Abstract
Glucocorticoids are commonly used in clinic, but the immunosuppression seriously hinders their usage. Herein, immunomodulatory effect of artesunate (AS) on hydrocortisone (HC)-induced immunosuppression was investigated. HC-induced immunosuppression mice (HC mice) were established by intramuscular administration with HC (20 mg/kg) once a day for 5 consecutive days. The results showed HC mice challenged with Escherichia coli on the sixth day presented a lower ability to clear bacteria, decreased TNF-α in blood, decreased spleen index and thymus index. Significantly, AS (20 mg/kg) treatment not only enhanced the ability of HC mice to clear bacteria, but also increased spleen index, the levels of pro-inflammatory cytokines from 78.7 ± 12.1 ng/ml (TNF-α) and 48.7 ± 8.6 pg/ml (IL-6) to 174.0 ± 90.5 ng/ml and 783.3 ± 90.5 pg/ml, number of white blood cells in blood, and sIgA in colon. Subsequently, HC-induced immunosuppression peritoneal macrophages model (HC cells) was established via addition of HC (0.5 μg/ml) for 0.5 h, and then LPS (100 ng/ml) was added to clarify the functional status of the cells. The results showed HC inhibited TNF-α and IL-6 mRNA expressions and their release, but AS (2.5 μg/ml) could increase TNF-α and IL-6 mRNA expressions and their release. AS inhibited GILZ mRNA up-regulated by HC and increases TLR4/NF-κB p65 expressions down-regulated by HC. Our findings revealed that AS's effect is closely related to the improvement of the TLR4/NF-κB signal transduction pathway via inhibiting the up-regulation of GILZ mRNA, demonstrating AS does possess immunomodulatory effects and is worth further investigation in the future.
Keywords: Artesunate, Glucocorticoids, Immunosuppression, Pro-inflammatory cytokines, GILZ, NF-κB p65
1. Introduction
Despite the development of many new drugs in the last decades, glucocorticoids (GCs) are still the most widely used treatment for inflammatory and autoimmune diseases (Cain and Cidlowski, 2017). Even in viral diseases such as Coronavirus Disease 2019 (COVID-19), glucocorticoids are still used due to the absence of effective and specific drugs (Alhazzani et al., 2020). However, both short-term and long-term use, especially excessive and/or prolonged use, can lead to serious adverse reactions in the body. Among them, secondary infection caused by immunosuppression seriously hinder the use of GCs. The prophylactic use of antimicrobial agents cannot solve the problem of secondary infection, and even lead to bacterial resistance and other serious adverse reactions. So it is of great significance to study new prevention and treatment strategies and drugs.
The innate immune system is the first defense line of human body against pathogen invasion to maintain a stable internal environment (Si-Tahar et al., 2009). It plays a pivotal role in the generation of inflammatory mediators and regulation of innate and adaptive immune responses, but also contributes to resolution of inflammation, not only by producing anti-inflammatory cytokines but also by removal of pro-inflammatory pathogens, cellular debris and apoptotic cells. However, after the application of GCs, the innate immune system is significantly suppressed, and the number and function of mononuclear/phagocytic cells are reduced, leading to a significant reduction in the ability to clear bacteria and the subsequent occurrence of secondary infection (Cain and Cidlowski, 2017).
GCs regulate the transcription and expression of its target genes by binding to receptor of GCs to form a hormone receptor-complex, and then binding to the glucocorticoid response elements (GRE) or negative glucocorticoid response elements (nGRE) after nuclear translocation. The important target gene such as glucocorticoid-induced leucine zipper (GILZ), a molecule closely related to the anti-inflammatory and immunosuppressive effects of GCs, is an important executor and can mediate many related effects of GCs (Yang et al., 2008). In addition, GILZ, like GCs, can bind to NF-κB p65 and inhibit the transcription and expression of pro-inflammatory cytokines mediated by NF-κB p65, resulting in decreased TNF-α and IL-6 expression (Xu et al., 2007) (Bereshchenko et al., 2019). However, TNF-α and IL-6 are tightly related to the ability of immune cells to remove bacteria, so their reduction will directly lead to the decreased bacterial clearance ability of cells. The level of GILZ expression elevated in macrophages in the late stage of inflammation, indicating GILZ is tightly related to anti-inflammation and immunosuppression (Hoppstadter and Kiemer, 2015).
Artesunate (AS), an anti-malarial drug, has been discovered to possess an immunomodulatory effect on cecum ligation and puncture (CLP)-induced immunosuppression mice (Shang et al., 2020). However, it is unclear whether AS has potent regulation on GCs-induced immunosuppression. Herein, hydrocortisone (HC), an active endogenous glucocorticoid secreted by the body, is used to establish an immunosuppression mice model, and then the effect and the mechanism of AS are investigated in cells treated with HC.
