Abstract
Objective
Half of all newly diagnosed patients with glioblastoma are > 65 years still with a poor prognosis. Preserving quality of life is of high importance. However, patient reported outcome (PRO) data in this patient group is rare. The aim was to compare health-related quality of life (HRQoL) and distress between elderly and younger patients with high-grade glioma (HGG).
Methods
We used baseline data of a prospective study where HGG patients were enrolled from 4 hospitals. Distress was measured using the distress thermometer (DT), HRQoL using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (QLQ-C30) plus brain module (BN20). We compared distress and HRQoL by age (≥ 65 vs. < 65 years), gender, performance score, and time since diagnosis using multivariate linear and logistic regressions.
Results
A total of n = 93 (30%) out of n = 309 patients were ≥ 65 years (mean 70 years, range 65–86 years). Mean DT score of elderly patients (5.2, SD 2.6) was comparable with younger patients (4.9, SD 2.6). Elderly patients reported significantly lower global health (GHS, mean elderly vs. younger; 50.8 vs. 60.5, p = 0.003), worse physical (56.8 vs. 73.3, p < 0.001) and lower cognitive functioning (51.1 vs. 63.2, p = 0.002), worse fatigue (52.5 vs. 43.5, p = 0.042), and worse motor dysfunction (34.9 vs. 23.6, p = 0.030). KPS and not age was consistently associated with HRQoL.
Conclusion
Physical functioning was significantly reduced in the elderly compared with younger HGG patients, and at the same time, emotional functioning and DT scores were comparable. KPS shows a greater association with HRQoL than with calendric age in HGG patients reflecting the particular importance for adequate assessment of HRQoL and general condition in elderly patients.
Keywords: Elderly patients, High-grade glioma, Glioma, Glioblastoma, Quality of life, Age, Karnofsky performance status, Distress
Introduction
High-grade gliomas (HGG) represent the majority of gliomas with glioblastomas showing an incidence of 3.19 (3.16–3.21) cases per 100,000 person years [1]. Half of all newly diagnosed patients with glioblastoma (GBM) are older than 65 years [2]. Although recently improved and cost-effective treatment schemes for elderly patients with GBM have been published, these patients are facing a particularly dismal prognosis with a median survival of less than 6 months [3–6] compared with younger healthier patients with more favorable outcomes and molecular marker profiles according to recent studies [2]. In many clinical trials, higher age is an exclusion criterion, and historically, many elderly patients received no tumor-specific therapy but best supportive care [7, 8]. Due to general condition (frailty) and comorbidities, treating elderly patients with HGG is challenging, and multidisciplinary approaches are required [9–11].
Therefore, preserving quality of life in elderly patients is of high importance particularly considering the short life expectancy. Distress and supportive care needs in HGG patients are high. They should be addressed early in the disease trajectory by psycho-oncologists but also supportive and palliative care services [12]. Additionally, quality of life during survival is equally important as the length of survival in the elderly population [12, 13]. Furthermore, it has been reported that lower performance status, higher age, female gender, and shorter time since diagnosis can be associated with distress and reduced quality of life [5]. However, health-related quality of life (HRQoL) data is rare for elderly people with HGG, and as soon as they experience clinical decline due to disease progression, assessment becomes difficult [2, 5].
Application of patient-reported outcomes (PROs) has become essential in assessing patients’ HRQoL, distress, and psychosocial burden, as well as supportive care needs [14]. Recently, it has been shown that monitoring symptoms via PRO measures can be very helpful for cancer patients and even influence survival [15–17]. As the elderly population in HGG in most of the studies is defined by an age ≥ 65 years, HRQoL and distress in this population might be different than in younger patients below than 65 years. Furthermore, so far, no data focusing especially on elderly patients’ HRQoL are available. Thus, a comparison might help clinicians in clinical daily practice to advise patients regarding this important issue.
Therefore, the aims of our study were to describe quality of life and distress in elderly patients with HGG and to compare the results with those of the younger ones focusing in psychological and physical issues. Furthermore, we aimed to investigate factors associated with HRQoL of HGG patients.
Patients and method
Patients
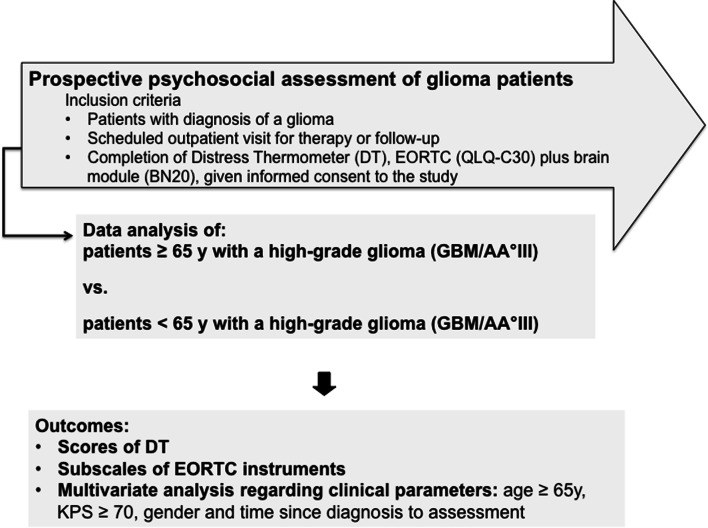
From March 2014 to October 2014 as well as April 2015 to June 2016, we conducted two prospective studies assessing HRQoL, distress, and supportive care needs in glioma patients. Patients at four German neuro-oncological centers were approached during their outpatient visits and asked to participate in the study as previously described [18–20]. Inclusion criteria data analysis was a diagnosis of glioma WHO grades III–IV regardless of disease stage (initial diagnosis or recurrent disease), absence of aphasia impairing communication or consent to the study, and given informed consent. Patients were asked to complete several PRO measures. Furthermore, demographic and clinical data were recorded in a database. If patients were assessed several times during the study, we only evaluated the first assessment per patient for this cross-sectional analysis. Figure 1 shows the course of the study. When patients declined the participation, gender, age, diagnosis, and if possible reason were documented.
