Abstract
Objectives
To determine the effect of vitamin D supplementation on disease progression and post-exposure prophylaxis for COVID-19 infection. We hypothesize that high-dose vitamin D3 supplementation will reduce risk of hospitalization/death among those with recently diagnosed COVID-19 infection and will reduce risk of COVID-19 infection among their close household contacts.
Methods
We report the rationale and design of a planned pragmatic, cluster randomized, double-blinded trial (N = 2700 in total nationwide), with 1500 newly diagnosed individuals with COVID-19 infection, together with up to one close household contact each (~1200 contacts), randomized to either vitamin D3 (loading dose, then 3200 IU/day) or placebo in a 1:1 ratio and a household cluster design. The study duration is 4 weeks. The primary outcome for newly diagnosed individuals is the occurrence of hospitalization and/or mortality. Key secondary outcomes include symptom severity scores among cases and changes in the infection (seroconversion) status for their close household contacts. Changes in vitamin D 25(OH)D levels will be assessed and their relation to study outcomes will be explored.
Conclusions
The proposed pragmatic trial will allow parallel testing of vitamin D3 supplementation for early treatment and post-exposure prophylaxis of COVID-19. The household cluster design provides a cost-efficient approach to testing an intervention for reducing rates of hospitalization and/or mortality in newly diagnosed cases and preventing infection among their close household contacts.
Keywords: Vitamin D, COVID-19, SARS-CoV-2, Early treatment, Prophylaxis, Cluster randomization
1. Introduction
Coronavirus disease (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presents a major threat to human health [1]. SARS-CoV-2 is highly infectious, associated with extensive morbidity and mortality, and household contacts of infected patients and healthcare personnel are at particularly high risk for infection [2]. As diagnoses and deaths continue to rise worldwide, COVID-19 is now widely recognized as a global pandemic.
Given this public health crisis, there is a critical need to have quickly deployable and efficient randomized trials to test promising treatments and prophylactic interventions to slow the pandemic. Development of pragmatic, adaptive, and cost-efficient trials is a high priority. In this setting, we identified vitamin D supplementation as a promising intervention to test. As described below and summarized in the Table 1 , several lines of evidence suggest that vitamin D deficiency is a risk factor for COVID-19 and that improvement in vitamin D status by supplementation may mitigate COVID-19 risk for clinical progression.
Table 1.
Lines of evidence that vitamin D deficiency is an important modifiable risk factor for COVID-19.
|
Table adapted from Manson and Bassuk [44].
Laboratory studies demonstrate that vitamin D is active in pathways relevant to immune function, stimulating the expression of vitamin D receptors in both the innate and the adaptive immune systems. Vitamin D metabolites support innate immune responses to several viruses in cultured human respiratory epithelial cells [[3], [4], [5], [6], [7]], including rhinovirus and respiratory syncytial virus (RSV). Vitamin D may reduce the burden of infections by lowering viral replication rates, through the induction of antimicrobial peptides [[8], [9], [10]] and by enhancing cellular immunity by reducing pro-inflammatory cytokines and increasing concentrations of anti-inflammatory cytokines [11,12].
Both epidemiological and clinical studies have shown that vitamin D, acting as an important immunomodulator, may reduce the incidence of respiratory tract infections in both adults and children [[13], [14], [15], [16], [17]]. Randomized trials have also provided supportive evidence that vitamin D supplementation reduces risk of acute respiratory infections in these populations (22–24). A meta-analysis of 11,321 participants in 25 randomized controlled trials of vitamin D supplementation to prevent acute respiratory tract infections (upper and lower respiratory tracts) demonstrated a protective effect of the intervention overall (12% reduction), but a particularly large magnitude of benefit (70% reduction) from supplementation in those with profound vitamin D deficiency at baseline [18]. However, results showed heterogeneity, with strong protective effects in some trials [[19], [20], [21]]⁎ and others with null findings [22,23]. This heterogeneity may reflect two factors: i) Variation in dose of vitamin D administered: some trials gave a low dose of ≤400 IU per day, and ii) Variation in participant characteristics: strongest protection effects against acute respiratory tract infection were seen among those with vitamin D deficiency at baseline. Given that SARS-CoV-2 is a novel virus, the potential role of vitamin D in affording protection against this specific infection is promising but unknown.
