Abstract
Objectives
Despite an initial success, Israel’s quarantine-isolation COVID-19 policy has abruptly collapsed. This study’s aim is to identify the causes that led to this exponential rise in the accumulation of confirmed cases.
Methods
Epidemiological investigation reports were used to reconstruct chains of transmission as well as assess the net contribution of local infections relative to imported cases, infected travelers arriving from abroad. A mathematical model was implemented in order to describe the efficiency of the quarantine-isolation policy and the inflow of imported cases. The model’s simulations included two scenarios for the actual time series of the symptomatic cases, providing insights into the conditions that lead to the abrupt change.
Results
The abrupt change followed a Jewish holiday, Purim, in which many public gatherings were held. According to the first scenario, the accumulation of confirmed cases before Purim was driven by imported cases resulting in a controlled regime, with an effective reproduction number, , of 0.69. In the second scenario, which followed Purim, a continuous rise of the local to imported cases ratio began, which led to an exponential growth regime characterized by an of 4.34.
It was found that the change of regime cannot be attributed to super-spreader events, as these consisted of approximately 5% of the primary cases, which resulted in 17% of the secondary cases.
Conclusions
A general lesson for health policymakers should be that even a short lapse in public responsiveness can lead to dire consequences.
Keywords: Infectious disease dynamics, Public health, Time series, Chains of transmission, Compartmental SEQIJR model
Introduction
Since its emergence, the impact of COVID-19 has been profound, and the public health challenge seems to be the most serious seen in a respiratory virus since the 1918 H1N1 influenza pandemic (Soper, 1919). Following its emergence in Wuhan, cases of COVID-19 were exported out of China, mainly by travelers using the global aviation networks (Wu et al., 2020). It should be noted that transboundary spread of viruses is quite common in veterinary medicine (Klausner et al., 2015, Klausner et al., 2017, Klausner et al., 2018).
Israel began its mitigation policy as early as January 24th, a month before the first COVID-19 case arrived in Israel. First, Israeli citizens were advised to avoid unnecessary travel to Hubei province in China. A week later, Israel closed all its border crossings (air, land, and sea) to individuals who were not citizens or residents of Israel and who visited China during the previous 14 days. On February 2nd Israel issued a warrant requiring Israeli citizens that returned to Israel after a stay in China during the previous 14 days to go into quarantine. Individuals who were in contact with a confirmed COVID-19 case were also required to go into quarantine. On February 17th, the quarantine requirement was expanded to citizens returning from Thailand, Hong-Kong, Singapore, and Macau. The first COVID-19 patients in Israel were two passengers that returned from the “Diamond Princess” cruise ship that was quarantined in Japan on February 21st and 23rd. On February 23rd, the quarantine requirement was expanded to include citizens returning from South Korea and Japan. On February 26th, the requirement was expanded to Italy, and a day later, a COVID-19 case was discovered that arrived from Italy on February 23rd. On March 4th, the quarantine requirement was expanded to other European countries: France, Germany, Switzerland, Spain, and Austria. On March 9th, the quarantine requirement was expanded for citizens and residents of Israel returning from any location abroad (Ministry of Health - News, 2020).
Aside from the quarantine requirement for individuals arriving from COVID-19 affected regions, Israel had also restricted large events and gatherings. On March 4th public events and gatherings were restricted to up to 5000 participants. On March 10th, this quota was lowered to 2000 participants, and two days later, it was lowered to 100 participants (Ministry of Health - News, 2020).
These mitigation measures seemed to be effective during the three weeks that followed the introduction of COVID-19 to Israel. Then, in mid-March, Israel experienced a surge in the number of daily new cases, which led to the closure of the education system on March 12th, and on March 25th, emergency regulations were issued in order to curb the spread of COVID-19 in Israel by minimizing social contact. These regulations required people to stay in their homes. They were only allowed to leave home for specific purposes and needs, such as receiving medical treatment, purchasing necessary groceries, and medicines (Ministry of Health - News, 2020).