2. Materials and methods
2.1. Animals
BALB/C mice (male, 4–6 weeks old, weighing 18–22 g) were obtained from Hunan Silaike Jingda Experimental Animal Co. KM mice (male, 4–6 weeks old, weighing 25–30 g) were obtained from Medical Experimental Center of Zunyi Medical University. Mice were housed in a pathogen-free facility with a 12-h artificial light-dark cycle. All the mice were provided with food and purified water at will. Each cage contained no more than 5 mice. The experimental procedures were approved by the ethical committee of Guizhou Medical University (license number: 1,804,102).
2.2. Isolation of peritoneal macrophages from mice
Mouse peritoneal macrophages (PMs) were isolated and cultured from male KM mice (4–6 weeks old, weighing 25–30 g). Each mouse was intraperitoneal injected with 3 ml of 3% thioglycolate (#T9032, Sigma-Aldrich) and killed using isoflurane on day 3. After intraperitoneal administration of 5 ml phosphate-buffered saline (PBS), the peritoneal cells were suspended with fresh DMEM free of FBS into cell culture dishes at 37 °C in 5% CO2. Two hours later, the floating cells were removed by washing the cells with PBS. The attached cells were considered to be PMs (purity is about 90%) and were subjected to further experiments. All the reagents and utensils used in the experiment were LPS-free.
2.3. Bacterial strain and preparation of bacterial suspension
Escherichia coli ATCC35218 (EC) was purchased from the American Type Culture Collection (ATCC, USA). EC were cultured in Mueller-Hinton Broth to the logarithmic phase and were collected and diluted in sterile normal saline (NS) to achieve the terminal concentration. The OD value of the bacterial solution was detected by ultraviolet spectrophotometer (λ = 600 nm), and colony-formation units (CFU)/ml was determined by 10-fold series dilution method. The regression equation of OD value of the bacterial solution and CFU was established.
2.4. Dose determination of HC for establishing HC-induce immunosuppression mice
Mice were divided into four groups: control group and three dosages of HC-induce immunosuppression mice (HC mice) (n = 6). Control group were administrated with normal saline (NS) via intramuscular administration each day for 5 days. HC mice were administrated with 5, 10 and 20 mg/kg of HC (Tianjin Jinyao Pharmaceuticals, Tianjin, China) via intramuscular administration each day for 5 days, respectively. At 12 h after the last HC administration, mice were intraperitoneally injected with Escherichia coli (EC, 1.0 × 108 CFU/kg), and were killed at 12 h after EC injection. Blood, spleen and thymus were collected for spleen index, thymus index, and TNF-α detection.
2.5. Dose determination of bacteria for establishment of HC mice model
Mice were divided into six groups: three control group and three HC mice (20 mg/kg) (n = 5). The mice administrated different three dosages of bacteria via intraperitoneal administration (EC1, 1.0 × 107 CFU/kg; EC2, 5.0 × 107 CFU/kg and EC3, 1.0 × 108 CFU/kg). Mice were killed at 6 h after bacterial challenge, the blood were collected for CFU count and TNF-α assay.
2.6. Therapy time determination of AS treatment in HC mice
HC mice were divided into three groups (n = 5): Control, HC and AS group. Control mice were intraperitoneally injected with normal saline (0.1 ml/mouse) for 6 days, HC group: HC (20 mg/kg) was intramuscularly injected once per day for 5 days. NS was administered on the first day, and respectively continued for 1,2,3,4, and 5 days (HC1, HC2,HC3, HC4 and HC5) after HC administration stopped. AS group: HC (20 mg/kg) was intramuscularly injected once per day for 5 days. At the same time of the first HC injection, mice were intraperitoneal injection of AS (20 mg/kg) twice a day, and respectively continued for 1, 2, 3, 4, and 5 days (AS1, AS2, AS3, AS4, and AS5) after HC administration stopped. Finally, EC (5.0 × 107 CFU/kg) was intravenously injected (i.v.). Mice were killed at 6 h after bacterial challenge, and the spleen and blood were collected for the spleen index calculation and IL-6 and TNF-α assay.
2.7. AS treatment in HC mice
Mice were divided into Control group, HC group and AS group (n = 10). Control group was only intramuscularly administrated with NS (0.1 ml/mouse) each day for 5 days. HC group was intramuscularly administrated with HC (20 mg/kg) each day for 5 days. AS group was intraperitoneally administrated with AS (20 mg/kg) twice per day for 7 days from Day 1 of HC administration. Bacterial challenge (5.0 × 107 CFU/kg) was given at 24 h after the last HC administration on Day 5. Mice were killed at 6 h after bacterial challenge; blood and colon were collected.
