8.0%.22 The most common comorbid conditions reported were diabetes mellitus (n = 17 [29%]) congestive heart failure (n = 11 [19%]), and prior stroke or transient ischemic attack (n = 7 [12%]). Nineteen participants (33%) were admitted to the ICU before follow-up screening and 9 (16%) underwent psychiatric consultation during hospital admission. At time of chart abstraction (July 2020), 38 (66%) of participants had a final discharge to home, 15 (26%) to a nursing home or rehabilitation facility, and 4 (7%) were deceased (Table 1 ).
Table 1.
Patient Characteristics
| Demographic characteristic | Study population (n = 58) |
|---|---|
| Gender | |
| Female | 21 (36%) |
| Male | 37 (64%) |
| Age (y) | |
| Mean (IQR) | 59 (46–70) |
| Range | 25–95 |
| <50 | 17 (29%) |
| 50–59 | 12 (21%) |
| 60–69 | 12 (21%) |
| 70–79 | 11 (19%) |
| ≥80 | 6 (10%) |
| Ethnic group | |
| Hispanic or Latino | 17 (29%) |
| Not Hispanic or Latino | 27 (47%) |
| Unknown | 14 (24%) |
| Race | |
| Asian | 1 (1.7%) |
| Black or African American | 24 (41%) |
| White | 14 (24%) |
| Other | 8 (14%) |
| Unknown | 11 (19%) |
| Residency | |
| Bronx | 19 (33%) |
| Manhattan | 29 (50%) |
| Queens | 1 (1.7%) |
| Staten Island | 1 (1.7%) |
| Suburban area (NY/NJ) | 7 (12%) |
| Undomiciled | 1 (1.7%) |
| Comorbidities (Charlson Index score) | |
| Mean (IQR) | 9.9 (8.0–11) |
| Range | 5.0–16 |
| Past psychiatric history | |
| Yes | 15 (26%) |
| No | 43 (74%) |
| Psychiatric diagnosis | |
| Major depressive disorder | 6 (10%) |
| Anxiety disorder | 5 (8.6%) |
| Schizophrenia/schizoaffective disorder | 2 (3.4%) |
| PTSD | 1 (1.7%) |
| Personality disorder | 1 (1.7%) |
| Other | 4 (6.9%) |
| Substance use disorder | |
| Yes | 11 (19%) |
| No | 47 (81%) |
| Substance | |
| Alcohol | 5 (8.6%) |
| Cannabis | 4 (6.9%) |
| Opioids | 2 (3.4%) |
| Crack/Cocaine | 2 (3.4%) |
| Tobacco | 2 (3.4%) |
| Methamphetamine | 1 (1.7%) |
| On psychotropics at home | |
| Yes | 9 (16%) |
| No | 49 (84%) |
| Psychotropic | |
| Antidepressant | 6 (10%) |
| Sedative/Hypnotic | 3 (5.1%) |
| Antipsychotic | 2 (3.4%) |
| Antiepileptic | 1 (1.7%) |
| Clinical characteristics | N = 58 |
|---|---|
| ICU admission | |
| Yes | 19 (33%) |
| No | 39 (67%) |
| Timing of ICU admission | |
| Before study entry | 12 (21%) |
| After study entry | 5 (8.6%) |
| Length of hospital admission (d) | |
| Mean | 15 (IQR 5.0–17) |
| Range | 1–65 |
| Final disposition | |
| Home | 38 (66%) |
| Nursing home/acute/subacute rehabilitation | 15 (26%) |
| Deceased | 4 (7%) |
| Remains hospitalized | 1 (1.7%) |
| Rehospitalized within 2 wk of follow-up interview | |
| Yes | 10 (17%) |
| No | 48 (83%) |
| Psychiatric consultation during hospitalization | |
| Yes | 9 (16%) |
| No | 49 (84%) |
| Inflammatory markers | Mean (IQR) |
|---|---|
| Mean C-reactive protein (ref 0–10 mg/L) | |
| Highest during admission (mg/L) n = 41 | 110 (9.6–190) |
| Before enrollment n = 38 | 56 (5.3–88) |
| Mean erythrocyte sedimentation rate (ref 0–15 mm/h) | |
| Highest during admission (mm/h) n = 25 | 73 (51–97) |
| Before enrollment n = 22 | 61 (29–90) |
| Mean interleukin-6 (Ref <7.0 pg/mL) | |
| Highest during admission (pg/mL) n = 26 | 83 (25–100) |
| Before enrollment n = 23 | 60 (13–70) |
ICU = intensive care unit; IQR= interquartile range; PTSD = posttraumatic stress disorder.