Fig. 1.
The course of the study
Instruments
Distress thermometer
The distress thermometer (DT) is a self-reporting screening instrument developed by the National Comprehensive Cancer Network to evaluate psychological distress on a visual analog scale (0–10 points). A problem list with 40 items is included for patients to indicate the area of concern (family, financial, and physical). Studies have proven its acceptance in oncological patients, and Goebel and colleagues evaluated the German version for brain tumor patients. A score ≥ 6 indicates a significant burden in brain tumor patients according to Goebel et al. [21].
European Organization for Research and Treatment of Cancer quality of life core questionnaire accompanied by the brain-specific module
The EORTC QLQ-C30 is a widely accepted questionnaire evaluating cancer patients’ quality of life. Functions’ symptom and the global health status (GHS) are investigated (physical (physf), role (rolef), emotional (emof), social (socf), and cognitive functioning (cogf), fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Its validity and reliability have been proven in numerous clinical studies, and it is available in 103 languages. The additional module for brain tumor patients (BN20) consists of 20 questions specifically assessing their symptoms (3 neurological deficit scales, 1 future uncertainty scale, treatment, and disease-related symptoms) [22, 23]. The scores were calculated according to the user manual. Each scale ranges from 0 to 100, with higher scores indicating better functioning for functional scales and worse symptoms for symptom scales.
Statistical analysis
We performed a descriptive explorative assessment comparing scores of DT and subscales of EORTC instruments between the elderly and the younger group. The following parameters were considered to be potentially associated with distress and quality of life: performance status (KPS) ≥ 70, age (≥ 65 years, according to clinical studies), gender, and time since diagnosis [5, 24]. These variables were selected content driven by clinical relevance. We tested their association with univariate and multivariate models using binary logistic regression for distress (DT ≥ 6) and linear regression for quality of life (all EORTC subscales).
Ethics
The study was in accordance with national law, institutional ethical standards, and the 1964 Helsinki Declaration and its later amendments. The ethic committees of all study centers approved the study (Mainz, Ludwigsburg, Stuttgart, and Ulm/Gunzburg, Germany, No: 837.349.15 (10117), and 837.472.13 (9157-F)). Patients provided written informed consent prior to data acquisition.
Results
Patients
A total of n = 93 (30%) out of n = 309 patients were ≥ 65 years (mean = 70 years, range 65–86 years). The majority of the elderly population harbored a glioblastoma (GBM, n = 77, 83%), n = 53 (57%) were male, median KPS was 70 (range 40–100), and mean time since diagnosis was 2.2 months. The younger population had a mean age of 48 years (range 19–64), n = 126 were male (57%), less patients had a GBM (n = 103, 47%), and the median KPS was 80 (range 40–100). Details are provided in Table 1.
Table 1.
Clinical and demographic data of the patient sample. All variables are indicated in numbers and % in brackets if not otherwise specified
| Variable | All patients n = 309 (100%) | Older patients n = 95 (100%) | Younger patients n = 214 (100%) |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 55 (14) | 70 (4.5) | 48 (10) |
| Median (range) | 55 (19–86) | 69 (65–86) | 50 (19–64) |
| Gender | |||
| Male | 179 (58) | 54 (57) | 127 (59) |
| Female | 130 (42) | 41 (43) | 87 (41) |
| Living situation | |||
| Single | 66 (21) | 16 (17) | 48 (23) |
| In relationship | 229 (74) | 76 (80) | 155 (72) |
| Missing | 14 (5) | 3 (3) | 11 (5) |
| Working situation | |||
| Working | 170 (55) | 11 (12) | 140 (65) |
| Retired or early retired | 93 (30) | 68 (71) | 44 (21) |
| Missing | 46 (15) | 16 (17) | 30 (14) |
| WHO grade | |||
| WHO III | 128 (41) | 17 (18) | 110 (51) |
| WHO IV | 181 (59) | 78 (82) | 104 (49) |
| Survival at assessment | |||
| < 1 year | 128 (41) | 52 (55) | 76 (36) |
| > 1 and < 2 years | 47 (15) | 17 (18) | 30 (14) |
| > 2 years | 134 (43) | 26 (27) | 108 (50) |
| Tumor localization | |||
| Frontal | 128 (41) | 37 (39) | 91 (43) |
| Temporal | 88 (28) | 28 (29) | 60 (28) |
| Parietal | 21 (7) | 7 (7) | 14 (6) |
| Occipital | 48 (16) | 16 (18) | 32 (15) |
| Other unknown | 20 (7) 4 (1) | 7 (7) 0 | 13 (6) 4 (2) |
| MGMT promotor methylation | |||
| Unmethylated | 128 (41) | 46 (48) | 82 (38) |
| Methylated | 86 (28) | 32 (34) | 54 (26) |
| Not analyzed | 73 (24) | 14 (15) | 59 (28) |
| Missing | 22 (7) | 3 (3) | 19 (8) |
| IDH status | |||
| IDH wild type | 127 (41) | 56 (59) | 71 (33) |
| IDH mutated | 84 (27) | 20 (21) | 64 (30) |
| Not analyzed | 76 (25) | 16 (17) | 60 (28) |
| Missing | 22 (7) | 3 (3) | 19 (9) |
| Ongoing chemotherapy | |||
| Yes | 125 (40) | 53 (56) | 72 (34) |
| No | 158 (51) | 39 (41) | 119 (56) |
| Missing | 26 (9) | 3 (3) | 23 (10) |
| Surgery for recurrent tumor | |||
| Yes | 87 (28) | 14 (15) | 73 (34) |
| No | 188 (61) | 67 (71) | 121 (57) |
| Missing | 34 (11) | 14 (15) | 20 (8) |
| Karnofsky index | |||
| Mean (SD) | 78 (16) | 74 (18) | 81 (15) |
| Median (range) | 80 (30–100) | 70 (40–100) | 80 (30–100) |
| Time since diagnosis in months | |||
| Mean (SD) | 42 (51) | 25 (44) | 49 (54) |
| Median (range) | 19 (0.5–298) | 10 (0.5–288) | 31 (0.5–296) |
Distress and health-related quality of life
Mean DT scores of elderly patients were comparable with those of younger patients (mean elderly patients vs. younger patients; 5.2 (SD = 2.6) vs. 4.9 (SD = 2.6), p = 0.42).
