Significance
Amyotrophic laterosclerosis (ALS) is a rapidly progressing neurological disease that robs patients’ motor functions. Despite intensive research, molecular events that initiate the disease are poorly understood. Expansion of G4C2 repeats in the C9orf72 gene causes ALS with frontotemporal dementia, one of the most common forms of ALS. Increasing evidence suggests that dipeptides translated from G4C2 repeat transcripts, especially the arginine-containing poly(GR) and poly(PR), are particularly toxic. We found that translation of poly(GR) can occur on mitochondrial surface and is frequently stalled, triggering ribosome-associated quality control and C-terminal extension, which promote poly(GR) aggregation and toxicity. Genetic studies uncovered conserved roles of mitochondrial protease YME1L and noncanonical Notch signaling in restraining poly(GR), offering insights into disease pathogenesis and targets for therapeutic intervention.
Keywords: C9-ALS/FTD, ribosome-associated quality control, CAT-tailing, YME1L, Notch
Abstract
Maintaining the fidelity of nascent peptide chain (NP) synthesis is essential for proteome integrity and cellular health. Ribosome-associated quality control (RQC) serves to resolve stalled translation, during which untemplated Ala/Thr residues are added C terminally to stalled peptide, as shown during C-terminal Ala and Thr addition (CAT-tailing) in yeast. The mechanism and biological effects of CAT-tailing–like activity in metazoans remain unclear. Here we show that CAT–tailing-like modification of poly(GR), a dipeptide repeat derived from amyotrophic lateral sclerosis with frontotemporal dementia (ALS/FTD)-associated GGGGCC (G4C2) repeat expansion in C9ORF72, contributes to disease. We find that poly(GR) can act as a mitochondria-targeting signal, causing some poly(GR) to be cotranslationally imported into mitochondria. However, poly(GR) translation on mitochondrial surface is frequently stalled, triggering RQC and CAT-tailing–like C-terminal extension (CTE). CTE promotes poly(GR) stabilization, aggregation, and toxicity. Our genetic studies in Drosophila uncovered an important role of the mitochondrial protease YME1L in clearing poly(GR), revealing mitochondria as major sites of poly(GR) metabolism. Moreover, the mitochondria-associated noncanonical Notch signaling pathway impinges on the RQC machinery to restrain poly(GR) accumulation, at least in part through the AKT/VCP axis. The conserved actions of YME1L and noncanonical Notch signaling in animal models and patient cells support their fundamental involvement in ALS/FTD.
Aberrant protein aggregation manifesting failed cellular protein homeostasis (proteostasis) is a defining feature of age-related neurodegenerative diseases (1, 2). These hallmark aggregates include amyloid plaque in Alzheimer’s disease, Lewy body in Parkinson’s disease (PD), neurofibrillary tangle in tauopathies, poly-Q aggregate in Huntington’s diseas, and TDP-43 aggregate in amyotrophic laterosclerosis (ALS). While genetic mutations associated with the familial forms of diseases may cause protein misfolding and thus promote protein aggregation, less well understood is how the protein aggregates form in the sporadic cases, which account for more than 90% of the disease and which do not contain the familial mutations.
Previous studies have focused heavily on aberrant folding and posttranslational modifications of fully synthesized, mature proteins in mediating protein aggregation in aging or age-related diseases (3). Nevertheless, ubiquitination and degradation of nascent peptides (NPs) still associated with ribosomes is a widespread phenomenon, indicating that quality control happens early in the life cycle of cellular proteins (4, 5). During NP synthesis, the translating ribosomes could be stalled by a number of factors, including mRNA defects, strong mRNA secondary structures, insufficient supply of aminoacyl-tRNAs, electrostatic interaction between NPs and the ribosome exit tunnel, or deficit in translation termination activity (6–8). In response, the conserved ribosome-associated quality control (RQC) complex is recruited to stalled ribosomes to target the aberrant translation products and the template mRNAs for degradation. Studies in yeast showed that NPs on stalled ribosomes can be modified while still attached to 60S subunits, in a C-terminal Ala and Thr addition (CAT-tailing) process (9). So far CAT-tailing has been studied mainly using artificial substrates and under conditions that interfere with the degradation of the stalled NPs. The physiological role of CAT-tailing in the context of intact RQC remains unsettled. It may induce the heat-shock response (10), push lysine residues of stalled NPs out of the ribosome exit tunnel (11), or drive degradation of stalled NPs on and off the ribosomes (12). Recent studies in yeast and metazoans emphasized the importance of RQC and CAT-tailing–like process in maintaining mitochondrial function by protecting the integrity of nuclear-encoded mitochondrial proteins that are cotranslationally imported (7, 13). Since failure in the timely removal of CAT-tailed proteins can cause proteotoxicity in yeast (14–16), and defective RQC is linked to neurodegeneration in mouse mutants (17, 18) and PD models (7), it is important to elucidate the regulation and function of RQC and CAT-tailing in disease settings. However, the CAT-tailing–like C-terminal extension (CTE) of stalled NPs, the compositions of the CTEs, and the pathological consequences of such CTE in disease settings have not been accessible for investigation due to the lack of identified CAT-tailed substrates in metazoans, except in the case of the mitochondrial complex-I 30-kDa protein in the context of PD (7).
Expansion of G4C2 repeats in the 5′-UTR of C9ORF72 is the most common genetic cause of ALS with frontotemporal dementia (C9-ALS/FTD). A number of mechanisms have been put forward to explain the pathogenesis of G4C2 repeat expansion (19, 20), including C9ORF72 haplo-insufficiency, toxicity associated with sense and antisense RNA foci, or proteotoxicity induced by dipeptide repeat (DPR) proteins (GA, GP, GR, PA, PR) translated from G4C2 repeat-carrying sense and antisense transcripts in all six possible reading frames. Increasing evidence emphasizes the pathogenic contribution of DPRs, with arginine-containing poly(GR) and poly(PR) exhibiting particular cytotoxicity (19–22). Despite intensive recent efforts, how the expression of the poly(GR) protein causes cellular toxicity remains unclear, and even less is known about cellular mechanisms that protect against such toxicity. Here we report a mechanism of poly(GR) toxicity that involves its translational stalling and CTE on the mitochondrial surface. We also describe cellular quality-control mechanisms protecting against poly(GR) toxicity uncovered through genetic analysis in Drosophila models and shown to be conserved in mammalian settings. Our results offer the initial insights into the regulation of RQC by cellular signaling pathways regulating development and metabolism, and highlight the pathogenic role of defective RQC in linking mitochondrial dysfunction and proteostasis failure.
Results
Genetic Identification of YME1L as a Conserved Regulator of Poly(GR) Abundance.
Drosophila has been an excellent model organism for investigating pathogenic mechanisms of human diseases, and a large body of work has been done on C9-ALS/FTD–related models in Drosophila (23–39). To elucidate the pathogenic mechanism of poly(GR), we used transgenic Drosophila expressing N-terminally Flag-tagged GR80 repeats (Flag-GR80) (40). When expressed in the muscle under Mhc-Gal4 control, Flag-GR80 caused prominent toxicity manifested as flightless flies with held up or droopy wing posture (Fig. 1A), resembling the phenotype caused by mitochondrial dysfunction and flight muscle degeneration seen in PINK1 mutant flies (41–43). Consistent with a mitochondrial toxicity of GR80 (40), in a candidate gene-based genetic modifier screen (SI Appendix, Table S1), we identified the mitochondrial intermembrane space ATPase associated with diverse cellular activities (i-AAA) protease YME1L, a major regulator of mitochondrial protein quality control (44). Overexpression of YME1L (YME1L-OE) rescued GR80-induced wing posture phenotype, whereas YME1L RNAi had the opposite effect (Fig. 1A and SI Appendix, Fig. S1A). Importantly, YME1L-OE dramatically reduced, whereas YME1L RNAi increased Flag-GR80 protein level (Fig. 1B). GR80 mRNA level was not affected (Fig. 1C), consistent with YME1L regulating poly(GR) at the protein level. Upon YME1L RNAi, GR80 formed more prominent puncta colocalizing with mitochondria (Fig. 1D). Examination of flight muscle mitochondria using the mito-GFP or mito-RFP reporter (Fig. 1D and SI Appendix, Fig. S1B) or by electron microscopy (SI Appendix, Fig. S1C) showed that Flag-GR80 caused severe defects (swollen or round-shaped mitochondria with marked vacuolization), which were rescued by YME1L-OE (SI Appendix, Fig. S1 B and C). Notably, YME1L did not affect the level of two other C9-ALS/FTD-associated DPRs—poly(PR) and poly(GA)—which were localized primarily to nonmitochondrial sites (SI Appendix, Fig. S1D), indicating specificity of YME1L regulation of poly(GR). Although most of the studies described above were done in male flies, female flies showed similar phenotypes and poly(GR) regulation by YME1L (SI Appendix, Fig. S1E). Moreover, manipulation of a number of other mitochondrial proteases, including Lon and Rhomboid, did not affect poly(GR) level (SI Appendix, Fig. S1F), demonstrating specificity of YME1L regulation of poly(GR).
