Abstract
Background
Disparities driven by socioeconomic factors have been shown to impact outcomes for cancer patients. We sought to explore this relationship among patients with multiple myeloma (MM) who were not considered for hematopoietic stem cell transplant in the first-line setting and how it varied over time.
Methods
We queried the National Cancer Database for patients diagnosed with MM between 2004 and 2016 and included only those who received systemic therapy as the first-line treatment. Enrollment rates for therapy were calculated as receipt of systemic therapy as the incident event of interest (numerator) over time to initiation of therapy (denominator) and used to calculate incident rate ratios that were further analyzed using Poisson regression analysis. A multivariate Cox proportional hazards model was constructed for survival analysis, and differences were reported as hazard ratios (HRs).
Results
We identified 56,102 patients for enrollment analysis and 50,543 patients for survival analysis. Therapy enrollment in a multivariate model was significantly impacted by race and sex (p < .005). Advanced age, earlier year of diagnosis, lack of insurance or Medicaid, and higher comorbidity were associated with poor survival (HR > 1), whereas female sex, non-Hispanic black race, higher income, and treatment at an academic center were associated with improved survival (HR < 1).
Conclusion
Disparities in treatment of MM exist and are caused by a complex interplay of multiple factors, with socioeconomic factor playing a significant role. Studies exploring such determinants may help in equitable distribution of resources to overcome such differences.
Keywords: Disparity, Multiple myeloma, Social determinants, Stem cell transplant, Systemic therapy
Introduction
Coronavirus disease 2019 (COVID-19) has brought attention to issues related to timeliness of treatment and impact of delay in treatments among cancer patients [1]. There is concern that disparity in health care outcomes driven by disparity in access to treatment would be magnified at the time of COVID-19 and that there is urgent need to understand and address them [2], [3]. Multiple myeloma (MM) is the second most common blood cancer diagnoses in the United States and accounted for 2% of all cancer-related deaths in 2016 [4]. While the outcomes for patients with this disease has improved over time, we aimed to explore if this improvement was impacted by social determinants of health [5].
The incidence of MM as well as its precursor monoclonal gammopathy of unknown significance is two- to three‐fold higher in African Americans (AA) than in other racial groups resulting in higher mortality overall from the disease [6], [7], [8], [9]. The treatment options in MM are variable and may involve chemotherapy, immune modulatory therapy, monoclonal antibodies, and hematopoietic stem cell transplant (HSCT) [10]. Prior studies found a variable impact of race and socioeconomic status on the outcome of patients with MM and that by providing equal access to therapy, different population groups may be able to achieve similar outcomes [11], [12], [13], [14], [15], [16], [17], [18]. However, survival improvement over the years has been less significant for AA compared with Caucasians when considering patients able to receive appropriate therapy [6]. Despite developments in stem cell transplantation which potentially offers the best outcome, a large number of patients remain ineligible for transplant [12], [13]. As novel non-transplant therapy options are being developed, factors impacting the treatment and outcomes of these patients are pertinent to be analyzed in the evolving health care paradigm. We hypothesized that socioeconomic factors impact the initiation of systemic therapy in patients with MM who receive systemic therapy as the first-line treatment. We also explored how these factors affected survival.
Methods
We conducted a retrospective analysis using de-identified data accessed from the National Cancer Database (NCDB). The study was exempted from Institutional Review Board (IRB) oversight and did not require ethics approval.
We queried the NCDB for patients diagnosed with MM between 2004 and 2016 (ICD-0-3 code 9732). We excluded patients who were considered for HSCT and included only those who received systemic therapy (chemotherapy, immunotherapy, or hormonal therapy) as the first-line treatment. Patients with active myeloma were defined as those who were treated within 120 days of diagnosis based on similar methodology of previous studies since the NCDB fails to distinguish active myeloma from smoldering myeloma at diagnosis [19]. Due to concern for reporting errors, we excluded patients diagnosed at one center but received further treatment at other centers.
For the purpose of the study, race was reclassified into four categories as non-Hispanic whites (NHW), non-Hispanic black (NHB), Hispanics, and other. Comorbidity was quantified using the Charlson/Deyo comorbidity index [20]. Socioeconomic data included educational status represented in terms of quartiles of the percentage of persons with less than a high school education according to the residents’ census tract and median household income. The facility type was assigned according to the Commission on Cancer accreditation category as used in the NCDB. Locations were assigned based on data provided by the US Department of Agriculture Economic Research Service. Insurance status is captured in the NCDB as it appears on the admission facesheet for the patient and was recoded as none, private, government, and other types of insurance. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Two groups were generated for analysis: (a) enrollment to therapy analysis group and (b) survival analysis group (excludes patients in 2016 as survival is not reported for patients diagnosed with disease in 2016).
Summary statistics are presented as percentage for categorical data and median value with interquartile range (IQR) for quantitative data.
