Abstract
Background
Neuroinflammation is an important pathological mechanism of depression that leads to an increase in indoleamine-2,3-dioxygenase (IDO) activity and NMDAR activation. This study aimed to observe the effects of electroacupuncture on depression-like behaviour in lipopolysaccharide (LPS)-treated rats and the underlying mechanism.
Methods
Wistar rats were intraperitoneally administered LPS (0.5 mg/kg) for 7 consecutive days to establish a depression model. Electroacupuncture treatment was administered 1 hour after daily LPS injection. The open field test (OFT), forced swimming test (FST), and sucrose preference test (SPT) were used to evaluate the depressive-like behaviours. IL-1β, IL-6, and TNF-α levels were determined by enzyme-linked immunosorbent assay (ELISA); Trp, 5-hydroxytryptamine (5-HT), kynurenine (Kyn) and quinolinic acid (Quin) were detected by ultra-high-performance liquid chromatography-tandem mass spectrometry; and N-methyl-D-aspartate receptor (NMDAR) protein and mRNA were assessed by Western blot and real-time qPCR.
Results
The results showed that electroacupuncture treatment successfully corrected LPS-induced depressive-like behaviour, reduced the inflammatory factor (IL-1β, IL-6 and TNF-α) levels in the blood and hippocampus, prevented IDO over-activation and recovered NR2B expression after challenge by LPS.
Conclusion
Electroacupuncture treatment provided protection against LPS-induced depressive-like behaviour, and the associated mechanisms may be related to inhibiting the inflammatory response, regulating the IDO-mediated tryptophan-degrading pathway, and inhibiting NR2B activation.
Keywords: depression, electroacupuncture, LPS, IDO
Introduction
Depression disorder is a common mental disease accompanied by manifestations of low mood, low interest, decreased energy, and inattention. Depression causes deteriorated physical function, increased disability, and decreased quality of life1,2 and leads to a significant increase in suicide risk.3 According to statistics by the World Health Organization, depression accounts for nearly 4.3% of the global disease burden and is expected to rank first in 2030.4,5 Such findings prompted researchers to explore the pathogenesis of depression from a broader perspective and find safer and more effective treatments.
Growing evidence suggests that inflammation is involved in the pathophysiological process of depression. Research shows that the levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in the peripheral blood of patients with depression are significantly increased;6–8 moreover, patients treated with cytokines for immunotherapy exhibit increased risk of depression disorder.9 Furthermore, patients with chronic inflammatory diseases are significantly more likely to suffer from depression.10,11 Similar indications have been found in related animal studies, which observed abnormal elevated levels of inflammatory cytokines in acute and chronic depressive stress models.12–14
Proinflammatory cytokines have been reported to activate indoleamine 2,3-dioxygenase (IDO) as a critical event in the switch from sickness to depression.15 IDO is a key enzyme responsible for tryptophan degradation along the kynurenine pathway.16 Excessive production of proinflammatory cytokines, such as IFN-γ, IL-6, TNF-α, and IL-1β, leads to increased IDO activity, which subsequently prompts the metabolism of Trp to shift towards kynurenine (Kyn) and eventually activates the N-methyl-D-aspartate receptor (NMDAR).17–19 Researchers have found that patients with depression have increased IDO activity in the blood, and depressive patients with suicidal tendencies have higher IDO activity than those without suicidal tendencies.20,21 Moreover, clinical studies have indicated that depressed patients could benefit from anti-inflammatory drugs and IDO inhibitors.22–24 Therefore, we speculated that inhibiting IDO activity is a viable therapeutic target for inflammation-associated depression.
Acupuncture is a widely used complementary and alternative therapy, and it has been reported to effectively alleviate the clinical symptoms of depression.25 Several existing meta-analyses also support the safety and significant clinical efficacy of acupuncture in ameliorating depressive symptoms.26–28 Although some studies have discussed the underlying mechanism of its anti-depressive effect,29–31 the mechanism of action is still not completely understood. In this study, lipopolysaccharide (LPS) was peripherally administered to establish a rat depression model to explore whether the antidepressant effect of acupuncture is related to the inhibition of the inflammatory response and regulation of the IDO-mediated tryptophan-degrading pathway.
Materials and Methods
Chemicals and Instruments
Trp, 5-hydroxytryptamine (5-HT), kynurenine (Kyn) and quinolinic acid (Quin), and LPS (Escherichia coli, serotype 0111:4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Benzoyl chloride and ammonium formate were obtained from Aladdin (Shanghai, China). LC-MS grade methanol, acetonitrile, and formic acid were purchased from Fisher Scientific. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Boster Biological Technology Co., Ltd. (Wuhan, China). NMDAR antibody was purchased from Abcam (Cambridge, UK). The real-time PCR system used in this study was the Thermo Fisher ABI StepOne Plus system. Ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) analysis was performed on an Agilent 1290 Infinity II UHPLC system coupled to a 6470A triple quadrupole mass spectrometer (Santa Clara, CA, United States).