COVID-19 patients with weakened innate immune systems may be susceptible to more severe symptoms and higher mortality [24]. An impaired host immune system response may lead to higher SARS-COV-2 viral load and subsequent overactivation of the adaptive immune system that results in cytokine release syndrome [25,26]. Vitamin D can modulate both the innate and adaptive immune responses [27] and suppress the hyperinflammatory response during infection [32].
Ecologic and demographic data also provide support for a protective role of vitamin D studies [28,29]. Inverse correlations between mean levels of vitamin D in each of 20 European countries and the rates of COVID-19 diagnoses and mortality have been observed [30]. Demographic groups known to be at elevated risk for vitamin D insufficiency— Black or Hispanic individuals, the elderly, nursing-home residents, and those with obesity, vascular comorbidities, or chronic kidney disease—are also those at higher risk of COVID-19 hospitalization and/or mortality. Finally, several observational studies have shown a significant association between a low serum 25(OH)D level status and worse clinical outcomes of COVID-19 patients [[31], [32], [33]].
The Vitamin D for COVID-19 (VIVID) trial is a pragmatic, cluster-randomized, placebo-controlled, double-blind trial to evaluate the efficacy of Vitamin D3 supplementation with 3200 IU/d daily for 4 weeks to reduce disease severity in persons with newly diagnosed COVID-19 infection and to prevent infection in their closest household members. In this article, we detail the rationale and design of this nationwide study that is in development and is subject to adaptive changes as needed.
2. Study design and methods
2.1. Overview of study design
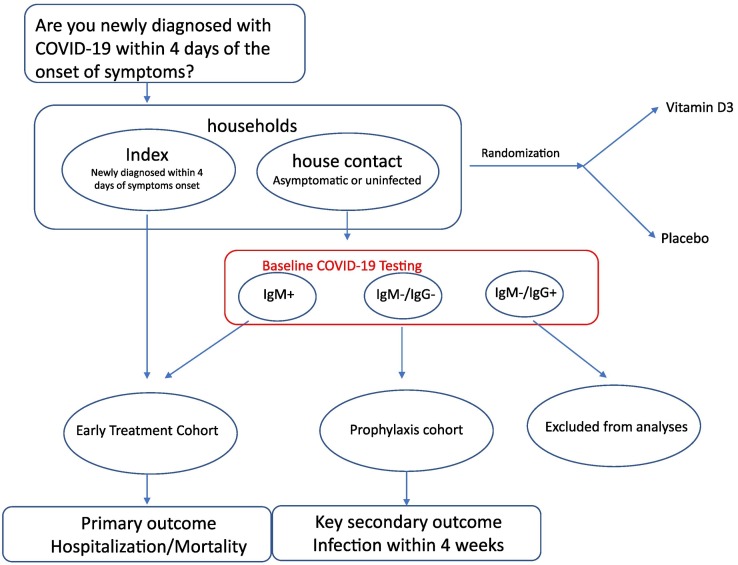
VIVID is based on a parallel group design (N = 2700 in total nationwide); the study population includes 1500 newly diagnosed individuals (referred to as “index cases”) as well as the closest household contacts of each index if such a person is identified and consents (~1200 in total). Each participant—whether index or contact—is randomized to either vitamin D3 or placebo in a 1:1 ratio in a household “cluster” design. The study duration for each participant is 4 weeks. The infected individuals will be followed to assess disease progression defined as the need for hospitalization and/or mortality. Their household members will be followed to assess infection status, either progression if seropositive at baseline, or seroconversion, if uninfected at baseline.