Enrollment rate ratios
Time to initiation of therapy (TTI) was defined as time in days from the date of diagnosis of cancer to earliest date of initiation of first-line cancer-directed therapy. The data was transformed into time series format with initiation into cancer-directed therapy as the incident event of interest (numerator) and TTI as the time horizon (denominator) to calculate rate of initiation of therapy or enrollment rate to therapy per 1000 person-days. In epidemiologic terms, an incident rate ratio is a relative difference measure used to compare the incidence rates of events (in this case, rate of enrollment into cancer-directed therapies) between various groups [21]. We utilized multivariate Poisson regression analysis to calculate the incident rate ratios (ratio of enrollment rates) and assess for significant impact of any variables on the enrollment to therapy [22]. This is reported as an enrollment rate ratio (ER). An ER < 1 indicates lower enrollment rate in comparison with the alternate group, whereas an ER > 1 suggests otherwise.
Survival analysis
Survival was measured in terms of months from the day of diagnosis to the day of censoring (last follow-up or day of death). Survival estimates were performed using the Kaplan–Meier method. Cox proportional hazard modeling assessed for significant independent variables impacting survival.
Data was analyzed using STATA version 15 (College Station, TX: StataCorp LLC). Adjusted effect size estimates and 95% confidence intervals are reported using an alpha level of 0.05 to indicate statistical significance. Extremely low values for p values are represented as <0.005. Most results are rounded to a decimal place of 1 except on occasion where we had to use decimal places up to two or three digits to represent the underlying differences or value for statistical significance.
Results
Cohort characteristics
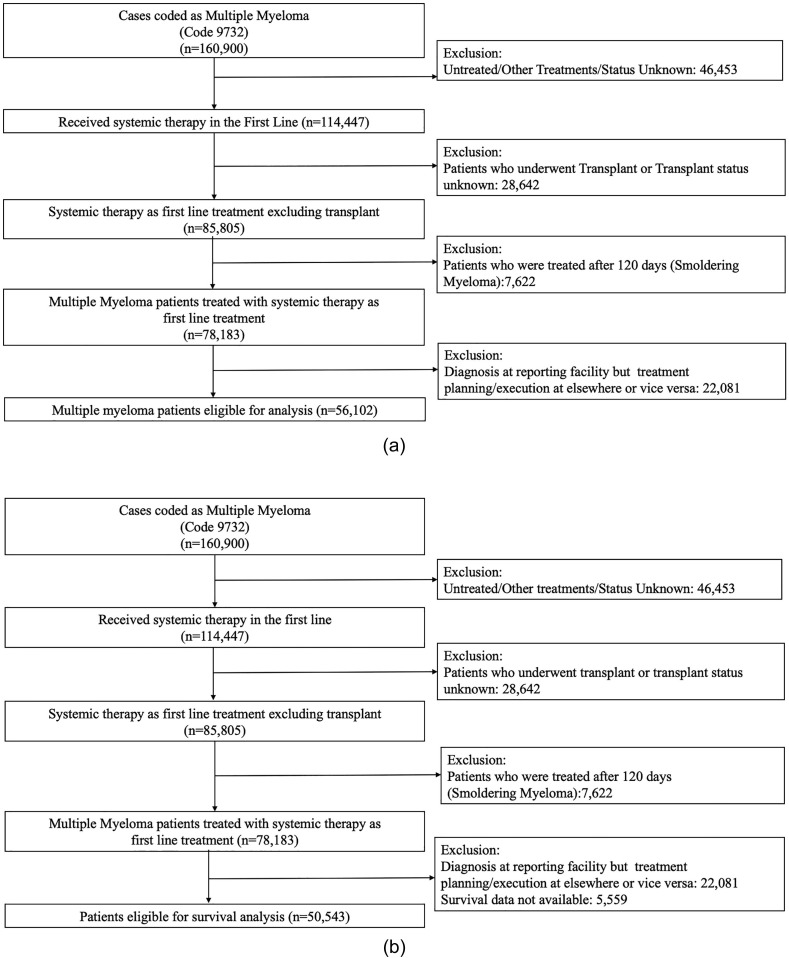
The dataset included 160,900 patients with MM. Of these, we identified 56,102 patients for enrollment analysis and 50,543 for survival analysis. Median age was 69 (IQR 60–77) years and 55% were males. The selection process is outlined in Fig. 1 A and B (CONSORT diagrams).
Fig. 1.
Flow diagram demonstrating selection of patients for the study for (A) enrollment to therapy analysis and (B) survival analysis.
Baseline characteristics of the cohorts are summarized in Table 1 . The majority were NHW, had insurance, belonged to metropolitan areas, and were treated at Comprehensive Community Cancer Centers or Academic Programs. Most patients had a comorbidity score of 0.
Table 1.