2.8. HC-induced immunosuppression cells and AS treatment
Confirmation of the dose of HC. Cells grown in 96-well plates (2.0 × 105 cells/well) were cultured with HC for only 0.5 h (HC cells). The addition of LPS O55:B5 (100 ng/ml, Sigma-Aldrich, St. Louis, MO, USA) was used to make sure whether the immunosuppression state was established in the cells.
Confirmation of the best effective dose of AS. Cells were cultured with AS (2.5–40 μg/ml) for 1 h, and then add HC (0.5 μg/ml) for 0.5 h. After LPS (100 ng/ml) was finally added for 4 h, the supernatant was collected for TNF-α detection.
The anti-inflammatory effect of AS. Cells were cultured with AS (2.5–40 μg/ml) for 1 h, and then LPS (100 ng/ml) was added for 4 h. The supernatant were collected for TNF-α detection.
Cells were cultured with AS (2.5 μg/ml) for 1 h, continues to add HC (0.5 μg/ml) for 0.5 h, and then add HC (0.5 μg/ml) for 0.5 h. After LPS (100 ng/ml) was finally added for 4 h, the supernatant was collected for TNF-α and IL-6 detection, and the cells were collected for mRNA detection of TNF-α, IL-6, TLR4, NF-κB P65, NF-κB P50 and GILZ.
2.9. Weight measurement of spleen and thymus
The weights of spleen and thymus were measured using an electronic scale (Mettler Toledo (ME 104), Columbus, Ohio, USA). The weights of spleen and thymus were recorded. The weight index of spleen or thymus calculated according to the following formula: the weight of spleen or thymus/body weight.
2.10. CFU count assay
Blood was collected and diluted in sterile NS to achieve the appropriate concentration. Subsequently, the diluents were added to nutrient agar plates and cultured for 18 h. Images of the agar plates and CFU counts were obtained using a G6 automated colony-counter (Shineso Science & Technology, Hangzhou, China).
2.11. Enzyme-linked immunosorbent assay
The levels of TNF-α and IL-6 in blood and the supernatant of cell culture were tested using respective ELISA kit (eBioscience, San Diego, Calif., USA). The level of NF-κB p65 was tested using Total NF-κB p65 Sandwich ELISA Kit (CST, USA). The level of sIgA in colon was detected using ELISA Kit (USCN, Wuhan, China).
2.12. Quantitative real-time RT-PCR
Total RNA was isolated from PMs using RNeasy mini kits (TaKaRa, Kusatsu, Shiga, Japan), and cDNA was synthesized using PrimeScriptTM RT Reagent Kit (TaKaRa, Kusatsu, Shiga, Japan). The primers (Table 1 ) were purchased from Sangon Biotech (Shanghai, China). Making a final 15 μl of reaction mixture [ 3 μl of cDNA, 7.5 μL of iQTM SYBR® Green Supermix (Bio-Rad, USA), 0.5 μl of primer and 4 μl of DEPC-H2O ] for analysis, the reaction was performed using CFX Connect Real-Time System (Bio-Rad, USA). The average transcript levels of genes were normalized to GAPDH using the following formula expression was calculated by the 2 (–ΔΔCt) method (Livak and Schmittgen, 2001), where Ct is the threshold cycle value.
Table 1.
Primer sequences for RT-qPCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| TLR4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| NF-κB p65 | CACCAAGGATCCACCTCACC | CTCTATAGGAACTATGGATACTGCG |
| GILZ | CTGTTGGCCTCGACTGCTG | GCCGAAAGTTGCTCACGAAG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
2.13. NF-kB proteins enzyme-linked immunosorbent assay
The whole protein were extracted with respective reagents (Solarbio, Beijing, China), and its concentration was determination using BCA kit (Beyotime, Jiangsu, China). The protein expression of NF-κB p65 was detected using respective Elisa kit (CST, USA).
2.14. Statistical analysis
Descriptive and analytical statistical analyses were performed using the SPSS (version 22.0) software package. Statistical significance between groups was determined by a two-tailed Student's t-test. Each experiment was repeated at least three times. The data are presented as the mean ± standard error of the mean (S.E.M.), and P < 0.05 indicates statistical significance.