Elderly patients reported significantly lower GHS (mean elderly vs. younger; 50.8 vs. 60.5, p = 0.003), lower physf (56.8 vs. 73.3, p < 0.001), lower cogf (51.1 vs. 63.2, p = 0.002), higher fatigue (52.5 vs. 43.5, p = 0.042), greater impairment by visual disorders (23.9 vs.15.0, p = 0.013), by motor dysfunction (34.9 vs. 23.6, p = 0.030), by weakness of legs (31.4 vs. 20.8, p = 0.03), and greater problems regarding bladder control (22.3 vs. 10.8, p = 0.001). Younger patients reported significantly higher symptom scores for insomnia (elderly patients vs. younger patients; 27.0 vs. 36.4, p = 0.003) and financial difficulties (18.8 vs. 33.8, p < 0.001), please see Table 2.
Table 2.
Comparison between patients ≥ 65 and < 65 years regarding score on DT and subscale or symptom scale on EORTC instruments
| Score on distress thermometer resp. subscale or symptom scale on EORTC instruments | ≥ 65 years mean (SD) | < 65 years mean (SD) | p |
|---|---|---|---|
| Distress score | 5.2 (2.6) | 4.9 (2.6) | 0.42 |
| Global health status (GHS) | 50.8 (22.1) | 60.5 (22.6) | 0.003 |
| Physical functioning | 56.8 (33.6) | 73.3 (27.1) | < 0.0001 |
| Role functioning | 52.6 (38.8) | 60.1 (34.4) | 0.21 |
| Emotional functioning | 62.3 (26.6) | 60.0 (26.5) | 0.66 |
| Social functioning | 52.5 (35.4) | 57.6 (34.8) | 0.35 |
| Cognitive functioning | 51.1 (33.8) | 63.2 (31.4) | 0.002 |
| Fatigue | 52.5 (30.6) | 43.5 (29.3) | 0.042 |
| Nausea | 7.7 (17.4) | 8.3 (16.5) | 0.49 |
| Pain | 24.9 (30.8) | 24.2 (29.8) | 0.702 |
| Insomnia | 27.0 (32.1) | 36.4 (35.2) | 0.003 |
| Appetite loss | 18.7 (28.8) | 19.2 (30.3) | 0.701 |
| Constipation | 18.2 (29.9) | 15.5 (27.6) | 0.69 |
| Financial difficulties | 18.8 (29.1) | 33.8 (37.5) | < 0.0001 |
| Future uncertainty | 45.3 (30.4) | 42.5 (29.2) | 0.58 |
| Visual disorder | 23.9 (26.1) | 15.0 (20.8) | 0.013 |
| Motor dysfunction | 34.9 (31.5) | 23.6 (25.7) | 0.030 |
| Communication deficit | 35.7 (33.8) | 28.4 (29.8) | 0.40 |
| Headaches | 24.6 (33.3) | 30.4 (33.9) | 0.06 |
| Seizures | 5.4 (34.6) | 8.5 (23.0) | 0.20 |
| Drowsiness | 48.9 (34.6) | 42.9 (31.6) | 0.57 |
| Itchy Skin | 23.1 (30.1) | 16.3 (26.2) | 0.25 |
| Hair loss | 19.7 (32.2) | 16.2 (31.1) | 0.38 |
| Weakness of legs | 31.4 (36.1) | 20.8 (31.3) | 0.03 |
| Bladder control | 22.3 (33.8) | 10.8 (25.3) | 0.001 |
Factors associated with distress and quality of life
In general, KPS was consistently associated with HRQoL regarding all functioning scales (physical functioning, p < 0.0001, 95% CI 30.73, 47.35; emotional functioning, p < 0.0001, 95% CI 9.29, 25.59; cognitive functioning, p < 0.0001, 95% CI 13.18, 33.72; social functioning, p < 0.0001, 95% CI 24.14, 45.48). This was true for most of the symptom scales as well as single item scales. Age same or above 65 years was associated with worse physical functioning (p = 0.029, 95% CI − 15.66, − 0.84), less financial difficulties (p < 0.0001, 95% CI − 29.99, − 9.36), visual disorders (p = 0.028, 95% CI 0.77, 13.48), and seizures (p = 0.030, 95% CI − 14.52, − 0.75). Details are provided in Table 3.
Table 3.