Fig. 1.
YME1L negatively regulates poly(GR) expression and toxicity. (A–C) Effects of YME1L overexpression (OE) or RNAi (RI) on wing-posture (A) and GR80 protein (B) and mRNA (C) expression in Mhc-Gal4 > Flag-GR80 flies. Examples of normal and droopy wing posture are shown in A. (D) Immunostaining showing increased GR80 accumulation on mitochondria (labeled with mito-GFP) after YME1L-RI. Compared to control flies, Flag-GR80 flies showed regions devoid of mito-GFP (arrows), which was exacerbated by YME1L-RI. (E and F) Immunoblots showing the effect of CRISPR-mediated YME1L knockdown on Flag-GR80 expression in HEK293T cells (E) and rescue with WT or E543Q forms of YME1L (F). Cotransfected GFP serves as control for transfection efficiency in this and subsequent figures. (G and H) Effect of cotransfection of YME1L-GFP, or Flag tagged YME1L, and YME1L-E543Q on Flag-GR80 expression in HEK293T cells. (I and J) In vitro YME1L protease activity assay using hexYME1L as protease and Flag-GR80 or Flag-C-I30 as substrates. **P < 0.01 and ***P < 0.001 in one-way ANOVA test followed by SNK test plus Bonferroni correction (multiple hypotheses correction); N.S., nonsignificant. Values under the Flag-GR80 blots indicate normalized levels relative to control in this and all subsequent figures. Western blots represent at least two biological repeats.
We further confirmed the YME1L effect on poly(GR) in mammalian cells. Flag-GR80 accumulated to higher levels in YME1L knockout HEK293 cells generated by CRISPR-Cas9 (Fig. 1E), a phenotype that was rescued by WT YME1L but not a catalytically inactive YME1L-E543Q (Fig. 1F). Conversely, overexpression YME1L-GFP or YME1L-Flag dramatically reduced GR80 level (Fig. 1 G and H), whereas YME1L-E543Q-Flag failed to do so (Fig. 1H). In C9-ALS/FTD patient fibroblasts containing expanded G4C2 repeats, poly(GR) but not poly(GA) level was reduced by YME1L cDNA and elevated by YME1L small-interfering RNA (siRNA) transfections (SI Appendix, Fig. S1G). Mitochondrial defects (swelling, vacuolization, and loss of cristae) seen in patient fibroblasts were also rescued by YME1L-OE (SI Appendix, Fig. S1H). These results implicate mitochondria as major sites of poly(GR) toxicity and YME1L, a key regulator of poly(GR) metabolism.
We next tested whether YME1L might regulate poly(GR) abundance by targeting it for degradation. Using an in vitro system that recapitulates the ATP-dependent protease activity of YME1L (45), we found that WT YME1L but not a catalytically inactive YME1L was able to degrade Flag-GR80. In contrast, YME1L failed to degrade a similarly prepared mitochondrial protein (Flag-C-I30), demonstrating substrate specificity of YME1L toward GR80 (Fig. 1 I and J). Thus YME1L, a mitochondrial i-AAA protease, protects against the build-up and toxicity of poly(GR).
Poly(GR) Is Cotranslationally Imported into Mitochondria.
Next we investigated how poly(GR) ended up in mitochondria to cause toxicity. GR80 may be synthesized in the cytosol and imported into mitochondria through the TOM complex. Import of nuclear-encoded proteins often involves a mitochondrial targeting sequence (MTS) rich in positively charged amino acids and other structural information, such as α-helical conformation (46). MTS mimicry by the poly(GR) may engage the TOM complex and lead to GR80 import into mitochondria. The TOM complex has also been shown to interact with MTS newly emerging from the exit tunnel, leading to recruitment of NP and associated mRNP/ribosome to mitochondria outermembrane (MOM) (47). Although GR80 could be found broadly in the cytosol including the nucleus, ∼60% of GR80 protein was found to colocalize with mitochondria (SI Appendix, Fig. S2A). Importantly, a portion of GR80 mRNA was present on percoll gradient-purified mitochondria (Fig. 2A), consistent with GR80 engaging in cotranslational import. To further test this idea, we purified mitochondria from HEK293 cells or fly muscle expressing Flag-GR80, and then treated them with hydroxylamine (HA), which releases NP from translating ribosomes (48), including MOM-associated ribosomes (49). Flag-GR80, but not mitochondrial proteins Tom40 or C-I30, was efficiently released from mitochondria by HA, supporting that it is translated on MOM (Fig. 2B). Treatment with puromycin, an antibiotic that resembles the 3′ end of aminoacylated tRNA, is another method used to terminate translation and release NP (50). We found that puromycin treatment could also release some GR80 from mitochondria (Fig. 2C). We also used an MS2-binding site (bs)-tagged poly(GR) mRNA reporter and MS2-GFP to track the localization of the mRNA. GR80-MS2-bs mRNA exhibited partial mitochondrial localization (Fig. 2D). These results, together with GR80 interaction with Tom40 (Fig. 2E), the constituent of the import channel (46), indicate that poly(GR) is cotranslationally imported. The engagement of GR80 with Tom40 may also explain why HA and puromycin treatment only resulted in partial release of GR80 from MOM (Fig. 2 B and C).
Fig. 2.
Stalled translation of poly(GR) mRNA on mitochondria. (A) RT-PCR analysis of Percoll gradient-purified mitochondrial fractions from GR80 transgenic fly muscle showing the presence of GR80 mRNA (Left) and GR80 mRNA levels in total and mitochondrial fractions (Right). Mitochondrial-encoded mtCo-1 and cytosolic tubulin serve as positive and negative controls. (B and C) Release of Flag-GR80 NP by HA (B) or puromycin (C) treatment of mitochondria purified from GR80-expressing HEK293 cells or fly muscle. Untreated samples (control), and the supernatant (HA-release) or mitochondrial pellet (HA-remaining) of HA-treated samples were analyzed. (D) HEK293 cells transfected with MS2-bs or GR60-MS2-bs reporter constructs were costained for the cotransfected MS2-GFP that binds to MS2-bs, and the mitochondrial marker Tom20. (E) Immunoblots showing co-IP between Flag-GR80 and Tom40, and the preferential binding of Flag-GR80 to Rpl3 over Rps6.
Stalled Translation of Poly(GR) mRNA on Mitochondrial Surface.
Given that GR80 is positively charged, and that electrostatic interaction between positively charged amino acidss in NP and negatively charged amino acids lining the ribosome exit tunnel can cause ribosome stalling (51), we further hypothesized that the translation of GR80 mRNA on MOM is frequently stalled, possibly explaining the difficulty in detecting long poly(GR) from patient cells or tissues by standard SDS/PAGE/Western blot. To test this idea, we repeated treatment of cells with puromycin, which can be incorporated into elongating as well as stalled NPs (52), but we also pretreated cells with homoharringtonine (HHT). HHT treatment inhibits new initiating events while allowing active/elongating ribosomes to run off (53), resulting in labeling of stalled NPs by puromycin in the presence of emetin (52). Intriguingly, while global translation was sensitive to HHT, mitochondria-associated GR80 was not (SI Appendix, Fig. S2 B and C), and nascent Flag-GR80 on MOM was translationally stalled, as shown by its puromycinylation in the presence of emetin (SI Appendix, Fig. S2C).