3. Results
3.1. HC mice model is established via administration of HC for consecutive 5 days
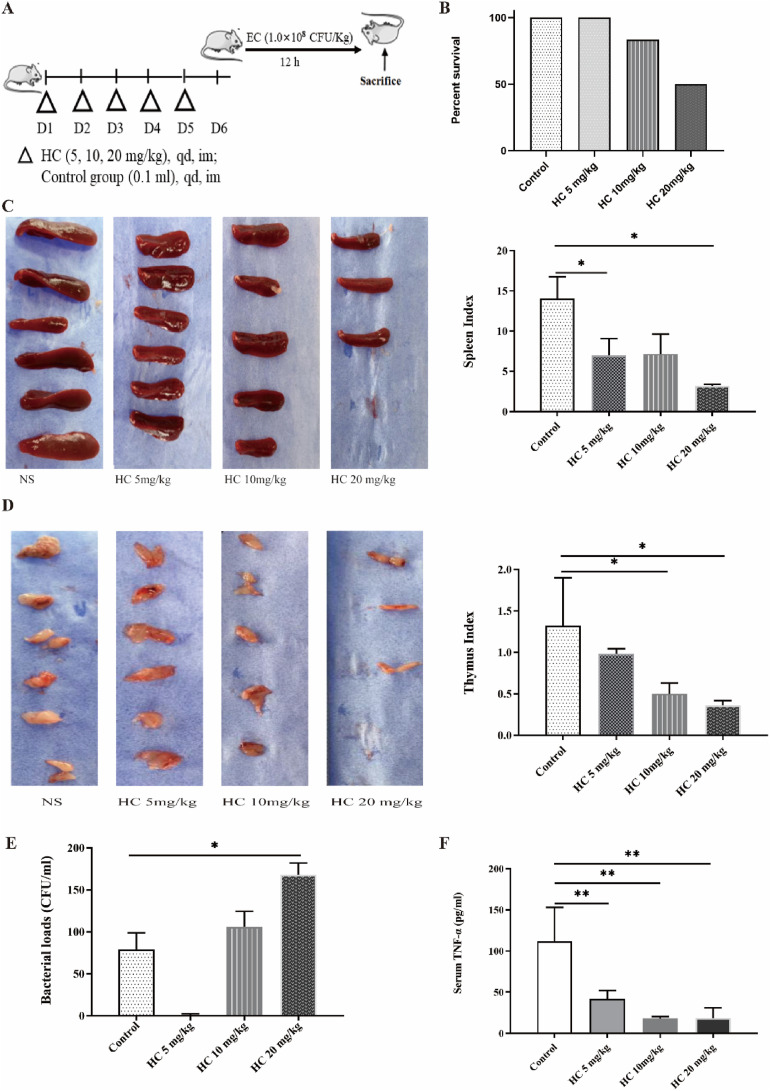
HC-induced immunosuppression mice model (HC mice) was established and used herein (Fig. 1 A). The results showed there was no statistical difference in mortality among the groups challenged with EC (1.0 × 108 CFU/kg) at 12 h after the last HC administration, but mice survival rates had a downward trend with the increase of HC dose. The survival rates of high dosage of HC group (20 mg/kg), middle dosage of HC group (10 mg/kg), and low dosage of HC group (5 mg/kg) were 50%, 83.3%, and 100%, respectively (Fig. 1B). Above results suggest HC can inhibit resistance of mice to bacterial challenge.
Fig. 1.
HC-induced immunosuppression mice model (HC mice) is established (n = 6). (A) Schematic diagram of HC mice challenged with EC (1.0 × 108 CFU/kg). (B) Survival of HC mice challenged with EC (n = 6). (C) Thymus index. (D) Spleen index. (E) The bacterial loads in serum. (F) The level of TNF-α in serum. *P < 0.05, **P < 0.01.
The spleen and thymus are important immune organs, so weight of the thymus was measured. The results showed spleen index and thymus index decreased with the increase of HC dosage, and there was a difference between Control group and high dosage of HC group (Fig. 1C and D). Above results suggest HC can lead to the decreased weights of spleen and thymus of mice.
HC-induced immunosuppression can lead to decreased bacterial clearance. The results from bacterial loads in blood showed bacterial numbers increased with the increase of HC dosage, and there was a difference between Control group and high dosage of HC group (Fig. 1E).
HC-induced immunosuppression can lead to decreased pro-inflammatory cytokines, which is tightly related to bacterial clearance. The results from TNF-α in blood showed decreased with the increase of HC dosage, and there was a significant difference between Control group and three dosages of HC group (Fig. 1F). Based above results, in the subsequent experiments, HC mice model was established via intramuscular administration with HC (20 mg/kg) once per day for 5 consecutive days.
