Abstract
Purpose
To overcome negative adverse effects and improve therapeutic index of dexamethasone (Dex) in rheumatoid arthritis (RA), we developed a novel sustained release formulation-intra-articular injectable dexamethasone-loaded thermosensitive hydrogel (DLTH) with chitosan-glycerin-borax as carrier for the remission of inflammation and pain. The focus of this article is to explore both anti-inflammatory and pain-relieving effects of DLTH joint injection in bovine type-II collagen-induced arthritis (CIA) rats.
Methods
Wistar rats were randomized into three groups, including the normal group (n=6), the model group (n=6) and the DLTH group (n=10). Joint injection of DLTH (1mg/kg Dex per rat) was injected on day 12 in the DLTH group twice a week for three weeks. Clinical signs of body weight, paw swelling and arthritis scores, histologic analysis, hind paw mechanical withdrawal threshold (MWT), plantar pressure pain threshold (PPT) were taken into consideration. Serum contents of IL-17A, prostaglandin E2 (PGE2), prostacyclin 2 (PGI2) and prostaglandin D2 (PGD2), real-time polymerase chain reaction (PCR) analysis of inflammatory factors and pain-related mediators in synovium and dorsal root ganglia (DRG), Western blotting of NF-κB in synovium were all evaluated.
Results
Paw swelling, arthritis scores and joint inflammation destruction were all attenuated in the DLTH-treated group. Results showed that DLTH not only down-regulated serum IL-17A, but also mRNA levels of inflammatory factors and NGF, and key proteins contents of the NF-κB pathway in synovium. Increases of MWT and PPT in DLTH-treated rats elucidated pain-reducing effects of DLTH. Elevated serum PGD2 levels and declines of serum PGE2 and PGI2, and inflammatory and pain-related genes in DRGs in the DLTH group were also recorded.
Conclusion
These data elucidated that DLTH joint injection impeded synovial inflammation processes through down-regulating transcription activity of NF-κB pathway, and intra-articular DLTH may aid in the regulation of RA pain through regulating inflammation and pain conduction process.
Keywords: anti-inflammatory, dexamethasone-loaded thermosensitive hydrogel; DLTH, pain-relieving, rheumatoid arthritis; RA
Introduction
Rheumatoid arthritis (RA) is a most common chronic articular synovial inflammatory disease with global prevalence of nearly 1%.1,2 Associated with continuous inflamed synovium, RA is characterized by chronic synovitis, cartilage destruction and pain, ultimately resulting in permanent joint deformity and reduced life expectancy.3 Synovial inflammation is considered to be the major pathophysiology of RA, and RA-related pain has often been suggested as solely a consequence of joint inflammation.4 However, accumulating studies have revealed that pain presented in RA patients may be not only nociceptive or inflammatory, but also neuropathic. Some studies reported that neuropathic pain-like symptoms, such as burning pain, numbness and allodynia accompanied by arthritis pain, were found in RA patients.5 In view of this background, anti-inflammatory activity and effective pain relief will be our main focus of RA.
Mounting data have supported an indispensable part of glucocorticoid in the control of RA.6 With potent anti-inflammatory and immunosuppressive effects, dexamethasone (Dex), remains the first-line medication for RA therapy.7,8 Due to its rapid elimination, the high and frequent dosing of Dex in a rather long period of time often causes undesirable side effects, including hyperglycemia, osteoporosis, and insulin resistance.9 Thus, formulations that can overcome these negative adverse effects and improve therapeutic index of glucocorticoids in inflamed target sites are urgently needed. Here, we developed a novel sustained release formulation-intra-articular injectable dexamethasone-loaded thermosensitive hydrogel (DLTH) with chitosan-glycerin-borax as carrier. As an excellent local drug delivery system, the in-situ chitosan-glycerin-borax hydrogel is syringeable solution of minimally invasion but becomes semisolid gels after administration at local sites in response to changes of temperature.10 Via direct injection into the affected joints, DLTH can not only achieve drug enrichment at target morbid joints and minimize systemic toxicity, but also prolong release duration time and reduce burst drug release, leading to improved Dex therapeutic index and less risks of adverse effects.11
Collagen-induced arthritis (CIA), is one of the most widely adopted animal models that shares similar clinical and histopathologic characteristics as that of human RA, such as swelling, pain, synovial hyperplasia, deformity in affected joints.4 Hence, it is an ideal model to illustrate the anti-inflammatory and pain-relieving effects of DLTH joint injection in studies of RA inflammation and RA-related pain research, and we aimed to explore the underlying mechanisms of DLTH in anti-inflammatory activity and effective pain relief.
Materials and Methods
Animals
In vivo animal experiments were performed on male Wistar rats (n = six to ten per group, aged seven to eight weeks) and were also in accordance with both the Guide for the Care and Use of Laboratory Animal (1996) and the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines.12,13 Ethical regulations were met in all studies by the ethical committee of animal experiments in Shanghai Ninth People’s Hospital (ID HKDL [2017] 207). Adult rats were housed on a 12-hour light/dark cycle with ad libitum food and water (LAD 0100; Trophy Feed Technology, NanTong, China). Rats were randomized into three groups, including the normal group (n=6), the model group (n=6) and the DLTH group (n=10).
Induction of CIA Rat
A mixture of 2mg/mL bovine type-II collagen (Chondrex, Redmond, WA) emulsified equally with incomplete Freund’s adjuvant (Chondrex) was administered intra-dermally around the base of each rat tail on day −7, followed by boosting injections in the same route with a week interval on day 0. Every rat received 0.1 mL mixture, which contained 100μg bovine type-II collagen.14,15
Intra-Articular DLTH Injection
To yield chitosan-glycerin-borax as carrier, chitosan was dissolved in 1% acetic acid for preparation of a 2% solution. Certain amount of glycerin was added into the mixture and the ratio of glycerin and chitosan by weight was 3:10. To make sure the pH of the solution at 7, borax worked as a pH adjuster. The concentration of Dex was 2.5 mg/mL. The gel was a flowable liquid at room temperature but a homogeneous semi-solid hydrogel at 37 °C (Figure 1A).16
Figure 1.