The aim of this study is to examine the dynamics during the first month of the COVID-19 outbreak in Israel, to identify the reasons for the sudden change from a controlled epidemic regime to an exponential growth regime, in light of policymakers' decisions and public behavior. To do so, we have analyzed a set of 406 epidemiological investigation reports that were released by the Israeli Ministry of Health (Israeli Ministry of Health, 2020). These reports were initially released in order to notify the public of the locations attended by confirmed COVID-19 cases. The reports were released from February 21st to March 21st and covered 40% of the 1023 laboratory-confirmed cases, until the end of the period. This study used these reports to reconstruct transmission chains and identify and separate the imported cases (which were required to quarantine on arrival) from the local cases, thus analyzing the net contribution of local infections.
An extended deterministic compartmental model, SEQIJR (Susceptible, Exposed, Quarantined, Infectious, Isolated, and Recovered) was constructed to simulate the disease dynamics. In particular, the model takes into account different degrees of infectivity by symptomatic (Infectious) and pre-symptomatic (Exposed) individuals. It also takes into account the efficiency of admitting pre-symptomatic individuals into quarantine and symptomatic individuals into isolation. The efficiency of each of these measures is manifested by a decrease in the infectivity of the individuals in these groups. Quarantine is designated for pre-symptomatic (Exposed) individuals, while isolation is for symptomatic (Infectious) individuals. The model also incorporates an inflow of imported cases entering the population undetected. By fitting the model’s parameters to the actual time series of the symptomatic cases, it is possible to provide insights regarding the conditions that led to the abrupt change.
Methods
The data analyzed in this study was obtained from the epidemiological investigation reports that were released by the Israeli Ministry of Health. The Ministry of Health began to issue these reports when COVID-19 was first introduced to Israel, with the arrival of a couple of passengers from the “Diamond Princess” cruise ship on February 21st and 23rd, 2020. The issuing of reports continued until March 21st, when the total number of PCR confirmed cases had reached 1023. Overall, the reports covered a total of 406 cases (Israeli Ministry of Health, 2020). Each epidemiological report consists of details on a given confirmed COVID-19 case, and the specific date and time they visited specific sites. Also, information is provided whether it is known if the person was in contact with another known COVID-19 case (See examples in the supplementary information). Sometimes the contact’s case number is provided. Also, if a case recently traveled abroad, the details of the departure and arrival flights are provided.
The preliminary analysis of the epidemiological reports consisted of the classification of each case to either category, imported or local infection. Imported cases are travelers arriving from abroad that were infected outside Israel. The Israeli mitigation policy required travelers from COVID-19 affected regions to go into quarantine on arrival. An imported case was considered as such if the details in its epidemiological report included a recent arrival from abroad. Otherwise, it was considered a local case.
For local cases, an attempt was made to identify an association of local infections with other cases, where a specific identification of the source of infection was not provided. The association was made by searching for other cases that visited the same locations. For example, cases 190 and 198 had both visited the same club on March 9th, and 198 and 252 had both visited the same banquet hall on March 10th (see supplementary information for details).
For cases with an established association with other cases, chains of transmission were reconstructed. This was made in order to examine whether there is evidence for super-spreading events and estimate their relative contribution to the overall COVID-19 spread in Israel during the first month of the outbreak. In several regions, it was reported that due to this phenomenon, 80% of the infections were caused by 5–20% of the index cases (Adam et al., 2020, Bi et al., 2020). The threshold for a person to be considered a super-spreader index case is infecting 6–8 secondary people (Adam et al., 2020).