Animal and LPS-Induced Model
Male Wistar rats weighing 180–200 g were obtained from the Animal Experiment Centre of Shanghai University of Traditional Chinese Medicine, China. Before the start of the experiment, the rats were fed adaptively for 1 week under standard feeding conditions (room temperature of 25 °C; 12-h/12-h light/dark cycle) and provided free access to food and water. The experiment was approved by the Experimental Animal Welfare and Ethics Committee of Shanghai University of Traditional Chinese Medicine. (animal ethical clearance number: PZSHUTCM190322027). Experiments were conducted according to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (publication 80–23), revised in 1996.
LPS was dissolved in sterile 0.9% saline at a concentration of 0.1 mg/mL and intraperitoneally injected into the Wistar rats at a dosage of 0.5 mg/kg daily for 1 week to induce depression-like behaviours. The dose was based on previously reported studies32,33 and verified in our preliminary studies.
Electroacupuncture Treatment
Rats were randomly divided into 3 groups (n = 8): (a) the control group, (b) the LPS group, and (c) the electroacupuncture group. The control group was intraperitoneally injected with physiological saline (5 mL/kg). The LPS group and electroacupuncture group were intraperitoneally injected with LPS (0.5 mg/kg). The Yintang (GV29) and Baihui (GV20) acupoints were selected for the electroacupuncture group. A disposable stainless-steel needle was inserted at depths of approximately 6–7 mm (GV20) and 3–4 mm (GV29). The needle was connected to an electroacupuncture device (frequency 2 Hz, intermittent wave, Hwato SDZ-V, Suzhou Medical Supplies, China). Treatment was initiated 1 hour after LPS injection, and each treatment session lasted for 30 min each time. Electroacupuncture was administered once a day for 7 consecutive days.
Behavioural Studies
To verify the effect of the LPS process on depression-like behaviour, the three groups were subjected to the forced swimming test (FST), open field test (OFT) and sucrose preference test (SPT) 24 hours after the last injection of LPS.
SPT
The SPT was conducted as described previously with minor modifications.33 For the first 24 hours, all rats were given two bottles containing 1% sucrose solution; for the second 24 hours, they were given a bottle of water and a bottle containing 1% sucrose solution. At the 12th hour, the positions of the bottle containing the sucrose solution and the water bottle were changed. Then, all rats were deprived of water for 24 hours, and the rats were given a bottle of 1% sucrose solution and a bottle of pure water. After 2 hours, the bottles were removed and weighed, and the consumption of sucrose solution and pure water was recorded.
OFT
The OFT was conducted to assess rat locomotor activity. The floor of a medical ABS plastic box (100×100×50 cm) was divided into 25 equal squares, and the number of gridlines crossed and the total distance of horizontal motion over a 5-min period were recorded. The test results were recorded with SuperMaze 2.0 (Xinruan Information and Technology, Shanghai, China).
FST
The FST was performed according to a previously reported method with slight modifications.34 The rats were placed in a bucket (50 cm high × 25 cm in diameter) filled with water (20–22 °C) to a depth of 35 cm. The rats were allowed to swim for 6 min, and immobility was recorded during the last 4 min. Immobility was defined as no movements other than those necessary to keep the head above the water. Immobility time was recorded by two observers who were unaware of the grouping.
ELISA
After the behavioural tests, the rats were anaesthetized via abdominal injection of pentobarbital sodium. Blood was collected from the abdominal aorta and centrifuged at 5000 rpm for 15 min after being allowed to stand for 4 hours. Then, the supernatant was collected and placed into a refrigerator at −80 °C until subsequent testing. According to the manufacturer’s instructions, the concentrations of IL-1β, IL-6, and TNF-α in the serum and hippocampus were determined by ELISA kits.
UHPLC-MS/MS Analysis
Sample preparation was performed as described by a previous publication with modifications.35 The tissue samples were weighed carefully, 10 volumes (μL/mg) of 80% acetonitrile solution was added, and the homogenates were ground and then centrifuged at 16,000 rcf and 4 °C for 15 min. Extraction of the supernatant was repeated, and then the supernatants were combined. A total of 100 μL supernatant was evaporated to dryness under nitrogen gas, and then 50 μL Na2CO3 (100 mmol/L) was added for redissolution. Derivatization was performed by adding 50 μL 2% benzoyl chloride in acetonitrile, and after centrifugation, the supernatant was isometrically mixed with isotope internal standards for quantification by UHPLC-MS/MS. All analytical standards were prepared individually at a concentration of 1 mM as stock solutions. A working solution containing 250 μM Trp, 50 μM Kyn, 5-HT and Quin was prepared by mixing standard stock solutions. After chemical derivatization by benzoyl chloride using the same protocol as that used for sample preparation, a derivatized working solution was obtained and serially diluted. The samples of the calibration curves were finally obtained by isometrically mixing the serially diluted standard mixture with an internal standard solution (benzoyl-13C6 chloride-derivatized standard mixture). Samples were injected into a Waters UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) at a flow rate of 0.25 mL/min. The mobile phase consisted of water in 2 mM ammonium formate and 0.1% formic acid (A) and acetonitrile (B). Chromatographic separation was conducted by a gradient elution programme as follows: 0–1 min, 15% B; 2 min, 40% B; 9 min, 60% B; 9.3 min, 100% B; 10.8 min, 100% B; 10.9 min, 15% B; 13 min, 15% B. The column temperature was 40 °C. The eluted analysts were ionized with an electrospray ionization source in positive mode (ESI+). The ESI+ source drying gas temperature was 300 °C, and the sheath gas temperature was 350 °C. The flow rates of the ESI+ source drying gas and sheath gas were 5 and 11 L/min, respectively. The pressure of the nebulizer was 40 psi, and the capillary voltage was 4000 V. Dynamic multiple reaction monitoring (dMRM) was performed to acquire data.