Baseline characteristics of patients selected for the study of enrollment to therapy analysis (n = 56,102) and survival analysis (n = 50,543).
| Characteristic | Enrollment analysis group Distribution Median (IQR) or n (%) |
Survival analysis group Distribution Median (IQR) or n (%) |
|---|---|---|
| Age, years | 69 (60–77) |
69 (60–77) |
| Days to systemic therapy | 17 (6–33) |
|
| Enrollment rate for therapy | 42.3 (42–42.7) |
|
| Sex | ||
| Male | 30,079 (54.7) | 27,619 (54.6) |
| Female | 25,393 (45.3) | 22,924 (45.4) |
| Race | ||
| Non-Hispanic whites | 36,889 (65.8) | 33,174 (65.6) |
| Non-Hispanic blacks | 11,402 (20.3) | 10,228 (20.2) |
| Hispanic | 3152 (5.6) | 2803 (5.6) |
| Others | 4659 (8.3) | 4338 (8.6) |
| Insurance | ||
| Uninsured | 2059(3.7) | 1941 (3.9) |
| Insured | 53,029 (96.3) | 47,673 (96.1) |
| Private | 16,241 (29.0) | 14,761 (29.2) |
| Medicare | 3399 (6.1) | 3005 (6.0) |
| Medicaid | 32,954 (58.7) | 29,518 (58.4) |
| Details unknown | 1449 (2.6) | 1318 (2.6) |
| Median income | ||
| <$38,000 | 11,871 (21.2) | 10,781 (21.4) |
| $38,000– $47,999 | 13,248 (23.7) | 11,939 (23.7) |
| $48,000–$62,999 | 14,638 (26.2) | 13,181 (26.2) |
| ≥$63,000 | 16,135 (28.9) | 14,445 (28.7) |
| Education | ||
| ≥21% | 10,628 (19.0) | 9548 (19.0) |
| 13.0–20.9% | 15,061 (26.9) | 13,581 (27.0) |
| 7.0–12.9% | 17,680 (31.6) | 15,931 (31.6) |
| <7.0% | 12,556 (22.4) | 11,312 (22.5) |
| Comorbidity score | ||
| 0 | 40,316 (71.9) | 36,509 (72.2) |
| 1 | 9902 (17.7) | 9103 (18.0) |
| 2 | 3927 (7.0) | 3421 (6.8) |
| ≥3 | 1957 (3.5) | 1510 (3.0) |
| Location | ||
| Metropolitan | 45,818 (83.7) | 41,277 (83.7) |
| Urban | 7817 (14.3) | 7048 (14.3) |
| Rural | 1124 (2.1) | 1013 (2.1) |
| Facility type | ||
| Community Cancer Center | 5482 (9.9) | 4944 (9.9) |
| Comprehensive Community Cancer Center | 24,395 (439) | 22,081 (44.1) |
| Academic/Research Program | 18,336 (33.0) | 16,340 (32.6) |
| Integrated Network Cancer Program | 7361 (13.3) | 6689 (13.4) |
| Year of diagnosis groups | ||
| 200–2005 | 5932 (10.6) | 5932 (12.0) |
| 2006–2007 | 6808 (12.1) | 6808 (14.1) |
| 2008–2009 | 7983 (14.2) | 7983 (15.8) |
| 2010–2011 | 8770 (15.6) | 8770 (17.4) |
| 2012–2013 | 9974 (17.8) | 9974 (19.7) |
| 2014–2015 | 11,076 (19.7) | 11,076 (21.9) |
| 2016– | 5559 (9.9) | |
| Implementation of Affordable Care Act (ACA) | ||
| Pre-ACA | 24,951 (44.5) | 24,951 (49.4) |
| Post-ACA | 31,151 (55.5) | 25,592 (50.6) |
IQR = interquartile range.
Rate of enrolment to chemotherapy and time to initiation of therapy
Overall rate of enrollment to systemic therapy was 42.3 per 1000 person-days, and median TTI was 17 (IQR 6–33) days. The temporal trend for the enrollment rate is depicted in Fig. 2 . Multivariate analysis of socioeconomic factors impacting enrollment to systemic therapy are summarized in Table 2 . Therapy enrollment in a multivariate model was significantly impacted by race and sex (p < .005) with ER < 1 for females versus males and NHB versus NHW.
Fig. 2.
Temporal trend for enrollment rate to systemic therapy (number per 1000 person-days).
Table 2.
Multivariate analysis of socioeconomic factors impacting enrollment to systemic therapy (n = 56,102).