Using the classification of cases into imported and local infections, the daily ratio of local infection to imported cases was calculated and compared to the accumulated number of confirmed cases. The confirmed cases' curve was modeled as a fit to an exponential growth function (de Silva et al., 2009; Zhao et al., 2020). Several serial interval distributions estimated for COVID-19 were examined (Nishiura et al., 2020, Ganyani et al., 2020, Zhao et al., 2020). As the number of daily PCR tests performed rose from 15 at the beginning of the period to more than 2500 by the end of the 1st month, the time series of the daily number of tests were also compared to the daily number of new cases and the daily percent of positive PCR results (see supplementary information).
To gain insights into conditions that governed the dynamics of the spread of epidemics and especially to quantify the effectiveness of the quarantine-isolation policy of Israel, a SEQIJR model (Gumel et al., 2004, Koonprasert et al., 2013, Siriprapaiwan et al., 2018) was constructed and implemented. This model is a deterministic compartmental model that allows the implicit inclusion of biological, epidemiological phases (including incubation period) and governmental interventions such as quarantine and their actual efficiency of implementation. A successful a posteriori implementation of this model to the transmission dynamics and control of the SARS epidemics in Toronto, Hong Kong, Singapore, and Beijing is given in Gumel et al., 2004. The model consists of a system of seven dynamical equations and 15 parameters. In this study, we implemented the model with parameters that reflect current knowledge of the COVID-19 pandemic. For details of the model and the parameters that were used, see supplementary information.
Results
The effective reproduction number, , was estimated for the daily new-cases data of the first month of the COVID-19 epidemic. The lowest estimated was 2.08 (95%CI 1.93–2.6) for the Gamma distributed serial interval with mean 4.4 and SD three days (Zhao et al., 2020). The highest estimate was 2.37 (95%CI 2.16–2.61) for the Gamma distributed serial interval with mean 5.2 and SD 2.8 days (Ganyani et al., 2020). The mean over all the serial interval distributions examined, was 2.19.
Another estimate for can be obtained from the reconstruction of chains of transmission (Figure 1 ). The mean number of secondary cases directly associated with an index case was 2.25 (95% CI 1.68–2.82). This number, which can be considered as an observed reproductive number (Bi et al., 2020), is quite close to the estimate described above.
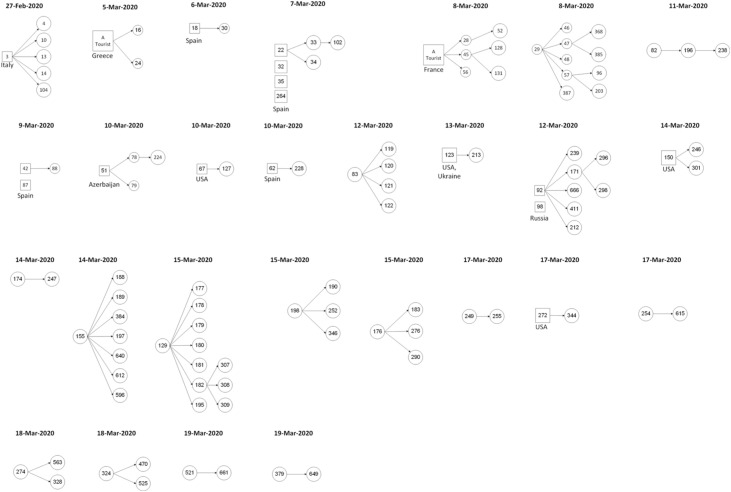
Figure 1.
Chains of transmission as reconstructed from epidemiological investigation reports. Each shape (circle or square) represents a single case. Locally infected cases are marked by a circle, and travel-associated infections (imported cases) are marked by a square. The chains are ordered by the date the primary case in each chain was announced by the Israeli Ministry of Health.
Two index cases could be considered super-spreaders (cases number 155 and 129), each of which resulted in seven secondary cases (Figure 1). These cases, which consist of 5.56% (95% CI 0.94 %–19.5%) of all index cases, are responsible of for 17.28% (95% CI 10.1 %–27.6%) of infections. While the CI bounds of the ratio of super-spreading events contain the ratios found in other regions (8.9–20% - Adam et al., 2020, Bi et al., 2020, Endo et al., 2020), the relative contribution of these events is much smaller than 80%.