A schematic of the design is displayed in the Fig. 1 , where vertical arrows show time sequences and horizontal arrows show randomization which will be done prior to COVID diagnostic testing for the household members, due to concerns regarding delays in receiving the contact's test results and the need to start treatment early. Subsequent events apply to both randomized groups. If the treatment works for both the early-treatment and prophylaxis cohorts, then this early-intervention approach could be implemented in practice.
Fig. 1.
Schematic of VIVID design.
2.2. Primary and secondary hypotheses
The primary hypothesis is that Vitamin D3 supplementation will reduce the risk of hospitalization and/or mortality among patients who are newly diagnosed with COVID-19 infection but do not require hospitalization at baseline. The primary outcome is the occurrence of hospitalization and/or mortality. Hospitalizations and deaths will be confirmed by chart review or interview of next of kin.
Secondary Hypotheses are:
For index cases:
-
•
Vitamin D3 supplementation will reduce self-reported disease severity over 4 weeks. Participants will self-report symptom severity and disease progression on weekly questionnaires: for each specified COVID-19 related symptoms (fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, diarrhea), participants will report one of the following 3 options: absence (score of 0), mild/moderate (score of 1), severe (score of 2). The total disease severity score will be the average across all potential symptoms.
-
•
Vitamin D3 supplementation will delay time to hospitalization/mortality.
-
•
Vitamin D3 supplementation will reduce the risk of ICU admission/ventilation support in index cases.
For the closest household contacts of index cases:
-
•
Vitamin D3 supplementation will prevent SARS-CoV-2 infection(seroconversion).
-
•
Vitamin D3 supplementation will reduce self-reported total disease severity defined in the same way as for the index participants.
2.3. Study population and inclusion/exclusion criteria
The study population will include individuals who tested positive for COVID-19 infection within 72 h of the COVID diagnostic testing and have experienced COVID-19 related symptoms for no longer than 4 days (for the “index cases”), and the closest cohabiting contact, if such a person is identified and consents, for each newly diagnosed individual.
For all participants, the ability to understand and provide informed consents, and to complete online questionnaire, and not participating in other COVID-19 trials are required.
For index cases, we will target a high-risk study population defined by adults ≥30 years of age with comorbidities. Specifically, we plan to enroll Age 30–49 with ≥ 2 comorbidities (diabetes, hypertension, BMI ≥30, chronic obstructive pulmonary disease (COPD) or emphysema, history of heart attack or stroke, history of coronary bypass surgery or coronary angioplasty or stent, diagnosed sleep apnea) and/or risk factors (smoking, being in a racial/ethnic minority group [African American, Hispanic, American Indian, South Asian]) OR Age 50–59 with at least 1 comorbidity or risk factor (as defined above) OR Age 60 or older regardless of comorbidities.
Key exclusion criteria include: 1) known pregnancy; 2) Current hospitalization; 3) Consume more than 400 IU/d of vitamin D from all supplemental sources combined in the past 4 weeks; 4) Known diagnosis of hypercalcemia or a condition associated with vitamin D hypersensitivity; 5) Having a diagnosis of advanced cancer or or or currently undergoing radiation, chemotherapy, or immunotherapy for cancer; 6) Kidney failure or dialysis, severe liver disease or cirrhosis, active tuberculosis, sarcoid or sarcoidosis, parathyroid disease; 7) Unstable, transient or group ((≥ 6 adults) living arrangement. (See Supplement for line listings regarding eligibility.)
For household members, we plan to recruit the closest contact, age 18 or older, living in the same household as the index case. Exclusion criteria that differ from the index individuals are: 1) History of SARS-CoV-2 diagnosis with onset of symptoms more than 4 days before study entry; and 2) Receipt of a SARS-CoV-2 vaccination or monoclonal antibody.