3.2. HC mice have very lower ability to clear bacteria
To make sure the degree of suppressed immune function, the ability of HC mice to clear bacteria was observed. Herein, different dose of E. coli (EC1, 1.0 × 107 CFU/kg; EC2, 5.0 × 107 CFU/kg; and EC3, 1.0 × 108 CFU/kg) were used to challenge HC mice. The results from bacterial loads showed HC mice had very lower ability to clear bacteria. And the higher the bacterial challenge dose, the lower the ability to clear bacteria (Fig. 2 A–C), indicating that HC mice were in the state of immunosuppression. Meanwhile, the results from blood TNF-α showed HC mice had the worst ability to release TNF-α after being challenged with the same amount of bacteria (Fig. 2D). The higher the amount of bacteria, the worse the ability to release TNF-α was, indicating that the level of TNF-α was closely related to the ability of the mice to clear bacteria.
Fig. 2.
HC mice have lower ability to clear bacteria (n = 5). (A) The bacterial loads in blood at 6 h after mice were challenged with EC1 (1.0 × 107 CFU/kg); (B) The bacterial loads in blood at 6 h after mice were challenged with EC2 (5.0 × 107 CFU/kg). (C) The bacterial loads in the blood at 6 h after mice were challenged with EC3 (1.0 × 108 CFU/kg). (D) The level of TNF-α in blood of mice challenged with different dosages of EC. †P > 0.05, *P < 0.05, **P < 0.01.
Based above results, mice were administrated with HC (20 mg/kg) for 5 days and then challenged with EC (5.0 × 107 CFU/kg) in the subsequent experiments to determine whether the model is successfully established.
3.3. AS enhances the ability of mice to clear bacteria and improved immune function
To determine the time point of AS to take effect, bacterial challenge was given after AS was administrated from Day 6 to Day 10, and then the cytokine levels and spleen index were measured (Fig. 3 A). The results showed there was no effect of 20 mg/kg of AS group on normal mice based on mice bacterial clearance and blood TNF-α and IL-6 levels (data not shown) but all of the levels of TNF-α and IL-6 of AS treatment mice were higher than those of HC mice (Fig. 3B and C), suggesting AS could increase he release of cytokines inhibited by HC. However, the spleen indexes of AS treatment mice only in long time treatment of AS (AS3, AS4, and AS5) were significant higher than those of HC mice (HC3, HC4, and HC5) (Fig. 3D), suggesting that the recovery of immune organs needs a longer time.
Fig. 3.
AS enhances the ability of mice to clear bacteria and improved immune function. (A) Schematic diagram of HC mice with AS treatment (20 mg/kg) (n = 6). AS1, continuing to give AS until one day after the cessation of HC. (B) Schematic diagram of HC mice with NS treatment (n = 6). HC1, continuing to give NS until one day after the cessation of HC. (C) Effect of AS treatment on spleen indexes (n = 6). (D) Effect of AS treatment on TNF-α (n = 6). (E) Effect of AS treatment on IL-6 (n = 6). (F) Effect of AS treatment on the bacterial load in HC mice challenged with EC (n = 10). (G) The number of white blood cells (WBC) (n = 10). (H) Effect of AS treatment on sIgA in colon (n = 10). (I) Effect of AS treatment on the bacterial load in Control mice challenged with EC (n = 10). (J) Effect of AS treatment on TNF-α in Control mice challenged with EC (n = 10). †P > 0.05, *P < 0.05, **P < 0.01.
Based on above results that the effect of AS on cytokine release was most significant in the AS2 group (AS treatment at the same time of the first HC injection twice a day, and continued for 2 days after HC administration stopped), the treatment in AS2 was used to observe the effect of AS in subsequent experiments. The results showed bacterial load in AS group significantly decreased than that in HC mice (Fig. 3E), demonstrating AS could significantly enhance the ability of mice to clear bacteria. For other indicators related to the body's removal of bacteria, HC markedly decreased the number of white blood cells but AS significantly increased the number of white blood cells (Fig. 3F); meanwhile, HC markedly decreased the level of sIgA but AS significantly increased the level of sIgA in colon (Fig. 3G). Above results demonstrated HC did inhibit the ability of mice to clear bacteria, but AS increased this ability in mice, which has been linked to an increase in immunological function.
3.4. AS increases the release of pro-inflammatory cytokines in HC cells
To further investigate the role of AS, a macrophages model treated by HC (HC cells) was established. Herein, mouse peritoneal macrophages (PMs) were used to establish HC cells. Firstly, cells were cultured with HC (0.02–43.74 μg/ml) for 0.5 h, and then LPS (100 ng/ml) was added. After cultured for another 4 h, the supernatant was collected for TNF-α detection to select suitable concentrations of HC and AS (Fig. 4 A). The results showed LPS very significantly increased TNF-α release while HC strongly inhibited LPS-induced TNF-α release even at a very low concentration such as 0.02 μg/ml. The maximum saturation effect could be reached at the concentration of 0.54 μg/ml, and the plateau period of the effect take place after this concentration (Fig. 4B). Thus, the concentrations of HC 0.5 μg/ml was used to establish HC cell model in subsequent experiments.