Therapeutic effects of DLTH against CIA rat. (A) The morphology of DLTH in soluble state at 25 °C and gel state at 37 °C. The gel was a flowable liquid at room temperature but a homogeneous semi-solid hydrogel at 37 °C. The photographs were taken on day 12. (B, C) Injections of DLTH in both knees of CIA rats. Forty-microliter joint injection of DLTH was injected on day 12 into each knee joints of the DLTH rat group twice a week for three weeks and each time every rat received 80-μL DLTH in total (1mg/kg Dex per rat). At 33 days after the second immunization, all rats were sacrificed by cervical dislocation. The photographs were taken on day 12. (D–F) Swelling degrees of hind paws in three groups of rats. Figures D, E and F were representative images of rats in the normal group, model group and DLTH group, respectively. The photographs were taken on day 33. (G–I) Paw volumes, arthritis scores and body weights of rats in all groups. The paw volumes and arthritis scores, indicating the intensity of arthritis, were determined by two independent blinded observers from the day of the second booster. Red arrows indicate the day of the first DLTH administration. Values are represented as the mean±S.E.M. (n= six to ten rats per group) and one-way analysis of variance (ANOVA) with Turkey’s test were adopted. All analyses were performed from day 0 to day 33. The significance between normal group and model group was presented as *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. The significance between model group and DLTH group was represented as #P<0.05 and ####P<0.0001.
Abbreviations: CIA, collagen-induced arthritis; DLTH, dexamethasone-loaded thermosensitive hydrogel.
Here, 40-μL joint injection of DLTH was injected on day 12 into each knee joints of the DLTH rat group twice a week for three weeks and each time every rat received 80-μL DLTH in total (1mg/kg Dex per rat) (Figure 1B and C). An equal volume of saline control was given to rats in both normal and model groups in the same way.
Behavior Assessments of Arthritis
The severities of arthritis in CIA rat were monitored by visual observation in weight-loss and swollenness of fore and hind paws. The clinical parameters of CIA rats were evaluated with articular index (AI) ranging from 0 to 4 for each paw, scored on a 0–16 scale by two observers blinded for the treatment regimen. AI scores were documented twice a week where 0 = normal, 1 = little swelling and/or redness, 2 = low to moderate edematous swelling, 3 = prominent edema with limited joint activity, and 4 = excess edema with spastic joints.17
Mechanical Withdrawal Threshold (MWT)
Von Frey sensitivity was evaluated after behavior assessments while the rat was in a mesh platform to adapt to the environment before started. A force of continuously elevating intensity was compressed in the hind paw with a serial of calibrated von Frey filaments (Stoelting, Wood Dale, IL) until a withdrawal reflex response appeared. Observers were unaware of animal grouping and average values of three repeated trials were adopted to calculate a 50% MWT.18
Pressure Pain Threshold (PPT) Measurement
PPTs were assessed by applying measured forces on the medial hind paw through a digital force gauge (ZSDichuang, YLS-3E), with a range of 0 to 2000g and a resolution of 0.1g. A round tip of the pressure algometer was applied in the inflamed hind paw with a digital recording of the increasing pressure.19 The corresponding pressure (in g) was recorded whenever there was a hind paw flection reflex or vocalization. Three measurements were carried out on the same location at intervals of more than ten minutes, and the average was calculated as the PPT and adopted for statistical analysis.
Sample Preparation
At 33 days after the second immunization, all rats were sacrificed by cervical dislocation. The right hind knee joint of each rat was carefully dissected and postfixed in 4% paraformaldehyde for over 48 hours. Decalcified with 0.5 M EDTA and dehydrated with graded ethanol, each specimen was paraffin embedded.19 Coronally sectioned at 4-μm thickness, slices were stained with H&E, photographed using a cool CCD camera connected to a Zeiss Axioskop 2 plus microscope (Carl Zeiss Microimaging) and evaluated by two blinded investigators. The L4-L6 dorsal root ganglias (DRGs) and synovial tissue in left joints of all the rats were excised and instantly frozen at −80°C for subsequent experiments. Peripheral blood was sampled from abdominal aorta and centrifuged at 3500 rpm for ten minutes at room temperature for serum. Serum samples were preserved at −80°C till assayed.
Histological Analysis of Synovial Joint
For objective assessments of pathologic changes in the hind legs, evaluations were made by two blinded observers for hyperplasia, pannus formation and joint erosion using a widely acknowledged modified semiquantitative scoring system. Hyperplasia was scored on a scale from 0 to 3 where 0 indicates normal cell structure without synovial lining cell thickness and 3 suggests excess synovial hyperplasia. Pannus formation was scored on a 0–3-point scale where 0 suggests no pannus formation and 3 indicates severe pannus formations. Joint erosion was scored on a scale from 0 to 3 where 0 shows no evidence of joint erosion and 3 displayed severe joint erosion in the observed sections.14,20
ELISA Detection of Inflammation Mediators in Serum
Serum contents of interleukin (IL)-17A (Multisciences, Hangzhou, China), prostaglandin E2 (PGE2) (Blue gene, Shanghai, China), prostacyclin 2 (PGI2) (Elabscience, Wuhan, China), prostaglandin D2 (PGD2) (Elabscience, Wuhan, China) in rats were measured by commercial ELISA kits in accordance with manufacturers’ protocols.21 The optical density (OD) values were determined by a microplate reader (Infinite M200 PRO NanoQuant; Tecan, Männedorf, Switzerland). All assays were carried out in duplicate and serum concentrations were generated by reference to standard curves. The highest detection concentration was 200 pg/mL for IL-17A, 25,000 pg/mL for PGE2, 2000 pg/mL for PGI2, and 1000 pg/mL for PGD2.
RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
DRGs and synovial tissues were both collected, minced and homogenized separately in 1mL of TRIzol Reagent (Ambion, Shanghai, China). A total amount of 1μg total RNA were reversely transcripted for first-strand cDNA with cDNA reverse transcription kit (Takara Bio, Tokyo, Japan) following the standard instructions. With a 7500 Real-Time PCR System (Thermo Fisher Scientific), SYBR Green RT-qPCR was performed using specific primers for analysis of IL-1β, IL-6, IL-17, tumor necrosis factor-(TNF-)α, nerve growth factor (NGF), transient receptor potential vanilloid 1 (TRPV1), voltage-gated sodium (Nav)1.3, Nav1.7, brain-derived neurotrophic factor (BDNF), Neuropeptide Y (NPY) with β-actin as a quantitative control (Table 1). Quantifications of the above gene relative expressions were calculated with the 2−ΔΔCT method and all assays were independently repeated more than three times in different groups.22
Table 1.