To further test the idea of translational stalling of GR80 and to estimate the approximate length of GR repeats required for ribosome stalling, we transfected HEK293 cells with Flag-(GR)n-GFP constructs expressing different-lengthed GR repeats flanked by N-terminal Flag tag and C-terminal GFP tag (SI Appendix, Fig. S2D). Detection of Flag− and GFP+ full-length proteins produced from these constructs revealed a gradual repeat length-dependent decrease of translation efficiency, with a dramatic decrease of translation efficiency occurring when more than 67 GR repeats was expressed (SI Appendix, Fig. S2D). Since the ribosome exit tunnel only accommodates a linear peptide of roughly 35-amino acids long, the longer GR peptide required to induce ribosome stalling suggested that electrostatic interactions within the tunnel was not sufficient to induce stalling, and that interaction of poly(GR) with factors outside of the tunnel was involved. For example, interaction with certain mitochondrial proteins may slow down the cotranslational import of GR80 and cause stalled translation. Supporting this notion, GR80 interacted with Tom40 of the TOM/TIM complex (Fig. 2E).
Activation of RQC on MOM by Poly(GR).
Ribosome stalling activates cotranslational quality-control mechanisms that target aberrant or incompletely synthesized/folded NPs for degradation. The first step toward resolving stalled ribosome is separation of 40S and 60S subunits, with the NPs still present in a peptidyl-tRNA/60S complex, forming a substrate for the RQC machinery. Consistent with RQC playing a physiological role in regulating mitochondrial homeostasis, RQC factors were found constitutively present in the mitochondrial fraction of control and GR80-expressing HEK293 cells (SI Appendix, Fig. S3A) or fly muscle (SI Appendix, Fig. S3B). In the mitochondrial fraction, GR80 associated with 60S and certain RQC factors, and this interaction was weakened by RNase treatment, suggesting the interactions were partially mediated by RNA (Fig. 3A). Ribosome-stalled NPs are modified by a nontemplated, 40S-independent CAT-tailing process catalyzed by Tae2 in yeast (9). We found that binding of RQC factor Clbn/NEMF, the metazoan homologs of Tae2, to GR80 mRNA could be detected by RNA-immunoprecipitation (IP) assays in HEK293 cells (Fig. 3B) or fly muscle (Fig. 3C). We note that the GR80 mRNA bound to Clbn/NEMF could be stalled GR80 mRNAs still bound to 60S before their degradation by the RNA quality-control machinery, or GR80 mRNA associated with 80S and polysomes, as previous studies showed that yeast Clbn/NEMF counterpart Tae2 can associate with 80S and polysomes (13). These results, together with the association of GR80 with Tom40 and 60S but not 40S (Fig. 2D), depicted a picture of active quality control of ribosome-stalled poly(GR) anchored to MOM by TOM (SI Appendix, Fig. S3C).
Fig. 3.
RQC of poly(GR) in fly tissues and mammalian cells. (A) Co-IP assays using extracts treated with or without RNase A to test the role of RNA in facilitating interactions between Flag-GR80 and the RQC factors. (B and C) RNA-IP assays showing binding of NEMF (B) or Clbn (C) to GR80 mRNA in HEK293T (B) or fly muscle (C) samples. Bar graphs show quantification of GR80 mRNA level in IP samples. ***P < 0.001 in Student’s t test. (D) Immunoblots showing co-IP between Flag-GR80 and RQC factors in fly muscle. (E–G) Immunoblots showing effects of overexpression or knockdown of RQC factors on Flag-GR80 level in fly muscle. (H) Immunostaining showing effects of overexpressing various RQC factors on Flag-GR80 level on muscle mitochondria. (I) Quantification showing effects of altered activities of RQC factors on GR80-induced wing posture phenotype. *P < 0.05 and **P < 0.01 in one-way ANOVA test followed by SNK test plus Bonferroni correction. Western blots represent at least two biological repeats.
Next, we examined the in vivo effect of cotranslational quality control, since GR80 also associated with RQC factors in the fly muscle (Fig. 3D). Overexpression of Pelo and ABCE1, ribosome-splitting factors needed for RQC (8), resulted in dramatic reduction of GR80 level (Fig. 3E). Overexpression of fly homologs of core components of RQC (Ltn1, valosin-containing protein [VCP]), involved in degradation of stalled NPs (6–8), also dramatically reduced GR80 level (Fig. 3F). Conversely, RNAi of Ltn1, VCP1, Pelo, or ABCE1 boosted GR80 level (Fig. 3 F and G). RNAi of RQC factors also promoted GR80 aggregate formation in the cytosol or associated with mitochondria (SI Appendix, Fig. S3D), whereas overexpression removed such aggregates (Fig. 3H). Correlating with effects on GR80 level, overexpression of the RQC factors rescued GR80-induced wing-posture defect, while their knockdown exacerbated that (Fig. 3I). These results support the notion that the RQC pathway is normally involved in restraining the expression of poly(GR). To test the specificity of the RQC effect, we examined FTD-related tau. The expression level of tau was not significantly affected by genetic manipulations of RQC factors (SI Appendix, Fig. S3E), suggesting that the RQC pathway preferentially affects GR80.
CAT-Tailing-like CTE of Poly(GR).
CAT-tailed substrates escaping the quality-control system can accumulate and form aggregates (14–16). Remarkably, we found that unlike the other RQC factors whose RNAi resulted in accumulation of GR80, as shown above, RNAi of fly or mammalian Tae2 homolog (Clbn/NEMF) decreased GR80 level (Fig. 4 A and B). Anisomycin treatment, which can inhibit CAT-tailing in vitro (54) and in vivo (7), showed similar but somewhat stronger effect (Fig. 4C), presumably because the Clbn/NEMF RNAi effect was not complete (SI Appendix, Fig. S4A) and thus not as potent as anisomycin in inhibiting CAT-tailing. Clbn/NEMF RNAi or anisomycin treatment led to not only reduced GR80 level but also removal of some slightly higher molecular weight smear signals (Fig. 4 A–C), presumably reflecting the elimination of CAT-tails. Conversely, Clbn-OE increased the relative proportion of poly(GR) smear signals (Fig. 4D). RNAi of a fly homolog of Vms1, a stress-responsive mitochondrial RQC factor that cleaves peptidyl-tRNA bond and thus antagonizes CAT-tailing–like activity (13, 55–58), increased GR80 smear signal and total GR80 abundance (Fig. 4E). Overexpression of mammalian Vms1 (ANKZF1) had the opposite effect (SI Appendix, Fig. S4B). In C9-ALS/FTD fibroblasts, ANKZF1 RNAi increased, whereas NEMF RNAi decreased poly(GR) level (SI Appendix, Fig. S4C). Thus steady-state poly(GR) level is positively correlated with a CAT-tailing–like process in fly tissues and patient fibroblasts.
Fig. 4.
CAT-tailing–like CTE of ribosome-stalled poly(GR). (A and B) Immunoblots showing effects of Clbn or NEMF RNAi on Flag-GR80 level in fly muscle (A) or HEK293 cells (B). (C) Immunoblots and immunostaining showing effect of anisomycin on Flag-GR80 level in HEK293T cells. (D–F) Immunoblots showing effects of Clbn-OE (D) or RNAi of fly Vms1 (E) and various ARSs (F) on Flag-GR80 level in fly muscle. (G) Immunostaining showing effects of RNAi of various ARSs on Flag-GR80 associated with muscle mitochondria. (H) Immunoblots showing effect of IARS RNAi on Flag-GR80 level in fly muscle. (I–K) MS/MS spectra of the parent ion of peptides generated by collision-induced dissociation fragmentation. Matched peptide sequence entries from the custom-built database were shown below each spectrum. The blue and yellow vertical lines in the MS spectra represent “y” and “b” ions, respectively, found in the BY matches. The BY matches are fragment ions found in the high-energy data that suggest a particular identification for a given precursor ion found in the low-energy data.
