Abstract
Background/Aims
Esophageal baseline impedance (BI) can be extracted from pH-impedance tracings as mean nocturnal baseline impedance (MNBI), and from high-resolution impedance manometry (HRIM), but it is unknown if values are similar between acquisition methods across HRIM manufacturers. We aim to assess correlations between MNBI and BI from HRIM (BI-HRIM) from 2 HRIM manufacturers in the setting of physiologic acid exposure time (AET).
Methods
HRIM and pH-impedance monitoring demonstrating physiologic AET (< 4%) off proton pump inhibitors were required. BI-HRIM was extracted as the average from 5 cm and 10 cm above the lower esophageal sphincter. Distal BI-HRIM (DBI-HRIM) was also extracted from the most distal channel (Medtronic studies). MNBI was extracted from 6 channels. Concordance between BI-HRIM across manufacturers with MNBI was analyzed.
Results
Thirty-six patients met the inclusion criteria (59.6 ± 1.7 years; 22% female; body mass index 30.5 ± 0.7; AET 1.6 ± 0.2%). Although MNBI was similar at all channels (P ≥ 0.18), Diversatek BI-HRIM was lower than Medtronic BI-HRIM (P = 0.003). Overall, BI-HRIM correlated with MNBI at corresponding recording sites, 7 cm and 9 cm (P < 0.05), but not at other sites (P ≥ 0.19). Pearson’s correlations > 0.5 were seen at MNBI at 7 cm for both systems, and at 9 cm for Medtronic. DBI-HRIM correlated with MNBI at 3 cm and 5 cm (P < 0.03), but not at other locations (P > 0.1).
Conclusions
While numeric differences exist between manufacturers, BI-HRIM correlates with MNBI from corresponding channels in patients with physiologic AET. Comparison with AET elevation is needed to determine correlations between pathologic MNBI with BI-HRIM across manufacturers. The optimal HRIM channels from which BI values should be extracted also warrants further study.
Keywords: Electric impedance, Esophageal mucosa, Esophageal pH monitoring, Gastroesophageal reflux
Introduction
Ambulatory reflux monitoring is often utilized when symptoms suspicious for gastroesophageal reflux disease (GERD) persist despite antisecretory therapy.1,2 Esophageal acid exposure time (AET) and reflux-symptom association (RSA) from ambulatory reflux monitoring can phenotype GERD and guide management.3-5 While AET from pH monitoring remains the most consistent predictor of symptom outcome, combination with impedance monitoring increases RSA yield and enhances confidence in a reflux mechanism for symptoms.4,6 However, catheter-based ambulatory reflux monitoring provides only a cross-sectional assessment of reflux burden and may not capture day-to-day variation in reflux burden.7
In contrast, esophageal baseline impedance (BI) is a novel marker for esophageal mucosal integrity, with lower values in erosive and non-erosive GERD compared to functional heartburn and healthy controls.8,9 Extracted from the nocturnal phase of ambulatory pH-impedance tracings, mean nocturnal baseline impedance (MNBI) can segregate reflux-related esophageal syndromes from controls,10-12 and predicts symptom improvement from antireflux therapy as a co-variate of distal AET.13,14 However, acquiring BI in this fashion still requires cumbersome ambulatory catheter-based monitoring and manual analysis of tracings.
High-resolution impedance manometry (HRIM), utilized to identify the lower esophageal sphincter (LES) for appropriate positioning of pH-impedance catheters, also incorporates impedance sensors to track esophageal bolus during pressure topography.15 BI acquired during the landmark phase of HRIM (BI-HRIM) performed with Medtronic HRIM equipment has been demonstrated to correlate with MNBI and reliably segregate patients with moderately pathologic AET from controls.16
Despite growing evidence supporting the value of BI from both pH-impedance monitoring and HRIM in identifying reflux-related esophageal syndromes, there is a paucity of similar data in patients with physiologic AET and functional esophageal syndromes. Evaluation of BI-HRIM has only been conducted using HRIM catheters from a single manufacturer (Medtronic, Minneapolis, MN, USA). In this study, we investigated BI-HRIM and MNBI values among patients with physiologic AET, across 2 HRIM device manufacturers to assess BI relationships with esophageal symptoms, and to determine if BI values are similar between HRIM devices in functional esophageal syndromes.
