Abstract
Background/Aims
Impaired esophageal motility and disrupted esophagogastric junction (EGJ) on high-resolution manometry (HRM) have been associated with increased reflux severity in gastroesophageal reflux disease (GERD) patients. However, there are limited data evaluating HRM parameters in proton pump inhibitors (PPI) non-responders.
Methods
Clinical and endoscopic data, HRM and multichannel intraluminal impedance-pH studies performed of PPI therapy in patients with typical GERD symptoms were reviewed from 3 international centers. Frequency of GERD symptoms was assessed on and off PPI therapy in both non-responders (< 50% symptom improvement on PPI therapy) and responders. Rome IV definitions identified non-erosive reflux disease, reflux hypersensitivity, and functional heartburn. Univariate and multivariate analyses were performed to determine predictors of non-response.
Results
Of 204 patients, 105 were PPI non-responders and 99 were responders. Non-responders showed higher EGJ contractile integral values, and a lower frequency of type II and III EGJ morphology (P ≤ 0.03 for each comparison). Esophageal body diagnoses on HRM (fragmented peristalsis, ineffective esophageal motility, or absent peristalsis) did not predict non-response. On multivariate analysis, non-pathological acid exposure time (OR, 2.5; 95% CI, 1.2-5.0; P < 0.001), normal mean nocturnal baseline impedance values (OR, 2.7-2.4; 95% CI, 1.0-6.1; P < 0.05), normal EGJ contractile integral values (OR, 3; 95% CI, 1.3-7.4; P = 0.012), and presence of type I EGJ morphology (OR, 1.9; 95% CI, 1.0-3.4; P = 0.044) were associated with an unfavorable response to PPIs.
Conclusions
Intact EGJ metrics on HRM complement normal reflux burden in predicting non-response to PPI therapy. HRM has value in the evaluation of PPI non-responders.
Keywords: Esophagogastric junction, Gastroesophageal reflux, Heartburn, Manometry, Proton pump inhibitors
Introduction
Gastroesophageal reflux disease (GERD), defined by the presence of esophageal and extra-esophageal symptoms due to pathological reflux of gastric content, represents one of the most common gastrointestinal disorders, with an increasing worldwide prevalence.1-3 However, as many as 40% of patients with GERD symptoms report an unsatisfactory response to acid suppression with proton pump inhibitor (PPI) therapy,4,5 and evaluating pathophysiology and mechanisms of symptom generation are critical to further investigation of these patients.
With the development of high-resolution manometry (HRM), more specific evaluation of esophageal motor function has expanded our knowledge of inter-relationships with GERD.6,7 HRM also allows precise characterization of esophagogastric junction (EGJ) morphology and identification of hiatus hernia.8 The esophagogastric junction contractile integral (EGJ-CI) is a HRM tool that assesses EGJ barrier function,9 and low EGJ-CI is associated with abnormal total and supine acid burden.10 Abnormal EGJ morphology is associated with a higher probability of positive multichannel intraluminal impedance pH (MII-pH) monitoring compared to normal EGJ morphology, and both EGJ morphology and EGJ-CI independently predict esophageal reflux burden.11
While EGJ disruption or dysfunction is a primary pathophysiologic factor in determining reflux occurrence, esophageal body motor function influences duration of contact of the refluxate with the esophageal mucosa. Consequently, impaired esophageal peristalsis may account for delayed bolus transit and reduced esophageal reflux clearance in patients with GERD.12 Early studies using conventional manometry demonstrated higher prevalence of low amplitude (< 30 mmHg) or non-transmitted esophageal body contractions in GERD patients, and these motility abnormalities increase in parallel with the severity of GERD.13,14 For instance, the presence of large breaks in esophageal peristaltic integrity on HRM is associated with significantly prolonged supine reflux clearance, higher acid exposure time (AET), and erosive esophagitis.15 Large breaks are often identified in the context of suspected GERD-related chronic cough,16,17 the presence of which can be associated with suboptimal benefit from antireflux therapy.16 Finally, failed swallows, which represent panesophageal breaks in peristaltic integrity, predict abnormal AET better than ineffective swallows without long breaks in peristaltic integrity.8
Despite these advances in assessment of esophageal motor function, no studies have evaluated HRM parameters in PPI non-responders. The present investigation is a multicenter, observational study aimed at evaluating and comparing HRM parameters between PPI responders and non-responders in patients with GERD symptoms.