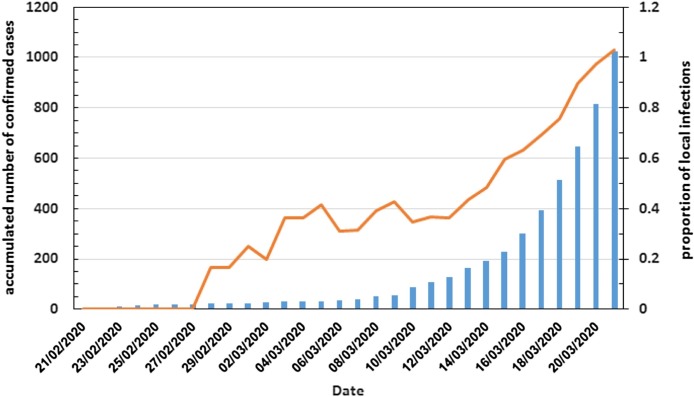
The chains of transmission are split evenly between those that began with travelers arriving from abroad (imported cases, represented by squares in Figure 1) and chains that began with individuals that were infected locally (local cases, represented by circles). This 1:1 ratio is compiled from transmission chains that span all of the studied period. To examine the dynamics of the ratio of local to imported cases during the studied period, the time series of the ratio of local to imported cases was calculated, based on the preliminary analysis of the epidemiological reports, as described in the methods section. This time series exhibits two distinct regimes (Figure 2 ). The first ended on March 12th, 2020, in which the local to imported cases ratio was around 1:3 (up to 1:2.5), i.e., for every three imported cases, there was one local infection. The second period began a day later. The first distinct sign for the change was evident on March 14th when the ratio rose to 1:2, then continued to rise on March 17th to 1:1.5 and finally, after a month into the epidemic in Israel, on March 21st, there were more local infections in Israel than imported cases (the ratio was above 1:1).
Figure 2.
Accumulated reported confirmed COVID-19 cases in Israel (blue bars) and the ratio of local infections to imported cases (orange line).
The examination of the epidemiological investigation reports provides a common reason for this distinct change, as it is possible to identify the locations that were simultaneously visited by several new COVID-19 cases. March 12th, 2020, is two days after the Jewish holiday of Purim, which this year was celebrated on March 10th. Purim customs usually include wearing costumes and holding public celebrations, parades as well as religious gatherings. Although many municipal authorities canceled public parades due to the COVID-19 outbreak, many privately organized parties and religious crowding occurred. Because Purim is also a school holiday, many youth celebrations were held a few days earlier. Some prominent examples of the effect of Purim are four new cases that participated on March 7th in a Purim party by a youth movement; the three new cases that attended a March 9th religious gathering and the four new cases that participated on March 10th in a Purim party organized by a students’ union of a college (Corona – Ministry of Health, 2020).
Two solutions were calculated for the SEQIJR model, according to two sets of parameters (Scenario 1 and 2; see supplementary information). The scenarios differ in two respects. The first is the ratio dividing the incubation time to the period before and in quarantine. The second concerns the modification factors of the effective contact rate related to the infectivity of the exposed-in-quarantine and infections-in-isolation classes.
According to the policy described in the SEQIJR model, from the pre-symptomatic phase, there are two possible paths. One is to proceed directly to the symptomatic phase. The other is a detour via a quarantine period and then, on the onset of symptoms, proceed to isolation. Therefore, the incubation period may be divided into two rates according to the time required to enter quarantine and the time to exit and proceed to isolation. In the scenarios examined in this study, scenario 1 describes a quick arrival into quarantine (corresponds to 20% of the incubation period), and scenario 2 describe a longer interval (80% of the incubation).