Materials and Methods
Patients
Adults with persistent esophageal symptoms who underwent esophageal HRIM using 1 of 2 device manufacturers (Diversatek, Highlands Ranch, CO, USA; and Medtronic, Minneapolis, MN, USA) and combined pH-impedance monitoring (Diversatek) at the performing institution over a 2-year period were eligible for inclusion into this retrospective observational cohort study. Exclusion criteria consisted of studies performed on antisecretory therapy, distal esophageal AET > 4%, achalasia, previous foregut surgery, inadequate studies (poor data quality precluding analysis), or incomplete studies (< 14 hours of recording time); patients with esophageal body motor disorders without outflow obstruction were not excluded. This study protocol was approved by the Institutional Review Board at the performing institution (IRB No. 02083); a waiver of informed consent was granted by the Institutional Review Board.
High-resolution Impedance Manometry Baseline Impedance
HRIM from 1 of 2 manufacturers (Diversatek and Medtronic) was used to localize the LES, identify motility disorders per Chicago classification version 3.0,17 and determine BI from esophageal manometry (BI-HRIM). BI-HRIM was extracted from HRIM studies as the average of impedance values from 5 cm and 10 cm above the LES during quiet rest (“landmark phase”) prior to initiation of water swallows.18 Distal BI-HRIM (DBI-HRIM) was also extracted from the distal-most esophageal impedance channel from Medtronic HRIM studies.
pH-impedance Monitoring
Patients were instructed to stop their proton pump inhibitor (PPI) medications for 7 days prior to the study, and any histamine-2 receptor antagonists, prokinetic medications, and antacids 3 days prior to the study. After an overnight fast, an experienced nurse positioned the pH-impedance catheter (Diversatek) such that the esophageal pH sensor was 5 cm proximal to manometrically-localized LES. Throughout the monitoring interval, patients logged their meals, position (supine vs upright), and symptom events by pressing appropriate buttons on the data recorder. Data uploaded from pH-impedance studies were analyzed with dedicated software (Bioview Analysis; Diversatek), with calculation of AET, reflux events, symptom events, and RSA parameters. AET was calculated as the percentage of time the pH was below 4 at the distal esophageal pH sensor. AET < 4% was considered physiologic,19 and was an entry criterion for this study.
Symptom events were considered associated with reflux episodes if they occurred within 2 minutes of a reflux episode. Symptom index was calculated as the ratio of symptom events associated with reflux episodes to total symptom events and considered positive if > 50%.20 Symptom association probability was calculated using the Ghillebert probability estimate,21 and designated positive if > 95%, corresponding to P < 0.05.
pH-impedance tracings were interrogated by one of the authors (A.H.) in a blinded fashion to calculate MNBI values. BI values were extracted at each impedance channel (3, 5, 7, 9, 15, and 17 cm above the LES) across stable nocturnal 10-minute periods (at or around 1 AM, 2 AM, and 3 AM) to avoid reflux events or swallows.22 The values from the 3 time periods for each channel were averaged to yield the MNBI for each channel. Distal MNBI was calculated as the average of MNBI values from the channels located at 3, 5, 7, and 9 cm above the LES.13
Symptom Assessment
Prior to esophageal function testing at the performing institution, all patients completed symptom surveys to rate their dominant and secondary symptom frequency and severity on 5-point Likert scales, as well as esophageal global symptom severity (GSS) on 100-mm visual analog scales, as previously described.4,5,23,24 Patients rate symptom frequency from 0 (no symptoms) to 4 (multiple daily episodes), and symptom severity from 0 (no symptoms) to 4 (very severe symptoms). Dominant symptom intensity (DSI) is then calculated as the product of symptom frequency and symptom severity (for a total score from 0 to 16). The DSI and GSS represented symptom burden metrics for this study. Dominant symptoms were also stratified into perceptive (heartburn and chest pain) and non-perceptive symptom groups.