Results of the multivariate analysis regarding the EORTC subscales and single items with content-driven-selected variables
| Subscale or symptom | Variable | Beta | p | 95% CI lower | 95% CI upper |
|---|---|---|---|---|---|
| Physf | Age ≥ 65 (yes/no) | − 0.126 | 0.029 | − 15.66 | − 0.84 |
| Time since diagnosis | 0.040 | 0.484 | − 0.53 | 1.12 | |
| KPS ≥ 70 (yes/no) | 0.526 | < 0.0001 | 30.73 | 47.35 | |
| gender | − 0.060 | 0.278 | − 10.58 | 3.05 | |
| Rolef | Age ≥ 65 (yes/no) | 0.015 | 0.815 | − 15.66 | − 0.84 |
| Time since diagnosis | 0.113 | 0.072 | − .53 | 1.12 | |
| KPS ≥ 70 (yes/no) | 0.416 | < 0.0001 | 30.73 | 47.35 | |
| Gender | − 0.033 | 0.592 | − 10.58 | 3.05 | |
| Emof | Age ≥ 65 (yes/no) | 0.102 | 0.130 | − 1.65 | 12.82 |
| Time since diagnosis | 0.030 | 0.650 | − 0.63 | 1.00 | |
| KPS ≥ 70 (yes/no) | 0.278 | < 0.0001 | 9.29 | 25.59 | |
| Gender | − 0.175 | 0.007 | − 15.86 | − 2.51 | |
| Cogf | Age ≥ 65 (yes/no) | − 0.084 | 0.205 | − 15.01 | 3.24 |
| Time since diagnosis | 0.071 | 0.280 | − 0.47 | 1.60 | |
| KPS ≥ 70 (yes/no) | 0.294 | < 0.0001 | 13.18 | 33.72 | |
| Gender | − 0.080 | 0.213 | − 13.75 | 3.08 | |
| Socf | Age ≥ 65 (yes/no) | 0.011 | 0.863 | − 8.60 | 10.24 |
| Time since diagnosis | 0.083 | 0.191 | − 0.37 | 1.77 | |
| KPS ≥ 70 (yes/no) | 0.408 | < 0.0001 | 24.14 | 45.48 | |
| Gender | − 0.065 | 0.294 | − 13.32 | 4.05 | |
| Fatigue | Age ≥ 65 (yes/no) | 0.057 | 0.391 | − 4.70 | 11.97 |
| Time since diagnosis | − 0.094 | 0.147 | − 1.61 | 0.24 | |
| KPS ≥ 70 (yes/no) | − 0.291 | < 0.0001 | − 30.51 | − 11.83 | |
| Gender | 0.120 | 0.061 | − 0.34 | 14.99 | |
| Nausea | Age ≥ 65 (yes/no) | − 0.060 | 0.388 | − 7.19 | 2.81 |
| Time since diagnosis | 0.014 | 0.840 | − 0.50 | 0.61 | |
| KPS ≥ 70 (yes/no) | − 0.021 | 0.757 | − 6.49 | 4.73 | |
| Gender | 0.156 | 0.021 | 0.84 | 10.01 | |
| Pain | Age ≥ 65 (yes/no) | 0.028 | 0.690 | − 6.93 | 10.45 |
| Time since diagnosis | 0.082 | 0.226 | − 0.37 | 1.56 | |
| KPS ≥ 70 (yes/no) | − 0.133 | 0.053 | − 19.38 | 0.120 | |
| Gender | 0.098 | 0.143 | − 2.03 | 13.92 | |
| Dyspnea | Age ≥ 65 (yes/no) | 0.087 | 0.212 | − 3.16 | 14.15 |
| Time since diagnosis | − 0.044 | 0.519 | − 1.28 | 0.65 | |
| KPS ≥ 70 (yes/no) | − 0.134 | 0.050 | − 19.39 | 0.02 | |
| Gender | 0.029 | 0.669 | − 6.23 | 9.69 | |
| Insomnia | Age ≥ 65 (yes/no) | − 0.125 | 0.074 | − 18.96 | 0.88 |
| Time since diagnosis | − 0.019 | 0.785 | − 1.25 | 0.95 | |
| KPS ≥ 70 (yes/no) | − 0.077 | 0.264 | − 17.44 | 4.80 | |
| Gender | 0.123 | 0.069 | − 0.65 | 17.59 | |
| Appetite loss | Age ≥ 65 (yes/no) | − 0.114 | 0.093 | − 16.87 | 1.31 |
| Time since diagnosis | − 0.130 | 0.053 | − 2.01 | 0.01 | |
| KPS ≥ 70 (yes/no) | − 0.210 | 0.002 | − 26.47 | − 6.08 | |
| Gender | 0.114 | 0.082 | − 0.95 | 15.73 | |
| Constipation | Age ≥ 65 (yes/no) | 0.022 | 0.754 | − 7.29 | 10.04 |
| Time since diagnosis | − 0.006 | 0.935 | − 1.02 | 0.94 | |
| KPS ≥ 70 (yes/no) | − 0.092 | 0.185 | − 16.35 | 3.17 | |
| Gender | 0.106 | 0.117 | − 1.61 | 14.35 | |
| Diarrhea | Age ≥ 65 (yes/no) | − 0.074 | 0.296 | − 9.41 | 2.88 |
| Time since diagnosis | − 0.058 | 0.399 | − 0.99 | 0.40 | |
| KPS ≥ 70 (yes/no) | − 0.065 | 0.349 | − 10.29 | 3.65 | |
| Gender | 0.011 | 0.868 | − 5.17 | 6.13 | |
| Financial difficulties | Age ≥ 65 (yes/no) | − 0.257 | < 0.0001 | − 29.95 | − 9.36 |
| Time since diagnosis | − 0.055 | 0.416 | − 1.64 | 0.68 | |
