Significance
Mitochondrial physiology affected by childhood maltreatment (CM) might be a biological link for the intergenerational transmission of CM-related consequences. As mitochondria are intergenerationally transmitted from mother to child, alterations in mitochondrial bioenergetics might influence how maternal CM affects physical and mental development as well as functioning in their children. Our study reports data on mitochondrial bioenergetics in peripheral mononuclear immune cells of mother-newborn dyads, which were positively correlated. Higher maternal maltreatment load was associated with higher mitochondrial respiration and density in mothers, but not in their newborns. Thus, we found no evidence for an intergenerational effect of maternal CM on mitochondrial bioenergetics in immune cells of their newborns.
Keywords: childhood maltreatment, mitochondria, bioenergetics, intracellular density, immune cells
Abstract
Childhood maltreatment (CM) comprises experiences of abuse and neglect during childhood. CM causes psychological as well as biological alterations in affected individuals. In humans, it is hardly explored whether these CM consequences can be transmitted directly on a biological level to the next generation. Here, we investigated the associations between maternal CM and mitochondrial bioenergetics (mitochondrial respiration and intracellular mitochondrial density) in immune cells of mothers and compared them with those of their newborns. In n = 102 healthy mother-newborn dyads, maternal peripheral blood mononuclear cells and neonatal umbilical cord blood mononuclear cells were collected and cryopreserved shortly after parturition to measure mitochondrial respiration and intracellular mitochondrial density with high-resolution respirometry and spectrophotometric analyses, respectively. Maternal CM was assessed with the Childhood Trauma Questionnaire. Maternal and neonatal mitochondrial bioenergetics were quantitatively comparable and positively correlated. Female newborns showed higher mitochondrial respiration compared to male newborns. Maternal CM load was significantly and positively associated with mitochondrial respiration and density in mothers, but not with mitochondrial respiration in newborns. Although maternal and neonatal mitochondrial bioenergetics were positively correlated, maternal CM only had a small effect on mitochondrial density in newborns, which was not significant in this study after adjustment for multiple comparisons. The biological relevance of our finding and its consequences for child development need further investigation in future larger studies. This study reports data on mitochondrial bioenergetics of healthy mother-newborn dyads with varying degrees of CM.
Childhood maltreatment (CM) has a strong impact on the development of psychological as well as biological functioning of affected individuals. CM includes physical, sexual, and emotional abuse as well as physical and emotional neglect during childhood and adolescence (1). A history of CM is associated with a statistically elevated risk for somatic and mental diseases throughout life such as diabetes, metabolic and cardiovascular diseases, depression, and posttraumatic stress disorder (2). A growing body of literature indicates that CM exposure exerts its lifelong detrimental effects on mental and physical health via impairing the physiological stress regulation (3, 4). However, specific pathways and underlying mechanisms linking CM to health outcomes in adulthood are hardly identified.
Not only women directly affected by CM suffer from the detrimental consequences of CM but also their children: CM exposure and psychosocial stress of mothers predicts an increased risk for adverse health outcomes among their children such as lower birth weight, asthma, or allergies (5, 6). Additionally, maternal CM experiences are linked to a higher prevalence of attention deficit hyperactivity disorder, autism, or emotional and behavioral problems among affected individuals’ descendants (7–9). However, not all affected individuals or their children develop adverse health outcomes later in life, suggesting that psychological and biological resilience factors may influence how deep psychological stress gets under the skin (10). There are different mechanisms through which risk and resilience factors might be conferred from parents to children, including (i) behavioral alterations, e.g., dysfunctional parenting behavior among parents with CM such as less maternal sensitivity or less emotional availability (11); (ii) inheritance of genetic risk or resilience factors (12); and (iii) the transmission of persistent biological alterations related to parental trauma exposure, termed biological intergenerational transmission (13).