To test the CAT-tailing model further, we examined the role of Ala- or Thr-tRNA synthetase (AARS or TARS) that are also required for CAT-tailing–like CTE in Drosophila and mammalian cells (7). AARS and TARS RNAi significantly reduced GR80 level, including the higher molecular weight smear signal (Fig. 4F), its aggregation (Fig. 4G), and muscle toxicity (SI Appendix, Fig. S4D). Interestingly, isoleucyl tRNA synthsetase (IARS) RNAi had similar effect (Fig. 4 G and H and SI Appendix, Fig. S4D), and like AARS and TARS RNAi, IARS RNAi effectively rescued GR80-induced mitochondrial morphology defect (SI Appendix, Fig. S4E). The slightly stronger effect of IARS RNAi on GR80 toxicity than AARS or TARS RNAi might be due to stronger knockdown of IARS mRNA level by the transgene line used (SI Appendix, Fig. S4A). The specificity of the A/T/I ARS effect was shown by the lack of effect of the other ARSs tested (Fig. 4F). Thus AARS/TARS, and possibly select other ARSs (e.g., IARS), appear to be involved in CAT-tailing–like CTE, and such modification confers stabilization, aggregation, and toxicity of GR80.
Further supporting this notion, we found that a fraction of poly(GR) from HEK293T cells or fly muscle tissue was present in SDS-resistant aggregates that were stuck in the stacking gel during SDS/PAGE (SI Appendix, Fig. S4F), and that poly(GR) containing an artificial CAT-tail (GR71-AT15) formed prominent aggregates on mitochondria on its own. As expected, GR71-AT15 level was not affected by NEMF RNAi or anisomycin treatment (SI Appendix, Fig. S4G). Moreover, while a Flag-GR11 protein was undetectable under conditions that allowed Flag-GR80 detection, presumably due to its short length and inability to induce CAT-tailing, artificially CAT-tailed Flag-GR11-AT16 was readily detectable and it formed SDS-resistant aggregates (SI Appendix, Fig. S4H).
To further test the CAT-tailing hypothesis, we performed mass spectrometry of Flag-GR80 purified from Mhc-Gal4 > Flag-GR80 flies by denaturing IP. Based on in vivo ARS requirements, we assumed that A/T/I might be the preferred amino acids added to the C terminus of GR repeats, and we built custom libraries with variations of A/T/I-containing CTEs. Our search of tandem mass spectra against the databases identified GR peptides with A/T/I-containing CTEs and spectra matching the collision-induced dissociation fragments of the peptides (Fig. 4 I–K and SI Appendix, Fig. S4I).
The Noncanonical Notch Signaling Pathway Restrains Poly(GR) Expression in Flies.
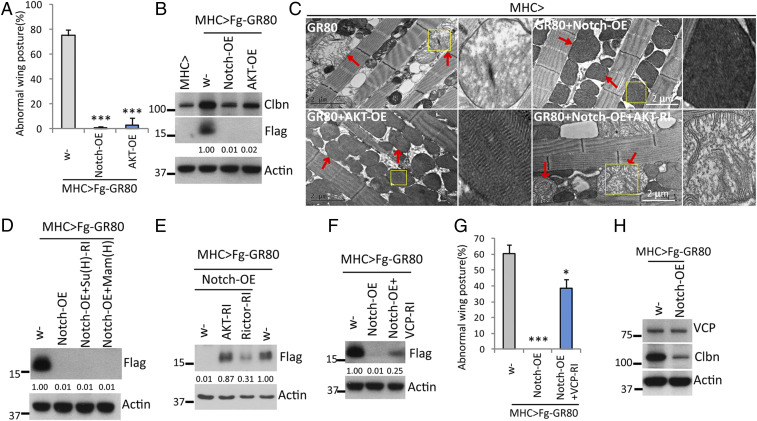
We sought to gain further insight into the in vivo regulation of poly(GR) RQC through analysis of genetic modifiers isolated from the screen that also led to YME1L (SI Appendix, Table S1). The strongest modifier we uncovered was Notch, whose overexpression resulted in the most complete suppression of GR80 toxicity (Fig. 5A). This was correlated with diminished GR80 level (Fig. 5B and SI Appendix, Fig. S5A), and full restoration of mitochondrial morphology (Fig. 5C). Although Notch is best known to act through conserved nuclear factors, such as mastermind (Mam) and Suppressor of Hairless [Su(H)], to regulate transcription via canonical Notch signaling (59), Su(H) and Mam were not required for the inhibition of GR80 by Notch (Fig. 5D). Consistently, overexpression of just Notch intracellular domain (NICD), which is critically involved in canonical Notch signaling, was not as effective as full-length Notch in inhibiting GR80 expression, whereas deletion of the Su(H)-interacting CDC10 repeats from the NICD of full-length Notch was without effect on Notch regulation of GR80 (SI Appendix, Fig. S5B).
Fig. 5.
Negative regulation of poly(GR) expression and toxicity by noncanonical Notch signaling in fly muscle. (A) Quantification of effect of Notch-OE or AKT-OE on GR80-induced wing posture defect. (B) Immunoblots showing effect of Notch-OE or AKT-OE on Flag-GR80 and Clbn expression. (C) TEM images showing effects of Notch-OE or AKT-OE, or Notch-OE + AKT-RI, on GR80-induced mitochondrial morphology defect. Arrows mark mitochondria. Insets show zoom-in view of selected areas of interest (yellow squares). GR-80 caused swelling and loss of cristae and electron-dense material, phenotypes rescued by Notch-OE or AKT-OE. AKT-RI blocked the Notch-OE effect. Magnification: 5×. (D) Immunoblots showing lack of effect of Su(H) RNAi or OE of dominant-negative Mam [Man(H)] on the inhibition of Flag-GR80 expression by Notch-OE. (E and F) Immunoblots showing the effects of RNAi of AKT or Rictor (E), or VCP RNAi (F) on the inhibition of Flag-GR80 expression by Notch-OE. (G) Quantification of effect of VCP RNAi on the suppression of GR80-induced wing posture defect by Notch-OE. (H) Immunoblots assessing effect of Notch-OE on endogenous VCP or Clbn expression. *P < 0.05; ***P < 0.001.
Full-length Notch has been shown to be present on muscle mitochondria, where Notch, PINK1, and mechanistic target of rapamycin (mTORC)2 are known to constitute a mitochondria-associated noncanonical Notch pathway, acting by influencing the phosphorylation and activity of the mTORC2 substrate AKT (60). We found that key components of this pathway, AKT (Fig. 5 A–C) and PINK1 (SI Appendix, Fig. S5 C and D), were as effective as Notch in restraining GR80. Consistent with these genes operating in a common pathway, the effect of Notch in inhibiting GR80 was abolished by knocking down mTORC2 component Rictor or AKT (Fig. 5 C and E).
The AKT-VCP Axis Mediates the Effect of Noncanonical Notch Signaling on Poly(GR).
We next explored the relationship between noncanonical Notch signaling and the RQC/CAT-tailing process. By manipulating individual RQC components, we found that VCP RNAi showed prominent effect in blocking the effect of Notch on GR80 (Fig. 5 F and G), suggesting that VCP is a key mediator of noncanonical Notch signaling. Since VCP protein level was not affected by Notch (Fig. 5H), we considered posttranslational modification of VCP by noncanonical Notch signaling and tested the potential role of AKT. We found robust physical interaction between AKT and VCP (Fig. 6A). Chemical activation of AKT with SC79 (61) increased VCP phosphorylation at consensus AKT-target sites, whereas inhibition of AKT with small-molecule inhibitors of AKT (AKTi) (62) had the opposite effect (Fig. 6B), suggesting that VCP is a genuine AKT substrate. Consistent with VCP being a key downstream effector of AKT, VCP RNAi also attenuated the inhibitory effect of AKT-OE on GR80 toxicity in fly muscle (Fig. 6 C and D). In HEK293T cells, overexpression of Notch1, one of the four mammalian Notch homologs, reduced Flag-GR80 level, an effect blocked by small-molecule inhibitors of VCP (VCPi) (SI Appendix, Fig. S6A). Treatment of HEK293T cells with ATK activator SC79 (SI Appendix, Fig. S6 B and C) also reduced Flag-GR80 level, whereas AKTi and VCPi increased Flag-GR80 level (SI Appendix, Fig. S6D). The observed effect of SC79 on GR80 expression was specific and dependent on AKT activity, as AKTi treatment (SI Appendix, Fig. S6E) or knockdown of AKT isoforms by RNAi (SI Appendix, Fig. S6F) blocked the SC79 effect.