| Characteristics | Enrollment per 1000 person-days (95% CI) | Incident rate ratios (95% CI) | p |
|---|---|---|---|
| Sex | |||
| Male | 43.7 (43.2–44.2) |
Reference | <0.005 |
| Female | 40.8 (40.3–41.3) |
0.94 (0.92–0.95) |
|
| Race | |||
| Non-Hispanic whites | 43.4 (42.9–43.8) |
Reference | |
| Non-Hispanic blacks | 39.5 (38.8–40.3) |
0.92 (0.90–0.95) |
<0.005 |
| Hispanic | 40.9 (39.5–42.5) |
0.97 (0.93–1.0) |
0.08 |
| Others | 42.5 (41.3–43.8) |
– | NS |
| Insurancea | |||
| Uninsured | 42.3 (40.5–44.2) |
– | NS |
| Insured | 42.3 (41.9–42.7) |
– | NS |
| Private | 42.6 (42.0–43.3) |
– | NS |
| Medicaid | 41.5 (40.1–42.9) |
– | NS |
| Medicare | 42.2 (41.8–42.7) |
– | NS |
| Details unknown | 43.5 (41.3–45.8) |
– | NS |
| Median income | |||
| <$38,000 | 41.9 (41.1–42.6) |
– | NS |
| $38,000–$47,999 | 41.9 (41.2–42.6) |
– | NS |
| $48,000–$62,999 | 42.4 (41.7–43.1) |
– | NS |
| ≥$63,000 | 42.9 (42.2–43.6) |
– | NS |
| Education | |||
| ≥21% | 41.4 (40.6–42.2) |
Reference | NS |
| 13.0–20.9% | 41.9 (41.2–42.6) |
– | NS |
| 7.0–12.9% | 42.1 (41.5–42.7) |
– | NS |
| <7.0% | 43.9 (43.2–44.7) |
1.04 (1.01–1.07) |
0.006 |
| Comorbidity score | |||
| 0 | 41.2 (40.8–41.6) |
Reference | |
| 1 | 44.3 (43.4–45.2) |
1.07 (1.05–1.1) |
<0.0005 |
| 2 | 48.8 (47.3–50.4) |
1.18 (1.15–1.22) |
<0.0005 |
| ≥3 | 46.8 (44.7–48.9) |
1.16 (1.11–1.22) |
<0.0005 |
| Location | |||
| Metropolitan | 42.0 (41.6–42.4) |
Reference | |
| Urban | 44.0 (43.1–45.0) |
1.05 (1.02–1.07) |
0.001 |
| Rural | 44.3 (41.8–47.0) |
– | NS |
| Facility type | |||
| Community Cancer Center | 39.8 (38.8–40.9) |
Reference | |
| Comprehensive Community Cancer Center | 43.8 (43.2–44.3) |
1.10 (1.07–1.21) |
<0.0005 |
| Academic/Research Program | 41.2 (40.6–41.8) |
1.05 (1.02–1.09) |
0.001 |
| Integrated Network Cancer Program | 42.7 (41.7–43.7) |
1.09 (1.05–1.13) |
<0.0005 |
| Year of diagnosis groups | |||
| 2004–2005 | 46.3 (45.1–47.5) |
Reference | |
| 2006–2007 | 43.5 (42.5–44.6) |
0.94 (0.91–97) |
<0.0005 |
| 2008–2009 | 40.6 (39.7–41.5) |
0.88 (0.85–0.91) |
<0.0005 |
| 2010–2011 | 42.1 (41.2–43.0) |
0.91 (0.88–0.93) |
<0.0005 |
| 2012–2013 | 42.9 (42.1–43.8) |
0.92 (0.89–0.95) |
<0.0005 |
| 2014–2015 | 41.7 (40.9–42.5) |
0.90 (0.87–0.93) |
<0.0005 |
| 2016– | 40.4 (39.3–41.5) |
0.87 (0.84–0.90) |
<0.0005 |
CI = confidence interval; NS = Not significant.
Survival by socioeconomic and demographic factors
The overall median survival improved by 5 months between 2004–2005 and 2014–2015 (28.5 vs. 33.4 months). The trend is depicted in Fig. 3 . The results for multivariate analysis of factors impacting the survival are summarized in Table 3 . Advanced age, earlier year of diagnosis, lack of insurance or Medicaid insurance, and higher comorbidity were associated with poor survival outcomes, whereas female sex, NHB race, higher income, and treatment at an academic center were associated with improved survival outcomes. The differential impact of race, income, and insurance on survival persisted over the years and are shown in Fig. 4 .
Fig. 3.
Temporal trend for median survival (months).
Table 3.
Multivariate analysis of socioeconomic factors impacting survival (n = 50,543).
| Characteristic | Hazards ratio (95% CI) |
p |
|---|---|---|
| Overall median survival | 33.4 (IQR 11.2–73.5) |
|
| Age | 1.03 (1.027–1.030) |
<0.0005 |
| Days to chemotherapy | 0.994 (0.994–0.995) |
<0.0005 |
| Year of diagnosis groups | ||
| 2004–2005 | Reference | |
| 2006–2007 | 0.95 (0.92–0.99) |
<0.0005 |
| 2008–2009 | 0.88 (0.85–0.92) |
<0.0005 |
| 2010–2011 | 0.86 (0.83–0.90) |
<0.0005 |
| 2012–2013 | 0.82 (0.79–0.85) |
<0.0005 |
| 2014–2015 | 0.84 (0.81–0.88) |
<0.0005 |
| Sex | ||
| Male | Reference | Reference |
| Female | 0.9 (0.90–0.94) |
<0.0005 |
| Race | ||
| Non-Hispanic whites | Reference | Reference |
| Non-Hispanic blacks | 0.90 (0.87–0.93) |
<0.0005 |
| Hispanic | 0.80 (0.76–0.85) |
<0.0005 |
| Others | 0.97 (0.94–1.01) |
NS |
| Insurance | ||
| Uninsured | Reference | Reference |
| Insured | ||
| Private | 0.83 (0.78–0.89) |
<0.0005 |
| Medicaid | 1.09 (1.01–1.17) |
0.03 |
| Medicare | 0.90 (0.84–0.96) |
0.003 |
| Details unknown | 0.89 (0.81–0.98) |
0.018 |
| Median income | ||
| <$38,000 | Reference | Reference |
| $38,000–$47,999 | 0.96 (0.93–0.99) |
0.040 |
| $48,000–$62,999 | 0.93 (0.90–0.97) |
<0.005 |
| ≥$63,000 | 0.91 (0.87–0.95) |
<0.005 |
| Location | ||
| Metropolitan | - | NS |
| Urban | - | |
| Rural | - | |
| Comorbidity score | ||
| 0 | Reference | Reference |
| 1 | 1.29 (1.26–1.33) |
<0.005 |
| 2 | 1.56 (1.50–1.63) |
<0.005 |
| ≥3 | 1.73 (1.63–1.84) |
<0.005 |
| Education | ||
| ≥21% | Reference | Reference |
| 13.0–20.9% | 0.99 (0.95–1.02) |
NS |
| 7.0–12.9% | 0.98 (0.94–1.02) |
NS |
| <7.0% | 0.94 (0.89–0.99) |
0.02 |
| Facility type | ||
| Community Cancer Center | Reference | Reference |
| Comprehensive Community Cancer Center | 1.01 (0.97–1.05) |
NS |
| Academic/Research Program | 0.95 (0.91–0.98) |
0.01 |
| Integrated Network Cancer Program | 1.01 (0.97–1.06) |
NS |
CI = confidence interval; NS = Not significant.