Statistical Methods
Data are reported here as mean ± standard error of the mean, unless otherwise indicated. Categorical data were compared using the χ-squared test, and continuous data were compared using ANOVA or the 2-tailed Student’s t test, as appropriate. Correlations between continuous variables (BI-HRIM, MNBI, and symptom burden) were quantified using Pearson’s correlation coefficient. In all cases, P < 0.05 was required for statistical significance. Statistical analyses were performed using IBM SPSS Statistics version 23 (Armonk, NY, USA).
Results
Study Cohorts
Inclusion criteria were met by 36 patients (age 59.6 ± 1.7 years, 22% female, body mass index 30.5 ± 0.7), with a mean total AET of 1.6 ± 0.2% (Table 1). Dominant perceptive symptoms were reported by 75% of the cohort, and symptom burden was modestly high (GSS 69.0 ± 4.5, DSI 10.4 ± 1.0). Esophageal body motor function was normal in 25 patients (69%); of the remainder, 5 had ineffective esophageal motility, 4 had jackhammer esophagus, and 1 each had absent contractility and diffuse esophageal spasm. HRIM was performed using Diversatek catheters in 14 (39%), and the remainder using Medtronic catheters. Demographics (age, gender, and body mass index), proportions with dominant perceptive symptoms, symptom burden metrics, proportions with normal esophageal body motor function on HRM, and total AET were similar between HRIM manufacturer cohorts (P ≥ 0.180; Table 1).
Table 1.
Comparisons Between High-resolution Impedance Manometry Systems
| Clinical characteristics | All HRIM (N = 36) |
Diversatek HRIM (n = 14) |
Medtronic HRIM (n = 22) |
P-valuea |
|---|---|---|---|---|
| Age (yr) | 59.6 ± 1.7 | 62.0 ± 2.2 | 58.1 ± 2.4 | 0.259 |
| Gender (female) | 8 (22%) | 3 (21%) | 5 (23%) | 0.927 |
| BMI | 30.5 ± 0.7 | 29.9 ± 1.1 | 30.9 ± 0.9 | 0.473 |
| Perceptive dominant symptom | 27 (75%) | 11 (79%) | 16 (73%) | 0.693 |
| GSS | 69.0 ± 4.5 | 62.9 ± 6.5 | 72.9 ± 6.0 | 0.279 |
| DSI | 10.4 ± 1.0 | 8.7 ± 1.7 | 11.4 ± 1.2 | 0.180 |
| Normal esophageal body motility on HRM | 25 (69%) | 10 (71%) | 15 (68%) | 0.837 |
| Total AET (%) | 1.6 ± 0.2 | 1.2 ± 0.3 | 1.8 ± 0.3 | 0.247 |
| BI-HRIM | 1564 ± 140 | 1062 ± 124 | 1884 ± 186 | 0.003 |
| MNBI-17 | 2184 ± 150 | 2387 ± 260 | 2055 ± 180 | 0.285 |
| MNBI-15 | 2083 ± 149 | 2151 ± 230 | 2039 ± 199 | 0.719 |
| MNBI-9 | 1553 ± 121 | 1703 ± 168 | 1458 ± 167 | 0.332 |
| MNBI-7 | 1589 ± 148 | 1699 ± 226 | 1519 ± 198 | 0.561 |
| MNBI-5 | 1474 ± 152 | 1713 ± 269 | 1322 ± 177 | 0.214 |
| MNBI-3 | 1376 ± 139 | 1614 ± 274 | 1225 ± 142 | 0.175 |
| Distal MNBIb | 1498 ± 124 | 1683 ± 204 | 1381 ± 154 | 0.241 |
aComparison between Diversatek and Medtronic high-resolution impedance manometry (HRIM) systems (performed with independent-samples t tests or Chi-square, as appropriate).
bDistal mean nocturnal baseline impedance (MNBI) represents the averaged MNBI values for 3,5,7, and 9 cm above the lower esophageal sphincter (LES).