Sequences of Specific Primers Used in RT-qPCR
| Primers | Sequences | |
|---|---|---|
| Forward | Reverse | |
| β-actin | 5ʹ-ACGGTCAGGTCATCACTATCG-3’ | 5ʹ-GGCATAGAGGTCTTTACGGATG-3’ |
| IL-1β | 5ʹ-AGCAGCTTTCGACAGTGAGG-3’ | 5ʹ-CTCCACGGGCAAGACATAGG-3’ |
| IL-6 | 5ʹ-CTCTCCGCAAGAGACTTCCAG-3’ | 5ʹ-TTCTGACAGTGCATCATCGCT-3’ |
| IL-17 | 5ʹ-CCATCCATGTGCCTGATGCT-3’ | 5ʹ-GTTATTGGCCTCGGCGTTTG-3’ |
| TNF-α | 5ʹ-ATGGGCTCCCTCTCATCAGT-3’ | 5ʹ-GCTTGGTGGTTTGCTACGAC-3’ |
| NGF | 5ʹ-CGCATCGCTCTCCTTCACAGA-3’ | 5ʹ-TACGCCGATCAAAAACGCTG-3’ |
| TRPV1 | 5ʹ-TTCACCGAATGGGCCTATGG-3’ | 5ʹ-TGACGGTTAGGGGTCTCACT-3’ |
| Nav 1.3 | 5ʹ-CTCAGAACAGGAAGCGGAGG-3’ | 5ʹ-GAATTGCCTGATGGAGAGCCT-3’ |
| Nav 1.7 | 5ʹ-ATGCTCTACTCTGCGGCTTC-3’ | 5ʹ-GCACGCAGAGTCTGTTGGTA-3’ |
| BDNF | 5ʹ-GTCGCACGGTCCCCATTG-3’ | 5ʹ-ACCTGGTGGAACTCAGGGT-3’ |
| NPY | 5ʹ-GTGTTTGGGCATTCTGGCTG-3’ | 5ʹ-TTCAAGCCTTGTTCTGGGGG-3’ |
Abbreviations: BDNF, brain-derived neurotrophic factor; IL-1β, interleukin-1β; IL-6, interleukin- 6; IL-17, interleukin-17; Nav1.3, voltage-gated sodium-1.3; Nav1.7, voltage-gated sodium-1.7; NGF, nerve growth factor; NPY, neuropeptide Y; TNF-α, tumor necrosis factor-α; TRPV1, transient receptor potential vanilloid 1.
Western Blotting Assay
Proteins were extracted for Western blot analysis from rat synovial tissues. Frozen synovium tissues were weighed and then grounded with eye scissors. Minced synovium was added with radio immunoprecipitation assay lysis buffer (Beyotime, Shanghai, China) along with halt protease and phosphatase inhibitor cocktail (100Ⅹ; Thermo Fisher Scientific). The mixture was put still on ice for 30 minutes and centrifugated at 14,000 rcf for 20 minutes. To determine total protein concentration, collected supernatant were measured with the Bradford assay (Bio-Rad). After adjustments of protein concentration, obtained protein samples (50 μg per well) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 100V and then transferred onto polyvinylidene difluoride membranes. Membranes were blotted with primary antibodies specific for NF-κB p65, inhibitor of NF-κB α (IκBα), IκB kinase (IKK) α/β, GAPDH, respectively (1:1000, Table 2) at 4°C for 16 hours followed by incubation of the corresponding secondary antibodies (1:10,000; both from Cell Signaling Technology) for one hour at room temperature. Developed with enhanced chemiluminescence, signals of target protein were quantified by Image-Pro Plus 6.0 software. Relative signal protein expressions were normalized to internal control of GAPDH. All assays were repeated three times.23
Table 2.
The Primary Antibodies Used in Western Blot Analysis
| Gene | Company | MW, kDa |
|---|---|---|
| NF-κB p65 | Cell Signaling Technology | 65 |
| Inhibitor of NF-κB α (IκBα) | Cell Signaling Technology | 39 |
| IKKα | Cell Signaling Technology | 85 |
| IKKβ | Cell Signaling Technology | 87 |
| GAPDH | Cell Signaling Technology | 37 |
Abbreviations: IκBα, inhibitor of NF-κB α; IKK α/β, IκB kinase α/β.
Statistical Analysis
All statistical analyses were performed with SPSS20.0 software and values were presented as mean±SEM. Statistical differences of multifactorial comparisons were analyzed via one-way analysis of variance (ANOVA) with Turkey’s test and comparisons between two groups were analyzed by Students’ t-test. The assumption of normality and the homogeneity of variance were also tested. Differences between each group were tested with post hoc multiple comparisons. If no heterogeneity was observed, the Bonferroni test was adopted to evaluate the differences between groups. If heterogeneity existed, the Welch test would be used to assess the equality of means and Dunnett’s T3 test to test the differences. A probability value of P<0.05 was taken as statistically significant.
Results
Anti-Arthritic Effects of DLTH Against CIA Rat
Rats in the DLTH group received intra-articular DLTH since day 12 twice a week whereas those in the normal group were administered equal amounts of normal saline. Representative images of swelling hind paws in three groups were displayed in Figure 1D–F. Clinical signs of body weight, paw swelling and arthritis scores were taken into consideration. CIA rats underwent increased paw swelling, erythema and spastic joints on day 12 as illustrated by paw volume and arthritis scores. However, compared with the model group, evident trends of paw edema and swollen shrinkage were observed in the DLTH group from day 12 onwards. Hence, treatment with DLTH displayed a strong and long-lasting therapeutic effect against CIA. See Figure 1G–I.
Intra-Articular DLTH Suppresses Systematic Inflammatory Responses of CIA Rats
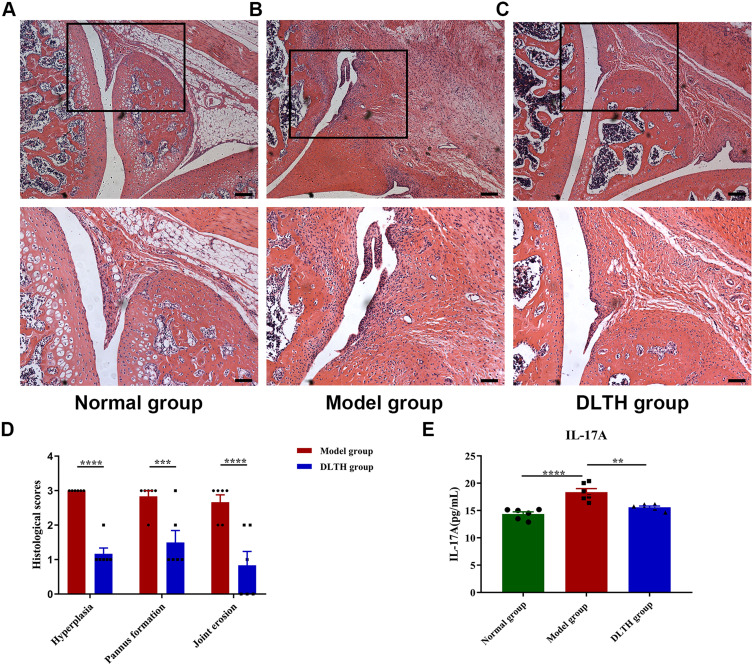
Consistent with their clinical behaviors, histological observations of arthritis, in terms of synovial hyperplasia, pannus formation and joint erosion, were more pronounced in the model group. Compared with the normal group, evidence of local synovial hyperplasia, pannus formation and joint erosion were presented in the model group, whereas these features were clearly ameliorated in the DLTH group. Results of semiquantitative histology scores also indicated that synovial hyperplasia, pannus formation and joint erosion were much severe in the model group while rats in the DLTH group exhibited mild inflammatory changes. There was no evidence of inflammatory activity or joint erosion in the normal group (Figure 2A–D, Figure S2).