2.4. Treatment assignment and randomization
Eligible index cases and their household contacts will be randomly assigned, in a cluster fashion, to one of the two study groups in a 1:1 ratio using a permuted block design (block size of 8), via a web-based randomization module after the index signs the informed consent.
2.4.1. Intervention
Daily oral softgel capsule containing 3200 IU vitamin D3 (with a loading dose of 9600 IU/day for day 1 and day 2), administered daily for 4 weeks. Participants will be mailed a supply of pills by an overnight courier service (due to the time sensitivity) and instructed to take 3 pills each day for the first two days and 1 pill daily thereafter.
2.4.2. Control
Daily oral placebo capsule of identical appearance and taste containing no vitamin D.
2.5. Study procedures
2.5.1. Recruitment
Participants will be recruited nation-wide via social media; community advocacy groups and equity initiatives; flyers and electronic communications distributed in healthcare centers, low income residential housing organizations, COVID-19 testing centers, and other avenues.
All recruitment will be conducted remotely and prospective participants will be asked to complete a web-based HIPAA-secure screening survey by the Research Electronic Data Capture (REDCap) system. Screen-eligible participants will be contacted by phone by research staff to complete the screening process using on-line consent forms. (Additional details are in the Supplement.)
2.5.2. Baseline and follow-up
Blood samples will be collected for all participants at baseline and at 4-week follow-up. The blood microsampling collection kit will be mailed to participants' home and will be returned through postal mail. The blood sample will be used for testing of 25-hydroxyvitamin D (25OHD) level as a marker of vitamin D status. For household members, week 4 samples will be tested for SARS-CoV-2 antibody positivity. For those who test positive at week 4, the baseline sample will also be tested to confirm absence of infection at baseline.
Participants will receive a daily text message as a reminder to take study pills. They will also be instructed to complete weekly online REDCap questionnaires for 4 weeks and an additional post-study follow-up questionnaire at week 8. The questionnaire will include items on adherence with randomized treatments, use of non-trial supplements of vitamin D, use of calcium supplements, development of symptoms and new illness, dietary intakes of vitamin D, and self-report of disease progression and severity status. Non-responders will be telephoned to collect study data. (Additional details are in the Supplement.)
Participants will be instructed to discontinue their study pills if, during follow-up, they receive a diagnosis of kidney stones, hypercalcemia, or other safety-exclusion conditions. Participants will be advised to discontinue the study treatment if, in the course of follow-up, they report taking supplementary vitamin D (not from diet) that amounts to greater than 400 IU per day or daily calcium supplements that amount to greater than 1200 mg per day.
2.6. Data management
We will use REDCap (Research Electronic Data Capture), a secure, HIPAA compliant web-based application hosted by the Partners HealthCare Research Computing, Enterprise Research Infrastructure & Services (ERIS) group. We will design a data dictionary and web-based surveys to capture data at each of the study timepoints: pre-baseline (screening/eligibility), consent form, baseline (demographics, health status, exposure history, etc.), post-randomization (interim symptom and Adverse Event (AE) assessments), and endpoint (testing data, symptoms, infection status). Participant surveys will be distributed using emails and/or text messages. (Additional details are in the Supplement.)
2.7. Statistical considerations
Analyses of treatment effects will be based on the intention-to-treat principle. Primary analyses will follow this principle; that is, individuals will be analyzed according to their assigned treatment group, whether or not they take the study treatment as assigned.
For endpoints (e.g., seroconversion) measured on individuals from the prophylaxis cohort (household members), because the study population consists of one individual from one household, the outcomes are independent and hence standard analysis methods for individual randomized trials apply. However, for endpoints (e.g., hospitalization/mortality, disease progression) measured on early treatment cohort (index cases and some of the household members who are already infected at baseline), because some of the household members may be included in the early treatment, it is necessary to take household clustering into account in the analysis. We will use the Generalized Estimating Equations approach with robust sandwich variance [34] to properly quantify the uncertainty of the treatment effect estimates for clustered outcomes.