Point Prevalence of Psychiatric Symptoms
At study entry, the HADS-A mean score was 5.4 points; 21 (36%) participants had anxiety symptom scores ≥ 8 points, a level denoting clinically significant symptoms. At 2-week follow-up, the HADS-A mean score was 3.4, with 4 (9%) participants scoring ≥ 8. The mean improvement in HADS-anxiety scores from study entry to 2 weeks was 1.3 points. On study entry, the HADS-D mean score was 6.3 points, and 17 (29%) participants had depression symptom scores ≥ 8, suggesting clinical significance; at 2 weeks, the HADS-D mean score was 4.2 with 9 participants (20%) scoring ≥ 8. The mean improvement in HADS-D was 1.2 points. At time of study entry, none of the enrollees screened positive for delirium by the short form of the Confusion Assessment Method. The mean National Stressful Events Survey Acute Stress Disorder Short Scale score at 2-week follow-up was 0.78 points with 11 individuals (25%) scoring as experiencing mild or moderate symptoms of acute stress disorder (Table 2 ).
Table 2.
Point Prevalence of Mood Symptoms
| Screening test | Mean (IQR) | Range (0–21) | Borderline abnormal (8–10) | Abnormal (≥11) | Total abnormal (≥8) | 95% confidence interval |
|---|---|---|---|---|---|---|
| HADS-A at study entry n = 58 | 5.4 (1.0–9.0) | 0–18 | 13 (22%) | 8 (14%) | 21 (36%) | [4.2,6.6] |
| HADS-A at 2 wk n = 44 | 3.4 (1.0–4.7) | 0–16 | 1 (1.7%) | 3 (5.2%) | 4 (9.0%) | [2.4,4.4] |
| Change in HADS-A n = 44 | −1.3 (−3.0 to 1.0) | −17 to 9 | ||||
| HADS-D at study entry n = 58 | 6.3 (3.0–8.3) | 0–21 | 5 (8.6%) | 12 (21%) | 17 (29%) | [5.0,7.6] |
| HADS-D at 2 wk n = 44 | 4.2 (1.0–6.0) | 0–19 | 5 (11%) | 4 (9.0%) | 9 (20%) | [3.0,5.4] |
| Change in HADS-D n = 44 | −1.2 (−3.8 to 1.8) | −13 to 8 |
| Mean (IQR) | Range (0–5.0) | Mild (1.0–1.99) | Moderate (≥2.0) | Total abnormal (≥1.0) | 95% confidence interval | |
|---|---|---|---|---|---|---|
| NSESSS at 2 wk n = 44 | 0.79 (1–6.8) | 0–2.1 | 10 | 1 | 11 (25%) | [0.4,1.2] |
HADS = Hospital Anxiety and Depression Scale; IQR= interquartile range; NSESSS = National Stressful Events Survey Acute Stress Disorder Short Scale.
Patient Characteristics and Psychiatric Symptoms
There were no demographic characteristics that predicted a statistically significant higher prevalence of psychiatric symptoms in the study cohort. Although more patients with a past psychiatric diagnosis screened positive for mood symptoms on admission than those without a psychiatric diagnosis, the difference was not statistically significant.
Regarding our hypothesis that severity of COVID-19 as indicated by admission to an ICU during index hospitalization would be associated with a more significant psychiatric symptom burden, we found that patients admitted to an ICU before study entry (n = 12) did not have a significantly higher prevalence of mood and acute stress disorder symptoms at either time point when compared with cohort peers not admitted to an ICU before study entry. However, ICU admission after study entry during the index hospitalization was significantly associated with a higher prevalence of depression symptoms at time of study entry (n = 5); the mean HADS-D score for this group at time of study entry was 10.8 points versus 5.9 for participants not admitted to the ICU during this same period (P = 0.027). Only 1 of the 5 patients admitted to the ICU after study entry was clinically stable enough to participate in follow-up assessment, so we were unable to ascertain the prevalence of mood symptoms in this group at 2 weeks. Length of stay was not associated with severity of psychiatric symptoms at either time point or mean improvement in mood symptoms.
Results did not confirm our hypothesis that final discharge to home would be associated with a higher mean improvement in mood symptoms at 2-week follow-up than among patients discharged to nursing homes or rehabilitation facilities.
With regard to inflammatory markers, the last interleukin-6 value measured before study entry was positively associated with a patient's HADS-A score at that time (r [21] = 0.443; P = 0.034); however, no associations between C-reactive protein or erythrocyte sedimentation rate and psychiatric symptoms at either time point were identified.