| KPS ≥ 70 (yes/no) | − 0.140 | 0.038 | − 23.91 | − 0.66 | |
| Gender | − 0.040 | 0.542 | − 12.42 | 6.55 | |
| GHS | Age ≥ 65 (yes/no) | − 0.125 | 0.058 | − 12.28 | 0.202 |
| Time since diagnosis | 0.003 | 0.960 | − 0.69 | 0.72 | |
| KPS ≥ 70 (yes/no) | 0.327 | < 0.0001 | 11.07 | 25.19 | |
| Gender | − 0.022 | 0.726 | − 6.78 | 4.73 | |
| Future uncertainty | Age ≥ 65 (yes/no) | − 0.072 | 0.287 | − 12.95 | 3.85 |
| Time since diagnosis | − 0.162 | 0.015 | − 2.07 | − 0.22 | |
| KPS ≥ 70 (yes/no) | − 0.238 | < 0.0001 | − 26.59 | − 7.68 | |
| Gender | 0.018 | 0.780 | − 6.60 | 8.78 | |
| Visual disorder | Age ≥ 65 (yes/no) | 0.149 | 0.028 | 0.77 | 13.48 |
| Time since diagnosis | − 0.048 | 0.465 | − 0.96 | 0.44 | |
| KPS ≥ 70 (yes/no) | − 0.221 | 0.001 | − 19.30 | − 4.94 | |
| Gender | 0.058 | 0.373 | − 3.18 | 8.45 | |
| Motor dysfunction | Age ≥ 65 (yes/no) | 0.059 | 0.335 | − 3.74 | 10.95 |
| Time since diagnosis | 0.004 | 0.943 | − 0.78 | 0.84 | |
| KPS ≥ 70 (yes/no) | − 0.493 | < 0.0001 | − 42.60 | − 26.13 | |
| Gender | 0.046 | 0.435 | − 4.07 | 9.41 | |
| Communication deficit | Age ≥ 65 (yes/no) | − 0.001 | 0.990 | − 8.88 | 8.76 |
| Time since diagnosis | 0.012 | 0.858 | − 0.89 | 1.06 | |
| KPS ≥ 70 (yes/no) | − 0.356 | < 0.0001 | − 37.21 | − 17.45 | |
| Gender | 0.000 | 0.999 | − 8.08 | 8.07 | |
| Headache | Age ≥ 65 (yes/no) | − 0.082 | 0.238 | − 15.76 | 3.94 |
| Time since diagnosis | 0.045 | 0.508 | − 0.72 | 1.45 | |
| KPS ≥ 70 (yes/no) | − 0.021 | 0.760 | − 12.77 | 9.33 | |
| Gender | 0.163 | 0.015 | 2.17 | 20.22 | |
| Seizures | Age ≥ 65 (yes/no) | − 0.151 | 0.030 | − 14.52 | − 0.75 |
| Time since diagnosis | 0.021 | 0.755 | − 0.63 | 0.87 | |
| KPS ≥ 70 (yes/no) | − 0.205 | 0.003 | − 19.36 | − 4.01 | |
| Gender | − 0.040 | 0.545 | − 8.22 | 4.36 | |
| Drowsiness | Age ≥ 65 (yes/no) | 0.060 | 0.378 | − 5.12 | 13.42 |
| Time since diagnosis | 0.020 | 0.761 | − 0.87 | 1.18 | |
| KPS ≥ 70 (yes/no) | − 0.255 | < 0.0001 | − 30.39 | − 9.60 | |
| Gender | 0.012 | 0.850 | − 7.68 | 9.32 | |
| Itchy skin | Age ≥ 65 (yes/no) | 0.093 | 0.185 | − 2.75 | 14.11 |
| Time since diagnosis | 0.023 | 0.740 | − 0.78 | 1.10 | |
| KPS ≥ 70 (yes/no) | − 0.111 | 0.109 | − 17.17 | 1.73 | |
| Gender | 0.079 | 0.242 | − 3.14 | 12.35 | |
| Hair loss | Age ≥ 65 (yes/no) | 0.020 | 0.768 | − 7.85 | 10.61 |
| Time since diagnosis | 0.070 | 0.290 | − 0.47 | 1.56 | |
| KPS ≥ 70 (yes/no) | − 0.042 | 0.535 | − 13.69 | 7.13 | |
| Gender | 0.291 | < 0.0001 | 20.71 | 27.64 | |
| Weakness of legs | Age ≥ 65 (yes/no) | 0.062 | 0.360 | − 5.23 | 14.33 |
| Time since diagnosis | − 0.053 | 0.426 | − 1.51 | 0.64 | |
| KPS ≥ 70 (yes/no) | − 0.307 | < 0.0001 | − 36.83 | − 14.88 | |
| Gender | 0.038 | 0.557 | − 6.25 | 11.56 | |
| Bladder control | Age ≥ 65 (yes/no) | 0.129 | 0.062 | − 0.43 | 17.32 |
| Time since diagnosis | 0.032 | 0.638 | − 0.74 | 1.21 | |
| KPS ≥ 70 (yes/no) | − 0.210 | 0.002 | − 25.75 | − 5.74 | |
| Gender | − 0.013 | 0.841 | − 8.95 | 7.30 |
Discussion
In our analysis comparing elderly and younger HGG patients, we found that the elderly population seems to be more affected by the disease than younger ones with regard to physical impairment. We found significant differences in scores between the two patient groups possibly highlighting the frailty of many elderly patients. However, regarding emotional function and DT, the results were comparable in both groups.