Fig. 6.
The AKT-VCP axis mediates the effects of noncanonical Notch signaling on poly(GR) expression and toxicity. (A) Immunoblots showing co-IP between VCP and AKT. (B) Immunoblots showing the effect of SC79 and AKTi on VCP phosphorylation at consensus AKT target site(s) in HEK293T cells. (C) Immunoblots showing effects of VCP RNAi on the inhibition of Flag-GR80 expression by AKT-OE. (D) Quantification of effect of VCP RNAi on the suppression of GR80-induced wing posture defect by AKT-OE. (E and F) Immunoblots showing the effects of VCP-WT, VCP-S745/747D, and VCP-S745/747A on Flag-GR80 protein level (E), and the effects of VCP-WT and VCP-S745/747D in the presence of AKTi (F). (G and H) Immunostaining showing the effect of Notch, AKT, VCP, and YME1L OE on the levels of Flag-GA80 (G) and Flag-PR80 (H) in fly muscle. Bar graphs show quantification of Flag-GA80 and Flag-PR80 immunofluorescent signals. *P < 0.05, **P < 0.01, and ***P < 0.001 in one-way ANOVA test followed by SNK test plus Bonferroni correction.
We next examined the biochemical relationship between VCP and AKT. VCP contains multiple potential AKT phosphorylation sites. Two adjacent sites (S745 and S747) had previously been implicated in phospho-regulation by AKT (63, 64). We found that a phospho-mimetic form of VCP (VCP-S745/747D) was more active than VCP-WT in reducing GR80 expression, whereas the nonphosphorylatable form (VCP-S745/747A) was inactive (Fig. 6E). Moreover, unlike VCP-WT, whose activity was inhibited by AKTi, VCP-S745/747D was resistant to AKTi (Fig. 6F). These results support the notion that AKT acts as an upstream kinase that positively regulates the activity of VCP.
Intriguingly, in addition to VCP, Clbn was also regulated by Notch signaling. Clbn protein level was significantly increased in GR80 fly muscle, an effect abolished by Notch-OE or AKT-OE (Fig. 5 B and H). Although the detailed mechanisms of Clbn up-regulation by GR80 and its subsequent down-regulation by Notch/AKT remain to be delineated, it appeared that VCP was involved (SI Appendix, Fig. S6G). Thus, through activation of VCP and inhibition of Clbn, noncanonical Notch signaling prevents the build-up of CAT-tailed poly(GR). Regulation of GR80 expression by the Notch/AKT/VCP axis and the RQC pathway is observed in female flies as well (SI Appendix, Fig. S6 H–J).
We also tested the specificity of the noncanonical Notch signaling and RQC/CAT-tailing pathways in regulating the expression of C9-ALS/FTD–associated DPRs. For reasons unclear to us, GA80 and PR80 proteins were undetectable by standard Western blots. We therefore resorted to immunostaining to detect these proteins upon genetic manipulations of RQC factors. We found that whereas GA80 was not affected by the various manipulations, PR80 responded to altered activities of Notch/AKT/VCP, but not Clbn (Fig. 6 G and H and SI Appendix, Fig. S6 K and L). The mechanism of PR80 regulation by Notch/AKT/VCP but not Clbn is currently unknown. YME1L-OE had no effect on GA80 or PR80 level (Fig. 6 G and H).
We also tested whether the YME1L and the Notch/RQC pathways identified from the muscle-based genetic screens work similarly in neuronal settings to regulate poly(GR). We used the photoreceptors as a neuronal system to test genetic interactions. Our results indicate that the genetic interactions detected in fly muscle are largely preserved in the photoreceptors (SI Appendix, Fig. S7).
Noncanonical Notch Signaling Regulates Poly(GR) in C9-ALS/FTD Patient Cells.
We further examined the effect of the noncanonical Notch signaling pathway in regulating poly(GR) expression in a more physiologically relevant setting using C9-ALS/FTD patient fibroblasts. Overexpressing noncanonical Notch pathway genes (Notch, AKT) or RQC factor (VCP) significantly reduced poly(GR) level (Fig. 7A) and restored mitochondrial morphology (Fig. 7B). On the other hand, AKTi or VCPi treatment increased poly(GR) level (Fig. 7C). Moreover, we found that poly(GR) was enriched in the mitochondrial fraction in C9-ALS/FTD patient cells, and SC79 treatment significantly reduced that (Fig. 7D), accompanied by restoration of mitochondrial morphology (Fig. 7B). As reported previously (65), C9-ALS/FTD patient fibroblasts exhibited elevated mitochondrial membrane potential (MMP) compared to cells from control subjects (SI Appendix, Fig. S8A). The increased MMP in C9-ALS fibroblasts is likely due to the alteration and tightening of cristae junctions caused by poly(GR), thereby impairing mitochondrial ion homeostasis, as reported in a recent publication (66). Overexpression of Notch and AKT rescued the MMP defect in patient fibroblasts (SI Appendix, Fig. S8B), and other conditions that reduced poly(GR) level, such as YME1L-OE (SI Appendix, Fig. S8C), also lowered MMP; thus, it is likely that Notch and AKT acted through poly(GR) to regulate MMP in patient cells.
Fig. 7.
Regulation of poly(GR) expression by noncanonical Notch signaling in C9-ALS/FTD patient fibroblasts. (A) Dot blots showing the effect of genetic manipulation of Notch and VCP on poly(GR) expression in C9-ALS/FTD patient fibroblasts. (B) TEM showing the effect of genetic manipulation or chemical treatment on mitochondrial morphology in patient fibroblasts. Arrows mark mitochondria. Insets show zoom-in view of selected areas of interest (yellow squares). The swelling and loss of electron-dense material and cristae structure was rescued by SC79 treatment or OE of Notch and AKT. Magnification: 5×. (C) Dot blots showing the effect of VCPi and AKTi treatment on poly(GR) level in C9-ALS/FTD patient fibroblasts. (D) Dot blots showing the enrichment of poly(GR) in the mitochondria of patient fibroblasts and the effect of SC79 treatment on poly(GR) level. Dot blots represent at least two biological repeats.
Discussion
Proteostasis failure increases with aging and is a common feature of age-related diseases. Not surprisingly, RQC—an important step in proteostasis control—is linked to neurodegeneration. Mitochondrial dysfunction is also intimately associated with aging and age-related diseases (2). How these hallmarks of disease are connected at the mechanistic level is not well defined. In this study we show that poly(GR) is cotranslationally imported into mitochondria and its translation is frequently stalled, leading to CAT-tailing–like modification and aggregation. We identify mitochondrial YME1L as a key factor in poly(GR) metabolism and noncanonical Notch signaling as a key pathway regulating the RQC and CAT-tailing of poly(GR). These results not only synthesize a new model connecting defective RQC/CAT-tailing with proteostasis failure and mitochondrial dysfunction, two pathological hallmarks of neurodegenerative diseases and other age-related diseases, but also identify other players regulating this process (SI Appendix, Fig. S9). The mechanism of mitochondrial dysfunction caused by poly(GR) is beginning to emerge. A recent study showed that poly(GR) can interact with components of the mitochondrial contact site and cristae organizing system (MICOS), altering MICOS dynamics and intrasubunit interactions (66). It is likely that poly(GR) interaction with other mitochondrial proteins, such as ATP5A1 (67), also contributes to its mitochondrial toxicity.