Fig. 4.
AS increases the release of pro-inflammatory cytokines in HC cells (n = 4). (A) Schematic diagram of HC cells with AS (2.5 μg/ml) treatment. (B) The confirmation of HC concentration. (C) The confirmation of AS concentration. (D) The anti-inflammatory effect of AS. (E) Effect of AS treatment on TNF-α and IL-6 mRNA expressions in HC cells treated with LPS. (F) Effect of AS (2.5 μg/ml) treatment on TNF-α and IL-6 release in HC cells treated with LPS. *P < 0.05, **P < 0.01.
Secondly, cells were pre-cultured with different concentrations of AS (2.5–20 μg/ml), and then 0.5 μg/ml of HC was added to the culture to make sure the optimum drug concentration. The results showed AS with concentration as low as 2.5 μg/ml already could increase TNF-α release from the cells treated with HC (Fig. 4C) although 2.5 μg/ml of AS had no inhibition on TNF-α release (Fig. 4D). Thus, the concentration of 2.5 μg/ml was used in subsequent experiments.
Lastly, under optimized experimental conditions, the effect of AS (2.5 μg/ml) on TNF-α and IL-6 mRNA expression and release were investigated. The results showed AS could increase not only TNF-α and IL-6 mRNA expression but also proteins’ release in HC cells (Fig. 4E and F), demonstrating AS could enhance the response of HC cells to LPS.
3.5. AS inhibits GILZ mRNA and increases TLR4/NF-κB p65 expressions in HC cells
GILZ is an important executor of the GCs' effects (Yang et al., 2008); it plays an important role for the anti-inflammatory and immunosuppressive effects of GCs. Herein, GILZ mRNA expression was investigated. The results showed HC dose-dependently increased GILZ mRNA expression (Fig. 5 A). However, AS could inhibit HC-induced higher GILZ mRNA expression both in HC cells and HC cells treated with LPS (Fig. 5B). Above results demonstrated AS's effect was tightly related to inhibition of higher GILZ mRNA expression induced by HC.
Fig. 5.
AS inhibits GILZ mRNA and increases TLR4/NF-κB p65 expressions in HC cells (n = 4). (A) Effect of HC on GILZ mRNA expression. (B) Effect of AS treatment on GILZ mRNA expression. (C) Effect of AS treatment on TLR4 mRNA expression. (D) Effect of AS treatment on NF-κB p65 mRNA expression. (E) Effect of AS treatment on NF-κB p65 protein expression. *P < 0.05, **P < 0.01.
TLR4/NF-κB signal transduction pathway plays a key and important role for LPS-induced pro-inflammatory cytokines release. GCs can inhibit this pathway via upregulating GILZ expression (Xu et al., 2007). Herein, the effect of AS on the key signal molecule (TLR4 and NF-κB) was investigated. The results showed LPS alone increased both TLR4 and NF-κB P65 mRNA expressions; HC significantly inhibited these mRNA expressions but AS can strongly increase these mRNA expressions (Fig. 5C and D). Importantly, HC could significantly inhibit NF-κB p65 protein expression induced by LPS but AS strongly increased its expression in HC cells (Fig. 5E), which was consistent with NF-κB mRNA expression. Above results demonstrated AS's effect was tightly related to increased TLR4 and NF-κB expressions.
4. Discussion
GCs-induced immunosuppression severely limits the application of GCs. So it is of great significance to investigate new prevention and treatment strategies and drugs. Herein, our results demonstrated that AS enhanced the ability to clear bacteria in HC mice, which was closely related to increased pro-inflammatory cytokines release via decreased GILZ expression and then increased TLR4/NF-κB pathway. This is the first report demonstrating the therapeutic effects of AS on GCs-induced immunosuppression.
Immunosuppression rodent models induced by GCs are commonly used to investigate the immunosuppression effects of GCs. Different drugs with different dosages were used in mouse or rat model. Previously, HC was intramuscularly administrated five times the next day. The dose of HC was 40 mg/kg per day, which is equivalent to the daily human dose for systemic lupus erythematosus (SLE) treatment. The phagocytic function of macrophages was one of observation marker (Yingjian et al., 2013). In another lab, dexmedetomidine (10 mg/kg) was intraperitoneal administrated to establish an immunosuppression mouse model. Spleen index, thymus index, blood routine index, and other markers were used. The dose of dexmedetomidine was 10 mg/kg per day, which is much higher than the daily human dose for SLE treatment (Li et al., 2017). Based on HC is widely applied for sepsis and autoimmune diseases, in this study, HC was used to establish GCs-induced immunosuppression mouse model via intramuscular administration of 20 mg/kg once a day for five consecutive days. Although the dose of 20 mg/kg is equivalent to half the equivalent dose of GCs used in SLE treatment of methylprednisolone, only five days usage of HC could induce immunosuppression in mice, presented by decreased thymus and spleen index, reduced TNF-α and IL-6 levels, and reduced bacterial clearance. Above results demonstrates that even in the case of dose such as HC 20 mg/kg for five days, HC could induce immunosuppression in a very short period of time.