Figure 2.
Intra-articular DLTH suppresses systematic inflammatory responses of CIA rats. (A–D) Histological analyses of synovial joints in rats. Representative photomicrographs of knee joint sections stained with hematoxylin and eosin (H&E), quantifications of hyperplasia, pannus formation and joint erosion using semiquantitative histology scoring system were shown. The black box represents the area of magnification in the corresponding figure below. Scale bar, 200μm (upper panel) and 100μm (lower panel). At 33 days after the second immunization, all rats were sacrificed by cervical dislocation. Slices of hind legs were stained with H&E and photographs were taken for objective assessments of pathologic changes. Students’ t-test was used in comparisons between two groups. (E) Serum profile of IL-17A in rats. Rats administered intra-articular DLTH in vivo contained less amounts of IL-17A in serum than those of untreated CIA rats. Values are represented as the mean±S.E.M. Serum IL-17A analysis was performed after the rats were sacrificed. One-way analysis of variance (ANOVA) with Turkey’s test were adopted. The significance between two groups indicated was presented as **P<0.01, ***P<0.001 and ****P<0.0001.
Abbreviations: CIA, collagen-induced arthritis; DLTH, dexamethasone-loaded thermosensitive hydrogel; IL-17, interleukin-17.
Evaluations of serum levels of IL-17A in the normal group, model group and the DLTH group were 14.36±0.87pg/mL, 18.35±1.45pg/mL, 15.59±0.51pg/mL, respectively, which indicated again DLTH has a potent anti-inflammatory effect (Figure 2E). As shown in Figure 2, ANOVA suggested remarkable statistical differences were found in the three groups. Comparisons between groups demonstrated that serum contents of IL-17A were notably higher in the model group than those either in the normal group or in the DLTH group. Enhanced levels of serum IL-17A were inhibited by DLTH treatment in CIA rat and no statistical difference was observed in IL-17A level in the normal group when compared with the DLTH group. These results demonstrated that intra-articular DLTH attenuated systematic inflammatory responses of CIA rats.
Inhibitory Effects of Intra-Articular DLTH in the Expressions of IL-1β, IL-6, IL-17, TNF-α, NGF in Synovial Tissues
RT-qPCR in synovial tissues was conducted to assess the expressions of IL-1β, IL-6, IL-17, TNF-α, as well as NGF. As shown in Figure 3, compared with those in the normal group, mRNA expressions of inflammatory factors and NGF in synovium were noticeably up-regulated in the model group. On the contrary, in comparison with the model group, expressions of IL-1β, IL-6, IL-17, TNF-α, NGF were apparently down-regulated by DLTH treatment. Therefore, treatment with intra-articular DLTH exhibited remarkable anti-inflammatory effects in mRNA levels of synovial tissues.
Figure 3.
Effects of intra-articular DLTH on mRNA levels of pro-inflammatory mediators and NGF in synovium. All data were presented as a value relative to those in the normal group. Intra-articular DLTH dampens productions of IL-1β, IL-6, IL-17, TNF-α, NGF in synovial tissues. Values are represented as the mean±S.E.M. All analyses were performed after the rats were sacrificed. One-way analysis of variance (ANOVA) were performed. The significance between two groups indicated was presented as **P<0.01, ***P<0.001 and ****P<0.0001.
Abbreviations: DLTH, dexamethasone-loaded thermosensitive hydrogel; IL-1β, interleukin-1β; IL-6, interleukin- 6; IL-17, interleukin-17; NGF, nerve growth factor; TNF-α, tumor necrosis factor-α.
Intra-Articular DLTH Exerts Anti-Inflammatory Effects Through Regulation of NF-κB Pathway
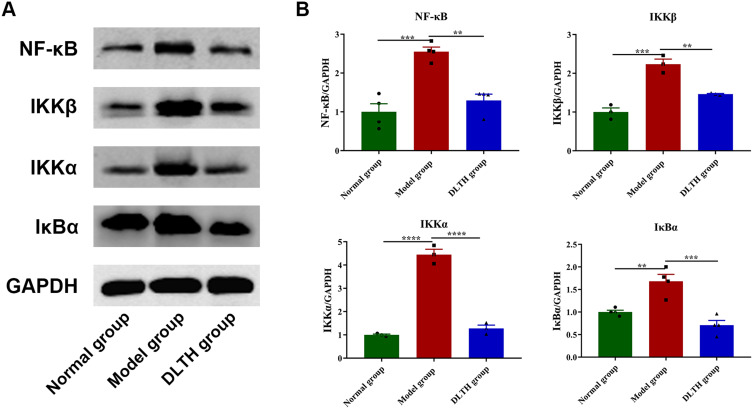
Western blotting was then adopted to evaluate contents of key proteins in the NF-κB pathway in synovium. As expected, relative protein expressions of NF-κB p65, IκBα, IKKα, IKKβ in the model group, were increased by different degrees in comparison with the normal group, whereas those in the DLTH group were remarkably down-regulated compared with the model group, which illustrated intra-articular DLTH ameliorated inflammation by inhibition of NF-κB p65, IκBα, IKKα, IKKβ upregulation. Detailed data are displayed in Figure 4.
Figure 4.