Fig. 4.
Temporal trend in differences in hazard ratios for survival by (A) race, (B) insurance, and (C) income from multivariate analysis.
Discussion
Studies have demonstrated improvement in the survival of patients with MM [10]. However, the improvement has not been experienced uniformly by all subsections of the society and was attributed to the disparity in the utilization of HSCT [12], [17]. However, the majority of patients with MM do not receive HSCT, and the present study aimed to evaluate the trends and disparities in the management of these patients [13]. We chose to measure the rate of enrollment to cancer-directed therapy rather than merely the therapy receipt status as this would reflect the receipt of therapy as well as the time to receiving the therapy, both of which may be impacted by socioeconomic and demographic factors. This is based on studies that used similar methodology to assess factors impacting the trends in treatment [23], [24], [25]. As far as we could gather from the literature, this is the first study looking at the impact of socioeconomic and demographic factors on the enrollment to systemic therapy for this group of patients as other studies focused on the receipt or lack of receipt of treatments [12], [13], [15], [17]. We found that disparities exist in enrollment to systemic therapy as well as outcomes for patients with MM. Moreover, the disparities were preserved over the years.
As mentioned previously, considerable variability existing in the therapies for MM and influenced by racial-ethnic and demographic characteristics has been revealed by other studies [15]. It has been observed that HSCT remains underutilized among AA [15]. Racial-ethnic minority status, older age, low socioeconomic status, residence in a metropolitan area, and lack of medical insurance were factors associated with lower likelihood of treatment with HSCT upfront [13]. Underutilization of treatments such as the proteasome inhibitor bortezomib has also been demonstrated among AA patients that was correlated to a 12% increased risk for mortality [26]. Even with improvement in the proportion of patients getting treatment, the differences based on racial characteristics were preserved and reflected in our study. This is discouraging from a public health standpoint [27].
Survival analyses in the present study also reveal several caveats. It is interesting that unlike other hematological malignancies, NHB achieved better odds for survival than Caucasians in this analysis model adjusted for income and insurance status [28], [29], [30]. This suggests that insurance, income, and comorbidities may be stronger drivers of health outcomes but not necessarily related to access to therapy. The finding in support of the argument that outcome disparity may not be fully explained in terms of access barriers is that NHB patients had higher survival rates despite lower enrollment rate to systemic therapy, whereas the trends were quite concordant when considering income and insurance status. Therefore, the study supports the idea that additional factors such as follow-up care and individual decision-making process are important in determining outcomes for these patients [14], [26], [27]. The better outcomes discrepant with enrollment rate for AA may also be due to favorable risk cytogenetics as hypothesized in recent studies [31], [32]. Notably, although better outcomes have been demonstrated historically for AA, the rate of improvement is still lower compared with Caucasians. This difference is most pronounced at times of development of novel therapies such as HSCT or the introduction of thalidomide [6].
The impact of income and socioeconomic status on survival in patients with MM is significant [27], [33], [34], [35], [36], [37]. Fiala et al. [26] reported a survival advantage associated with higher socioeconomic status that was partially accounted by the higher rates of HSCT. In the present study, this variability was minimized as we excluded patients who underwent HSCT reported as the first-line treatment although it is possible some of these patients did receive HSCT downstream. In addition, multiple surrogates for socioeconomic status (income, education, and geographical location) were used, and their respective adjustments during analysis suggested income to be the dominant variable. An improved survival in patients with MM belonging to a higher socioeconomic status has been demonstrated in other countries as well including those with universal health care and 2-week referral system for suspected cancer patients [38], [39], [40], [41]. This implies that increasing access and reducing costs alone may not eliminate outcome disparities. Possible mechanisms of poor survival may include poor tolerance of therapies or poor adherence but is an area of active investigation. Factors such as private insurance, academic center, and favorable geographical location also appeared to impact survival [42], [43]. These should be matters of consideration while designing health care policies in the future.