The primary endpoint is the occurrence of hospitalization or death by the end of study week 4 among subjects with newly diagnosed COVID-19 status at baseline (index participants and their household contacts with serology tests that are IgM positive but IgG negative).
The primary analysis will use a logistic regression model regressing the occurrence of hospitalization or mortality as outcome on the treatment indicator. The secondary endpoints for the index cases are: 1) occurrence of ICU admission/ventilator support; 2) time to hospitalization/death; 3) self-reported disease progression score over 4 weeks; and 4) change in vitamin D 25(OH)D levels from baseline to week 4.
The secondary endpoints for the household members who are seronegative at baseline are: 1) COVID-19 infection during the course of the study; 2) self-reported disease severity or disease progression (hospitalization or identified deaths) by the end of week 4; and 3) change in vitamin D 25(OH)D levels from baseline to week 4. (Additional details regarding the data analysis are in the Supplement.)
2.7.1. Pre-specified subgroup analyses
Pre-specified subgroup analyses include the evaluation of the potential modification of treatment effect by age, sex, race/ethnicity, baseline 25OHD levels (<12, 12–20, and ≥ 20 ng/mL), obesity status, whether or not taking a vitamin D supplement in addition to study pills.
2.7.2. Sample size and power considerations
2.7.2.1. For index cases
Although the primary analysis is based on data from the early treatment cohort which includes both the index cases and some of their household members, because we do not know the fraction of index cases who will have a household member enrolled, the fraction of household members who are already infected at baseline, and the intraclass correlation coefficient, sample size calculation for the early treatment cohort is based on the index cases only. Assuming a four-week hospitalization/mortality rate of 20% in the control arm, a total sample size of 1500 (the target number of index cases) achieves ≥95% power to detect a reduction of 40% in event rate with a type I error of 5%, based on a Fisher's exact test. This sample size has 80% power to detect a 28% reduction from 20% to 14.4%. In a meta-analysis assessing the effect of vitamin D supplementation on risk of acute respiratory tract infection, among those who received daily or weekly vitamin D, Martineau et al. reported an adjusted odds ratio of 0.3 or 0.75 in those with baseline 25-hydroxyvitamin D levels <25 nmol/L or ≥ 25 nmol/L respectively [18]. Because the study duration for each participant is only four weeks, we anticipate a low lost-to follow-up rate. Recognizing that a proportion of the household contacts will already be infected at baseline and will be included in the primary analysis of early treatment cohort, the actual power will be greater and can offset power loss due to the loss to follow-up.
2.7.2.2. For household contacts
Assuming 80% of newly diagnosed participants will also have a close co-habiting contact enrolled into the study, we anticipate enrolling 1200 into the household contact cohort. Jing et al. estimated a 12.4% secondary attack rate among household contacts [35]. In a systematic review, Shah et al. found that the reported secondary attack rate of COVID-19 in household contacts ranged from 4.6% to 49.6% [36]. Assuming 20% of these individuals are already seropositive for COVID-19 at baseline, the number of evaluable participants for the key secondary hypothesis that the household members of the infected individual will have a lower seroconversion rate is n = 1000. A sample size of 500 in each comparison arm achieves 80% power to detect a 40% reduction in serological positive rate from 15% to 9% based on a Fisher's exact test.
2.8. Data and safety monitoring
We will establish an external DSMB for the trial. Given the short duration of the study, an interim meeting is planned when half of the participants have been enrolled, or at 6 months, whichever occurs earlier. At the meeting, the DSMB will assess whether study progress, data integrity and safety monitoring warrant continuation of the study and make recommendations accordingly. (Additional details are in the Supplement.)