Regarding the modification factors, in scenario 1 people in quarantine and isolation were not infectious; in scenario 2, 48% and 20% of the people supposed to be in quarantine and isolation (respectively) were infectious. These measures were based on the estimated proportion of pre-symptomatic transmission in Singapore (Ganyani et al., 2020) and on the proportion of individuals in the infectious-in-isolation class which, despite the precautions and restrictions that are associated with isolation, were de facto infectious during the first phase of the SARS outbreak in Singapore (Gumel et al., 2004). Modification factors can be considered measures of the effectiveness of quarantine and isolation. In this context, efficiency is defined as the fractional decrease of the possibility of infecting compared to the infectivity of a symptomatic patient. Scenario 1 considered 100% effectiveness of these measures, while scenario 2 considered the quarantine of exposed individuals as 52% effective and the isolation of infectious individuals as 80% effective. The change between the regimes that, in the model, signifies the change between scenario 1 (Figure 3 , solid line) and scenario 2 (Figure 3, dashed line) was set to March 13th, based on the change identified in the local to imported cases ratio (Figure 2). To assess the effect of regime change that followed Purim, the time series of scenario 1 was continued after March 13th (Figure 3, dotted line).
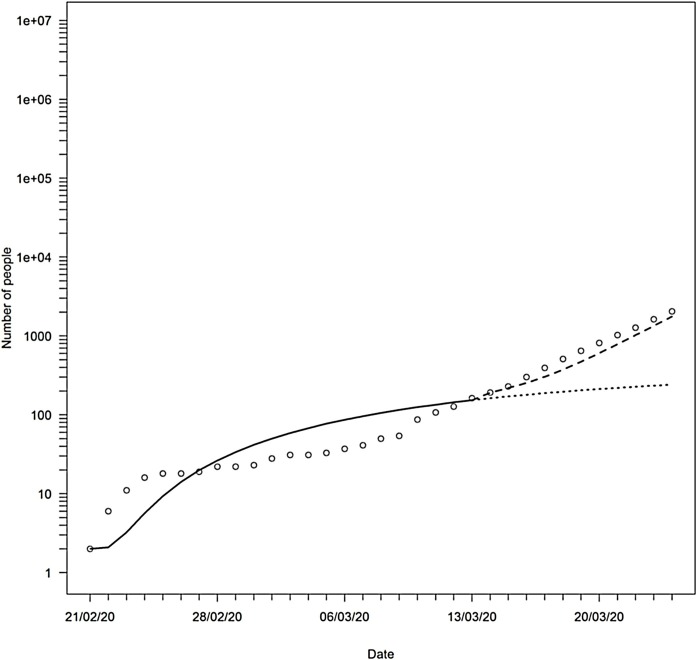
Figure 3.
The total number of symptomatic cases in Israel: observed (circles), and 2 scenarios from the SEQIJR model: 1 - solid line until March 13th and then continues in dotted line; 2 – dashed line beginning on March 13th.
To examine the SEQIJR model's ability to reconstruct the general behavior of the observed total number of symptomatic cases, FAC2, the fraction of a model’s predictions that are within a factor of two of the observations, was calculated. This measure is used in many applications to evaluate the degree of agreement between a model’s predictions and observations (De Schamphelaere et al., 2003, Duijm, 1999, Efthimiou et al., 2011, Hanna and Chang, 2012, Juraske et al., 2009, Kadiyala and Kumar, 2012, McGrath et al., 2005). The FAC2 of the SEQIJR model, with the parameters under the two scenarios, was 82%, which is considered to be excellent agreement (Efthimou et al., 2011; Hanna and Chang, 2012).
Based on the two sets of parameters, it is possible to calculate , the effective reproduction number from the model, for the two regimes (Eq. 9 in the supplementary information). Up until the abrupt change, the effective reproduction number was 0.69 (scenario 1), and then it changed to 4.34 (scenario 2). This shows that the estimates that were based on the overall data from the first month of the COVID-19 epidemic in Israel, 2.19 (based on a fit to an exponential growth function) or 2.25 (based on the reconstructed chains of transmission), are actually a combination of two regimes, first a controlled regime and then an exponential growth.