BMI, body mass index; GSS, global symptom severity; DSI, dominant symptom intensity; HRM, high-resolution manometry; AET, acid exposure time; BI-HRIM, baseline impedance from HRIM.
Mean Nocturnal Baseline Impedance and Baseline Impedance From High-resolution Impedance Manometry Values
Those with abnormal esophageal body motor function on HRM had similar BI-HRIM and MNBI values to those with normal body motor function (P > 0.369). Individual MNBI values at each impedance site as well as composite distal MNBI were similar between the 2 HRIM manufacturer cohorts (P ≥ 0.175; Table 1). In contrast, BI-HRIM acquired from Diversatek catheters was significantly lower than that from Medtronic catheters (1062 ± 124 Ω vs 1884 ± 186 Ω, P = 0.003), despite similar symptom burden and AET in the 2 cohorts.
Correlations Between Mean Nocturnal Baseline Impedance (MNBI) and Baseline Impedance From High-resolution Impedance Manometry (BI-HRIM)
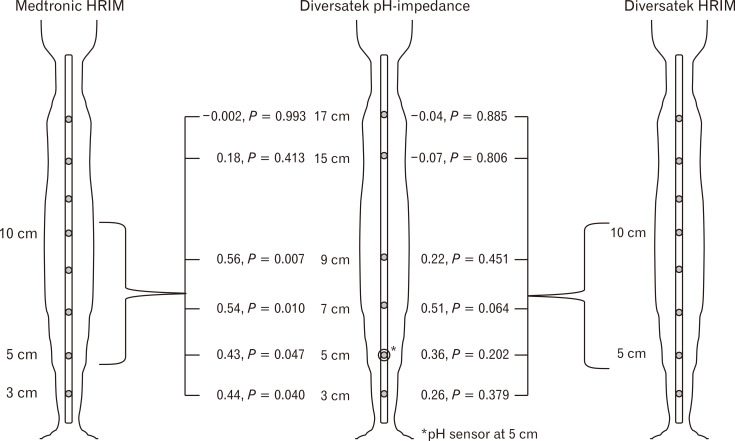
Across the total cohort, there were statistically significant correlations between BI-HRIM and MNBI at comparable channels––7 cm (r = 0.40, P = 0.015) and 9 cm (r = 0.33, P = 0.047) proximal to the LES––but not at other sites (P ≥ 0.19; Table 2 and Figure). Diversatek BI-HRIM numerically trended with MNBI at 7 cm (r = 0.51, P = 0.064), but not at the other MNBI sites (P ≥ 0.202). Medtronic BI-HRIM correlated with MNBI at 3, 5, 7, and 9 cm above the LES (r ≥ 0.43 and P ≤ 0.047 for all sites), but not at the proximal esophageal channels located at 15 cm and 17 cm above the LES (P ≥ 0.413).
Table 2.