Western Blot Analysis
After blood was drawn from the abdominal aorta, the rats were immediately decapitated. The hippocampal tissues were separated and placed in liquid nitrogen for quick freezing and then placed in a refrigerator at −80 °C until analysis. The hippocampal tissue was cut into small pieces, ground in a homogenizer with RIPA lysis buffer, and then centrifuged at 4 °C for 5 min at 12,000 rpm. The supernatant was collected, and the protein concentration of the supernatant was measured using a BCA protein concentration determination kit. Total protein (30 μg) was electrophoretically separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. NMDAR2A (Abcam 1:1000) and NMDAR2B (Abcam 1:1000) antibodies were added, and the membranes were incubated at 4 °C overnight. β-actin was used as an internal reference. The next day, the membranes were washed with TBST three times for 5 min each, and then the blots were incubated with secondary antibody for 2 hours at room temperature and subsequently washed five times in TBST for 15 min each. ECL chemiluminescence detection reagent was applied to visualize the signals on the blot, and the optical densities of the bands were analysed by ImageJ.
Real-Time qPCR (RT-qPCR)
According to the manufacturers’ instructions, total RNA was extracted from the hippocampus with TRIzol reagent (Gibco, Invitrogen) and RNA was transcribed into cDNA using a reverse transcription kit. Then, the cDNA was amplified with primers. The primer sequences for the genes were as follows: (NMDAR2A: forward, 5ʹ-GGATCCGACATCCACGTTCT-3ʹ; reverse, 5ʹ-GGAGAAGACGTGCCAGTCGTA-3ʹ; NMDAR2B: forward, 5ʹ-GTGGATATGCAAGCGAGAAGA-3ʹ; reverse, 5ʹ-GCTGGGCTTCATCTTCAGCTA-3ʹ; GAPDH: forward, 5ʹ-CGCATTGCAGAGGATGGTAG-3ʹ; reverse, 5ʹ-CACCGACCTTCACCATCTTG-3ʹ). The results were analysed using the 2−ΔΔCT method for relative quantitative analysis.
Statistical Analysis
GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. The data were expressed as the mean ± SEM, and differences between groups were analysed by one-way ANOVA with Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
Effects of Electroacupuncture on Depression-Like Behaviours
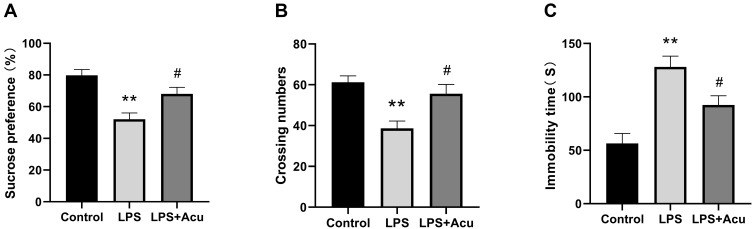
Distinctly significant differences among these groups were observed in the SPT [F (2, 27) = 12.37, P = 0.0002, Figure 1A], OFT [F (2, 27) = 9.387, P = 0.0008, Figure 1B] and FST [F (2, 27) = 14.58, P < 0.0001, Figure 1C]. Multiple comparisons suggested that the sucrose preference percentage (P < 0.0001, Figure 1A) and the number of crossings in the OFT (P = 0.0008, Figure 1B) exhibited an evident decrease and the immobility duration increased considerably (P < 0.0001 in Figure 1C) in the LPS-administered group compared with the control group. In contrast, the electroacupuncture treatment substantially ameliorated the sucrose consumption (P = 0.0218, Figure 1A) and the number of crossings in the OFT (P = 0.0113, Figure 1B) and decreased the immobility duration (P = 0.0315, Figure 1C) compared with the LPS group.
Figure 1.
Effects of electroacupuncture on depressive-like behaviours. Sucrose preference test (A), crossing numbers in open field test (B), and immobility time in the forced swimming test (C). Data are expressed as the mean ± SEM, (n = 10). **P < 0.01 compared to the control group; #P < 0.05 compared to the LPS group.