Treatments with intra-articular DLTH inhibit NF-κB pathway in synovial tissues of CIA rats, detected by Western blot assay. (A) Grey values of NF-κB p65, IκBα, IKKα, IKKβ bands. (B) A graphic figure of protein expressions in NF-κB pathway. Relative expression of each protein in the normal group was defined as 1. Values are represented as the mean±S.E.M. All analyses were performed after the rats were sacrificed. One-way analysis of variance (ANOVA) were performed. The significance between two groups indicated was presented as **P<0.01, ***P<0.001 and ****P<0.0001.
Abbreviations: CIA, collagen-induced arthritis; DLTH, dexamethasone-loaded thermosensitive hydrogel; IκBα, inhibitor of NF-κB α; IKK α/β, IκB kinase α/β.
Pain-Relieving Effects of Intra-Articular DLTH in CIA Rats Detected by MWT and Plantar PPT
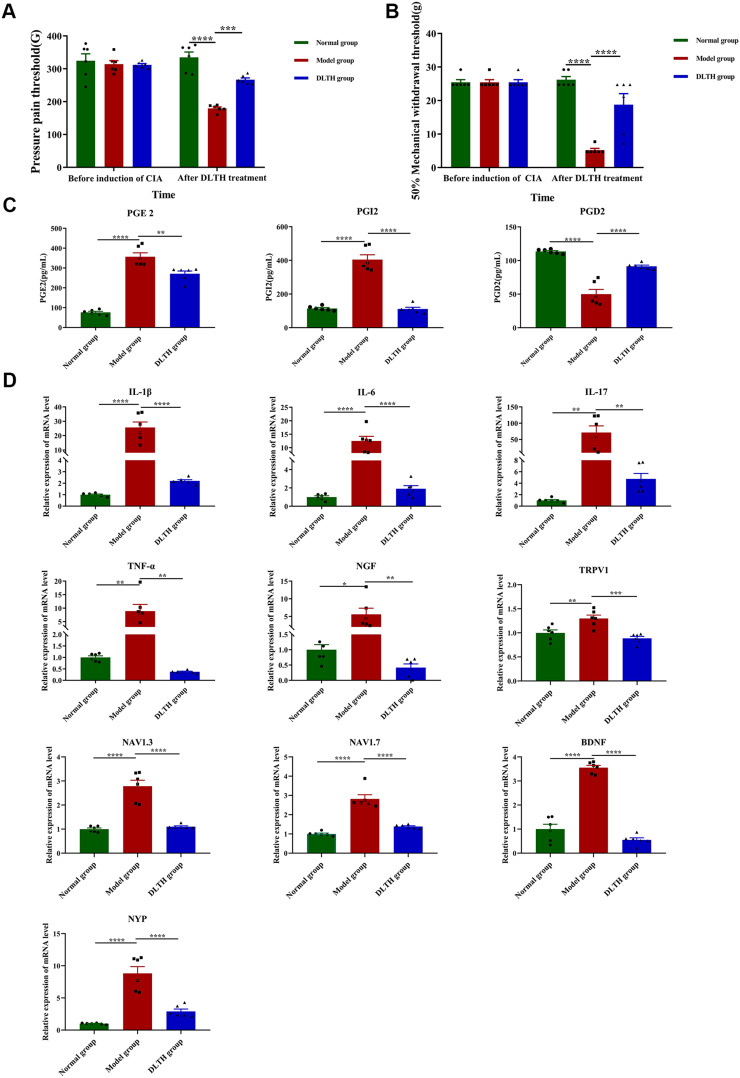
To explore the in vivo pain-relieving effects of DLTH, MWT and plantar PPT were performed in all groups of rats. Systemic application of the three groups had no significance on MWT and PPT before treatments in the three groups; whereas after successful induction of CIA, mean MWT and plantar PPT in the model group were much less than those measured in the normal group with marked differences between groups indicated. However, ANOVA tests showed that in comparison with the arthritis-induced group, MWT and PPT prominently recovered in the DLTH group after 21 days of DLTH joint injection (Figure 5A and B). Therefore, we draw the conclusion that reduced MWTs and PPTs were exhibited in CIA but DLTH was able to relieve pain to a certain extent.
Figure 5.
Pain-relieving effects of intra-articular DLTH in CIA rats. (A, B) MWT and PPT of rats in the three groups before induction of CIA and after treatment of DLTH. MWT and PPT measurements were performed before the first immunization and at 33 days after the second immunization (day 33) before cervical dislocation. One-way analysis of variance (ANOVA) were performed. (C) Serum profiles of PGE2, PGI2 and PGD2 in all groups. Compared with those in the normal group, levels of serum PGE2 and PGI2 were much higher while serum PGD2 saw a significant decrease in the model group. Rats receiving DLTH in vivo contained less amounts of PGE2 and PGI2 and more PGD2 in serum than those of untreated CIA rats. One-way ANOVA were performed. (D) Pain-relieving effects of intra-articular DLTH on mRNA levels of pro-inflammatory and pain-related mediators in DRGs. All data were presented as a value relative to those in the normal group. DLTH compromised the productions of IL-1β, IL-6, IL-17, TNF-α, NGF, TRPV1, Nav1.3, Nav1.7, BDNF, NPY in DRGs. Values are represented as the mean±S.E.M. All analyses were performed after the rats were sacrificed. One-way ANOVA were performed. The significance between two groups indicated was presented as *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Abbreviations: BDNF, brain-derived neurotrophic factor; CIA, collagen-induced arthritis; DLTH, dexamethasone-loaded thermosensitive hydrogel; IL-1β, interleukin-1β; IL-6, interleukin- 6; IL-17, interleukin-17; MWT, mechanical withdrawal threshold; Nav1.3, voltage-gated sodium-1.3; Nav1.7, voltage-gated sodium-1.7; NGF, nerve growth factor; NPY, neuropeptide; PGE2, prostaglandin E2; PGI2, prostacyclin 2; PGD2, prostaglandin D2; PPT, pressure pain threshold; Y; TNF-α, tumor necrosis factor-α; TRPV1, transient receptor potential vanilloid 1.
Suppressive Effects of Intra-Articular DLTH in Serum Inflammatory Mediators and Pain-Related Gene Expressions in DRGs
Commercial ELISA kits were adopted to determined serum profile of PGE2, PGI2 and PGD2 in three group of rats. The standard curves for PGE2, PGI2 and PGD2 were measured with R2 is 0.9965, 0.9857 and 0.9498, respectively (Figure S1). The concentrations of PGE2 in the normal group, model group, DLTH group were 76.06±11.53 pg/mL, 356.81±43.87 pg/mL, 270.83±31.44 pg/mL, respectively. Serum levels of PGI2 in the normal group, model group, DLTH group were 114.48±11.86 pg/mL,404.95±63.47 pg/mL, 111.26±22.53 pg/mL, respectively and PGD2 contents in the serum in the normal group, model group, DLTH group were 113.51±2.91pg/mL, 50.07±15.74 pg/mL, 91.48±3.79 pg/mL, respectively. Intra-articular DLTH therapy diminished the production of serum PGE2, PGI2 and increased PGD2 level, which confirmed that treatment with DLTH can mitigate arthritis pain. See Figure 5C.