Discussion and summary
As described in the introduction section, Israel had gradually implemented different mitigations steps, weeks before the arrival of the first COVID-19 cases in Israel, on February 21st and 23rd. This policy succeeded in keeping the rate of daily new cases small, up until March 9th. Then, four days later, a sudden change of regime occurred, which was manifested by the distinct change of the confirmed cases’ curve of Israel towards exponential growth. March 15th marks the first time that the daily new locally infected cases were higher than the new travel-associated cases.
The timing of this abrupt change is not coincidental. Regarding the cases arriving from abroad, the requirement for home quarantine affecting all travelers arriving began on March 9th. Moreover, from March 9th to 11th, a Jewish holiday, Purim, was celebrated. This holiday is characterized by big parades organized by local municipalities, as well as religious gatherings and privately organized parties. Although authorities canceled the public parades, many privately organized and religiously-crowded events occurred. Regrettably, these drove Israel from a controlled, mitigated regime to exponential growth, as described in the results section. Therefore, despite its intense efforts, Israel’s effective reproduction number, , for the period ending in March 20th, was 2.19, slightly smaller than the of 2.6–3.2 estimated for the republic of Korea and Italy for the period ending on March 5th (Zhuang et al., 2020). Such an abrupt transition based on social behavior emphasizes the fragility of mitigation policies. The values of the effective of the two regimes were calculated for the two sets of fitted parameters of the SEQIJR model. These two values illustrate the gravity of the effect of the Purim celebrations. Israel has changed from of 0.69 before Purim to 4.34 following the holiday.
Similar to the effect of the Purim holiday, there are reports of events associated with many infections during the beginning of the COVID-19 pandemic. These are the Champions League football match, which was held between Italy’s Atalanta and Spain’s Valencia in the San Siro Stadium in Milan on February 19th, 2020, the Muay Thai match that took place at the Lumpinee Boxing Stadium on March 6th, and the Sri Petaling mass religious gathering that took place on February 27th to March 1st (Rudan, 2020, Koga, 2020, Mat et al., 2020). However, Purim is fundamentally different from these events because all these were mass gatherings that occurred at a specific location. Contrary to these, the rise in the new cases associated with Purim involved different communities and diverse groups of Israeli society, all geographically dispersed.
Therefore, we emphasize the importance of early fine-tuned but intense directives for social distancing and isolation measures. This study clearly demonstrates the lesson learned from the Israeli policy, that even a short lapse in public responsiveness can have a dramatic effect on public health during a pandemic outbreak.
The analysis of the reconstructed chains of transmission has shown that while super-spreading events did occur during the first month of the COVID-19 outbreak in Israel, we have found no evidence that their contribution is as dramatic as discussed in other studies (Adam et al., 2020, Bi et al., 2020, Endo et al., 2020, Miller et al., 2020). Specifically, our analysis does not support the conclusion of Miller et al. (2020), which modeled the COVID-19 outbreak in Israel up until April 22, 2020. Their dynamic SEIR model assumes two classes of infectious individuals, one with low transmissibility and one with high transmissibility. It does not take into account quarantine and isolation, thus assumes that all infectious individuals interact freely with members of the susceptible populations. Their model is a factor of two agreement (FAC2) with the observations 36% of the time (compared to 82% in our study); however, this agreement occurs only in the last part of the modeled period (beginning on March 31th). Before that period, their model provides an over-prediction, first by a factor of 100, then gradually declines to less than 25, by March 12th. The ratio of the actual number of infections to the reported number of cases was estimated for different regions (Böhning et al., 2020, Cowling et al., 2020, Hao et al., 2020, Havers et al., 2020). As this ratio spans between 1.25 and 10, the over-prediction until March 17th (close to half the modeled period) cannot be attributed to low reporting rates, let alone cannot support the claim that the dynamics of the COVID-19 epidemic in Israel during the first month were profoundly influenced by super-spreading events.