3. Discussion
The coronavirus disease 2019 (COVID-19) is an escalating global pandemic associated with the potential for severe respiratory complications. There is an urgent public health need for effective therapeutic and prophylactic strategies that have proven to be safe in humans—which is especially acute given the rapid spread of SARS-CoV-2 and lack of vaccines or highly effective treatments for infected individuals who do not require hospitalization to slow down the disease progression. To investigate the effect of vitamin D and potential alternative agents requires studies that can be completed expeditiously, conducted safely, use resources efficiently, and produce reliable results.
The VIVID trial will assess the effect of Vitamin D supplementation for early treatment and post-exposure prophylaxis of COVID-19. The study is highly pragmatic and will be conducted remotely with no study visits required, which facilitates national recruitment, limiting infection control challenges. Salient features of the study include the cluster randomization design which both offers logistic convenience and avoids treatment contamination: both household members will receive the same treatment; and permits answering two important questions: early treatment and prophylaxis, in the same study.
The trial is innovative in that it allows for participants in the same cluster to be in two different cohorts, which have different endpoints—while maintaining valid randomization and therefore preservation of the type I error control. The design is cost-efficient in that testing for COVID-19 among household contacts need not be done until the end of the study; hence, baseline samples need only be tested for those who test seropositive at end of follow-up. The nationwide recruitment allows for nimble and targeted recruitment in geographic areas with higher infection rates (‘hot spots’), the locations of which will change over time. Another innovative aspect of the trial design is that enrollment of household members occurs before they are tested for COVID-19. This is possible because the same agent is under study for both prophylaxis and treatment components; hence vitamin D can be initiated as quickly as possible after participants have consented to randomization.
In searching for a treatment and prevention agent for COVID-19, many drugs and chemical compounds are being considered (see for example, Jeon et al. [37]). The everchanging landscape of prevention policies, pandemic dynamics, and rapidly accruing knowledge requires studies to be sufficiently flexible. Our study design is customizable and can be tailored to test other candidates in a rolling platform fashion [38]. It can also be extended to efficiently test two drugs at the same time through a factorial design by adding a second agent (e.g., inhaled corticosteroids).
The study has limitations. First, it is possible that vitamin D blood levels would not improve rapidly enough to affect the course of the infection. Although a higher loading dose (9600 IU/day) is incorporated for the first two days of the study, it may take a week or longer to eliminate vitamin D deficiency. We plan to measure vitamin D levels to better understand the trajectory of 25(OH)D increase over time in this study population. We are avoiding massive bolus dosing because it is not physiologic and has not shown benefits for reducing respiratory infections or other adverse outcomes in previous vitamin D trials [[39], [40], [41]]. Calcifediol can more rapidly increase serum 25(OH)D level and a recent study evaluated its effect when added to best available therapy among patients hospitalized for COVID-19 [42]; future studies may be designed to assess its effect in slowing disease progression to hospitalization. Second, despite considerable uncertainty regarding the role of vitamin D for the treatment and prevention of COVID-19, some participants may choose to take out-of-study vitamin D supplements; vitamin D is readily available in pharmacies and stores. Also, recruitment may be challenging due to unwillingness to participate in a randomized trial with a 50% chance of receiving placebo. However, our study pills will have stricter quality control monitoring than most over-the counter dietary supplements. In a recent study, LeBlanc et al. found that the cholecalciferol content of over-the-counter and compounded vitamins was highly variable [43]. We will provide this information to potential participants to increase the attractiveness of the study. Third, the initiation of treatment is very time sensitive. Due to the lag time in receiving COVID test results by participants and contacting the study, it may be challenging to initiate treatment within the short time window allowed after the onset of symptoms. However, enrolling their asymptomatic close household contacts at baseline–some of whom are likely already to have been infected and will contribute information to the early treatment cohort–will improve our ability to assess the effect of vitamin D on progression when treatment begins early.