Materials and Methods
Patients
Adult patients (age > 18 years) with suspected GERD evaluated with HRM and 24-hour MII-pH monitoring at 3 centers (2 in Europe and 1 in the United States) over a 2-year period (2017-2019) for symptoms unresponsive to acid suppressive therapy or prior to anti-reflux surgery were eligible for inclusion in this retrospective observational cohort study. Further inclusion criteria consisted of the presence of dominant esophageal symptoms (heartburn, regurgitation, and chest pain),18 ambulatory MII-pH studies performed off acid-suppressive therapy (at least 7 days pharmacological wash-out),19 and 10 acceptable supine water swallows for HRM analysis using Chicago classification version 3.0 (CC v3.0).20 Patients with inadequate studies (equipment malfunction, poor study quality, and artifacts) and/or incomplete HRM studies were excluded. Patients with achalasia spectrum disorders (integrated relaxation pressure > 15 mmHg), connective tissue disease, history of neoplasia and prior foregut surgery were also excluded. The study protocol was approved by the Institutional Review Board of the 3 University Centers (IRB No. 201607083), and each collaborating institution completed data sharing agreements for analysis of deidentified demographic, clinical, MII-pH and HRM data. Written informed consent was obtained from all patients.
While esophageal symptoms were required for study inclusion, the presence of extra-esophageal symptoms (chronic cough, asthma, hoarseness, and globus) was also recorded. Symptom frequency (based on number of symptom episodes/week) of esophageal symptoms was assessed before and after at least 8 weeks of standard dose PPI therapy (Esomeprazole 40 mg once daily [od], Pantoprazole 40 mg od, Lansoprazole 30 mg od, and Omeprazole 20 mg od) within the previous year on validated institutional self-report Likert scales on patient questionnaires at each study site.21-24 Patients were categorized as non-responders if symptom improvement while on therapy, using these scales, was < 50% compared to symptom assessment off therapy.25
Esophageal High-resolution Manometry
A catheter with 36 circumferential solid state pressure sensors, located at 1-cm intervals (Medtronic, Minneapolis, MN, USA) was inserted, after an overnight fast, through an anesthetized nostril such that at least 3 distal pressure sensors positioned in the stomach. The manometric study was performed using ten 5 mL swallows of ambient temperature fluid at 30-second intervals in a semi-recumbent position.26
Each HRM study was evaluated using the following CC v3.0 criteria20: (1) intact swallow: distal contractile integral (DCI) > 450 mmHg∙cm∙sec; (2) fragmented swallow: DCI > 450 mmHg∙cm∙sec with > 5 cm breaks; (3) weak swallow: DCI 100-450 mmHg∙cm∙sec; AND (4) failed swallow: DCI < 100 mmHg∙cm∙sec. CC v3.0 diagnoses consisted of the following: (1) fragmented peristalsis: ≥ 50% fragmented swallows; (2) ineffective esophageal motility (IEM): ≥ 50% of any combination of weak or failed swallows; and (3) absent contractility: 100% failed swallows. EGJ barrier function and morphology were recorded. EGJ-CI was evaluated by recording the EGJ barrier vigor (using a DCI like tool) during a period of quiet rest over exactly 3 respiratory cycles, and divided by the duration of the respiratory cycles to make the metric independent of respiration. EGJ-CI was considered low when < 39.1 mmHg∙cm.8,9
EGJ morphology was determined by the relationship between lower esophageal sphincter (LES) and crural diaphragm; type I when LES and crural diaphragm were superimposed, type II when separated < 3 cm, and type III when separated ≥ 3 cm.7
24-Hour Multichannel Intraluminal Impedance pH Monitoring