Pro-inflammatory cytokines such as IL-1β, IL-6, IL-17, TNF-α at mRNA levels in dissected DRGs were analyzed, either. As shown in Figure 5D, relative expression values of IL-1β, IL-6, IL-17, TNF-α were distinctly elevated in DRGs of CIA rats, but DLTH could enormously decrease the secretions of these inflammatory cytokines. In addition, NGF, TRPV1, Nav1.3, Nav1.7, BDNF and NPY, pain-related key factors, were also tested. Noticeable upregulations of NGF, TRPV1, Nav1.3, Nav1.7, BDNF, NPY were observed in the model group and treatment of DLTH suppressed the expression levels of above pain-related mediators. These data demonstrated that DLTH joint injection alleviated pain behavior by depressing IL-1β, IL-6, IL-17, TNF-α in DRGs of CIA rats, as well as NGF, TRPV1, Nav1.3, Nav1.7, BDNF, NPY expressions.
Discussion
RA is a chronic autoimmune arthritis disease with manifestations of inflammation, synovial hyperplasia and bone destruction. Among them, synovial hyperplasia, eventually resulting in synovitis and pannus, is the most destructive prevalent pathogenesis in RA.24 Considering the risks of adverse effects, we are able to increase efficacy of Dex at a given dose and reduce its systemic side effects by twice a week joint injection of DLTH. As expected, with utilization of DLTH joint injection, declines of paw volumes, arthritis scores, serum contents of IL-17A, and histological observation of decreased hyperplasia of synovial lining, alleviated pannus formation and joint erosion in CIA rats were recorded, elucidating that synovitis and systemic inflammation could be blunt by the long-lasting effects of DLTH. Our study presented that DLTH can not only achieve Dex enrichment at target inflamed joints but prolong release duration and reduce burst drug release, leading to increased efficacy.
Mechanisms of intra-articular DLTH in preventing inflammation exacerbation were then explored at mRNA levels and protein expressions. The consequent inflammation in synovium and pannus formation are major manifestations of RA, thereby, synovium, a key effector tissue, is our study target.25 In mRNA levels, gene expressions of IL-1β, IL-6, IL-17, TNF-α, NGF, were all increased in synovium of CIA rats but all mitigated with intra-articular DLTH treatment. Studies reported that IL-17 enhanced production of IL-1 and TNF-α, and stimulated synovial cells to secret IL-6, IL-8, PGE2. It is, therefore, an upstream inflammatory mediator in the etiological origin of RA.26,27 Stimulated synovium, consequently, becomes a main source of pro-inflammatory mediators, like IL-1, IL-6 TNF-α, PGE2, and these various inflammatory factors in turn account for synovial inflammation and bone destruction.25 The vicious pro-inflammatory loop, involving IL-1, TNF, and IL-6, will in the end contribute to pathogenic RA.28 In accordance with our study, inflammatory gene expression increments in the model group and reduction of above genes in the DLTH group revealed that treatment with intra-articular DLTH exhibited remarkable anti-inflammatory effects in mRNA levels of synovial tissues.
It is generally acknowledged that action mechanism of glucocorticoids is mostly built on repression of some pro-inflammatory genes. In RA, NF-κB, a pleiotropic transcriptional mediator, plays a regulatory role in multiple pro-inflammatory cytokines and thereby is capable of accelerating synovial hyperplasia.29 Herein, we are attracted to the regulatory role of DLTH on NF-κB signaling pathway owing to its significance in inflammatory process. Up-regulations of NF-κB target protein contents (eg.NF-κB, IκBα, IKKα, IKKβ) in the model group and down-regulations of the above protein expressions in the DLTH group were both noted in Western blot assay. Mediated by the separation of IKKβ from IKKα, complexes of IκBα and NF-κB will break down under some cellar stimuli. Once NF-κB has been activated and transferred into nucleus, the production of inflammatory factors, such as TNF-α, IL-1β, will be promoted, resulting in deterioration of arthritis.30 Consistently, NF-κB was activated in the model group whereas DLTH joint injection in CIA rats could impede synovial inflammation processes through reversely down-regulating transcription activity of NF-κB pathway.
Multiple lines of evidences revealed that RA pain originates from inflammatory joints and pain conduction processes with multiple regulatory mechanisms.31 Treatment with intra-articular DLTH may be a therapeutic approach for managements of RA pain. MWT and plantar PPT are the common indicators that reflect pain sensitivity to pressure stimulation. In our study, CIA rats displayed reduced MWT and PPT, but after DLTH joint injection treatment, MWT and PPT values of CIA rats were prominently elevated, illustrating that CIA rats exhibited pain behavior and treatment with intra-articular DLTH were able to relieve pain to a certain extent. Furthermore, we explored the potential regulatory inflammatory mediator underlying RA pain. PGE2, as a well-known prostanoid modulator of inflammatory pain, servers as an algogenic substance in the whole progression of RA pain.32 Other than PGE2, PGI2 and PGD2 are also involved in the inflammatory process of RA pain. Sugita et al evaluated the involvement of PGE2 and PGI2 in a rat inflammatory pain model and found that both PGE2 and PGI2 were adequate to induce inflammatory hyperalgesia and concurrent inhibition of PGE2 and PGI2 could relieve inflammatory pain at a level comparable to cyclooxygenase-2 inhibitor.32 It’s believed that PGD2 exerts an anti-inflammatory effect in arthritis. Elevated PGD2 is considered to trigger anti-inflammatory cascade as it is spontaneously converted into 15-deoxy-Δ12, 14-prostaglandinJ2 (15d-PGJ2) which has been proven to be to anti-inflammatory by inhibition of NF-κB pathway.33,34 Minami et al reported that PGD2 suppressed PGE2-induced allodynia, demonstrating an antinociceptive role of PGD2.35 In line with our study, we observed the remarkable increases of serum PGE2 and PGI2 and an obvious decrease of serum PGD2 in the model group, and there were noticeable decreases of PGE2 and PGI2 and a prominent increase of PGD2 in the DLTH group, confirming that intra-articular DLTH therapy does ease arthritis pain at some levels.