GCs-induced immunosuppression is closely related to the decreased function of the mononuclear/phagocytic system in organism. Mononuclear macrophages, an important cell of the innate immune system, play a critical role for eliminating bacteria. Activated macrophages produce various proinflammatory cytokines such TNF-α and IL-6 et al., contributing to the clearance of invading pathogens (Zhang and Wang, 2014). Herein, our results showed that the level of TNF-α were significantly reduced in HC mice, suggesting the level of TNF-α was indeed related to the body's ability to clear bacteria.
Since the anti-inflammatory and immunosuppressive effect of GCs are not the same but they are closely related. Therefore, it is a very important problem how to reduce the inhibition of GCs on the immune system while ensuring the therapeutic effect of glucocorticoids. In theory, it is reliably to investigate immunomodulatory agents to improve immune functions based on the molecular mechanism of the immunosuppression of GCs (Cain and Cidlowski, 2017). Previously, in our lab AS was found not only inhibit excessive inflammation during sepsis (Kuang et al., 2018) but also possess an immunomodulatory effect on CLP-induced immunosuppression mice (Shang et al., 2020). Herein, in HC mice, our results further demonstrated AS has a significant protective effect on HC mice via increasing pro-inflammatory cytokines release and enhancing bacterial clearance, suggesting AS is indeed a bidirectional immunomodulator, which is worthy of further investigation.
GCs-mediated immune inhibition has classically been attributed to glucocorticoid receptor (GR)-induced alterations of gene expression. The complex of GCs and GR binds glucocorticoid response elements (GREs) or negative glucocorticoid response elements (nGREs) to regulate target genes’ expression (such as GILZ). As well-known, the TLR4/NF-κB pathway plays a key role in the inflammatory response (Cruz-Topete and Cidlowski, 2015). Till now, GCs exert immunosuppressive effects primarily through GR tethering of transcription factors such as NF-κB and AP-1, reduces the expression of pro-inflammatory genes (transrepression) (Mittelstadt and Ashwell, 2001; Di Marco, Massetti et al., 2007). Interestingly, not only the complex of GCs and GR but also GILZ binds NF-κB and AP-1 to inhibit TNF-α and IL-6 genes expression. Herein, the results showed HC up-regulated the mRNA expression of GILZ in a dose-dependent manner, and AS could inhibit the up-regulation of GILZ mRNA. The mRNA expressions of TLR4, TNF-α, and IL-6, the target genes of NF-κB, was downregulated in HC cells, but AS could upregulate the expressions of these genes. Above results demonstrated AS could reduce immunosuppression of GCs via inhibiting the up-regulation of GILZ mRNA.
Activation of toll-like receptors (TLRs) plays a pivotal role in the host defense against bacteria and results in the activation of NF-κB - mediated transcription of pro-inflammatory mediators. Therefore, NF-κB plays a critical role during inflammation and immune regulation (Deretic and Levine, 2018). It also is very important for GCs to play its immunosuppressive effect because of tethering by GCs and GILZ (Ratman et al., 2013). Herein, our results demonstrated that NF-κB p65 mRNA and protein expression downregulated in HC cells, and LPS couldn't regulate its expression after HC treatment. However, AS could significantly regulate its expression, demonstrating AS could reduce the immunosuppressive effect by HC and increase the cells' response to LPS. Therefore, our findings indicated that the protective effect of AS is closely related to the improvement of the TLR4/NF-κB signal transduction pathway, which then enhances pro-inflammatory cytokines release and bacterial clearance.
In summary, herein both HC-induced immunosuppression mouse model and macrophage model were established. AS significantly enhanced bacterial clearance of HC mice challenged with Escherichia coli and increase TNF-α and IL-6 release and other immune function-related markers. In HC cells, AS significantly increase TNF-α and IL-6 mRNA expressions and their release. Mechanically, AS inhibited the up-regulation of GILZ mRNA expression induced by HC, and upregulated NF-κB p65 mRNA and protein expression induced by HC. Our findings revealed that AS's effect is closely related to the improvement of the TLR4/NF-κB signal transduction pathway via inhibiting the up-regulation of GILZ mRNA and then reduced GCs-induced immunosuppression. Of course, the effect of AS to increase pro-inflammatory cytokines release needs further investigation in the future.