While timely initiation of therapy is an important factor to improve outcomes, it is interesting to note that in studies on hematological malignancies, there is paradoxically worse survival in those who started therapy earlier which is contradictory to that from solid tumors [44], [45]. Although the findings may appear paradoxical, the variability likely reflects the fact that sicker patients and patients with advanced stage MM associated with poor prognosis are enrolled into therapy earlier [44]. Therefore, it is proposed that clinical trials should accommodate patients who need urgent therapy to detect treatment effects in high‐risk groups [44].
The present study has the advantage that it utilized the NCDB that included patients with private and government insurance, thereby allowing for comparison between the groups. The impact of other parameters, such as geographical location and educational status of the patients, was also included in the multivariate analysis. Use of multiple surrogates for socioeconomic status (income, education, and geographical location) and their respective adjustments during analysis helped improve the robustness and generalizability of the results. Advancements in the pharmacological therapy for MM in the form of proteasome inhibitor bortezomib (2003), thalidomide and lenalidomide (2006: early part of study), and pomalidomide (2013: late part of study) could have certainly influenced the outcomes at various time periods but were not studied separately. The NCDB database fails to distinguish active MM from smoldering MM—a limitation of previous studies [13]. We overcame this limitation by defining patients with active myeloma as those treated within120 days from diagnosis similar to previous studies [19].
A major limitation of the study is that we were unable to identify the type of systemic therapy employed and to assess for variations in terms of the type of therapy. At the same time, the present study is applicable to majority of the patients with MM as only a minority of the patients are eligible for HSCT despite the advancements in the field [26]. By choosing to assess the enrollment rate to therapy, we were able to capture the impact of various factors on the timeline of diagnosis of the disease to initiation of myeloma-directed therapy. While this adds to the strength of the study, it should be noted that factors related to lack of receipt of therapy, which are important in determining outcomes, are not considered in the present study but have been evaluated previously [12], [13], [15], [17]. Another limitation is the lack of data on progression of the disease and that overall survival may not be an adequate marker for assessing outcomes in these patients. Disease characteristics such as stage of the disease and quality of life are factors to be considered which was not captured in the database and therefore not amenable to analysis [46]. The racial and ethnic variations in the incidence of second primary malignancies in treated MM have been demonstrated with uncertain impact on the long-term outcomes. This could not be adjusted in the present study and is an area for further research [47]. The inability of our study to capture the impact of monoclonal antibody therapy (which is now indicated in the front-line setting) on the outcome measures is another limitation.
While this study is representative of real-world data over a decade, the concern also applies to clinical trials. Studies show that minorities, older individuals, and persons with more advanced disease are underrepresented in MM trials, compromising external validity of results [12]. It has been demonstrated that when disparities are mitigated, the outcome disparity may be obliterated to some extent. Therefore, efforts to ensure improved accrual of disadvantaged sections of the society (lower socioeconomic groups, patients with higher comorbidities, and patients on government insurance according to the present study) should be a priority. There have been campaigns aimed toward eliminating outcome disparities in cancer, but those focused on hematologic malignancies specifically may be needed [26]. The efforts should also include creating awareness among disadvantaged sections of the population and health care providers involved in treating them. The impact of the Affordable Care Act that includes measures to eliminate disparity based on insurance, increased health care access, and improved preventive visits remains to be seen.
Conclusions
Disparities in MM exist and are caused by a complex interplay of multiple factors, with socioeconomic factors such as insurance and income playing a dominant role. The disparities not only exact high human cost but also negatively impact the economics of health care. As we navigate the care of special patient populations such as these during a pandemic and design health care policies, social determinants of health are important factors to be kept in mind.
Funding
This study received no funding.
Ethics approval
This study does not contain any identifiable data and was exempt from institutional review board oversight and ethics approval process.
Authors’ contributions
Study concept and design: TJ, VB, RW, SS. Analysis and interpretation of data: TJ, VB, RW, SS. Drafting of the manuscript: TJ, VB, ZC, JL, RW, SS. Critical revision of the manuscript for important intellectual content: TJ, VB, ZC, JL, RW, SS. Administrative, technical, or material support: RW, SS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
None.
Data statement
Authors TJ and SS had full access to all the data in the study. We take full responsibility for the integrity of the data and the accuracy of the analysis as well as sharing the data with any interested investigators.
Footnotes
Prior presentation: American Society of Hematology Annual Meeting, December 2019, Orlando Florida.
References
- 1.COVID-19 Pandemic Delaying Treatments of Hematologic Cancers. Hematology Advisor. Published April 1, 2020. [accessed May 3, 2020]. https://www.hematologyadvisor.com/home/topics/general-hematology/coronavirus-2019-disease-precautions-may-delay-hematologic-cancer-treatment/.
- 2.Snapshot. [accessed May 3, 2020]. https://news.harvard.edu/gazette/story/2020/04/health-care-disparities-in-the-age-of-coronavirus/.
- 3.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 4.SEER Cancer Statistics Review; 1975–2016. SEER. [accessed November 10, 2019]. https://seer.cancer.gov/csr/1975_2016/index.html.
- 5.Definitions|Social Determinants of Health|NCHHSTP|CDC. Published April 30, 2019. [accessed November 11, 2019]. https://www.cdc.gov/nchhstp/socialdeterminants/definitions.html.