Conflict of interest
All authors declare no conflicts of interest.
Funding source
There was no external funding. The research was a part of the institutional research program on COVID-19.
Ethical approval
The study is a theoretical analysis that was based on publicly available data. The study involved theoretical analysis, so ethical consent and animal care issues were not applicable.
Acknowledgment
We would like to thank Gadi Eidelheit and Liat Markus for their assistance with collecting the epidemiological investigation reports and time series of the PCR tests. We would also like to thank Ruth Hashkes and Idan Dardikman of the https://covid19data.co.il/ website for organizing the new COVID-19 cases time series.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.016.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adam D., Wu P., Wong J., Lau E., Tsang T., Cauchemez S., Leung G., Cowling B. Clustering and superspreading potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in Hong Kong. Nat Med. 2020 doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., Liu X., Wei L., Truelove S.A., Zhang T., Gao W., Cheng C., Tang X., Wu X., Wu Y., Sun B., Huang S., Sun Y., Zhang J., Ma T., Lessler J., Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhning D., Rocchetti I., Maruottic A., Holling H. Estimating the undetected infections in the Covid-19 outbreak by harnessing capture-recapture methods. Int J Infect Dis. 2020;97:197–201. doi: 10.1016/j.ijid.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona – Ministry of Health . 2020. Israel Ministry of Health Telegram channel of news and updates.https://t.me/s/MOHreport [Google Scholar]
- Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.W.Y.K., Li J.C.M., Fong M.W. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schamphelaere K.A., Vasconcelos F.M., Heijerick D.G., Tack F.M., Delbeke K., Allen H.E., Janssen C.R. Development and field validation of a predictive copper toxicity model for the green alga Pseudokirchneriella subcapitata. Environ Toxicol Chem. 2003;22:2454–2465. doi: 10.1897/02-499. [DOI] [PubMed] [Google Scholar]
- De Silva U.C., Warachit J., Waicharoen S., Chittaganpitch M. A preliminary analysis of the epidemiology of Influenza A(H1N1)v virus infection in Thailand from early outbreak data. Euro Surveill. 2009;14(31) doi: 10.2807/ese.14.31.19292-en. [DOI] [PubMed] [Google Scholar]
- Duijm N.J. Estimates of roughness parameters for arrays of obstacles. Boundary Layer Meteorol. 1999;91:1–22. doi: 10.1023/A:1001794831176. [DOI] [Google Scholar]
- Efthimiou G.C., Bartzis J.G., Koutsourakis N. Modelling concentration fluctuations and individual exposure in complex urban environments. J Wind Eng Ind Aerodyn. 2011;99:349–356. doi: 10.1016/j.jweia.2010.12.007. [DOI] [Google Scholar]
- Endo A., Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group, Abbott S., Kucharski A.J., Funk S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganyani T., Kremer C., Chen D., Torneri A., Faes C., Wallinga J. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.17.2000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumel A.B., Ruan S., Day T., Watmough J., Brauer F., van den Driessche P. Modelling strategies for controlling SARS outbreaks. Proc Royal Soc B. 2004;271:2223–2232. doi: 10.1098/rspb.2004.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna S., Chang J. Acceptance criteria for urban dispersion model evaluation. Meteorol Atmospheric Phys. 2012;116:133–146. doi: 10.1007/s00703-011-0177-1. [DOI] [Google Scholar]
- Hao X., Cheng S., Wu D., Wu T., Lin X., Wang C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nat. 2020;584:420–424. doi: 10.1038/s41586-020-2554-8. [DOI] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- Updates (Press Releases). https://www.gov.il/en/departments/news/?OfficeId=104cb0f4-d65a-4692-b590-94af928c19c0&limit=10&startDate=2020-02-01&endDate=2020-03-31&skip=0, 2020 (accessed 12 July 2020).