MII-pH was recorded using a 2.3 mm diameter polyvinyl catheter assembly containing a series of impedance electrodes, each 4 mm in axial length, spaced at 2-cm intervals, and a distal antimony pH electrode (Sandhill Scientific Inc, Highlands Ranch, CO, USA). The pH electrodes were calibrated using pH 4.0 and pH 7.0 buffer solutions before pH-impedance monitoring. Following HRM, the MII-pH assembly was passed through the anesthetized nostril, and positioned with the pH electrode 5 cm above the LES, and impedance electrodes at 3, 5, 7, 9, 15, and 17 cm proximal to the LES. Event markers, corroborated with paper diaries, were used to record symptoms, meal times, and supine periods. AET was defined as pathological if the time pH < 4 exceeded 6% of the total recording time,19,27 and non-pathologic if < 6%. Reflux-symptom association was assessed using symptom association probability (SAP) for all reflux episodes using previously described methodology.28 Mean nocturnal baseline impedance (MNBI) was calculated by measuring baseline impedance values at 3 cm and 5 cm above LES, across stable nocturnal 10-minute periods (at or around 1, 2, and 3 AM). The values from the 3 time periods for both levels were averaged to yield the mean nocturnal baseline impedance (MNBI) for each channel. Values < 2292 Ω defined abnormal studies. 29
Gastroesophageal Reflux Disease Phenotypes
Endoscopy negative patients with abnormal AET were defined as having non-erosive reflux disease (NERD).30 In the context of esophageal symptoms, patients with normal AET but positive SAP were diagnosed as reflux hypersensitivity (RH), and those with normal AET and negative SAP were classified as functional heartburn (FH).27,30
Statistical Methods
Data are presented as mean ± standard deviation. Comparisons between groups were assessed using the Fisher’s exact test. Group means were compared using ANOVA with Bonferroni correction. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated to assess the association between AET, MNBI at 3 cm and 5 cm above the LES, EGJ-CI, EGJ morphology, and the PPI response. Multivariate regression models were generated to evaluate if HRM and 24-hour MII-pH parameters were independent predictors for PPI response or non-response. Significance was achieved when the P-value was < 0.05. Statistical analysis was performed using SPSS version 16.0 software (SPSS Inc, Chicago, IL, USA).
Results
Study Population
A total of 204 patients with esophageal symptoms fulfilled inclusion criteria and formed the study cohort (Table 1). Among the included patients, 105 (54%) non-responders to prior PPI therapy while 99 (46%) were responders; these did not differ in terms of sex, age, and body mass index. Extra-esophageal symptoms were reported by approximately a third of both non-responders and responders, with similar proportions of chronic cough (24% vs 30%, respectively), asthma (6% vs 4%), hoarseness (10% vs. 11%), and globus (6% vs 5%, P > 0.05 for each comparison). A total of 21 patients had erosive reflux disease (ERD) (15 grade A, 5 grade B, and 1 grade C according to Los Angeles classification). Proportions of ERD patients and endoscopically identified hiatus hernia were also similar (Table 1).
Table 1.
Demographic and Clinical Characteristics in Non-responders and Responders
| Demographic/clinical characteristics | Non-responders (n = 105) |
Responders (n = 99) |
P-value |
|---|---|---|---|
| Male/female | 46/59 | 43/56 | 0.891 |
| Age (yr) | 50 (21.1) | 47 (18.3) | 0.807 |
| BMI (kg/m2) | 23.1 (1.9) | 22.4 (2.1) | 0.774 |
| Presence of atypical symptoms | 36 (34%) | 29 (29%) | 0.452 |
| Erosive esophagitis | 10 (9%) | 11 (11%) | 0.816 |
| Hiatal hernia | 19 (18%) | 16 (16%) | 0.847 |
BMI, body mass index.
Data are presented as number, mean (SD), or number (%).
Multichannel Intraluminal Impedance pH Data in Non-responders and Responders
Esophageal acid burden, as measured by AET and MNBI, was lower in non-responders (Table 2). Non-responders demonstrated significantly lower mean total and supine AET compared to responders (P ≤ 0.03 for each comparison), despite similar mean upright AET values (P = 0.229). MNBI values at both the 3 cm and 5 cm locations were significantly higher in non-responders (P ≤ 0.03 for each comparison). Non-responders and responders reported similar numbers of reflux episodes (P= 0.347).
Table 2.