- 6.Waxman A.J., Mink P.J., Devesa S.S., Anderson W.F., Weiss B.M., Kristinsson S.Y., et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010;116:5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O., Weiss B.M. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691–1697. doi: 10.1038/leu.2009.134. [DOI] [PubMed] [Google Scholar]
- 8.Brown L.M., Pottern L.M., Silverman D.T., Schoenberg J.B., Schwartz A.G., Greenberg R.S., et al. Multiple myeloma among Blacks and Whites in the United States: role of cigarettes and alcoholic beverages. Cancer Causes Control. 1997;8:610–614. doi: 10.1023/a:1018498414298. [DOI] [PubMed] [Google Scholar]
- 9.Lewis D.R., Pottern L.M., Brown L.M., Silverman D.T., Haves R.B., Schoenberg J.B., et al. Multiple myeloma among blacks and whites in the United States: the role of chronic antigenic stimulation. Cancer Causes Control. 1994;5:529–539. doi: 10.1007/bf01831381. [DOI] [PubMed] [Google Scholar]
- 10.Kazandjian D. Multiple myeloma epidemiology and survival, a unique malignancy. Semin Oncol. 2016;43:676–681. doi: 10.1053/j.seminoncol.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatnagar V., Wu Y., Goloubeva O.G., Ruehle K.T., Milliron T.E., Harris C.G., et al. Disparities in black and white patients with multiple myeloma referred for autologous hematopoietic transplantation: a single center study. Cancer. 2015;121:1064–1070. doi: 10.1002/cncr.29160. [DOI] [PubMed] [Google Scholar]
- 12.Costa L.J., Huang J.-X., Hari P.N. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21:701–706. doi: 10.1016/j.bbmt.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hamadani M., Hashmi S.K., Go R.S. Use of autologous hematopoietic cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol. 2014;89:825–830. doi: 10.1002/ajh.23753. [DOI] [PubMed] [Google Scholar]
- 14.Ailawadhi S., Bhatia K., Aulakh S., Meghji Z., Chanan-Khan A. Equal treatment and outcomes for everyone with multiple myeloma: are we there yet? Curr Hematol Malig Rep. 2017;12:309–316. doi: 10.1007/s11899-017-0393-y. [DOI] [PubMed] [Google Scholar]
- 15.Ailawadhi S., Frank R.D., Advani P., Swaika A., Temkit M.H., Menghani R., et al. Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: a SEER-medicare analysis. Cancer Med. 2017;6:2876–2885. doi: 10.1002/cam4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ailawadhi S., Jacobus S., Sexton R., Stewart A.K., Dispenzieri A., Hussein M.A., et al. Disease and outcome disparities in multiple myeloma: exploring the role of race/ethnicity in the Cooperative Group clinical trials. Blood Cancer J. 2018;8:67. doi: 10.1038/s41408-018-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailawadhi S., Azzouqa A.G., Hodge D., Cochuyt J., Jani P., Ahmed S., et al. Survival trends in young patients with multiple myeloma: a focus on racial-ethnic minorities. Clin Lymphoma Myeloma Leuk. 2019;19:619–623. doi: 10.1016/j.clml.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Verma P.S., Howard R.S., Weiss B.M. The impact of race on outcomes of autologous transplantation in patients with multiple myeloma. Am J Hematol. 2008;83:355–358. doi: 10.1002/ajh.21139. [DOI] [PubMed] [Google Scholar]
- 19.Ravindran A., Bartley A.C., Holton S.J., Gonsalves W.I., Kapoor P., Siddiqui M.A., et al. Prevalence, incidence and survival of smoldering multiple myeloma in the United States. Blood Cancer J. 2016;6:e486. doi: 10.1038/bcj.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Compare Two Crude Rates (Incidence Rate Ratio) – StatsDirect. [accessed August 2, 2020]. https://www.statsdirect.com/help/rates/compare_crude_incidence_rates.htm.
- 22.Poisson Regression|R Data Analysis Examples. [accessed August 2, 2020]. https://stats.idre.ucla.edu/r/dae/poisson-regression/.
- 23.Whitney R.L., Bell J.F., Tancredi D.J., Romano P.S., Bold R.J., Joseph J.G. Hospitalization Rates and predictors of rehospitalization among individuals with advanced cancer in the year after diagnosis. J Clin Oncol. 2017;35:3610–3617. doi: 10.1200/JCO.2017.72.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panchal H., Pilewskie M.L., Sheckter C.C., Albornoz C.R., Razdan S.N., Disa J.J., et al. National trends in contralateral prophylactic mastectomy in women with locally advanced breast cancer. J Surg Oncol. 2019;119:79–87. doi: 10.1002/jso.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ukoha E.P., Feinglass J., Yee L.M. Disparities in electronic patient portal use in prenatal care: retrospective cohort study. J Med Internet Res. 2019;21:e14445. doi: 10.2196/14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiala M.A., Wildes T.M. Racial disparities in treatment utilization for multiple myeloma. Cancer. 2017;123:1590–1596. doi: 10.1002/cncr.30526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ailawadhi S., Frank R.D., Sharma M., Menghani R., Temkit M.H., Paulus S., et al. Trends in multiple myeloma presentation, management, cost of care, and outcomes in the Medicare population: a comprehensive look at racial disparities. Cancer. 2018;124:1710–1721. doi: 10.1002/cncr.31237. [DOI] [PubMed] [Google Scholar]
- 28.Pulte D., Redaniel M.T., Brenner H., Jeffreys M. Changes in survival by ethnicity of patients with cancer between 1992–1996 and 2002–2006: is the discrepancy decreasing? Ann Oncol. 2012;23:2428–2434. doi: 10.1093/annonc/mds023. [DOI] [PubMed] [Google Scholar]
- 29.Giri S., Shrestha R., Pathak R., Bhatt V.R. Racial differences in the overall survival of hairy cell leukemia in the united states: a population-based analysis of the surveillance, epidemiology, and end results database. Clin Lymphoma Myeloma Leuk. 2015;15:484–488. doi: 10.1016/j.clml.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Bulatao RA, Anderson NB, National Research Council (US) Panel on Race E. Health Care. National Academies Press (US); 2004. [accessed November 28, 2019]. https://www.ncbi.nlm.nih.gov/books/NBK24693/.