Study population and generalizability of the data
Although this is one of the first studies describing HRQoL in HGG with a focus on age, we have to take into account that according to a former drop-out analysis of the study, 30% of all patients seen within the screening period declined the assessment [25]. Most of the decliners harbored a GBM and were in a significantly worse general condition; age was not found to be associated with study decline. Therefore, we probably present positively biased data for the elderly, but also for the younger population due to the selection bias of the study. Furthermore, we observed significant differences between the younger and the older patient group regarding WHO grade resulting in a higher percentage of patients in the younger patient group with a longer survival at the assessment than the older ones. However, it has been shown that DT and HRQoL are not influenced by WHO grade, and even in patients with meningioma, a significant burden can be observed [26–29]. Due to its greater incidence with higher age, GBM is diagnosed in older people more frequently than in the younger population [1]. Similarly, there were more patients under chemotherapy in the older patient group (56 vs. 34% in the younger patient group). This should be considered influencing the HRQoL.
Results of younger and elderly patients with HGG
Both patient groups reported relatively low social and emotional functioning compared with the normal population reflecting the significance of the disease and the relevance of the topic [30]. In comparison with other cancer diagnoses, where younger patients are more distressed than elderly patients in general, our findings further emphasize how much more burdening a brain tumor diagnosis is for all age groups (REFs). Further, we found a significant association between longer time since diagnosis and lower future uncertainty in the regression analysis underlining the initial shock of the diagnosis and a certain adaption to the situation and the symptoms over time.
Elderly patients’ HRQoL was more affected than the younger one’s regarding physical impairment and motor dysfunction. However, emotional functioning was comparable. This is interesting and probably due to accentuated neurocognitive deficits in older patients, which permit full recognition of the severity of the situation. On the other hand, older patients may be more satisfied with their life lived thus far and less anxious about life-time lost due to the disease. Furthermore, elderly people could hesitate to allow depressive thoughts and try to present themselves strong, compliant, and positive in order to preserve their strength but also the support of the doctor as long as advanced care planning is not early enough provided [31, 32].
Yet, elderly patients reported significantly lower GHS, physical and cognitive functioning, greater fatigue, and impairment due to visual disorders as well as greater motor dysfunction meaning that they definitely perceived critical impairment, even though some may not be disease related. Although HRQoL assessed by the EORTC instruments is a subjective estimation, it remains sometimes unclear what the item or function does mean for the individual patient. Elderly patients may recognize their problems well but seem emotionally not as burdened as we would probably assume. Assessing quality of life by structured questionnaires like the EORTC instruments is a standardized approach where an individual weighting of issues is not intended in order to facilitate inter-individual comparisons. However, the assessment with individual consideration of the variety of topics influencing HRQoL (individualized approach) is complex, hard to implement in clinical routine or even in studies, and data are challenging to compare between patients [33–35]. However, regarding the brain tumor patient population, it may be worth to develop alternative approaches in order to assess the meaning of HRQoL for different individuals, e.g., by developing instruments more focusing on their situation or signaling questions implementable into clinical routine in order to facilitate, refine, and individualize the assessment.
In line with results of emotional functioning, the DT was comparable between the two groups. Finally, to admit a psychological burden may be more difficult than a physical impairment. Nonetheless, since some of the deficits older patients experience, such as loss in motor function, visual disturbances, weakness of legs, and loss of bladder control, may not be solely disease related and already persistent for a longer period of time, patients may have gotten used to them and find them less worrisome. Additionally, losing bodily functions may be more acceptable when growing old, in general. Greater fatigue in older patients may explain lower rates of insomnia compared with younger patients. On the other hand, cancer patients can develop symptoms of fatigue and present with sleeping disorder at the same time, which occur in brain tumor patients frequently and are suboptimal detected and treated by the neuro-oncologists [36].
Younger patients reported more often financial difficulties than elderly people, which is a significant problem in HGG patients. They often lose their higher executive functions and are not able to work anymore as they used to before the disease, leading to financial restrictions with significant consequences for their family and caregivers, who often have to play a new part in the relationship. Older patients, receiving pension or living off of retirement funds, do not need to worry as much anymore about providing for themselves and their family.
Significance of age and KPS
Our regression analysis revealed that after applying several models, KPS was associated stronger with HRQoL than calendric age. This is probably not surprising. However, given the fact that the newer studies led to different treatment schemes based on age, e.g., for GBM, it seems to be debatable to assign patients to therapy schemes considering solely their age in light of our findings: deciding on a more or less aggressive treatment should be based not only on KPS or calendric age alone, but also on patient perceived HRQoL. Undoubtedly, especially in elderly HGG patients, we should include geriatric assessment what seems to be helpful in geriatric cancer patients in general [37, 38].
Limitations and strengths of the study
Of note, we have to discuss several flaws of the study. First, we report a post hoc analysis, what gives a retrospective character to the prospectively collected data. Therefore, findings have to be interpreted carefully. Second, as already mentioned, due to the time consuming assessment, we observed a selection bias, as patients in a worse clinical condition in both groups independently of age declined participation in the study. Therefore, we report data of a well-functioning cohort which may influence mean scores, yet, since patients are assumed to have dropped out in both groups, the general findings regarding between group differences should be valid. Further, as mentioned above, the two patient populations exhibited differences regarding WHO grade, ongoing chemotherapy, and mean time since diagnosis. However, in a recent published review including glioblastoma patients, the authors found that chemotherapy and radiotherapy may not have harmful effects on HRQoL in fit elderly cancer patients [39]. A further limitation of the study is that we did not assess the comorbidities of the patients, which may have also influenced the results in the two different groups overestimating the influence of the HGG diagnosis. Furthermore, quality of life is influenced by multiple factors in this group, of which not all could be assessed as we analyzed content-driven variables to reduce multiple testing. Therefore, results and comparisons have to be interpreted carefully.