Effect of Electroacupuncture on Proinflammatory Factors in the Serum and Hippocampus
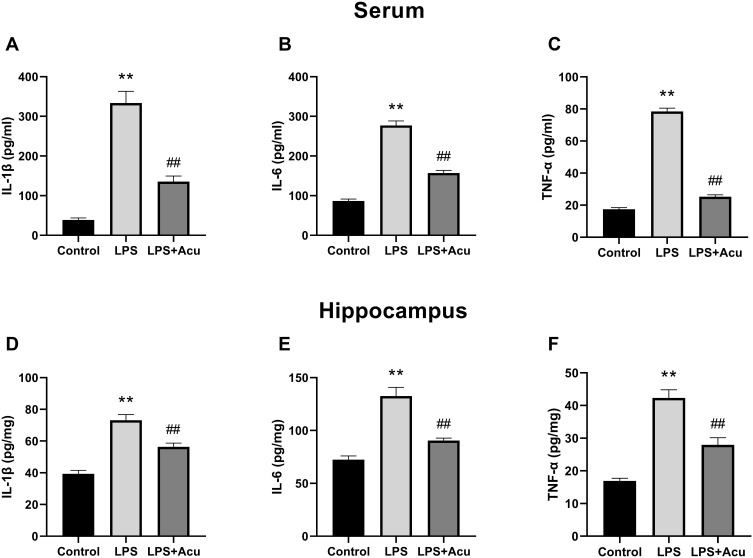
Overall statistically significant differences were obtained for IL-1β [F (2, 24) = 61.67; P < 0.0001, Figure 2A], IL-6 [F (2, 24) = 144.3; P < 0.0001, Figure 2B], and TNF-α [F (2, 24) = 513.9, P < 0.0001, Figure 2C] in the serum, and significant differences were observed for IL-1β [F (2, 18) = 36.42; P < 0.0001, Figure 2D], IL-6 [F (2, 18) = 33.80; P < 0.0001, Figure 2E], and TNF-α [F (2, 18) = 42.19, P < 0.0001, Figure 2F] in the hippocampus. A post hoc analysis indicated that LPS administration strongly increased the levels of proinflammatory factors IL-1β (P < 0.0001), IL-6 (P < 0.0001), and TNF-α (P < 0.0001) in the serum and hippocampus (Figure 3A–E). Treatment with electroacupuncture reversed the increase of IL-1β (P < 0.0001), IL-6 (P < 0.0001), and TNF-α (P < 0.0001) in the serum and IL-1β (P = 0.0013), IL-6 (P < 0.0001), and TNF-α (P = 0.0002) in the hippocampus (Figure 3A–E).
Figure 2.
Effect of electroacupuncture on proinflammatory factors in the serum and hippocampus. IL-1β, IL-6, and TNF-α levels in the plasma (A–C), IL-1β, IL-6, and TNF-α levels in the hippocampus (D–F). Data are expressed as the mean ± SEM, (n = 8–10). **P < 0.01 compared to the control group; ##P < 0.01 compared to the LPS group.
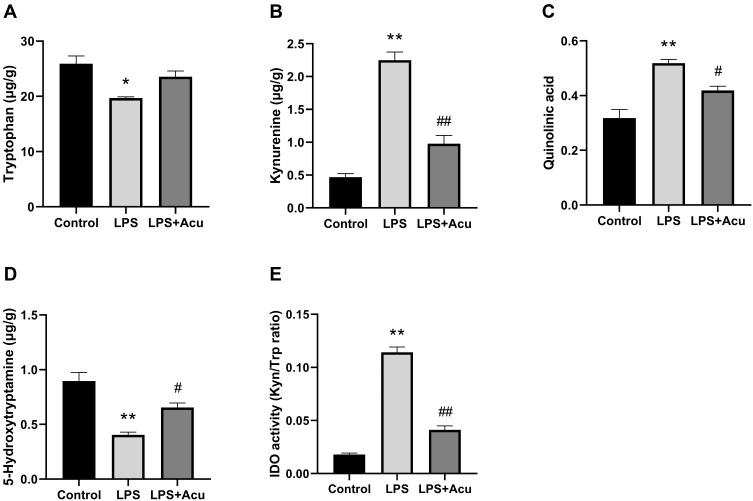
Figure 3.
Effect of electroacupuncture on the tryptophan degrading pathway and IDO activity in hippocampus. Tryptophan (A), kynurenine (B), quinolinic acid (C), 5-Hydroxytryptamine (D), and IDO activity (Kyn/Trp radio) (E). Data are expressed as the mean ± SEM, (n = 6). *P < 0.05, **P < 0.01 compared to the control group; #P < 0.05; ##P < 0.01 compared to the LPS group.