Quality control starts when NPs are still associated with ribosomes. Our finding of frequent ribosome stalling associated with poly(GR) translation offers one explanation of translational stress associated with poly(GR) (68). Our results support the model of positively charged poly(GR) mimicking MTS and directing nascent poly(GR) and the ribosome/mRNP complex to MOM, causing stalled translation in a GR length-dependent manner, presumably facilitated by poly(GR) interaction with mitochondrial factors such as Tom40. This may explain the poly(GR) length (∼67 GR) required to induced translational stalling. Although shorter GR may not be able to induce stalled translation, they could still cause cytotoxicity by other mechanisms, including alteration of nucleolar function (31). It is also possible that delivering of nascent poly(GR)/ribosome/mRNP complex to MOM represents a cellular attempt to use mitochondria and mitochondrial protein quality-control machinery to handle aggregating proteins, as previously shown in yeast (69). In either case, inefficient resolution of stalled ribosomes on MOM will led to CAT-tailing–like CTE and accumulation and aggregation of CAT-tailed poly(GR), which can either enter mitochondria to disrupt mitochondrial homeostasis or be released from MOM to form cytosolic aggregates and cause proteotoxic stress. One could also envision that ribosome-stalled GR80 may sequester RQC factors away from their normal cellular substrates, creating a deficiency of cellular RQC activity, which may also lead to neurodegeneration (17, 18). Besides identifying poly(GR) as the first disease-causing protein that is subjected to CAT-tailing–like CTE modification, this study further emphasizes the essential role of RQC to mitochondrial proteome integrity and organelle homeostasis (7, 13).
Through genetic analysis in Drosophila models, we identified two mechanisms, mediated by noncanonical Notch signaling and YME1L, in the quality control of poly(GR) at the cotranslational and posttranslational levels. Although by necessity the Drosophila models for studying the toxicity of individual DPRs invariably involve overexpression, the fact that we could validate the roles of Notch and YME1L in patient fibroblasts strongly supports the relevance of our findings to human disease conditions. Future studies will explore the specific features in poly(GR) that make it a substrate for YME1L and possible interplay between the noncanonical Notch and YME1L pathways. The mechanisms by which Clbn is up-regulated when poly(GR) is expressed, and how this is blocked by Notch and VCP, also warrant further study.
Other important issues regarding the role of RQC in C9-ALS/FTD remain: For example, the cross-talk between RQC and mitochondrial functional state, as mitochondrial stress can directly influence the RQC machinery (7); the general role of mitochondrial dysfunction in ALS/FTD, as previous studies have implicated a pathogenic role of defective mitochondria in ALS (70, 71); the relationship between RQC/CAT-tailing and stress granule formation, a process broadly implicated in the pathogenesis of C9-ALS/FTD and other diseases (72). Future studies addressing these questions will offer new insights into ALS pathogenesis.
Materials and Methods
Drosophila Genetics.
The fly stocks were obtained from the following sources: UAS-Flag-GR80, UAS-Flag-GA80, UAS-Flag-PR80, and GMR-Gal4 > Flag-GR80; Gal80ts (Fen-Biao Gao, University of Massachusetts, Worcester, MA), UAS-mito-GFP (William Saxton, University of California at Santa Cruz, Santa Cruz, CA), UAS-YME1L-GFP (Hong Xu, NIH, Bethesda, MD), UAS-VCP (Paul Taylor, St. Jude Children’s Hospital, Memphis, TN), UAS-Notch-V5 (Mark Fortini, NIH, Bethesda, MD), PINK1B9 (Jongkeong Chung, Seoul National University, Seoul, Republic of Korea), UAS-Clbn (Xiaolin Bi, Dalian Medical University, Dalian, China), UAS-Tau (Mel Feany, Harvard Medical School, Harvard, MA), UAS-Notch-NICD, and UAS-Notch-∆cdc10 (Ed Giniger, NIH, Bethesda, MD). The YME1L RNAi (#51752), UAS-Pelo (#68150), ABCE1 RNAi (#31601), ABCE1-EP (#27945), Ltn-EP (#30116), Ltn RNAi (#41866), Clbn RNAi (#62402), Vms1 RNAi (#62861), IARS RNAi (#58176), UAS-AKT (#8191), AKT RNAi (#31701), Rictor RNAi (#31527), Su(H) RNAi (#28900), UAS-Mam(H) (#26673), CG6512 RNAi (#50524), CG6512 RNAi (#34343), Lon RNAi (#34586), Rhomboid-7 RNAi (#35617), and Rhomboid-7 RNAi (#67309) fly stocks were obtained from the Bloomington Drosophila Stock Center. VCP RNAi (v24354), Pelo RNAi (v34770), AARS RNAi (v17171), TARS RNAi (V7752), DARS RNAi (v7750), YARS RNAi (v105615), LARS RNAi (v45048), SARS RNAi (v41928), VARS RNAi (v21782), CARS RNAi (v45611), FARS RNAi (v107079), and MARS RNAi (v106493) stocks were purchased from the Vienna Drosophila Resource Center Stock Center. Other stocks were generated in our laboratory.
Fly stocks were raised at room temperature and crosses were performed with standard procedures. In general, flies were raised at 25 °C and with 12/12-h dark/light cycles. Fly food was prepared according to the standard receipt (water, 17 L; agar, 93 g; cornmeal, 1,716 g; brewer’s yeast extract, 310 g; sucrose, 517 g; dextrose, 1,033 g). Unless otherwise indicated, male flies at 1 to 2 wk of age were used for the experimental procedures.
For all wing posture assays, 7-d-old male flies were visually scored and all of the experimental groups were aged at 25 °C. Around 20 male flies were collected/raised in one vial and 3 ∼ 4 independent vials were counted per genotype.
Immunostaining.
For immunostaining analysis of adult fly muscles, 7- to 10-d-old male flies raised at 25 °C were analyzed. Images shown were representative of at least five individuals for each genotype. Briefly, fly thoraxes were dissected and quickly washed with 1× PBS and fixed with 4% formaldehyde in 1× PBS for 20 min at room temperature. After fixation, muscle tissues were blocked with 1× PBS containing 5% normal goat serum for 30 min. Indicated primary antibodies were added and incubated overnight at 4 °C. After three steps of washing with 1× PBST containing 0.1% Triton X-100, each for 15 min at room temperature, Alexa Fluor 568-conjugated and Alexa Fluor 488-conjugated second antibodies (1:500, Molecular Probes) and Alexa Fluor 633 Phalloidin (1:500, Invitrogen, A22284) were mixed with tissues for 2 h at room temperature and subsequently washed and mounted in SlowFade Gold buffer (Invitrogen).
For immunohistochemical analysis in mammalian cells, cells were cultured on ethanol-cleaned cover glasses. After washing with 1×PBS three times and fixing with 4% formaldehyde in 1× PBS for 30 min at room temperature, cells were washed and permeabilized with 1× PBS containing 0.25% Triton X-100 for 15 min. The fixed samples were subsequently blocked with 1× PBS containing 5% normal goat serum and incubated for 1 h at room temperature followed by incubation with primary antibodies at 4 °C overnight. After washing, samples were incubated with Alexa Fluor 488-, 594-, and 633-conjugated secondary antibodies (1:500; Molecular Probes).
Generation of YME1L Knockout HEK293 Cell Lines.
To perform lentiviral CRISPR/Cas9-mediated knockout manipulation (73), single-guide RNA (sgRNA) sequences with a minimal number of off-target sites in human YME1L gene were selected and insert into the LentiCRISPR vector. sgRNA sequences are as follows: YME1L-KO-1-5: 5′CACCGTAAAGACTTACCTCACTGCT3′; YME1L-KO-1-3: 5′AAACAGCAGTGAGGTAAGTCTTTAC3′; YME1L-KO-2-5: 5′CACCGCTCTTCGTT CTGCTGCTATT3′; YME1L-KO-2-3: 5′AAACAATAGCAGCAGAACGAAGAGC3′; YME1L-KO-3-5: 5′CACCGGCAGAACGAAGAGAATCAGA3′; YME1L-KO-3-3: 5′AAACTCTGATTCTCTTCGTTCTGCC3′. To establish YME1L KO HEK293T cell lines, LentiCRISPR-YME1L constructs with different sgRNAs were transfected with the packaging plasmids pVSVg and psPAX2 into HEK293(F)T cells. Seventy-two hours posttransfection, viral supernatant was collected to infect HEK293T cells, followed by puromycin (1 µg/mL) selection. Several batches of knockout cell pool were picked and confirmed by immunostaining and Western blot with YME1L antibody.