Yet, the strength of this study is that rare data are available for this patient group, and our study may on the one hand provide a basis for decision-making in daily clinical practice and on the other hand motivate to conduct further clinical studies assessing HRQoL in elderly HGG patients.
Conclusion
Physical functioning was significantly reduced in the elderly compared with younger HGG patients. Emotional functioning and DT scores were comparable. KPS shows a greater association with HRQoL than with calendric age in HGG patients reflecting the particular importance for adequate assessment of HRQoL and general condition in elderly patients.
Acknowledgments
We acknowledge all patients and caregivers for their study participation. Further, we acknowledge all medical students of our study group Karoline Kohlmann, Mareile Janko, Anne-Katrin Reuter, Linda Stöckelmaier, Katrin Nickel, Dorothea Maurer, and Heike Lahr for their work as the manuscript contents of parts of their dissertation. Further, we thank Sonja Grüninger, Monika Deininger, and Christoph Richter for their tremendous support.
Funding information
Open Access funding provided by Projekt DEAL. The Friedhelm Frees Stiftung, Mainz, Germany, funded this project.
Compliance with ethical standards
The study was in accordance with national law, institutional ethical standards, and the 1964 Helsinki Declaration and its later amendments. The ethic committees of all study centers approved the study (Mainz, Ludwigsburg, Stuttgart, and Ulm/Gunzburg, Germany, No: 837.349.15 (10117), and 837.472.13 (9157-F)). Patients provided written informed consent prior to data acquisition.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ironside S, Das S, Sahgal A, Moroney C, Mainprize T, Perry JR. Optimal therapies for newly diagnosed elderly patients with glioblastoma. Curr Treat Options in Oncol. 2017;18(11):66. doi: 10.1007/s11864-017-0508-7. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, Baker S, de Castro DG, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, Dyttus-Cebulok K, Rosenblatt E, Fidarova E, Roa W. Improved cost-effectiveness of short-course radiotherapy in elderly and/or frail patients with glioblastoma. Radiother Oncol. 2018;127(1):114–120. doi: 10.1016/j.radonc.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Moroney C, Perry JR, Tsang DS, Bilodeau D, Mueller C, Soliman H, Myrehaug S, Sahgal A, Tseng CL, Tsao MN. Hospitalizations in elderly glioblastoma patients. Ann Palliat Med. 2017;6(Suppl 2):S161–S1S9. doi: 10.21037/apm.2017.06.02. [DOI] [PubMed] [Google Scholar]
- 5.Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 6.Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 7.Amsbaugh MJ, Yusuf MB, Gaskins J, Burton EC, Woo SY. Patterns of care and predictors of adjuvant therapies in elderly patients with glioblastoma: an analysis of the National Cancer Data Base. Cancer. 2017;123(17):3277–3284. doi: 10.1002/cncr.30730. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 9.Harris G, Jayamanne D, Wheeler H, Gzell C, Kastelan M, Schembri G, et al. Survival outcomes of elderly patients with glioblastoma multiforme in their 75th year or older treated with adjuvant therapy. Int J Radiat Oncol Biol Phys. 2017;98(4):802–810. doi: 10.1016/j.ijrobp.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Tsang DS, Khan L, Perry JR, Soliman H, Sahgal A, Keith JL, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol) 2015;27(3):176–183. doi: 10.1016/j.clon.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Zouaoui S, Darlix A, Fabbro-Peray P, Mathieu-Daude H, Rigau V, Fabbro M, et al. Oncological patterns of care and outcomes for 265 elderly patients with newly diagnosed glioblastoma in France. Neurosurg Rev. 2014;37(3):415–423. doi: 10.1007/s10143-014-0528-8. [DOI] [PubMed] [Google Scholar]
- 12.Pace A, Villani V. Palliative and supportive care of patients with intracranial glioma. Prog Neurol Surg. 2018;31:229–237. doi: 10.1159/000467383. [DOI] [PubMed] [Google Scholar]
- 13.Harrison RA, de Groot JF. Treatment of glioblastoma in the elderly. Drugs Aging. 2018;35(8):707–718. doi: 10.1007/s40266-018-0568-9. [DOI] [PubMed] [Google Scholar]
- 14.Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJ. Health-related quality of life in high-grade glioma patients. Chin J Cancer. 2014;33(1):40–45. doi: 10.5732/cjc.013.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 18.Hickmann AK, Hechtner M, Nadji-Ohl M, Janko M, Reuter AK, Kohlmann K, Haug M, Grüninger S, Deininger M, Ganslandt O, König J, Wirtz CR, Coburger J, Renovanz M. Evaluating patients for psychosocial distress and supportive care needs based on health-related quality of life in primary brain tumors: a prospective multicenter analysis of patients with gliomas in an outpatient setting. J Neuro Oncol. 2017;131(1):135–151. doi: 10.1007/s11060-016-2280-0. [DOI] [PubMed] [Google Scholar]
- 19.Renovanz M, Hickmann AK, Coburger J, Kohlmann K, Janko M, Reuter AK et al (2018) Assessing psychological and supportive care needs in glioma patients - feasibility study on the use of the Supportive Care Needs Survey Short Form (SCNS-SF34-G) and the Supportive Care Needs Survey Screening Tool (SCNS-ST9) in clinical practice. Eur J Cancer Care 27(1) [DOI] [PubMed]