Discussion
We evaluated the severity of symptoms of anxiety and depression shortly after hospital admission or transfer out of intensive care in a prospective cohort of patients hospitalized with COVID-19 infection early in the course of the pandemic in New York City. We subsequently reassessed symptoms of anxiety, depression, and assessed symptoms of acute stress disorder approximately 2 weeks after the initial assessment. We found a substantial prevalence of clinically significant anxiety (36%) and depression (29%) symptoms on initial assessment. On follow-up, there was a significant reduction in anxiety symptoms but a relative persistence of depressive symptoms. Twenty-five percent of patients assessed at the follow-up point had mild-or-greater symptoms of acute stress disorder. Our results did not support our hypotheses that more severe illness, as indexed by prior ICU admission or elevated inflammatory markers, would be associated with more severe psychiatric symptoms, except for an association of interleukin-6 level with anxiety symptoms. Likewise, we did not find evidence that discharge to home rather than to another care facility was associated with greater improvement in psychiatric symptoms over the course of the study.
Our results extend those previously identified in the literature. In an Italian study of 402 patients with COVID-19 evaluated in the emergency department and then screened for psychopathology approximately 1 month after initial emergency department evaluation, approximately 56% of patients screened positive in at least 1 psychiatric domain. Of these, approximately 31% screened positively for depression, 42% for anxiety, and 28% for obsessive-compulsive symptoms.17 Based on the high prevalence of psychiatric symptoms reported in ICU survivors in existing literature,23 we expected that participants who were admitted to the ICU during index hospitalization would have more mood and acute stress disorder symptoms than patients not requiring ICU admission; however, our study found no significant difference in symptom burden between cohorts. This finding may be due to the low power of the study or to eligibility criteria that excluded nonverbal and delirious patients, such that patients recruited from the ICU in our study were less severely ill than COVID-19 ICU admissions generally. In the data reported by Mazza et al.,17 systemic inflammation as measured by the systemic immune inflammation index was correlated with depression and anxiety scores at follow-up. While our study did not detect a correlation between depressive symptoms and inflammation, this may have been due to our small sample size, and we did detect some degree of correlation between anxiety and inflammation.
Our data are also consistent with psychiatric presentations of other severe coronavirus infections including Middle East respiratory syndrome and severe acute respiratory syndrome-1 (SARS). Rogers et al.24 conducted a systematic review and meta-analysis of such presentations and identified depression and anxiety rates of 32.6% and 35.7%, respectively. As in our data, rates of depression and anxiety prevalence were lower among individuals in the postillness phase (12.3% and 10.5%, respectively). Because we were interested in understanding the epidemiology of mood symptoms in hospitalized patients with COVID-19, a follow-up period of 2 weeks was chosen to assess the progression of psychiatric symptoms in the acute care setting. This is in contrast to existing data, such as that presented by Mazza et al.,17 which focused on patients in the posthospitalization period.
Our results suggest the need for systematic psychiatric screening for individuals diagnosed with COVID-19 infection. This conclusion is buttressed by the body of less common but more severe neuropsychiatric complications of COVID-19 identified in the literature but for which our study did not screen, including psychosis and persistent agitated delirium.11 , 25 , 26 Screening for common psychiatric manifestations of COVID-19 can be performed using standard psychiatric screening tools, including the Hospital Anxiety and Depression Scale (used in this study), the Patient Health Questionnaire (for depression), the Generalized Anxiety Disorder Scale (for anxiety), the Confusion Assessment Method (for delirium), and the Primary Care Posttraumatic Stress Disorder Screen for DSM-5 (for posttraumatic stress disorder). Many of these screening tools can be used by clinicians across a range of disciplines and may help identify patients in need of specialist psychiatric consultation. For patients in whom psychiatric symptoms are confounded by ongoing medical complications, evaluation and management by a consultation-liaison psychiatrist remains the gold standard.27
Our study represents a contribution to the understanding of psychiatric complications of COVID-19 infection. We identified a prospective cohort that were followed up longitudinally between time of hospital admission over a 2-week period (during which many were discharged). We used validated measures to screen for depression, anxiety, acute stress disorder, and delirium. Despite restrictions on in-person research involving individuals infected with COVID-19, we were able to maintain safety and progress in our data collection using telephone contact.