Effect of Electroacupuncture on the IDO-Mediated Tryptophan-Degrading Pathway
As shown in Figure 3, the levels of Trp [F (2, 6) = 9.644, P = 0.0134, Figure 3A], kyn [F (2, 6) = 75.14, P < 0.0001, Figure 3B], Quin [F (2, 6) = 21.31, P = 0.0019, Figure 3C], and 5-HT [F (2, 6) = 20.71, P = 0.0020, Figure 3D] and the activity of IDO [F (2, 6) = 183.9, P < 0.0001, Figure 3E] were significantly different among these groups. Post hoc analysis indicated after LPS-administration, the levels of Trp (P = 0.0115, Figure 3A) and 5-HT (P = 0.0016, Figure 3D) were significant decreased while the levels of kyn (P < 0.0001, Figure 3B) and Quin (P = 0.0015, Figure 3C) and activity of IDO (P < 0.0001, Figure 3E) were significantly increased in the hippocampus compared with the control group. Treatment with electroacupuncture reversed the decreasing trend for Trp (P = 0.0777, Figure 3A) and 5-HT (P = 0.0388, Figure 3D) and reduced the level of kyn (P = 0.0004, Figure 3B) and Quin (P = 0.0395, Figure 3C) and the activity of IDO (P < 0.0001, Figure 3E).
Effect of Electroacupuncture on NMDAR Protein and mRNA Levels
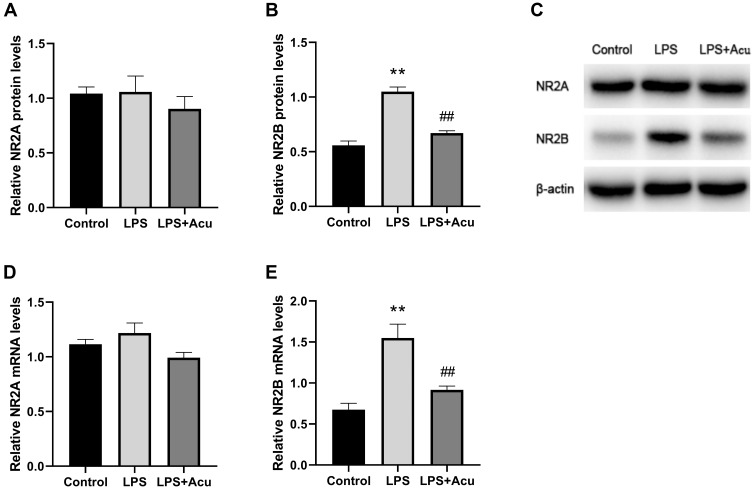
A one-way ANOVA indicated significant interaction effects between LPS and electroacupuncture administration for the levels of relative protein [F (2, 6) = 52.13, P = 0.0002, Figure 4B] and mRNA [F (2, 9) = 16.69, P = 0.0009, Figure 4E] of NR2B. A post hoc analysis indicated that LPS administration strongly increased the expression of the NR2B protein (P = 0.0002, Figure 4B and C) and mRNA (P = 0.0009, Figure 4E) in the hippocampus. Treatment with electroacupuncture significantly downregulated the NR2B protein (P = 0.0007, Figure 4B and C) and mRNA (P = 0.0073, Figure 4E). However, significant differences were not observed in the relative protein and mRNA levels of NR2A (Figure 4A and D) between the three groups.
Figure 4.
Effect of electroacupuncture on NMDAR protein and mRNA levels. Relative protein expression of NR2A and NR2B (A, B), Western blot bands (C), and relative mRNA expression of NR2A and NR2B (D, E). Data are expressed as mean ± SEM, (n = 5). **P < 0.01 compared to the control group; ##P < 0.01 compared to the LPS group.
Discussion
A growing number of studies have indicated the satisfactory effectiveness of acupuncture when treating depression. Studies have reported that acupuncture can reduce the level of inflammatory factors and relieve depressive symptoms in chronic unpredictable mild stress depression model rats.36,37 Meanwhile, in various LPS-induced models, acupuncture has shown a good anti-inflammatory effect.38–40 This study is the first to explore the effect of electroacupuncture on a LPS-induced depression model, and the results showed that electroacupuncture has a good antidepressant effect. In addition, electroacupuncture can effectively reduce the levels of proinflammatory cytokines (IL-1 β, IL-6 and TNF-α), inhibit the activity of IDO, and downregulate the protein and mRNA expression of NR2B. All of these changes may be partly attributed to the antidepressant effect of electroacupuncture.
Intraperitoneal injection of LPS is commonly used to induce inflammatory depression models. Based on previous research, a rat model of depression was successfully replicated, and then the SPT, OFT, and FST, which are classical tests, were used to assess the depression-like behaviour. The results showed that the depression model rats exhibited reduced sucrose water consumption in the SPT, crossed the gridlines significantly fewer times in the OFT, and exhibited a significantly longer immobility time in the FST. These behavioural changes were reversed by electroacupuncture treatment, indicating that electroacupuncture has a good antidepressant effect.
Hippocampal neuroinflammation is closely related to depression. Multiple clinical studies and animal experiments have suggested that hippocampal neuroinflammation is involved in the pathological process of depression.41,42 Anti-inflammatory therapy can help relieve depression and stress-related mood disorders.43 As an immune activator and cytokine inducer, LPS can lead to immune activation and inflammatory cytokine release, thereby causing depression-like behaviour in rats.33,44 A previous study found that acupuncture could effectively suppress neuroinflammation in the hippocampus of a stress-induced depression rat model.45 Therefore, the contents of IL-1β, IL-6, and TNF-α in the plasma and hippocampus were measured in this study. We found that intraperitoneal injection of LPS induced a significant increase in the inflammatory factors IL-1β, IL-6, and TNF-α in the rat serum and hippocampus compared with the control animals while the electroacupuncture treatment effectively reduced the levels of inflammatory factors while improving the symptoms of depressed rats.