Pearson Correlations With Baseline Impedance From High-resolution Impedance Manometry
| MNBI, cm above the LES | Total cohort (N = 36) | Diversatek (n = 14) | Medtronic (n = 22) |
|---|---|---|---|
| MNBI-17 | –0.10 (P = 0.564) | –0.04 (P = 0.885) | –0.002 (P = 0.993) |
| MNBI-15 | 0.08 (P = 0.663) | –0.07 (P = 0.806) | 0.18 (P = 0.413) |
| MNBI-9 | 0.33 (P = 0.047) | 0.22 (P = 0.451) | 0.56 (P = 0.007) |
| MNBI-7 | 0.40 (P = 0.015) | 0.51 (P = 0.064) | 0.54 (P = 0.010) |
| MNBI-5 | 0.22 (P = 0.190) | 0.36 (P = 0.202) | 0.43 (P = 0.047) |
| MNBI-3 | 0.17 (P = 0.314) | 0.26 (P = 0.379) | 0.44 (P = 0.040) |
| Distal MNBIa | 0.32 (P = 0.058) | 0.39 (P = 0.167) | 0.55 (P = 0.008) |
aDistal mean nocturnal baseline impedance (MNBI) represents the averaged MNBI values for 3,5,7, and 9 cm above the lower esophageal sphincter (LES).
Figure.
Pearson correlations and corresponding P-values for baseline impedance from high-resolution impedance manometry (HRIM) by HRIM system (Medtronic or Diversatek) with mean nocturnal baseline impedance (MNBI) calculated at each Diversatek pH-impedance channel.
DBI-HRIM from Medtronic catheters was also calculated from the most distal esophageal impedance sensor (1471 ± 172 Ω). There were significant correlations between DBI-HRIM and the closest corresponding MNBI values at 3 cm (r = 0.58, P = 0.005) and 5 cm (r = 0.48, P = 0.024; Table 3). However, these correlations incrementally decreased as MNBI was acquired from progressively proximal channels.
Table 3.
Pearson Correlations With Medtronic Distal Baseline Impedance From High-resolution Impedance Manometry
| MNBI, cm above the LES | Medtronic DBI-HRIMa (n = 22) |
|---|---|
| MNBI-17 | 0.03 (P = 0.883) |
| MNBI-15 | 0.16 (P = 0.475) |
| MNBI-9 | 0.24 (P = 0.288) |
| MNBI-7 | 0.34 (P = 0.123) |
| MNBI-5 | 0.48 (P = 0.024) |
| MNBI-3 | 0.58 (P = 0.005) |
| Distal MNBIb | 0.44 (P = 0.038) |
aDistal baseline impedance from high-resolution impedance manometry (DBI-HRIM) represents the baseline impedance from high-resolution impedance manometry (BI-HRIM) obtained from the most distal esophageal impedance sensor using the Medtronic HRIM catheter.
bDistal mean nocturnal baseline impedance (MNBI) represents the averaged MNBI values for 3,5,7, and 9 cm above the lower esophageal sphincter (LES).
Correlations Between Baseline Impedance and Symptoms
Patients presenting with dominant perceptive symptoms had no significant differences in distal composite MNBI values, compared to those with dominant non-perceptive symptoms (perceptive: 1481 ± 125 Ω, non-perceptive: 1549 ± 341 Ω; P = 0.816). Further, there were no differences in BI-HRIM values based on dominant perceptive versus non-perceptive symptoms (Diversatek: 992 ± 90 Ω vs 1315 ± 530 Ω; P = 0.606; Medtronic: 1886 ± 217 Ω vs 1877 ± 395 Ω; P = 0.983). Although GSS and DSI correlated with each other (r = 0.65, P < 0.001), neither symptom burden metric correlated with distal composite MNBI (r < 0.05, P > 0.785), Medtronic BI-HRIM (r < 0.38, P > 0.081), or Diversatek BI-HRIM (r < 0.40, P > 0.158).
Discussion
In this study, we demonstrate that BI acquired from HRIM studies correlates with corresponding MNBI acquired from 24-hour pH-impedance tracings in patients with physiologic AET tested off antisecretory therapy, with numerical differences noted between HRIM manufacturers. Although they shared similar MNBI values across all channels, BI-HRIM acquired from Diversatek catheters was significantly lower than that from Medtronic catheters. Further, BI-HRIM from Medtronic catheters correlated with MNBI across more measurement sites in the distal esophagus, with stronger Pearson correlation values, compared to Diversatek catheters. There were no apparent differences in MNBI or BI-HRIM in patients presenting with dominant perceptive versus non-perceptive symptoms. Finally, no significant associations were noted between symptom burden metrics and BI values, regardless of method of acquisition or manufacturer.