Meanwhile, in RA, neuropathic pain is often accompanied and emerging evidences support a pivotal role of inflammation in the occurrence and maintenance of neuropathic pain. DRG is a place predominately innervating pain conduction and perceiving potential noxious stimulation in the hind limbs.36 Sensory neurons accumulate in the DRGs, and sensitization of DRG is proven to be one mechanism that underlies RA pain influenced by interaction of inflammatory cells with DRG neurons.37 Hence, DRG is our targeted tissue to study RA neuropathic pain.
Pro-inflammatory mediators such as IL-1β, TNF-α, and IL-6 were regarded as contributors of the development of RA neuropathic pain.38 Yu et al found that pain-mediated cytokines, such as TNF-α, IL-1β and IL-6, were associated with neuropathic pain in the DRGs.23 Moreover, TNF‑α is able to enhance the synthesis of PGE2 and the elevated levels of PGE2 and TNF‑α concurred with other inflammatory mediators in sensitization of sensory neurons and pain responses.23 NGF, implicated as a crucial role in acute and persistent inflammatory pain recently, is expressed in inflamed synovial tissue and may contribute to nociception and pro-algesic effects.39 Hefti et al observed reduced pain sensation by inhibition of NGF in rodent pain models and Djouhri et al also verified that inhibition of NGF or its receptors led to attenuate chronic inflammatory pain.40,41 Nevertheless, NGF alone is barely an inflammogen but working synergistically with other inflammatory factors, like TNF-α, IL-1β, it will make a difference in chronic neuropathic pain.42 Not only NGF, but also BDNF, play a key regulatory part of pain in the periphery.43 Ion channel genes such as TRPV1, Nav1.3 and Nav1.7, are also investigated in RA pain. Synthesized in DRGs, TRPV1 is linked to inflammatory pain and peripheral neuropathy. Release of NGF would enhance TRPV1-induced nociception and IL-1β could also mediate pain hypersensitivity by activation of TRPV1.44 Studies discovered that Nav 1.3 and Nav1.7 are promising targets in neuropathic pain treatments since up-regulation of Nav1.3 and Nav1.7 were implicit in expression of neuropathic pain.45 In consistent with our findings, elevated mRNA expressions of TNF-α, IL-1β, IL-6, IL-17, NGF, TRPV1, Nav1.3, Nav1.7, BDNF, NPY were noted in CIA DRGs and CIA rats receiving intra-articular DLTH exhibited lower mRNA expressions in comparison with the model group. This observation means that DLTH joint injection is capable of reliving pain through regulating inflammation and pain conduction process.
Conclusion
In conclusion, the data presented in this study illustrated that intra-articular injection of DLTH had anti-inflammatory and pain-relieving effects in collagen-induced arthritis. The involvement of NF-κB pathway accounts for the anti-inflammatory activity of intra-articular DLTH, and modulatory ability of inflammation and pain conduction process are responsible for the pain-relieving effects of DLTH. This newly developed DLTH might serve as a new modified formulation for anti-inflammatory activity and effective pain relief in the treatments of RA in the near future.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grants numbers 81874011, 81572104 and 81301531 (to T.-Y. W)] and the Shanghai Municipal Science and Technology Commission [Innovation Grant numbers 18140903502 (to T.-Y. W.)].
Disclosure
All the authors have no conflicts of interest to disclose for this work.
References
- 1.Song D, DuBois DC, Almon RR, Jusko WJ. Modeling sex differences in anti-inflammatory effects of dexamethasone in arthritic rats. Pharm Res. 2018;35(11):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Xu B, Fan K, Wu J, Wang T. Inflammation suppression by dexamethasone via inhibition of CD147-mediated NF-κB pathway in collagen-induced arthritis rats. Mol Cell Biochem. 2020. doi: 10.1007/s11010-020-03808-5 [DOI] [PubMed] [Google Scholar]
- 3.Shen B, Sun Q, Chen H, et al. Effects of moxibustion on pain behaviors in patients with rheumatoid arthritis: a meta-analysis. Medicine. 2019;98(30):e16413. doi: 10.1097/MD.0000000000016413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bas DB, Su J, Wigerblad G, Svensson CI. Pain in rheumatoid arthritis: models and mechanisms. Pain Manag. 2016;6(3):265–284. doi: 10.2217/pmt.16.4 [DOI] [PubMed] [Google Scholar]
- 5.Noda K, Tajima M, Oto Y, et al. How do neuropathic pain-like symptoms affect health-related quality of life among patients with rheumatoid arthritis?: a comparison of multiple pain-related parameters. Modern Rheumatol. 2019;1–7. [DOI] [PubMed] [Google Scholar]
- 6.van der Goes MC, Jacobs JW, Bijlsma JW. Rediscovering the therapeutic use of glucocorticoids in rheumatoid arthritis. Curr Opin Rheumatol. 2016;28(3):289–296. doi: 10.1097/BOR.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 7.Scherholz ML, Schlesinger N, Androulakis IP. Chronopharmacology of glucocorticoids. Adv Drug Deliv Rev. 2019;151–152:245–261. doi: 10.1016/j.addr.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenen M, Culemann S, Vettorazzi S, et al. Glucocorticoid receptor in stromal cells is essential for glucocorticoid-mediated suppression of inflammation in arthritis. Ann Rheum Dis. 2018;77(11):1610–1618. doi: 10.1136/annrheumdis-2017-212762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorscheider M, Tsapis N, Ur-Rehman M, et al. Dexamethasone palmitate nanoparticles: an efficient treatment for rheumatoid arthritis. J Control Release. 2019;296:179–189. doi: 10.1016/j.jconrel.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Qin X, Yang R, et al. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. J Pharm Sci. 2016;105(1):122–130. doi: 10.1016/j.xphs.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Wang K, Wang H, et al. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy. Colloids Surf B Biointerfaces. 2017;149:97–104. doi: 10.1016/j.colsurfb.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Resources IoLA. Guide for the Care and Used of Laboratory Animals. National Academies Press; 1996. [PubMed] [Google Scholar]