IDO is the rate-limiting enzyme in the tryptophan-kynurenine metabolic pathway,16 and although its expression is low under normal conditions, in the case of immune activation under the action of IFN-γ, TNF-α and other inflammatory factors, its activity is greatly increased.17–19 Increased IDO catalyses the conversion of tryptophan to Kyn, increases the levels of neurotoxic metabolite Quin, and reduces the bioavailability of tryptophan, resulting in insufficient synthesis of 5-HT.46,47 IDO activity is closely related to depression, and the ratio of Kyn/Trp is often used as an index to evaluate the activity of IDO. The higher the ratio, the greater the activity of IDO.48 In this study, we used UHPLC-MS/MS to determine the levels of Trp, Kyn, Quin and 5-HT in the hippocampi of rats and evaluated IDO activity by measuring the ratio of Kyn to Trp. The results showed that the 5-HT levels were significantly reduced, and Quin and IDO activity were significantly increased in the model group compared with the control group. Electroacupuncture reversed the increases in the concentrations of Quin and increased the 5-HT level in the hippocampus as well as reduced the activity of IDO.
NMDAR is an ionic glutamate receptor involved in learning and memory, synaptic plasticity, excitotoxicity, and so on, and it is closely related to depression.49,50 NMDARs are heterooligomeric complexes that consist of essential NR1 subunit and regulatory NR2 (NR2A-D) subunits,51 NR2A- and NR2B-containing NMDARs are considered the major isoforms of functional NMDARs in neurons.52 NR2B-containing NMDARs mainly reside at extrasynaptic sites and are involved in cell death.53,54 A previous study found that NR2B expression was significantly upregulated in animal models of depression.55 In addition, compared with depressed patients who did not die of suicide, the expression level of NR2B mRNA was higher in depressed patients who died by suicide.56 Quin is an endogenous agonist of NMDAR, when NMDAR is activated, ion channels are opened. Then, the influx of a large amount of Ca2+ into the extracellular fluid causes calcium overload in neurons, triggering a series of downstream pro-death signalling events, such as calpain activation, reactive oxygen species generation, and mitochondrial damage, further aggravating the symptoms of depression.57–59 We detected the protein and mRNA expression of NMDAR subunits in hippocampal homogenates by Western blotting and q-PCR analysis. The results showed that the increased protein and mRNA expression of NR2B in the LPS group was reversed by electroacupuncture treatment. Similar to our study, an increase in NR2B expression but not in NR2A expression was observed in response to LPS challenge in previous reports.60 Combining these findings, we can speculate that antidepressant effects of electroacupuncture may be related to the inhibition of inflammatory response as well as IDO over-activation, thereby inhibiting the activation of NR2B.
Conclusions
In conclusion, we provide relevant evidence to suggest that electroacupuncture relieves LPS-induced depression-like behaviour. The underlying mechanisms may involve in inhibiting inflammatory response, regulating IDO-mediated tryptophan-degrading pathway, inhibiting the activation of NR2B. It is suggested that electroacupuncture can be an effective treatment for depression disorder.
Acknowledgments
This research was funded by grants from the Science and Technology Commission of Shanghai Municipality, china (Grant No. 18401970600) and Shanghai Municipal Health and Family Planning Commission (Grant No. ZY(2018-2020)-CCCX-1005).