Ambulatory pH-impedance monitoring, which combines esophageal pH monitoring with impedance sensors to define reflux events regardless of acidity, aids in the diagnosis and phenotyping of GERD.19,25,26 Recently, interest in impedance monitoring has focused on assessing esophageal mucosal integrity using baseline or mucosal impedance analysis.12 Specifically, esophageal acid exposure in both animals and humans induces changes in the mucosal integrity of the esophagus, as seen histologically by increased numbers of dilated intracellular spaces, which corresponds with lower mucosal impedance values.8,9,27,28 BI values acquired from pH-impedance tracings (MNBI) have been described to negatively correlate with AET,9 predict symptomatic response with antireflux therapy,13 and improve with the healing of esophagitis.13,29,30 MNBI has also been shown to distinguish non-erosive reflux disease (NERD) and reflux hypersensitivity from functional heartburn and healthy controls.10-12 Compared to PPI-refractory NERD, PPI-responsive NERD is associated with lower MNBI.11,22 Even when AET is borderline or inconclusive, MNBI values correlate with symptomatic outcome with antireflux therapy.14
Since HRIM catheters incorporate technology to measure esophageal impedance,15 BI-HRIM has potential to provide BI data without subjecting patients to ambulatory transnasal catheter-based monitoring. We demonstrate that there are differences between BI recorded from the 2 manufacturers studied, and thresholds defined for one manufacturer may not apply to the other. Recent work from the Mayo group demonstrated that BI acquired from Medtronic HRIM studies correlated with MNBI (r = 0.59), and segregated patients with pathologic AET ≥ 5% from controls (AET ≤ 3%), with a threshold of 1582 Ω having a sensitivity of 86.2% and specificity of 88.5% for GERD.16 While this proposed cutoff for BI-HRIM applies for Medtronic HRIM catheters, this may not be applicable for Diversatek HRIM catheters.
The HRIM catheters differ in sensor morphology between these manufacturers––the Diversatek pressure sensors protrude from the catheter, while the Medtronic pressure sensors are more embedded along the catheter. Prior work has demonstrated variability in HRM software metrics (including integrated relaxation pressures) between solid-state manometry systems,31-35 and the Chicago classification therefore acknowledges differences in threshold values for HRM metrics based on manometry systems (particularly for integrated relaxation pressures).17 Although these differences in solid-state manometry pressure sensors and metrics are established, similar investigations into potential differences in impedance sensing between manometry systems have not been performed, to our knowledge.
Moreover, correlations between MNBI and BI-HRIM differed between Medtronic and Diversatek catheters. However, the optimal HRIM channels from which to extract BI values needs further investigation. Further, impedance values can be influenced by the presence of fluid in the esophageal lumen, and the absence of liquid content within the esophageal lumen cannot be completely excluded during a 10-15 minutes HRIM study, especially if there is concurrent esophageal hypomotility.36
Limitations in our study design temper our conclusions. This was a retrospective cohort study; patients were not randomized to undergo HRIM with a particular catheter manufacturer (or both, to serve as internal controls). Optimally, sequential studies with HRIM catheters from both manufacturers would have provided better comparison. Further, the strict parameters for study inclusion resulted in relatively small cohort sizes that could have resulted in insufficient power to detect potential differences. This study was performed at a tertiary care center among a population of veterans, with a skewed population of more male and older patients than most published esophageal motility cohorts, and thus generalizability to community gastroenterology practices may be limited. However, we believe that better understanding esophageal symptoms and disorders in this sometimes-overlooked population is an important consideration. Finally, treatment approaches and symptom outcome data were unavailable for the cohort.