- 13.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Zhao FT, Fan KJ, et al. Dihydromyricetin inhibits inflammation of fibroblast-like synoviocytes through regulation of nuclear factor-kappab signaling in rats with collagen-induced arthritis. J Pharmacol Exp Ther. 2019;368(2):218–228. doi: 10.1124/jpet.118.253369 [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Wang J, Chu Y, Zhou X. Serum based fluorescent assay for evaluating dipeptidyl peptidase I activity in collagen induced arthritis rat model. Mol Cell Probes. 2017;32:5–12. doi: 10.1016/j.mcp.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Chen ZP, Liu W, Liu D, et al. Development of brucine-loaded microsphere/thermally responsive hydrogel combination system for intra-articular administration. J Control Release. 2012;162(3):628–635. doi: 10.1016/j.jconrel.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 17.Kuo WS, Weng CT, Chen JH, et al. Amelioration of experimentally induced arthritis by reducing reactive oxygen species production through the intra-articular injection of water-soluble fullerenol. Nanomaterials (Basel, Switzerland). 2019;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Huang J, Zhao L, Fan Y, et al. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun. 2019;10(1):2876. doi: 10.1038/s41467-019-10753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa K, Kajiwara Y, Sakamoto J, et al. Low-intensity muscle contraction exercise following the onset of arthritis improves hyperalgesia via reduction of joint inflammation and central sensitization in the spinal cord in a rat model. Neurosci Lett. 2019;706:18–23. doi: 10.1016/j.neulet.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 20.Choi JK, Kim SW, Kim DS, et al. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol Appl Pharmacol. 2016;290:1–9. doi: 10.1016/j.taap.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Li Q, Jin L, et al. Kirenol inhibits the function and inflammation of fibroblast-like synoviocytes in rheumatoid arthritis in vitro and in vivo. Front Immunol. 2019;10:1304. doi: 10.3389/fimmu.2019.01304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiang J, Lv T, Wu Z, Yang X. Down-regulation of microRNA-142-3p inhibits the aggressive phenotypes of rheumatoid arthritis fibroblast-like synoviocytes through inhibiting nuclear factor-kappaB signaling. Biosci Rep. 2019;39:7. doi: 10.1042/BSR20190700 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yu HM, Wang Q, Sun WB. Silencing of FKBP51 alleviates the mechanical pain threshold, inhibits DRG inflammatory factors and pain mediators through the NF-kappaB signaling pathway. Gene. 2017;627:169–175. doi: 10.1016/j.gene.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 24.Fearon U, Mullan R, Markham T, et al. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum. 2006;54:3152–3162. doi: 10.1002/art.22161 [DOI] [PubMed] [Google Scholar]
- 25.Hong R, Sur B, Yeom M, et al. Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag in IL-1beta-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception. Phytomedicine. 2018;38:45–56. doi: 10.1016/j.phymed.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Wang Y, Wang Q, et al. The bispecific antibody aimed at the vicious circle of IL-1beta and IL-17A, is beneficial for the collagen-induced rheumatoid arthritis of mice through NF-kappaB signaling pathway. Immunol Lett. 2016;179:68–79. doi: 10.1016/j.imlet.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 27.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001 [DOI] [PubMed] [Google Scholar]
- 28.Ruscitti P, Cipriani P, Liakouli V, et al. The emerging role of IL-1 inhibition in patients affected by rheumatoid arthritis and diabetes. Rev Recent Clin Trials. 2018;13(3):210–214. doi: 10.2174/1574887113666180314102651 [DOI] [PubMed] [Google Scholar]
- 29.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641 [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Li S, Yuan L, Yang Y, Pan MH. Effect of nobiletin on the MAPK/NF-kappaB signaling pathway in the synovial membrane of rats with arthritis induced by collagen. Food Funct. 2017;8(12):4668–4674. [DOI] [PubMed] [Google Scholar]
- 31.Zhou LL, Zhu YM, Qian FY, Yuan CC, Yuan DP, Zhou XP. MicroRNA1433p contributes to the regulation of pain responses in collagen-induced arthritis. Mol Med Rep. 2018;18(3):3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugita R, Kuwabara H, Kubota K, et al. Simultaneous inhibition of PGE2 and PGI2 signals is necessary to suppress hyperalgesia in rat inflammatory pain models. Mediators Inflamm. 2016;2016:9847840. doi: 10.1155/2016/9847840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frederick ED, Hausburg MA, Thomas GW, Rael LT, Brody E, Bar-Or D. The low molecular weight fraction of human serum albumin upregulates COX2, prostaglandin E2, and prostaglandin D2 under inflammatory conditions in osteoarthritic knee synovial fibroblasts. Biochem Biophys Rep. 2016;8:68–74. doi: 10.1016/j.bbrep.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minami T, Okuda-Ashitaka E, Mori H, Ito S, Hayaishi O. Prostaglandin D2 inhibits prostaglandin E2-induced allodynia in conscious mice. J Pharmacol Exp Ther. 1996;278(3):1146–1152. [PubMed] [Google Scholar]
- 36.Nieto FR, Clark AK, Grist J, Chapman V, Malcangio M. Calcitonin gene-related peptide-expressing sensory neurons and spinal microglial reactivity contribute to pain states in collagen-induced arthritis. Arthritis Rheumatol. 2015;67(6):1668–1677. doi: 10.1002/art.39082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren S, Yezierski RP, Capra NF. Chapter 18 – the somatosensory system II: nociception, thermal sense, and touch. Fund Neurosci Basic Clin Appl. 2018. [Google Scholar]
- 38.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. doi: 10.1016/j.jneuroim.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 39.Miyagi M, Ishikawa T, Kamoda H, et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord. 2017;18(1):428. doi: 10.1186/s12891-017-1792-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27(2):85–91. doi: 10.1016/j.tips.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 41.Djouhri L. PG110, A Humanized Anti-NGF antibody, reverses established pain hypersensitivity in persistent inflammatory pain, but not peripheral neuropathic pain, rat models. Pain Med. 2016;17(11):2082–2094. doi: 10.1093/pm/pnw007 [DOI] [PubMed] [Google Scholar]
- 42.Ashraf S, Bouhana KS, Pheneger J, Andrews SW, Walsh DA. Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res Ther. 2016;18(1):97. doi: 10.1186/s13075-016-0996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun S, Diggins NH, Gunderson ZJ, Fehrenbacher JC, White FA, Kacena MA. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone. 2019;131:115109. doi: 10.1016/j.bone.2019.115109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol. 2009;220:383–390. doi: 10.1016/j.expneurol.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie MX, Yang J, Pang RP, et al. Bulleyaconitine A attenuates hyperexcitability of dorsal root ganglion neurons induced by spared nerve injury: the role of preferably blocking Nav1.7 and Nav1.3 channels. Mol Pain. 2018;14:1744806918778491. doi: 10.1177/1744806918778491 [DOI] [PMC free article] [PubMed] [Google Scholar]