Esophageal Physiologic Test Results in Responders and Non-responders
| Esophageal physiologic test variables | Non-responders (n = 105) |
Responders (n = 99) |
P-value |
|---|---|---|---|
| MII-pH | |||
| AET (%) | 4.2 (1.3) | 7.8 (2.4) | 0.034 |
| Upright AET (%) | 6.2 (2.1) | 6.7 (2.3) | 0.225 |
| Supine AET (%) | 2.1 (0.9) | 9.0 (3.4) | 0.003 |
| Reflux episodes | 51 (17) | 64 (23) | 0.344 |
| MNBI 3 cm (Ω) | 2108 (412) | 1607 (235) | 0.027 |
| MNBI 5 cm (Ω) | 2057 (436) | 1654 (258) | 0.024 |
| HRM Basal LES pressure (mmHg) |
23.7 (3.3) | 20.4 (2.8) | 0.033 |
| EGJ-CI (mmHg∙cm) | 30 (4.2) | 22 (2.1) | 0.011 |
| IRP (mmHg) | 8.2 (1.5) | 7.7 (1.2) | 0.448 |
| DCI (mmHg∙cm∙sec) | 1641 (158) | 1236 (116) | 0.029 |
| Absent peristalsis | 7 (7%) | 8 (8%) | 0.789 |
| DES | 1 (1%) | 0 (0.%) | 0.884 |
| Fragmented peristalsis | 10 (9%) | 5 (5%) | 0.278 |
| IEM | 11 (10%) | 15 (15%) | 0.402 |
| Normal peristalsis | 76 (73%) | 71 (72%) | 0.896 |
MII-pH, multichannel intraluminal impedance pH; AET, acid exposure time; MNBI, mean nocturnal baseline impedance; HRM, high-resolution manometry; LES, lower esophageal sphincter; EGJ-CI, esophagogastric junction contractile integral; IRP, integrated relaxation pressure; DCI, distal contractile integral; DES, distal esophageal spasm; IEM, ineffective esophageal motility.
Data are presented as mean (SD) or number (%).
High-resolution Manometry Data in Non-responders and Responders
Non-responders demonstrated significantly higher mean basal LES pressure and EGJ-CI values compared to responders (P ≤ 0.03 for each comparison), while mean integrated relaxation pressure values were comparable between the 2 groups (P = 0.438). Mean DCI values were significantly higher in non-responders compared to responder patients (P = 0.029). Proportions of patients with absent peristalsis, distal esophageal spasm (DES), fragmented peristalsis and IEM were comparable between non-responders and responders (P ≥ 0.3 for each comparison) (Table 2).
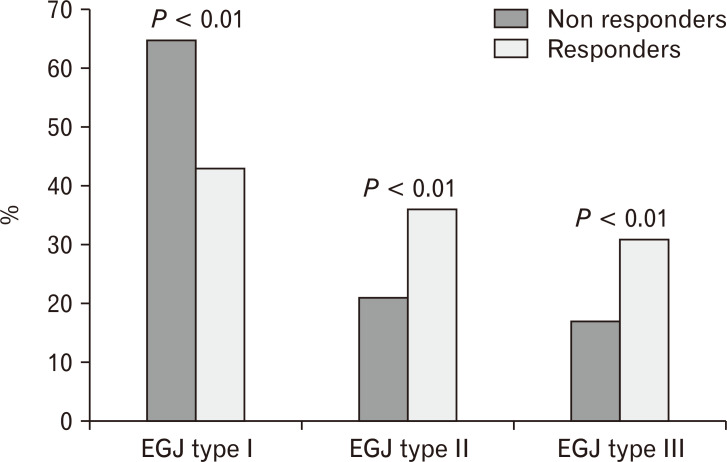
There was a significantly lower frequency of type II and III EGJ morphology in non-responders compared to responders (31% and 36% vs 17% and 21%, respectively; P < 0.01) (Figure).
Figure.
Proportion of responders and non-responders in patients with Type I, II, and III esophagogastric junction (EGJ). There were statistically more non-responders among patients with Type 1 EGJ. In contrast, with EGJ disruption, the likelihood of response was statistically higher, indirectly indicating that the presence of EGJ disruption was a marker for abnormal reflux burden.
On univariate analysis, non-pathologic AET (OR [95% CI], 3 [1.5-5.9]; P = 0.001), normal MNBI values at both the 3 cm and 5 cm locations (OR [95% CI], 1.9 [1.1-3.1]; P = 0.041 and 1.8 [1.1-2.9]; P = 0.043), normal EGJ-CI values (OR [95% CI]: 3.4 [1.4-8.0]; P = 0.006), and type I EGJ morphology were independent predictors of non-response to PPI (OR [95% CI], 1.9 [1.0-3.4], P = 0.046). In contrast, presence of type II and III EGJ was associated with a significantly higher probability of PPI response (OR [95% CI], 2 [1.4-2.7]; P < 0.01).