Mammalian Cell Culture.
HEK293T cells (ATCC) were cultured under standard conditions (1× DMEM, 5% FBS, 5% CO2, 37 °C). HEK 293T cell transfections were performed using Lipofectamine 3000 (cat#: L3000015, Invitrogen), and siRNA knockdown experiments were performed using Lipofectamine RNAiMAX reagent (cat#: 13778150, Invitrogen), according to manufacturer’s instructions. Briefly, HEK293T cells and patient fibroblast cells were transfected with lipofectamine RNAiMAX reagent according to standard protocol. After 72-h transfection, cells were washed with warm PBS, followed by lysis and Western blot analysis. Invitrogen siRNAs used for individual genes are as follows: siCON (cat#: 12935-400), siNEMF (HSS113541, HSS113540), siYME1L (cat#: AM16708), siVCP (HSS111263, HSS111264), siANKZF1 (HSS123962), siAKT1 (VHSS40082), siAKT2 (VHS41339), siAKT3 (cat#: AM51331). C9-ALS/FTD patient fibroblasts, and matched control fibroblasts were described previously ( 74) and kindly provided by Aaron Gitler (Stanford University, Stanford, CA).
Drugs and their concentrations used in cell culture studies are: Anisomycin (A9789, Sigma; 50 µM); AKT activator sc79 (S7863, Selleckchem; 2 µM); AKT inhibitor MK-2206 (S1078; Selleckchem; 2 µM); VCP inhibitor NMS-873 (cat#: S7285, Selleckchem; 1 µM); Puromycin (P9620, Sigma; 100 µM); Emetine (E2375, Sigma; 200 µM); HHT (H0635, Sigma; 5 µM). MMP of C9-ALS/FTD patient fibroblasts was measured using Image-iT TMRM reagent (Invitrogen, cat# I34361) following the manufacturer’s instructions.
Puromycin Labeling of Stalled NPs.
HEK293T cells transfected with pcDNA-Flag-GR80 were treated with puromycin (100 µM, Sigma) and emetine (200 µM, Sigma) at 37 °C for 5 min before harvesting. Cells were then placed on ice, washed with cold HBS, and subjected to mitochondrial purification. To preferentially label stalled NPs, cells were treated with HHT (5 µM; Tocris Bioscience) for 10 min to prevent new translation initiation and to allow active ribosomes to run off before the addition of puromycin/emetine. NPs on MOM were subjected to Western blot or IP analysis. For in vitro puromycin labeling of NPs on MOM, purified mitochondria were suspended in 10 mM Tris (pH 7.4), 400 mM KCl, 3 mM MgCl2, and 2 µM biotin-linked puromycin (Jena Bioscience). Puromycylation reactions were performed at 37 °C for 90 min, and postreaction, biotin-puromycin labeled NPs were purified with Pierce Neutravidin agarose beads and subjected to further analysis.
RT-PCR.
For RT-PCR analysis, total RNAs were extracted from HEK293T cells or fly thoraces by using the RNeasy Minikit (Qiagen), followed by cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad). For RT-PCR analysis of fly muscle samples, 7-d-old male flies were used. For RT-PCR analysis of GR80 expression in HEK293T cells, RNAs were extracted after 72 h posttransfection with pcDNA3-Flag-GR80 plasmid.
Sequences of RT-PCR primers used in fly studies are as follows: GR80 sense: 5′GGATTACAAGGACGACGACGAT3′; GR80 antisense: 5′ATTCCACCACTGCTCCCATTC3′; Actin42A sense: 5′TCTTACTGAGCGCGGTTACAG3′; Actin42A antisense: 5′ATGTCGCGCACAATTTCAC3′; α-Tubulin sense: 5′CGTATACGCTCTCTGAGTCAGACCTC3′; α-Tubulin antisense: 5′GCAGACCGGTGCACTGATCGGCCAGC3′; Tfam sense: 5′GGCTCAGGTGGATCGATAAG3′; Tfam antisense: 5′GAGTGGCACCAAAAGACCAC3′; YME1L sense 5′-GAGTCGGCCACACAGATCG-3′; YME1L antisense 5′-GAGAAGAGCGGACGAAGAAGA -3′; Clbn sense 5′-GCGCAAGACGCAGCAGACG-3′; Clbn antisense 5′-TCTGCTGGGCATCTCTTC-CTCC-3′; IARS sense 5′-CTAGAGCGGAACGACGTGTG-3′; IARS antisense 5′TCAAAGATATTTTCGTGTCGCCA-3′; AARS sense 5′-GCACATCTATGTTCACTC-GTCC-3′; AARS antisense 5′-CCAGTTTCCCAGCATTTCAAAG-3′; TARS sense 5′-AGGGTCTCGCTGACAACAC-3′; and TARS antisense 5′-GCAGGGTGCAGTTTC-CCTC-3′.
Sequences of RT-PCR primers used in mammalian cell studies are: GR80 sense: 5′ATGGATTACAAGGACGACGACGAT3′; GR80 antisense: 5′ACACCTACTCAGACAATGCGATGC3′; GAPDH sense: 5′AGAAGGCTGGGGCTCATTTG3′; and GAPDH-antisense: 5′AGGGGCCATCCACAGTCTTC3′.
For qRT-PCR, RNA was purified using RNeasy mini kit (Qiagen), and subjected to reverse transcription using the iScript cDNA synthesis kit (Bio-Rad). cDNA templates were mixed with PowerUp SYBR Green Master Mix (Applied Biosystems) and detected by StepOnePlus real-time PCR system according to standard PCR thermal cycling protocol. Calculated data were collected using StepOne software V2.3. Data were exported into Excel and further processed for quantification and statistical analysis. Relative mRNA levels shown in the graph were normalized by actin. Relative GR80 mRNA levels shown in the RNA-IP graph were normalized by Tfam.
Quantification and Statistical Analysis.
All analyses were performed with SPSS (IBM). Error bars represent SD. For pair-wise comparisons, we used two-tailed Student’s t test. For comparing multiple groups, we used a one-way ANOVA test followed by Student Newman–Keuls test (SNK test) plus Bonferroni correction (multiple hypotheses correction). Images and Western blots shown were representatives of three independent repeats. Quantification of signal intensity on immunoblots for normalization was performed using NIH ImageJ. For wing posture analysis, three groups were used, with each group n = 15. For qRT-PCR analysis, seven flies were used to extract RNA, followed by cDNA transcription and qRT-PCR. Three biological repeats were performed. For the quantification of GA, PR, and TMRM intensity, three independent experiments were performed, with five technical repeats used for each genotype.
Supplementary Material
Acknowledgments
We thank Drs. William Saxton, Fen-Biao Gao, Paul Taylor, Hong Xu, Xiaolin Bi, the Vienna Drosophila RNAi Center, FlyORF, and the Bloomington Drosophila Stock Center for fly stocks; Drs. Fen-Biao Gao and Luke Wiseman for plasmids; Xiaolin Bi for antibody; Dr. Aaron Gitler for C9-amyotrophic lateral sclerosis with frontotemporal dementia patient fibroblasts and discussions; Jennifer Gaunce for maintaining flies and providing technical support; and members of the B.L. laboratory for discussions. This work was supported by NIH Grants R01NS083417, R01NS084412, and R01AR0748750 (to B.L.), and R01GM115898 (to S.G.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005506117/-/DCSupplemental.
Data Availability.
All study data are included in the main text and SI Appendix.