In summary, we report that BI-HRIM correlates with MNBI extracted from corresponding impedance channels in patients with physiologic esophageal acid burden on testing off antisecretory therapy. Our study is unique in assessment of BI-HRIM between 2 different HRIM manufacturers, demonstrating numeric differences in BI-HRIM based on HRIM manufacturer. Further work is needed to determine the optimal HRIM channels from which to extract BI for maximal clinical value, and comparison in patients with corresponding AET elevation is warranted to better understand the associations between MNBI, BI-HRIM, and esophageal acid exposure.
Footnotes
Financial support: None.
Conflicts of interest: C Prakash Gyawali discloses consulting and speakers’ bureau relationships with Medtronic and Diversatek. The other authors do not declare any pertinent conflicts.
Author contributions: Anthony Horton: study design, data collection and analysis, manuscript preparation, and critical review of manuscript; Brian Sullivan, Katie Charles, Thasha McIntosh, Andrea Davis, Ziad Gellad, and Rahul Shimpi: data collection and critical review of manuscript; C Prakash Gyawali: study design, manuscript preparation, and critical review of manuscript; and Amit Patel: study concept and design, data analysis, manuscript preparation, and final approval of manuscript.
References
- 1.Gyawali CP, Carlson DA, Chen JW, Patel A, Wong RJ, Yadlapati RH. ACG clinical guidelines: clinical use of esophageal physiologic testing. Am J Gastroenterol. 2020;115:1412–1428. doi: 10.14309/ajg.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, Gyawali CP. Gastroesophageal reflux monitoring. JAMA. 2018;319:1271–1272. doi: 10.1001/jama.2018.1144. [DOI] [PubMed] [Google Scholar]
- 3.Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29:1–15. doi: 10.1111/nmo.13067. [DOI] [PubMed] [Google Scholar]
- 4.Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2015;13:884–891. doi: 10.1016/j.cgh.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, Sayuk GS, Kushnir VM, Chan WW, Gyawali C. GERD phenotypes from pH-impedance monitoring predict symptomatic outcomes on prospective evaluation. Neurogastroenterol Motil. 2016;28:513–521. doi: 10.1111/nmo.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredenoord AJ, Wuesten BL, Timmer R, Conchillo JM, Smout AJ. Addition of esophageal impedance monitoring to pH monitoring increases the yield of symptom association analysis in patients off PPI therapy. Am J Gastroenterol. 2006;101:453–459. doi: 10.1111/j.1572-0241.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 7.Penagini R, Sweis R, Mauro A, Domingues G, Vales A, Sifrim D. Inconsistency in the diagnosis of functional heartburn: usefulness of prolonged wireless pH monitoring in patients with proton pump inhibitor refractory gastroesophageal reflux disease. J Neurogastroenterol Motil. 2015;21:265–272. doi: 10.5056/jnm14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farré R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892. doi: 10.1136/gut.2010.233049. [DOI] [PubMed] [Google Scholar]
- 9.Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093–2097. doi: 10.1038/ajg.2011.276. [DOI] [PubMed] [Google Scholar]
- 10.de Bortoli N, Martinucci I, Savarino E, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. 2015;13:1082–1088.:e1. doi: 10.1016/j.cgh.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. 2015;13:1075–1081. doi: 10.1016/j.cgh.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. 2016;14:40–46. doi: 10.1016/j.cgh.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890–898. doi: 10.1111/apt.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rengarajan A, Savarino E, Della Coletta M, Ghisa M, Patel A, Gyawali CP. Mean nocturnal baseline impedance correlates with symptom outcome when acid exposure time is inconclusive on esophageal reflux monitoring. Clin Gastroenterol Hepatol. 2020;18:589–595. doi: 10.1016/j.cgh.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Patel A, Gyawali CP. How to Optimally Apply Impedance in the Evaluation of Esophageal Dysmotility. Curr Gastroenterol Rep. 2016;18:60. doi: 10.1007/s11894-016-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravi K, Geno DM, Vela MF, Crowell MD, Katzka DA. Baseline impedance measured during high-resolution esophageal impedance manometry reliably discriminates GERD patients. Neurogastroenterol Motil Published Online First. 2016 Oct 24; doi: 10.1111/nmo.12974. doi: 10.1111/nmo.12974. [DOI] [PubMed] [Google Scholar]