On multivariate analysis, non-pathologic AET, normal MNBI values at both 3 cm and 5 cm above the LES, normal EGJ-CI values and presence of type I EGJ morphology were associated with non-response to PPIs (Table 3). On the other hand, pathologic AET, abnormal MNBI at both 3 cm and 5 cm above the LES, abnormally low EGJ-CI values and presence of type II-III EGJ morphology were associated with PPI response (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Predictors of Proton Pump Inhibitors Non-response
| Predictors of PPIs non-response | OR (95% CI) | P-value |
|---|---|---|
| AET < 6% | 2.5 (1.2-5.0) | 0.011 |
| Number of reflux episodes | 1.3 (0.6-2.9) | 0.608 |
| MNBI 3 cm > 2292 Ω | 2.7 (1.0-6.1) | 0.047 |
| MNBI 5 cm > 2292 Ω | 2.4 (1.0-5.6) | 0.046 |
| EGJ-CI > 39.1 mmHg∙cm | 3 (1.3-7.1) | 0.012 |
| Type I EGJ | 1.8 (1.0-3.4) | 0.047 |
| IEM | 1.8 (0.7-5.2) | 0.225 |
| Fragmented peristalsis | 0.5 (0.2-1.6) | 0.324 |
| Absent peristalsis | 0.7 (0.4-2.7) | 0.778 |
PPIs, proton pump inhibitors; AET, acid exposure time; MNBI, mean nocturnal baseline impedance; EGJ-CI, esophagogastric junction contractile integral; IEM, ineffective esophageal motility.
Gastroesophageal Reflux Disease Phenotypes
Among the 183 endoscopy-negative patients, 50 (27%) were phenotyped as NERD according to MII-pH findings. Fifty-six (31%) had RH, and the remaining 77 patients (42%), with normal AET and negative SAP, were characterized as FH. Sixteen patients out of the 105 non-responders (15%) and 34 patients out of the 99 responders (34%) fulfilled criteria for NERD (P = 0.002).
Mean basal LES pressure, EGJ-CI and DCI values were significantly lower in ERD and NERD compared to FH and in RH patients, but other HRM parameters did not differ among the 3 groups. In the NERD and ERD group, a significantly higher proportion of patients had evidence of esophageal body hypomotility features (absent peristalsis, fragmented peristalsis, and IEM) compared to FH (P ≤ 0.003 for each comparison, Table 4).
Table 4.
High-resolution Manometry Findings in ERD, NERD, RH and FH patients
| HRM findings | ERD (n = 21) | NERD (n = 50) | RH (n = 56) | FH (n = 77) |
|---|---|---|---|---|
| Basal LES pressure (mmHg) | 20.8 (2.4)a | 20.9 (2.5)a | 28.6 (3.2) | 29.4 (2.8) |
| EGJ-CI (mmHg∙cm) | 17.5 (4.9)a | 21.0 (4.5)a | 30.6 (5.9) | 32.4 (6.6) |
| IRP (mmHg) | 6.8 (1.2) | 7.1 (1.1) | 8.2 (1.5) | 9.1 (1.9) |
| DCI (mmHg∙cm∙sec) | 1246 (123)a | 1123 (128)a | 1769 (158) | 1989 (201) |
| Absent peristalsis | 4 (19%)b | 6 (12%)b | 4 (7%) | 1 (1%) |
| DES | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Fragmented peristalsis | 2 (10%)b | 9 (18%)b | 3 (5%) | 1 (1%) |
| IEM | 4 (19%)a | 9 (18%)a | 8 (15%)a | 3 (4%) |
| Normal peristalsis | 30 (55%) | 26 (52%) | 38 (71%) | 72 (94%) |
aP < 0.05 vs functional heartburn (FH).
bP < 0.01 vs FH.
HRM, high-resolution manometry; ERD, erosive reflux disease; NERD, non-erosive reflux disease; RH, reflux hypersensitivity; LES, lower esophageal sphincter; EGJ-CI, esophagogastric junction contractile integral; IRP, integrated relaxation pressure; DCI, distal contractile integral; DES, distal esophageal spasm; IEM, ineffective esophageal motility.
Data are presented as mean (SD) or number (%).