Abbreviations
IDO, indoleamine 2,3-dioxygenase; 5-HT, 5-hydroxytryptamine; UHPLC-MS/MS, Ultra-high-performance liquid chromatography-tandem mass spectrometry; FST, forced swimming test; OFT, open field test; SPT, sucrose preference test; Trp, tryptophan; LPS, lipopolysaccharide; Kyn, kynurenine; Quin, quinolinic acid.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002;53(4):859–863. doi: 10.1016/S0022-3999(02)00313-6 [DOI] [PubMed] [Google Scholar]
- 2.Talarowska M, Szemraj J, Kowalczyk M, Gałecki P. Serum KIBRA mRNA and protein expression and cognitive functions in depression. Med Sci Monit. 2016;22:152–160. doi: 10.12659/MSM.895200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca-Garcia E, Perez-Rodriguez MM, Diaz Sastre C, Saiz-Ruiz J, de Leon J. Suicidal behavior in schizophrenia and depression: a comparison. Schizophr Res. 2005;75(1):77–81. doi: 10.1016/j.schres.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 4.Docherty AR, Edwards AC, Yang F, et al. Age of onset and family history as indicators of polygenic risk for major depression. Depress Anxiety. 2017;34(5):446–452. doi: 10.1002/da.22607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 6.Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25(6):1301–1311. doi: 10.1038/s41380-018-0096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rush G, O’Donovan A, Nagle L, et al. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J Affect Disord. 2016;205:60–68. doi: 10.1016/j.jad.2016.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su KP, Lai HC, Peng CY, Su WP, Chang JP, Pariante CM. Interferon-alpha-induced depression: comparisons between early- and late-onset subgroups and with patients with major depressive disorder. Brain Behav Immun. 2019;80:512–518. doi: 10.1016/j.bbi.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 10.Jacob L, Rockel T, Kostev K. Depression risk in patients with rheumatoid arthritis in the United Kingdom. Rheumatol Ther. 2017;4(1):195–200. doi: 10.1007/s40744-017-0058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812–820. doi: 10.1001/jamapsychiatry.2013.1111 [DOI] [PubMed] [Google Scholar]
- 12.Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22(4):573–589. doi: 10.1016/j.bbi.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Chen HJ, Spiers JG, Sernia C, Lavidis NA. Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Radic Biol Med. 2016;90:219–229. doi: 10.1016/j.freeradbiomed.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Gao Z, Zhang H, et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr Dis Treat. 2015;11:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laugeray A, Launay JM, Callebert J, et al. Chronic treatment with the IDO1 inhibitor 1-methyl-D-tryptophan minimizes the behavioural and biochemical abnormalities induced by unpredictable chronic mild stress in mice - comparison with fluoxetine. PLoS One. 2016;11(11):e0164337. doi: 10.1371/journal.pone.0164337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christmas DM, Potokar J, Davies SJ. A biological pathway linking inflammation and depression: activation of indoleamine 2,3-dioxygenase. Neuropsychiatr Dis Treat. 2011;7:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobos N, de Vries EF, Kema IP, et al. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis. 2012;28(4):905–915. doi: 10.3233/JAD-2011-111097 [DOI] [PubMed] [Google Scholar]
- 18.Zunszain PA, Anacker C, Cattaneo A, et al. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6(10):e918. doi: 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doolin K, Allers KA, Pleiner S, et al. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology. 2018;95:8–17. doi: 10.1016/j.psyneuen.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 21.Messaoud A, Mensi R, Douki W, et al. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J Biol Psychiatry. 2019;20(9):703–711. doi: 10.1080/15622975.2018.1468031 [DOI] [PubMed] [Google Scholar]
- 22.Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21(4):227–231. doi: 10.1097/00004850-200607000-00005 [DOI] [PubMed] [Google Scholar]
- 23.Müller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680–684. doi: 10.1038/sj.mp.4001805 [DOI] [PubMed] [Google Scholar]
- 24.Ogyu K, Kubo K, Noda Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. doi: 10.1016/j.neubiorev.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 25.Zhao B, Li Z, Wang Y, et al. Can acupuncture combined with SSRIs improve clinical symptoms and quality of life in patients with depression? Secondary outcomes of a pragmatic randomized controlled trial. Complement Ther Med. 2019;45:295–302. [DOI] [PubMed] [Google Scholar]
- 26.Armour M, Smith CA, Wang L-Q, et al. Acupuncture for depression: a systematic review and meta-analysis. J Clin Med. 2019;8(8):1140. doi: 10.3390/jcm8081140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):Cd004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Qu SS, Zhang JP, et al. Rapid onset of the effects of combined selective serotonin reuptake inhibitors and electroacupuncture on primary depression: a meta-analysis. J Altern Complement Med. 2016;22(1):1–8. doi: 10.1089/acm.2015.0114 [DOI] [PubMed] [Google Scholar]
- 29.Tu CH, MacDonald I, Chen YH. The effects of acupuncture on glutamatergic neurotransmission in depression, anxiety, schizophrenia, and alzheimer’s disease: a review of the literature. Front Psychiatry. 2019;10:14. doi: 10.3389/fpsyt.2019.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo D, Ma R, Wu Y, et al. Mechanism underlying acupuncture-ameliorated depressive behaviors by enhancing glial glutamate transporter in Chronic Unpredictable Mild Stress (CUMS) Rats. Med Sci Monit. 2017;23:3080–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan DM, Tu Y, Liu P, Jiao S. Antidepressant effect of electroacupuncture regulates signal targeting in the brain and increases brain-derived neurotrophic factor levels. Neural Regen Res. 2016;11(10):1595–1602. doi: 10.4103/1673-5374.193238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, Yang Y, Ni Z, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554(7692):323–327. doi: 10.1038/nature25752 [DOI] [PubMed] [Google Scholar]