In summary, this study will provide reliable randomized evidence about the effect of vitamin D on both early treatment and post-exposure prophylaxis of COVID-19. The data collected from this study will also permit us to develop and validate a COVID-19 symptom score for disease progression. Given that COVID-19 disproportionately affects race/ethnicity minority groups and high prevalence of vitamin D deficiency in these groups, vitamin D efficacy may provide opportunities for reducing health disparities. This innovative, pragmatic, and timely RCT will address a critical unmet need in the prevention and clinical management of SARS-CoV-2 in the community. The results will be of immediate clinical relevance by providing evidence to inform measures to control the pandemic and mitigate disease occurrence and severity, as well as to reduce personal suffering, mortality, and health care costs.
Funding
VIVID is supported by Harvard interinstitutional funding, charitable trust foundation support (anonymous), and philanthropic support from Jon R. Sabes of Minneapolis, MN. The vitamin D supplements and matched placebos were provided by Tishcon Corporation at a discounted price.
Disclosure statement
No authors reported competing interests. Dr. Samia Mora provided consulting to Quest Diagnostics for work unrelated to the current study. Dr. Susan Redline received grant and consulting fees from Jazz Pharmaceuticals, and received consulting fees from RespirCardia Inc and Eisai Pharma, unrelated to the current study.
Footnotes
Camargo Jr. and D. Ganmaa are equally contributed.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2020.106176.
Appendix A. Supplementary data
Additional information about the trial.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - Navigating the uncharted. N. Engl. J. Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greiller C.L., Suri R., Jolliffe D.A., Kebadze T., Hirsman A.G., Griffiths C.J., Johnston S.L., Martineau A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019;187:152–159. doi: 10.1016/j.jsbmb.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Telcian A.G., Zdrenghea M.T., Edwards M.R., Laza-Stanca V., Mallia P., Johnston S.L., Stanciu L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017;137:93–101. doi: 10.1016/j.antiviral.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Hansdottir S., Monick M.M., Lovan N., Powers L., Gerke A., Hunninghake G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010;184(2):965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F., Borregaard N., Modlin R.L., Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laaksi I. Vitamin D and respiratory infection in adults. Proc. Nutr. Soc. 2012;71(1):90–97. doi: 10.1017/S0029665111003351. [DOI] [PubMed] [Google Scholar]
- 10.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., Kamen D.L., Wagner M., Bals R., Steinmeyer A., Zugel U., Gallo R.L., Eisenberg D., Hewison M., Hollis B.W., Adams J.S., Bloom B.R., Modlin R.L. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 11.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1) doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belderbos M.E., Houben M.L., Wilbrink B., Lentjes E., Bloemen E.M., Kimpen J.L., Rovers M., Bont L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 13.de Sa Del Fiol F., Barberato-Filho S., Lopes L.C., de Cassia Bergamaschi C. Vitamin D and respiratory infections. J. Infect. Dev. Ctries. 2015;9(4):355–361. doi: 10.3855/jidc.5711. [DOI] [PubMed] [Google Scholar]
- 14.Gibney K.B., MacGregor L., Leder K., Torresi J., Marshall C., Ebeling P.R., Biggs B.A. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin. Infect. Dis. 2008;46(3):443–446. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz J.L., Jones B.G., Penkert R.R., Gansebom S., Sun Y., Tang L., Bramley A.M., Jain S., McCullers J.A., Arnold S.R. Low retinol-binding protein and vitamin D levels are associated with severe outcomes in children hospitalized with lower respiratory tract infection and respiratory syncytial virus or human metapneumovirus detection. J. Pediatr. 2017;187:323–327. doi: 10.1016/j.jpeds.2017.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laaksi I., Ruohola J.P., Tuohimaa P., Auvinen A., Haataja R., Pihlajamaki H., Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 17.Roth D.E., Shah R., Black R.E., Baqui A.H. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99(3):389–393. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 18.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Jr., Stelmach I., Kumar G.T., Urashima M., Camargo C.A., Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo C.A., Jr., Ganmaa D., Frazier A.L., Kirchberg F.F., Stuart J.J., Kleinman K., Sumberzul N., Rich-Edwards J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561–e567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 20.Bergman P., Norlin A.C., Hansen S., Rekha R.S., Agerberth B., Bjorkhem-Bergman L., Ekstrom L., Lindh J.D., Andersson J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodall E.C., Granados A.C., Luinstra K., Pullenayegum E., Coleman B.L., Loeb M., Smieja M. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: a randomized controlled trial. BMC Infect. Dis. 2014;14:273. doi: 10.1186/1471-2334-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo C.A., Sluyter J., Stewart A.W., Khaw K.T., Lawes C.M.M., Toop L., Waayer D., Scragg R. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin. Infect. Dis. 2019;71(2):311–317. doi: 10.1093/cid/ciz801. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch D.R., Slow S., Chambers S.T., Jennings L.C., Stewart A.W., Priest P.C., Florkowski C.M., Livesey J.H., Camargo C.A., Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S., Giovannucci E., Mugusi F.M., Spiegelman D., Aboud S., Hertzmark E., Msamanga G.I., Hunter D., Fawzi W.W. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5(1):e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird E., Rhodes J., Kenny R.A. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir. Med. J. 2020;113(5):81. [PubMed] [Google Scholar]
- 29.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alipio M. 2020. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-19) SSRN. [Google Scholar]
- 32.Lau F., Majumher R., Torabi R., Saeg F., Hoffman R., Cirillo J.D., Greiffenstein P. Vitamin D insufficiency is prevalent in severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.04.24.20075838. [DOI] [Google Scholar]
- 33.Raharusun P., Priambada S., Budiarti C., Agung E., Budi C. Patterns of COVID-19 mortality and vitamin D: an Indonesian study. SSRN. 2020 (Available at SSRN 3585561. 2020 Apr 26.) [Google Scholar]
- 34.Zeger S.L., Liang K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 35.Jing Q.L., Liu M.J., Zhang Z.B., Fang L.Q., Yuan J., Zhang A.R., Dean N.E., Luo L., Ma M.M., Longini I., Kenah E., Lu Y., Ma Y., Jalali N., Yang Z.C., Yang Y. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect. Dis. 2020;20(10):1141–1150. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah K., Saxena D., Mavalankar D. Secondary attack rate of COVID-19 in household contacts: systematic review. QJM. 2020 doi: 10.1093/qjmed/hcaa232. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020;64(7) doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ventz S., Alexander B.M., Parmigiani G., Gelber R.D., Trippa L. Designing clinical trials that accept new arms: an example in metastatic breast Cancer. J. Clin. Oncol. 2017;35(27):3160–3168. doi: 10.1200/JCO.2016.70.1169. [DOI] [PubMed] [Google Scholar]
- 39.Latham N.K., Anderson C.S., Reid I.R. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J. Am. Geriatr. Soc. 2003;51(9):1219–1226. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 40.Leventis P., Kiely P.D. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand. J. Rheumatol. 2009;38(2):149–153. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 41.Sanders K.M., Stuart A.L., Williamson E.J., Simpson J.A., Kotowicz M.A., Young D., Nicholson G.C. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 42.Castillo M.E., Costa L.M.E., Barrios J.M.V., Diaz J.F.A., Miranda J.L., Bouillon R., Gomez J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203(105751) doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeBlanc E.S., Perrin N., Johnson J.D., Jr., Ballatore A., Hillier T. Over-the-counter and compounded vitamin D: is potency what we expect? JAMA Intern. Med. 2013;173(7):585–586. doi: 10.1001/jamainternmed.2013.3812. [DOI] [PubMed] [Google Scholar]
- 44.Manson J.E., Bassuk S.S. Commentary. Eliminating vitamin D deficiency during the COVID-19 pandemic: a call to action. Metabolism. 2020;112:154322. doi: 10.1016/j.metabol.2020.154322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about the trial.