Discussion
In this observational, international multicenter study, we demonstrate that esophageal HRM is more likely to show intact EGJ metrics in non-responders to acid suppressive therapy, suggesting that the generation of esophageal symptoms occur without evidence of abnormal GERD pathophysiology at the EGJ. In contrast, abnormal EGJ morphology and barrier function were strongly associated with PPI response, indicating that these features are primary HRM markers of abnormal reflux burden in the esophagus. Indeed, similar EGJ disruption is demonstrated in patients with characteristics of NERD, in contrast to FH. We conclude that evaluation of esophageal motor function, and interpretation of HRM using CC v3.0 as well as novel EGJ metrics is useful in the investigation of PPI non-responders.
We made efforts to select a large group of patients with esophageal symptoms across multiple centers in order to evaluate a representative patient cohort, with meticulous characterization into responders and non-responders based on patient questionnaires and therapeutic outcome evaluation. Indeed, our designation into responder status appears appropriate, since responders had higher AET as well as lower MNBI, both features that have been documented to predict treatment response.31-33 While it is conceivable that non-responders may have had higher proportions of weakly acid reflux episodes, the overall proportions of reflux episodes were similar between responders and non-responders (Table 2). Therefore, it is more likely that non-responders may have non-reflux mechanisms of symptom generation.34
Our findings demonstrate that PPI non-responders have a higher likelihood of having intact EGJ barrier function, and intact esophageal body contraction vigor, compared to responders. We demonstrate a higher likelihood of PPI response in the presence of a hiatus hernia (EGJ morphology types II and III), which is a known marker for abnormal esophageal reflux burden compared to normal EGJ morphology (type I EGJ, no hiatus hernia).8 Our findings therefore concur with data that demonstrate that abnormal esophageal acid burden predicts PPI response.35 Further, our MNBI data also supports the fact that abnormal values of MNBI at 3 cm and 5 cm above the LES are characterized by a favorable response to acid-suppressive therapy.33 The EGJ-CI is a novel HRM tool that is gaining acceptance as a metric for assessment of EGJ barrier function. This has been demonstrated to be associated with abnormal reflux burden, especially when esophageal body evaluation shows IEM, since IEM is another HRM metric that predicts abnormal reflux burden.36 Supporting these conclusions, non-responders in our study demonstrate higher basal LES pressure, EGJ-CI, and DCI mean values, as well as lower total and supine mean AET values. Consistent with previous reports, the manometric finding of a hiatus hernia (EGJ morphology types II and III, 44% of patients) was higher than the endoscopic recording of a hiatus hernia (17% of patients, P < 0.01). It is now well recognized that HRM is more accurate than endoscopy in the identification of a hiatus hernia,37,38 which adds to the value of HRM in the context of GERD, particularly since our data and other reports demonstrate that a manometric hiatus hernia is predictive of abnormal reflux burden,36,39 and our current findings show a higher proportion of PPI responders with a manometric hiatus hernia.
While EGJ disruption or dysfunction is a primary pathophysiologic factor in determining reflux occurrence, esophageal body motor function influences duration of contact of the refluxate with the esophageal mucosa. Consequently, impaired esophageal peristalsis may account for delayed bolus transit and reduced esophageal reflux clearance in patients with GERD.9 Large breaks are often identified in the context of suspected GERD-related chronic cough,16,17 the presence of which can be associated with suboptimal benefit from antireflux therapy.16 Finally, failed swallows, which represent panesophageal breaks in peristaltic integrity, predict abnormal AET better than ineffective swallows without long breaks in peristaltic integrity.10 In this study, we did not identify any of these esophageal body motor findings as predictors of PPI non-response.