Our study has a number of limitations. Because only patients with capacity to provide informed consent were eligible to enroll, our data likely underestimate the prevalence of delirium. Of the 247 patients screened for eligibility, 80 could not be approached to consent to study participation because of cognitive impairment. We are unable to assess how many of these patients had new cognitive difficulty or delirium specifically associated with COVID-19 infection versus underlying dementia. Because the study protocol required that screening be performed remotely, patients with respiratory symptoms requiring invasive support measures that rendered them nonverbal were underrepresented making it difficult to ascertain fully the relationship between illness severity and psychiatric symptoms. Given non–English-speaking patients were excluded, our sample is not representative of the diverse population our hospital system serves; a large portion of our patient population is predominantly Spanish-speaking. Because of a rapid decline in the COVID-19 hospitalization rate that coincided with the period in which we enrolled patients, we were unable to achieve our target enrollment of 100 subjects; the small sample size increases the risk of type 2 error in hypothesis testing. Finally, our ability to follow up participants beyond 2 weeks was constrained by available time and resources; a prospective cohort study that follows up hospitalized patients with COVID-19 months and years after discharge is needed to better characterize the psychiatric sequelae of this novel virus.
Conclusions
Among patients hospitalized with COVID-19 infection, there is a high prevalence of clinically significant symptoms of anxiety and depression. These symptoms are largely unrelated to severity of illness or ultimate disposition, though some inflammatory markers may correlate with anxiety symptoms. Over the 2 weeks after hospital admission, the burden of anxiety symptoms decreases significantly. However, depressive symptoms are more persistent. In addition, at 2 weeks after study enrollment, approximately a quarter of patients experience at least mild symptoms of acute stress disorder. These data suggest that clinicians should screen acutely ill patients with COVID-19 for anxiety and depression both at initial evaluation and during follow-up and should be aware of the risk of acute stress disorder or posttraumatic stress disorder as patients recover from their acute illness.
Acknowledgments
The authors thank the primary teams involved in care of patients with COVID-19 at our institution for their help in identification of potential subjects. Supported in part by the Nathaniel Wharton Fund.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mapping the Covid-19 Outbreak Globally. Bloomberg.com Available from:
- 3.Brooks S.K., Webster R.K., Smith L.E. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucci V., Moukaddam N., Meadows J., Shah S., Galwankar S.C., Kapur G.B. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9:151–156. doi: 10.4103/jgid.jgid_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress after SARS. Emerg Infect Dis. 2005;11:1297–1300. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S.K.W., Wong C.W., Tsang J., Wong K.C. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychol Med. 2004;34:1187–1195. doi: 10.1017/s0033291704002272. [DOI] [PubMed] [Google Scholar]
- 7.Druss B.G. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77:891–892. doi: 10.1001/jamapsychiatry.2020.0894. [DOI] [PubMed] [Google Scholar]
- 8.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shechter A., Diaz F., Moise N. Psychological distress, coping behaviors, and preferences for support among New York healthcare workers during the COVID-19 pandemic. Gen Hosp Psychiatry. 2020;66:1–8. doi: 10.1016/j.genhosppsych.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J., Dong L., Wang T. Psychological symptoms among frontline healthcare workers during COVID-19 outbreak in Wuhan. Gen Hosp Psychiatry. 2020 doi: 10.1016/j.genhosppsych.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach S.R., Praschan N.C., Hogan C. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellul M.A., Benjamin L., Singh B. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varatharaj A., Thomas N., Ellul M.A. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rheenen T.E., Meyer D., Neill E. Mental health status of individuals with a mood-disorder during the COVID-19 pandemic in Australia: initial results from the COLLATE project. J Affect Disord. 2020;275:69–77. doi: 10.1016/j.jad.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Q., Feng H., Huang J. The mental health of frontline and non-frontline medical workers during the coronavirus disease 2019 (COVID-19) outbreak in China: a case-control study. J Affect Disord. 2020;275:210–215. doi: 10.1016/j.jad.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazza M.G., De Lorenzo R., Conte C. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Inouye S. Hospital Elder Life Program; Boston: 2014. The short confusion assessment method (Short CAM): training manual and coding guide. [Google Scholar]
- 20.Kilpatrick D.G., Resnick H.S., Friedman M.J. American Psychiatric Association Publishing; Washington DC: 2013. Severity of acute stress symptoms—adult (national stressful events survey acute stress disorder short scale [NSESSS]) [Google Scholar]
- 21.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Quan H., Li B., Couris C.M. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 23.Dijkstra-Kersten S.M.A., Kok L., Kerckhoffs M.C. Neuropsychiatric outcome in subgroups of intensive care unit survivors: implications for after-care. J Crit Care. 2020;55:171–176. doi: 10.1016/j.jcrc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Rogers J.P., Chesney E., Oliver D. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambar Akkaoui M., Lejoyeux M., Geoffroy P.A. Chloroquine-induced first-episode psychosis in a patient self-medicated for COVID-19. Biol Psychiatry. 2020 doi: 10.1016/j.biopsych.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrando S.J., Klepacz L., Lynch S. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61:551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalev D., Shapiro P.A. Epidemic psychiatry: the opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68–71. doi: 10.1016/j.genhosppsych.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