- 33.Song Q, Fan C, Wang P, Li Y, Yang M, Yu SY. Hippocampal CA1 βCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J Neuroinflammation. 2018;15(1):338. doi: 10.1186/s12974-018-1377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naserzadeh R, Abad N, Ghorbanzadeh B, Dolatshahi M, Mansouri MT. Simvastatin exerts antidepressant-like activity in mouse forced swimming test: role of NO-cGMP-K(ATP) channels pathway and PPAR-gamma receptors. Pharmacol Biochem Behav. 2019;180:92–100. doi: 10.1016/j.pbb.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Wong JM, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J Chromatogr A. 2016;1446:78–90. doi: 10.1016/j.chroma.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, Shao RH, Hu L, Tu Y, Guo JY. Potential antiinflammatory effects of acupuncture in a chronic stress model of depression in rats. Neurosci Lett. 2016;618:31–38. doi: 10.1016/j.neulet.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Shao RH, Jin SY, Hu L, Tu Y, Guo JY. Acupuncture ameliorates inflammatory response in a chronic unpredictable stress rat model of depression. Brain Res Bull. 2017;128:106–112. doi: 10.1016/j.brainresbull.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Chen T, Xiong Y, Long M, et al. Electro-acupuncture pretreatment at Zusanli (ST36) acupoint attenuates lipopolysaccharide-induced inflammation in rats by inhibiting Ca(2+) Influx associated with cannabinoid CB2 receptors. Inflammation. 2019;42(1):211–220. doi: 10.1007/s10753-018-0885-5 [DOI] [PubMed] [Google Scholar]
- 39.Han YG, Qin X, Zhang T, et al. Electroacupuncture prevents cognitive impairment induced by lipopolysaccharide via inhibition of oxidative stress and neuroinflammation. Neurosci Lett. 2018;683:190–195. doi: 10.1016/j.neulet.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 40.Ramires CC, Balbinot DT, Cidral-Filho FJ, Dias DV, Dos Santos AR, da Silva MD. Acupuncture reduces peripheral and brainstem cytokines in rats subjected to lipopolysaccharide-induced inflammation. Acupunct Med. 2020;964528420938379. [DOI] [PubMed] [Google Scholar]
- 41.Mahajan GJ, Vallender EJ, Garrett MR, et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:177–186. doi: 10.1016/j.pnpbp.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Zhang Q, Yuan L, et al. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav Brain Res. 2014;274:282–290. doi: 10.1016/j.bbr.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 44.Voss OH, Murakami Y, Pena MY, et al. Lipopolysaccharide-induced CD300b receptor binding to toll-like receptor 4 alters signaling to drive cytokine responses that enhance septic shock. Immunity. 2016;44(6):1365–1378. doi: 10.1016/j.immuni.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue N, Li B, Yang L, et al. Electro-acupuncture alleviates chronic unpredictable stress-induced depressive- and anxiety-like behavior and hippocampal neuroinflammation in rat model of depression. Front Mol Neurosci. 2018;11:149. doi: 10.3389/fnmol.2018.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x [DOI] [PubMed] [Google Scholar]
- 47.Gabbay V, Klein RG, Katz Y, et al. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry. 2010;51(8):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang B, Lian Y-J, Dong X, et al. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behav Brain Res. 2019;180:250–257. doi: 10.1016/j.bbr.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 49.Gonçalves-Ribeiro J, Pina CC, Sebastião AM, Vaz SH. Glutamate transporters in hippocampal LTD/LTP: not just prevention of excitotoxicity. Front Cell Neurosci. 2019;13:357. doi: 10.3389/fncel.2019.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amidfar M, Woelfer M, Réus GZ, Quevedo J, Walter M, Kim YK. The role of NMDA receptor in neurobiology and treatment of major depressive disorder: evidence from translational research. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109668. doi: 10.1016/j.pnpbp.2019.109668 [DOI] [PubMed] [Google Scholar]
- 51.Vyklicky V, Korinek M, Smejkalova T, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–S203. doi: 10.33549/physiolres.932678 [DOI] [PubMed] [Google Scholar]
- 52.Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104 [DOI] [PubMed] [Google Scholar]
- 53.Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13(3):366–371. doi: 10.1016/S0959-4388(03)00073-4 [DOI] [PubMed] [Google Scholar]
- 54.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li SX, Han Y, Xu LZ, et al. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry. 2018;23(3):597–608. doi: 10.1038/mp.2017.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20(9):1057–1068. [DOI] [PubMed] [Google Scholar]
- 57.Dong Y, Kalueff AV, Song C. N-methyl-d-aspartate receptor-mediated calcium overload and endoplasmic reticulum stress are involved in interleukin-1beta-induced neuronal apoptosis in rat hippocampus. J Neuroimmunol. 2017;307:7–13. doi: 10.1016/j.jneuroim.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Ming H, Chen R, et al. CIH-induced neurocognitive impairments are associated with hippocampal Ca(2+) overload, apoptosis, and dephosphorylation of ERK1/2 and CREB that are mediated by overactivation of NMDARs. Brain Res. 2015;1625:64–72. doi: 10.1016/j.brainres.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 59.Lovelace MD, Varney B, Sundaram G, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112(Pt B):373–388. doi: 10.1016/j.neuropharm.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 60.Deng YT, Zhao MG, Xu TJ, Jin H, Li XH. Gentiopicroside abrogates lipopolysaccharide-induced depressive-like behavior in mice through tryptophan-degrading pathway. Metab Brain Dis. 2018;33(5):1413–1420. [DOI] [PubMed] [Google Scholar]