Our results also show that mean basal LES pressure and EGJ-CI values were significantly lower in ERD and NERD patients compared to FH and in RH patients. Moreover, a significantly higher proportion of esophageal body hypomotility (fragmented peristalsis, IEM, and absent contractility) was also observed in these cohorts compared to FH. This supports the argument that the modern definition of NERD, which requires abnormal esophageal reflux burden in addition to absence of erosive disease, demonstrates abnormal EGJ and esophageal body motor characteristics that have been shown to be associated with abnormal reflux burden. This is consistent with existing evidence that suggests that PPI non-response may be a marker for non-GERD mechanisms of symptom generation,34 wherein motor features associated with GERD pathophysiology are identified less often, and MII-pH demonstrates features of FH. Our findings support the classification of motor findings proposed by the GERD consensus group, where hierarchical reporting of EGJ and esophageal body motor features is recommended in HRM studies performed in the context of GERD.7
To our knowledge, this is the first study evaluating the role of the HRM parameters and their association with PPI response in a large series of responders and non-responders. Strengths of the present study are the number of patients included and rigorous selection process. However, some limitations temper the strength of our findings, the predominant limitation relating to retrospective patient identification and data analysis for the purpose of this multicenter study, despite the fact that data collection was prospectively performed independent of the current study across the 3 sites. Questionnaire data can be subject to recall bias. Patients were evaluated after at least 8-week PPI treatment, however some patients have been evaluated after a longer period of standard dose PPI therapy. Additional psychological and psychosocial factors influencing symptom presentation and PPI non-response, as well as alternate medications (such as neuromodulators) contributing to symptom response were not addressed. Moreover, it is well established that multiple factors contribute to non-response, and our designation of non-response could have been biased by patient related factors that were not evaluated in this study. Finally, we could not address further outcome following the performance of these esophageal tests and due to the retrospective nature of the study. Nevertheless, despite these limitations, our results demonstrate the value of assessing esophageal motor function in patients with persisting esophageal symptoms, and identify a clear role for HRM in these clinical settings.
In summary, our results demonstrate that impaired esophageal function on HRM associates with increased reflux burden, and consequently, a better response to PPI therapy. On the other hand, patients not responding to acid suppressive therapy are characterized by a less severe reflux burden and better HRM metrics, implying a non-GERD mechanism for symptom persistence. These results lead to the conclusion that HRM should be performed and taken into account before subjecting patients with symptoms suggestive of GERD to anti-reflux therapies, and that the future iteration of the CC should incorporate this information in the context of assessment of patients with GERD.
Footnotes
Financial support: None.
Conflicts of interest: Edoardo Savarino participated in consulting (Allergan, MSD, Takeda, Sofar, and Janssen) and teaching and speaking (Medtronic, Reckitt-Benckiser, Malesci, and Zambon); and C Prakash Gyawali participated in consulting (Ironwood, Torax, and Quintiles) and Teaching and speaking (Medtronic and Diversatek).
Author contributions: Mentore Ribolsi: study concept and design, data analysis, and manuscript preparation; Edoardo Savarino: study design, data interpretation, and manuscript preparation; Benjamin Rogers, Arvind Rengarajan, Marco Della Coletta, and Matteo Ghisa: data collection, data interpretation, and critical review of manuscript; Michele Cicala: data interpretation and critical review of manuscript; and C Prakash Gyawali: study concept and design, data interpretation, manuscript preparation, critical review, and final approval of manuscript.
References
- 1.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1156. doi: 10.1016/S0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21:953–956. doi: 10.1007/BF01071906. [DOI] [PubMed] [Google Scholar]
- 4.Fass R. Proton pump inhibitor failure--what are the therapeutic options? Am J Gastroenterol. 2009;104(suppl 2):S33–S38. doi: 10.1038/ajg.2009.50. [DOI] [PubMed] [Google Scholar]
- 5.Bytzer P, van Zanten SV, Mattsson H, Wernersson B. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis - a post hoc analysis of 5796 patients. Aliment Pharmacol Ther. 2012;36:635–643. doi: 10.1111/apt.12007. [DOI] [PubMed] [Google Scholar]
- 6.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 7.Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29:e13104. doi: 10.1111/nmo.13104. [DOI] [PubMed] [Google Scholar]