- 31.Marinac C.R., Ghobrial I.M., Birmann B.M., Soiffer J., Rebbeck T.R. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020;10:1–8. doi: 10.1038/s41408-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg A.J., Philip S., Paner A., Velinova S., Badros A., Catchatourian R., et al. Racial differences in primary cytogenetic abnormalities in multiple myeloma: a multi-center study. Blood Cancer J. 2015;5:e271. doi: 10.1038/bcj.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baris D., Brown L.M., Silverman D.T., Hayes R., Hoover R.N., Swanson G.M., et al. Socioeconomic status and multiple myeloma among US blacks and whites. Am J Public Health. 2000;90:1277–1281. doi: 10.2105/ajph.90.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koessel S.L., Theis M.K., Vaughan T.L., Koepsell T.D., Weiss N.S., Greenberg R.S., et al. Socioeconomic status and the incidence of multiple myeloma. Epidemiol Camb Mass. 1996;7:4–8. doi: 10.1097/00001648-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Savage D., Lindenbaum J., Van Ryzin J., Struening E., Garrett T.J. Race, poverty, and survival in multiple myeloma. Cancer. 1984;54:3085–3094. doi: 10.1002/1097-0142(19841215)54:12<3085::aid-cncr2820541246>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Weston B., Grufferman S., MacMillan J.P., Cohen H.J. Effects of socioeconomic and clinical factors on survival in multiple myeloma. J Clin Oncol. 1987;5:1977–1984. doi: 10.1200/JCO.1987.5.12.1977. [DOI] [PubMed] [Google Scholar]
- 37.Lenhard R.E., Enterline J.P., Crowley J., Ho G.Y. The effects of distance from primary treatment centers on survival among patients with multiple myeloma. J Clin Oncol. 1987;5:1640–1645. doi: 10.1200/JCO.1987.5.10.1640. [DOI] [PubMed] [Google Scholar]
- 38.Renshaw C., Ketley N., Møller H., Davies E.A. Trends in the incidence and survival of multiple myeloma in South East England 1985–2004. BMC Cancer. 2010;10:74. doi: 10.1186/1471-2407-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristinsson S.Y., Derolf A.R., Edgren G., Dickman P.W., Björkholm M. Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in sweden. J Clin Oncol. 2009;27:2073–2080. doi: 10.1200/JCO.2008.18.2006. [DOI] [PubMed] [Google Scholar]
- 40.Pasqualetti P., Colantonio D., Collacciani A., Casale R. Socioeconomic status and survival in multiple myeloma. Minerva Med. 1990;81:713–716. [PubMed] [Google Scholar]
- 41.Suspected cancer: recognition and referral. Clinical Practice Guidelines. Guideline Central. [accessed November 28, 2019]. https://www.guidelinecentral.com/summaries/suspected-cancer-recognition-and-referral/#section-420.
- 42.Shah N.N., Xi Y., Liu Y., Koff J.L., Flowers C.R., Behera M., et al. Racial and socioeconomic disparities in mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:e312–e320. doi: 10.1016/j.clml.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Han H., Shah G., Giralt S., Landgren C.O., He J., et al. Significant nationwide variability in the costs and hospital mortality rates of autologous stem cell transplantation for multiple myeloma: an analysis of the nationwide inpatient sample database. Biol Blood Marrow Transplant. 2019;25:41–46. doi: 10.1016/j.bbmt.2018.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olszewski A.J., Ollila T., Reagan J.L. Time to treatment is an independent prognostic factor in aggressive non-Hodgkin lymphomas. Br J Haematol. 2018;181:495–504. doi: 10.1111/bjh.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khorana A.A., Tullio K., Elson P., Pennell N.A., Grobmyer S.R., Kalady M.F., et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS ONE. 2019;14:e0213209. doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyle R.A., Rajkumar S.V. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ailawadhi S., Swaika A., Razavi P., Yang D., Chanan-Khan A. Variable risk of second primary malignancy in multiple myeloma patients of different ethnic subgroups. Blood Cancer J. 2014;4 doi: 10.1038/bcj.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]