- 20.Renovanz M, Maurer D, Lahr H, Weimann E, Deininger M, Wirtz CR, et al. Supportive care needs in glioma patients and their caregivers in clinical practice: results of a multicenter cross-sectional study. Front Neurol. 2018;9:763. doi: 10.3389/fneur.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel S, Mehdorn HM. Measurement of psychological distress in patients with intracranial tumours: the NCCN distress thermometer. J Neuro Oncol. 2011;104(1):357–364. doi: 10.1007/s11060-010-0501-5. [DOI] [PubMed] [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 23.Taphoorn MJ, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, Stupp R, Mirimanoff RO, van den Bent M, Bottomley A, EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. doi: 10.1016/j.ejca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Coomans M, Dirven L, K Aaronson N, Baumert BG, van den Bent M, Bottomley A, et al. The added value of health-related quality of life as a prognostic indicator of overall survival and progression-free survival in glioma patients: a meta-analysis based on individual patient data from randomised controlled trials. Eur J Cancer. 2019;116:190–198. doi: 10.1016/j.ejca.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Renovanz MHMK, Kohlmann K, Janko M, Nadji-Ohl M, Singer S, Ringel F, Coburger J, Hickmann AK (2017) Compliance with patient-reported outcome assessment in glioma patients: predictors for drop out. Neuro Oncol Practice [DOI] [PMC free article] [PubMed]
- 26.Goebel S, Mehdorn HM. Development of anxiety and depression in patients with benign intracranial meningiomas: a prospective long-term study. Support Care Cancer. 2013;21(5):1365–1372. doi: 10.1007/s00520-012-1675-5. [DOI] [PubMed] [Google Scholar]
- 27.Boele FW, Zant M, Heine EC, Aaronson NK, Taphoorn MJ, Reijneveld JC, Postma TJ, Heimans JJ, Klein M. The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neurooncol Pract. 2014;1(2):40–46. doi: 10.1093/nop/npu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fountain DM, Allen D, Joannides AJ, Nandi D, Santarius T, Chari A. Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: a systematic review. Neuro Oncol. 2016;18(11):1475–1486. doi: 10.1093/neuonc/now107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, Lobatto DJ, Groen JL, Broekman MLD, Peerdeman SM, Peul WC, Taphoorn MJB, van Furth W. Impaired health-related quality of life in meningioma patients-a systematic review. Neuro Oncol. 2017;19(7):897–907. doi: 10.1093/neuonc/now250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37(11):1345–1351. doi: 10.1016/S0959-8049(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 31.Koekkoek JA, Chang S, Taphoorn MJ. Palliative care at the end-of-life in glioma patients. Handb Clin Neurol. 2016;134:315–326. doi: 10.1016/B978-0-12-802997-8.00019-0. [DOI] [PubMed] [Google Scholar]
- 32.Giammalva GR, Iacopino DG, Azzarello G, Gaggiotti C, Graziano F, Guli C et al (2018) End-of-life care in high-grade glioma patients. The palliative and supportive perspective. Brain Sci 8(7) [DOI] [PMC free article] [PubMed]
- 33.Hamidou Z, Baumstarck K, Chinot O, Barlesi F, Salas S, Leroy T, Auquier P. Domains of quality of life freely expressed by cancer patients and their caregivers: contribution of the SEIQoL. Health Qual Life Outcomes. 2017;15(1):99. doi: 10.1186/s12955-017-0672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGee HM, O’Boyle CA, Hickey A, O’Malley K, Joyce CR. Assessing the quality of life of the individual: the SEIQoL with a healthy and a gastroenterology unit population. Psychol Med. 1991;21(3):749–759. doi: 10.1017/S0033291700022388. [DOI] [PubMed] [Google Scholar]
- 35.Joyce CR, Hickey A, McGee HM, O’Boyle CA. A theory-based method for the evaluation of individual quality of life: the SEIQoL. Qual Life Res Int J Qual Life Asp Treat Care Rehab. 2003;12(3):275–280. doi: 10.1023/a:1023273117040. [DOI] [PubMed] [Google Scholar]
- 36.Jeon MSD, Dhillon HM, Descallar J, Lam L, Allingham S, Koh E-S, Currow DC, Agar MR (2019) Prevalence and severity of sleep difficulty in patients with a CNS cancer receiving palliative care in Australia. Neurooncol Pract:npz005 [DOI] [PMC free article] [PubMed]
- 37.Goldberg RJ, Cullen LO. Depression in geriatric cancer patients: guide to assessment and treatment. Hosp J. 1986;2(2):79–98. doi: 10.1080/0742-969X.1986.11882560. [DOI] [PubMed] [Google Scholar]
- 38.Kirkhus L, Saltyte Benth J, Gronberg BH, Hjermstad MJ, Rostoft S, Harneshaug M, et al. Frailty identified by geriatric assessment is associated with poor functioning, high symptom burden and increased risk of physical decline in older cancer patients: prospective observational study. Palliat Med. 2019;33(3):312–322. doi: 10.1177/0269216319825972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng KK, Lim EY, Kanesvaran R. Quality of life of elderly patients with solid tumours undergoing adjuvant cancer therapy: a systematic review. BMJ Open. 2018;8(1):e018101. doi: 10.1136/bmjopen-2017-018101. [DOI] [PMC free article] [PubMed] [Google Scholar]