- 8.Rengarajan A, Bolkhir A, Gor P, Wang D, Munigala S, Gyawali CP. Esophagogastric junction and esophageal body contraction metrics on high resolution manometry predict esophageal acid burden. Neurogastroenterol Motil. 2018;30:e13267. doi: 10.1111/nmo.13267. [DOI] [PubMed] [Google Scholar]
- 9.Gor P, Li Y, Munigala S, Patel A, Bolkhir A, Gyawali CP. Interrogation of esophagogastric junction barrier function using the esophagogastric junction contractile integral: an observational cohort study. Dis Esophagus. 2016;29:820–828. doi: 10.1111/dote.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–1063. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil. 2015;27:1175–1182. doi: 10.1111/nmo.12606. [DOI] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Dodds WJ, Hogan WJ, Kern M, Arndorfer RC, Reece A. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91:897–904. doi: 10.1016/0016-5085(86)90692-X. [DOI] [PubMed] [Google Scholar]
- 13.Leite LP, Johnston BT, Barrett J, Castell JA, Castell DO. Ineffective esophageal motility (IEM): the primary finding in patients with nonspecific esophageal motility disorder. Dig Dis Sci. 1997;42:1859–1865. doi: 10.1023/A:1018802908358. [DOI] [PubMed] [Google Scholar]
- 14.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476–486. doi: 10.1111/j.1365-2036.2011.04742.x. [DOI] [PubMed] [Google Scholar]
- 15.Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46–51. doi: 10.1038/ajg.2013.373. [DOI] [PubMed] [Google Scholar]
- 16.Bennett MC, Patel A, Sainani N, Wang D, Sayuk GS, Gyawali CP. Chronic cough is associated with long breaks in esophageal peristaltic integrity on high-resolution manometry. J Neurogastroenterol Motil. 2018;24:387–394. doi: 10.5056/jnm17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almansa C, Smith JA, Morris J, et al. Weak peristalsis with large breaks in chronic cough: association with poor esophageal clearance. Neurogastroenterol Motil. 2015;27:431–442. doi: 10.1111/nmo.12513. [DOI] [PubMed] [Google Scholar]
- 18.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group, author. The montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 19.Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29:1–15. doi: 10.1111/nmo.13067. [DOI] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsson R, Dent J, Bolling-Sternevald E, et al. Bolling-Sternevald E, et al. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 22.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 24.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 25.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340–1354. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 26.Savarino E, de Bortoli N, Bellini M, et al. Practice guidelines on the use of esophageal manometry - a GISMAD-SIGE-AIGO medical position statement. Dig Liver Dis. 2016;48:1124–1135. doi: 10.1016/j.dld.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741–1745. doi: 10.1016/0016-5085(94)90815-X. [DOI] [PubMed] [Google Scholar]
- 29.Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546–555. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 30.Aziz Q, Fass R, Gyawali CP, et al. Functional esophageal disorders. Gastroenterology. 2016;150:1368–1379. doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2015;13:884–891. doi: 10.1016/j.cgh.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890–898. doi: 10.1111/apt.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frazzoni L, Frazzoni M, de Bortoli N, et al. Postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance can link PPI-responsive heartburn to reflux better than acid exposure time. Neurogastroenterol Motil. 2017;29:e13116. doi: 10.1111/nmo.13116. [DOI] [PubMed] [Google Scholar]
- 34.Herregods TV, Troelstra M, Weijenborg PW, Bredenoord AJ, Smout AJ. Patients with refractory reflux symptoms often do not have GERD. Neurogastroenterol Motil. 2015;27:1267–1273. doi: 10.1111/nmo.12620. [DOI] [PubMed] [Google Scholar]
- 35.Patel A, Sayuk GS, Gyawali CP. Acid-based parameters on pH-impedance testing predict symptom improvement with medical management better than impedance parameters. Am J Gastroenterol. 2014;109:836–844. doi: 10.1038/ajg.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rengarajan A, Gyawali CP. High-resolution manometry can characterize esophagogastric junction morphology and predict esophageal reflux burden. J Clin Gastroenterol. 2020;54:22–27. doi: 10.1097/MCG.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 37.Weijenborg PW, van Hoeij FB, Smout AJ, Bredenoord AJ. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil. 2015;27:293–299. doi: 10.1111/nmo.12507. [DOI] [PubMed] [Google Scholar]
- 38.Tolone S, Savarino E, Zaninotto G, et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: a comparison with surgical in vivo evaluation. United European Gastroenterol J. 2018;6:981–989. doi: 10.1177/2050640618769160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciriza-de-los-Ríos C, Canga-Rodríguez-Valcárcel F, Castel-de-Lucas I, Lora-Pablos D, de-la-Cruz-Bértolo J, Castellano-Tortajada G. How useful is esophageal high resolution manometry in diagnosing gastroesophageal junction disruption: causes affecting this disruption and its relationship with manometric alterations and gastroesophageal reflux. Rev Esp Enferm Dig. 2014;106:22–29. doi: 10.4321/S1130-01082014000100004. [DOI] [PubMed] [Google Scholar]