Authorship contribution statement
Yan Wang: Conceptualization, Methodology, Data curation, and Writing - original draft. Mengling Liao: Data curation. Yu Zhang: Methodology. Fei Deng: Data curation. Jing Luo: Methodology. Nuoyan Wang: Methodology. Min Liu: Methodology. Lin Ao: Methodology. Qi-Mei Fang: Methodology. Qing-Chun Wang: Methodology. Hong Zhou: Conceptualization, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was sub-project of National Natural Science Foundation of China-Guizhou Provincial People's Government Joint Fund Project [NSFC-U1812403-4-1], the National Natural Science Foundation of China [NSFC- 81872914 and 82073902], and the fourth batch of “Thousand People Innovation and Entrepreneurship Talents Fund” in Guizhou Province.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2020.173630.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., supported S., Oczkowski b, Levy M.M., Derde L., Dzierba A., Du B., Aboodi M., Wunsch H., Cecconi M., Koh Y., Chertow D.S., Maitland K., Alshamsi F., Belley-Cote E., Greco M., Laundy M., Morgan J.S., Kesecioglu J., McGeer A., Mermel L., Mammen M.J., Alexander P.E., Arrington A., Centofanti J.E., Citerio G., Baw B., Memish Z.A., Hammond N., Hayden F.G., Evans L., Rhodes A. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus disease 2019 (COVID-19) Crit. Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko O., Migliorati G., Bruscoli S., Riccardi C. Glucocorticoid-induced leucine zipper: a novel anti-inflammatory molecule. Front. Pharmacol. 2019;10:308. doi: 10.3389/fphar.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018;14(2):243–251. doi: 10.1080/15548627.2017.1402992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco B., Massetti M., Bruscoli S., Macchiarulo A., Di Virgilio R., Velardi E., Donato V., Migliorati G., Riccardi C. Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Res. 2007;35(2):517–528. doi: 10.1093/nar/gkl1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppstadter J., Kiemer A.K. Glucocorticoid-induced leucine zipper (GILZ) in immuno suppression: master regulator or bystander? Oncotarget. 2015;6(36):38446–38457. doi: 10.18632/oncotarget.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang M., Cen Y., Qin R., Shang S., Zhai Z., Liu C., Pan X., Zhou H. Artesunate attenuates pro-inflammatory cytokine release from macrophages by inhibiting TLR4-mediated autophagic activation via the TRAF6-beclin1-PI3KC3 pathway. Cell. Physiol. Biochem. 2018;47(2):475–488. doi: 10.1159/000489982. [DOI] [PubMed] [Google Scholar]
- Li Y., Zheng B., Tian H., Xu X., Sun Y., Mei Q., Lin X., Liu L. Yupingfeng Powder relieves the immune suppression induced by dexamethasone in mice. J. Ethnopharmacol. 2017;200:117–123. doi: 10.1016/j.jep.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mittelstadt P.R., Ashwell J.D. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J. Biol. Chem. 2001;276(31):29603–29610. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- Ratman D., Vanden Berghe W., Dejager L., Libert C., Tavernier J., Beck I.M., De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol. Cell. Endocrinol. 2013;380(1–2):41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Shang S., Wu J., Li X., Liu X., Li P., Zheng C., Wang Y., Liu S., Zheng J., Zhou H. Artesunate interacts with the vitamin D receptor to reverse sepsis-induced immunosuppression in a mouse model via enhancing autophagy. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tahar M., Touqui L., Chignard M. Innate immunity and inflammation--two facets of the same anti-infectious reaction. Clin. Exp. Immunol. 2009;156(2):194–198. doi: 10.1111/j.1365-2249.2009.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jagannath C., Liu X.D., Sharafkhaneh A., Kolodziejska K.E., Eissa N.T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27(1):135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Zhang W., Shi X.M. Glucocorticoid-induced leucine zipper (GILZ) mediates glucocorticoid action and inhibits inflammatory cytokine-induced COX-2 expression. J. Cell. Biochem. 2008;103(6):1760–1771. doi: 10.1002/jcb.21562. [DOI] [PubMed] [Google Scholar]
- Yingjian L., Junming H., Min C., Chenyue L., Dachao Z., Yuanhua H., Zhi L. A health food high-peptide meal alleviates immunosuppression induced by hydrocortisone and cyclophosphamide in mice. Food Funct. 2013;4(9):1352–1359. doi: 10.1039/c3fo30230j. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang C.C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat. Dis. Int. 2014;13(2):138–152. doi: 10.1016/s1499-3872(14)60024-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.