- Juraske R., Castells F., Vijay A., Muñoz P., Antón A. Uptake and persistence of pesticides in plants: Measurements and model estimates for imidacloprid after foliar and soil application. J Hazard Mater. 2009;165:683–689. doi: 10.1016/j.jhazmat.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Kadiyala A., Kumar A. Evaluation of indoor air quality models with the ranked statistical performance measures using available software. Environ Prog Sustain Energy. 2012;31:170–175. doi: 10.1002/ep.11642. [DOI] [Google Scholar]
- Klausner Z., Klement E., Fattal E. Modeling long distance dispersal of airborne foot-and-mouth disease virus as a polydisperse aerosol–Application to the emergence of a new strain from Egypt to Israel. Atmospheric Environ. 2015;122:332–342. doi: 10.1016/j.atmosenv.2015.09.067. [DOI] [Google Scholar]
- Klausner Z., Fattal E., Klement E. Using synoptic systems’ typical wind trajectories for the analysis of potential atmospheric long‐distance dispersal of lumpy skin disease virus. Transbound Emerg Dis. 2017;64:398–410. doi: 10.1111/tbed.12378. [DOI] [PubMed] [Google Scholar]
- Klausner Z., Klement E., Fattal E. Source–receptor probability of atmospheric long‐distance dispersal of viruses to Israel from the eastern Mediterranean area. Transbound Emerg Dis. 2018;65:205–212. doi: 10.1111/tbed.12649. [DOI] [PubMed] [Google Scholar]
- Koga K. Japan-southeast Asia relations: great disruption: Uncertainty over the Indo-Pacific. Comparative Connections. 2020;22:137–148. [Google Scholar]
- Koonprasert S., Janreung S., Moore E.J. An analysis of quarantine and isolation in an SEQIJR model. Far East J Math Sci. 2013:152–172. [Google Scholar]
- Mat N.F.C., Edinur H.A., Razab M.K.A.A., Safuan S. A single mass gathering resulted in massive transmission of COVID-19 infections in Malaysia with further international spread. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J.A., Parkerton T.F., Hellweger F.L., Di Toro D.M. Validation of the narcosis target lipid model for petroleum products: gasoline as a case study. Environ Toxicol Chem. 2005;24:2382–2394. doi: 10.1897/04-387r.1. [DOI] [PubMed] [Google Scholar]
- Miller D., Martin M.A., Harel N., Kustin T., Tirosh O., Meir M. Full genome viral sequences inform patterns of SARS-CoV-2 spread into and within Israel. medRxiv. 2020 doi: 10.1101/2020.05.21.20104521. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I. A cascade of causes that led to the COVID-19 tragedy in Italy and in other European Union countries. J Glob Health. 2020;10 doi: 10.7189/jogh.10.010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriprapaiwan S., Moore E.J., Koonprasert S. Generalized reproduction numbers, sensitivity analysis and critical immunity levels of an SEQIJR disease model with immunization and varying total population size. Math Comput Simul. 2018;146:70–89. doi: 10.1016/j.matcom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper G.A. The lessons of the Pandemic. Science. 1919;49:501–506. doi: 10.1126/science.49.1274.501. [DOI] [PubMed] [Google Scholar]
- Zhao S., Gao D., Zhuang Z., Chong M.K.C., Cai Y., Ran J. Coronavirus disease (COVID-19): A statistical analysis using the public data in Hong Kong from January 16 to February 15, 2020. Front Phys. 2020;8:347. doi: 10.3389/fphy.2020.00347. [DOI] [Google Scholar]
- Zhuang Z., Zhao S., Lin Q., Cao P., Lou Y., Yang L. Preliminary estimating the reproduction number of the coronavirus disease (COVID-19) outbreak in Republic of Korea and Italy by 5 March 2020. Int J Infect Dis. 2020;95:308–310. doi: 10.1016/j.ijid.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.