References
- 1.Klaips C. L., Jayaraj G. G., Hartl F. U., Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 217, 51–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou Y. et al., Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A., Brundin P., The ubiquitin proteasome system in neurodegenerative diseases: Sometimes the chicken, sometimes the egg. Neuron 40, 427–446 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Duttler S., Pechmann S., Frydman J., Principles of cotranslational ubiquitination and quality control at the ribosome. Mol. Cell 50, 379–393 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F., Durfee L. A., Huibregtse J. M., A cotranslational ubiquitination pathway for quality control of misfolded proteins. Mol. Cell 50, 368–378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joazeiro C. A. P., Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 20, 368–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z. et al., MISTERMINATE mechanistically links mitochondrial dysfunction with proteostasis failure. Mol. Cell. 75, 835–848.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandman O., Hegde R. S., Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 23, 7–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen P. S. et al., Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347, 75–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandman O. et al., A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostova K. K. et al., CAT-tailing as a fail-safe mechanism for efficient degradation of stalled nascent polypeptides. Science 357, 414–417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitron C. S., Brandman O., CAT tails drive degradation of stalled polypeptides on and off the ribosome. Nat. Struct. Mol. Biol. 26, 450–459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izawa T., Park S. H., Zhao L., Hartl F. U., Neupert W., Cytosolic protein Vms1 links ribosome quality control to mitochondrial and cellular homeostasis. Cell 171, 890–903.e18 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Choe Y. J. et al., Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature 531, 191–195 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Yonashiro R. et al., The Rqc2/Tae2 subunit of the ribosome-associated quality control (RQC) complex marks ribosome-stalled nascent polypeptide chains for aggregation. eLife 5, e11794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defenouillère Q. et al., Rqc1 and Ltn1 prevent C-terminal alanine-threonine tail (CAT-tail)-induced protein aggregation by efficient recruitment of Cdc48 on stalled 60S subunits. J. Biol. Chem. 291, 12245–12253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu J. et al., A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 2097–2103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishimura R. et al., RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 345, 455–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor J. P., Brown R. H. Jr., Cleveland D. W., Decoding ALS: From genes to mechanism. Nature 539, 197–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendron T. F., Petrucelli L., Disease mechanisms of C9ORF72 repeat expansions. Cold Spring Harb. Perspect. Med. 8, a024224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balendra R., Isaacs A. M., C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuva-Aydemir Y., Almeida S., Gao F. B., Insights into C9ORF72-related ALS/FTD from Drosophila and iPSC models. Trends Neurosci. 41, 457–469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z. et al., Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 110, 7778–7783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizielinska S. et al., C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 345, 1192–1194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D. et al., FTD/ALS-associated poly(GR) protein impairs the Notch pathway and is recruited by poly(GA) into cytoplasmic inclusions. Acta Neuropathol. 130, 525–535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K. et al., The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freibaum B. D. et al., GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129–133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran H. et al., Differential toxicity of nuclear RNA foci versus dipeptide repeat proteins in a Drosophila model of C9ORF72 FTD/ALS. Neuron 87, 1207–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burguete A. S. et al., GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. eLife 4, e08881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeynaems S. et al., Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci. Rep. 6, 20877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K. H. et al., C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell 167, 774–788.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry S., Han Y., Das A., Dickman D., Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Hum. Mol. Genet. 26, 4153–4167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simone R. et al., G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol. Med. 10, 22–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moens T. G. et al., Sense and antisense RNA are not toxic in Drosophila models of C9orf72-associated ALS/FTD. Acta Neuropathol. 135, 445–457 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W., Xu J., C9orf72 dipeptide repeats cause selective neurodegeneration and cell-autonomous excitotoxicity in Drosophila glutamatergic neurons. J. Neurosci. 38, 7741–7752 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moens T. G. et al., C9orf72 arginine-rich dipeptide proteins interact with ribosomal proteins in vivo to induce a toxic translational arrest that is rescued by eIF1A. Acta Neuropathol. 137, 487–500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Gonzalez R. et al., Partial inhibition of the overactivated Ku80-dependent DNA repair pathway rescues neurodegeneration in C9ORF72-ALS/FTD. Proc. Natl. Acad. Sci. U.S.A. 116, 9628–9633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berson A. et al., Drosophila Ref1/ALYREF regulates transcription and toxicity associated with ALS/FTD disease etiologies. Acta Neuropathol. Commun. 7, 65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He H. et al., Amyotrophic lateral sclerosis-associated GGGGCC repeat expansion promotes Tau phosphorylation and toxicity. Neurobiol. Dis. 130, 104493 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Gonzalez R. et al., Poly(GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron 92, 383–391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J. et al., Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Clark I. E. et al., Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Yang Y. et al., Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl. Acad. Sci. U.S.A. 103, 10793–10798 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacVicar T., Langer T., OPA1 processing in cell death and disease—The long and short of it. J. Cell Sci. 129, 2297–2306 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Shi H., Rampello A. J., Glynn S. E., Engineered AAA+ proteases reveal principles of proteolysis at the mitochondrial inner membrane. Nat. Commun. 7, 13301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neupert W., Herrmann J. M., Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Lesnik C., Golani-Armon A., Arava Y., Localized translation near the mitochondrial outer membrane: An update. RNA Biol. 12, 801–809 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziehr D. R., Ellis J. P., Culviner P. H., Cavagnero S., Production of ribosome-released nascent proteins with optimal physical properties. Anal. Chem. 82, 4637–4643 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Gold V. A., Chroscicki P., Bragoszewski P., Chacinska A., Visualization of cytosolic ribosomes on the surface of mitochondria by electron cryo-tomography. EMBO Rep. 18, 1786–1800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adelman M. R., Sabatini D. D., Blobel G., Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J. Cell Biol. 56, 206–229 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu J., Deutsch C., Electrostatics in the ribosomal tunnel modulate chain elongation rates. J. Mol. Biol. 384, 73–86 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graber T. E. et al., Reactivation of stalled polyribosomes in synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 110, 16205–16210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tujebajeva R. M., Graifer D. M., Karpova G. G., Ajtkhozhina N. A., Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett. 257, 254–256 (1989). [DOI] [PubMed] [Google Scholar]
- 54.Osuna B. A., Howard C. J., Kc S., Frost A., Weinberg D. E., In vitro analysis of RQC activities provides insights into the mechanism and function of CAT tailing. eLife 6, e27949 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma R. et al., Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent chains from stalled ribosomes. Nature 557, 446–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zurita Rendón O. et al., Vms1p is a release factor for the ribosome-associated quality control complex. Nat. Commun. 9, 2197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuroha K., Zinoviev A., Hellen C. U. T., Pestova T. V., Release of ubiquitinated and non-ubiquitinated nascent chains from stalled mammalian ribosomal complexes by ANKZF1 and Ptrh1. Mol. Cell. 72, 286–302.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yip M. C. J. et al., Mechanism for recycling tRNAs on stalled ribosomes. Nat. Struct. Mol. Biol. 26, 343–349 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Kopan R., Ilagan M. X., The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 137, 216–233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K. S. et al., Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 27, 2642–2647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jo H. et al., Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. U.S.A. 109, 10581–10586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirai H. et al., MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 9, 1956–1967 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Klein J. B. et al., Akt-mediated valosin-containing protein 97 phosphorylation regulates its association with ubiquitinated proteins. J. Biol. Chem. 280, 31870–31881 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Vandermoere F. et al., The valosin-containing protein (VCP) is a target of Akt signaling required for cell survival. J. Biol. Chem. 281, 14307–14313 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Onesto E. et al., Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol. Commun. 4, 47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S. et al., Altered MICOS morphology and mitochondrial ion homeostasis contribute to poly(GR) toxicity associated with C9-ALS/FTD. Cell Rep. 32, 107989 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi S. Y. et al., C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat. Neurosci. 22, 851–862 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y. J. et al., Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. Nat. Med. 24, 1136–1142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruan L. et al., Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J. et al., FUS interacts with HSP60 to promote mitochondrial damage. PLoS Genet. 11, e1005357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang P. et al., TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 15, e1007947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan V. H., Fawzi N. L., Physiological, pathological, and targetable membraneless organelles in neurons. Trends Neurosci. 42, 693–708 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cong L. et al., Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kramer N. J. et al., Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science 353, 708–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.