- 17.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blonski W, Hila A, Vela MF, Castell DO. An analysis of distal esophageal impedance in individuals with and without esophageal motility abnormalities. J Clin Gastroenterol. 2008;42:776–781. doi: 10.1097/MCG.0b013e31806daf77. [DOI] [PubMed] [Google Scholar]
- 19.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiener GJ, Richter JE, Copper JB, Wu WC, Castell DO. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83:358–361. [PubMed] [Google Scholar]
- 21.Ghillebert G, Janssens J, Vantrappen G, Nevens F, Piessens J. Ambulatory 24 hour intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31:738–744. doi: 10.1136/gut.31.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546–555. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 23.Patel A, Sayuk GS, Gyawali CP. Acid-based parameters on pH-impedance testing predict symptom improvement with medical management better than impedance parameters. Am J Gastroenterol. 2014;109:836–844. doi: 10.1038/ajg.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A, Cassell B, Sainani N, et al. Comparison of motor diagnoses by Chicago Classification versions 2.0 and 3.0 on esophageal high-resolution manometry. Neurogastroenterol Motil Published Online First. 2017 Feb 23; doi: 10.1111/nmo.13042. doi: 10.1111/nmo.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–1043. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 26.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55:1398–1402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ates F, Yuksel ES, Higginbotham T, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. 2015;148:334–343. doi: 10.1053/j.gastro.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48:601–610. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1815–1823.:e2. doi: 10.1016/j.cgh.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Frazzoni M, de Bortoli N, Frazzoni L, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil Published Online First. 2016 Sep 12; doi: 10.1111/nmo.12947. doi: 10.1111/nmo.12947. [DOI] [PubMed] [Google Scholar]
- 31.Rengarajan A, Drapekin J, Patel A, Gyawali CP. Comparison of two high-resolution manometry software systems in evaluating esophageal motor function. Neurogastroenterol Motil. 2016;28:1836–1843. doi: 10.1111/nmo.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herregods TV, Roman S, Kahrilas PJ, Smout AJ, Bredenoord AJ. Normative values in esophageal high-resolution manometry. Neurogastroenterol Motil. 2015;27:175–187. doi: 10.1111/nmo.12500. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Xiao Y, Peng S, Lin J, Xiong L, Chen M. Normative data of high-resolution impedance manometry in the Chinese population. J Gastroenterol Hepatol. 2013;28:1611–1615. doi: 10.1111/jgh.12285. [DOI] [PubMed] [Google Scholar]
- 34.Kuribayashi S, Iwakiri K, Kawada A, et al. Variant parameter values-as defined by the Chicago criteria-produced by manoscan and a new system with unisensor catheter. Neurogastroenterol Motil. 2015;27:188–194. doi: 10.1111/nmo.12446. [DOI] [PubMed] [Google Scholar]
- 35.Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Normal values for esophageal high-resolution manometry. Neurogastroenterol Motil. 2013;25:762–e579. doi: 10.1111/nmo.12167. [DOI] [PubMed] [Google Scholar]
- 36.Horton A, Posner S, Sullivan B, et al. Esophageal contractile segment impedance from high-resolution impedance manometry correlates with mean nocturnal baseline impedance and acid exposure time from 24-hour pH-impedance monitoring. Dis Esophagus Published Online First. 2020 Jul 1; doi: 10.1093/dote/doaa063. doi: 10.1093/dote/doaa063. [DOI] [PubMed] [Google Scholar]