On a biological scale, studies have tried to improve the understanding of how CM affects health by targeting (epi)genetics, gene expression, as well as endocrine and immunological changes (14, 15). Specifically, the immune system has become a topic of interest as chronic and traumatic psychological stress has been related to chronic low-grade inflammation (16–18) and impaired wound healing (19). Additionally, there is evidence for altered mitochondrial functioning in individuals with CM (16). We provided the first empirical evidence for an association between CM and mitochondrial oxygen consumption rates as well as the production of reactive oxygen species (ROS) in postpartum women with CM (16, 20). Human studies assessing the association between early life stress such as CM and mitochondrial density revealed mixed results: In our previous study, mitochondrial density was not altered in postpartum women with CM (16), whereas nonpregnant, nonpostpartum adults with CM showed significantly higher leukocyte mitochondrial DNA copy number (mtDNAcn) than those without CM (21). Another study also reported a significantly higher mtDNAcn with the experience of childhood sexual abuse in adult women with current major depression, however not in healthy controls (22). Chronic caregiving stress, another kind of chronic stress, was associated with lower mitochondrial respiration but not with mtDNAcn in chronically stressed women (23).
Mitochondria are intracellular organelles present in almost all eukaryotic cells. Mitochondria are essentially responsible for the production of biochemical energy in the form of adenosine triphosphate (ATP). To a great extent, ATP synthesis depends on cellular oxygen consumption through the oxidative phosphorylation (OXPHOS) system located at the inner mitochondrial membrane. Mitochondria possess their own circular DNA, mitochondrial DNA (mtDNA), which encodes protein subunits of the OXPHOS system. Additionally, mitochondria play a key role in regulating calcium homeostasis (24), redox balance (25), apoptosis (26), steroid synthesis (27), and inflammatory processes (28). Moreover, mitochondria are key components of the physiological stress response in humans (29). They are primarily responsible for meeting the increased energy demand in stressful situations challenging the homeostasis of cells, tissues, and organs (30). Therefore, mitochondrial functioning and the intracellular density of mitochondria are highly reactive to stress (29, 31).
Notably, among humans, mitochondria and mtDNA are predominantly inherited from mothers to the next generation (32). However, there is an ongoing debate about the possibility of biparental transmission of mtDNA in some exceptional cases (33). Nevertheless, stress and trauma-associated alterations in mitochondrial functioning can be expected to be predominantly inherited from mothers to their children. On the biological level, the findings of lower placental mtDNAcn in mothers reporting more traumatic events over their lifespan can be seen as a first hint on possible intergenerational effects of trauma-associated mitochondrial alterations (34, 35), while the physiological consequence of these findings remain undetermined.
To address a direct intergenerational transmission of CM-associated mitochondrial alterations from mother to child, we investigated mitochondrial bioenergetics (i.e., mitochondrial respiration and density) with respect to maternal CM experiences in mother-newborn dyads (n = 102, SI Appendix, Table S1). First, we characterized mitochondrial respiration and density in umbilical cord blood mononuclear cells (UBMCs) of newborns and in peripheral blood mononuclear cells (PBMCs) of their mothers to test for possible associations of maternal and neonatal mitochondrial parameters. Second, based on our previous results (16), we hypothesized to find maternal CM exposure associated with higher mitochondrial bioenergetics in women in the early postpartum period (n = 105). Third, we hypothesized an association of neonatal mitochondrial bioenergetics (n = 104) with maternal CM exposure as an indicator of a possible intergenerational transmission of mitochondrial alterations from mother to child.
Results
Mitochondrial Respiration and Density in Mother-Newborn Dyads.
Mitochondrial respiration and intracellular density (citrate synthase activity; CSA) values were in a comparable range for mothers and their newborns (Table 1). Maternal and neonatal mitochondrial respiration parameters showed small- to medium-sized positive correlations (τ = 0.10−0.29), which were all significant with the exception for Leak respiration and Leak control ratio (Table 1 and SI Appendix, Fig. S1). Maternal and neonatal CSA showed a small-sized positive correlation (τ = 0.13, 95% CI [−0.01, 0.26], P = 0.072; PFDR = 0.090; Table 1 and SI Appendix, Fig. S1). Furthermore, female newborns showed significantly higher Routine respiration (η2p = 0.053, P = 0.018, PFDR = 0.036) and ATP-turnover–related respiration (η2p = 0.094, P = 0.003, PFDR = 0.008) than male newborns (SI Appendix, Tables S3 and S4). Moreover, maximal respiratory capacity was also higher in female newborns than in male newborns. However, this effect was small (η2p = 0.032) and no longer significant after the adjustment for multiple comparisons (P = 0.034, PFDR = 0.051, SI Appendix, Tables S3 and S4).
Table 1.
Biological data of mothers and their newborns with correlation between maternal and neonatal mitochondrial bioenergetics
| Mothers (n = 105) | Newborns (n = 104) | Correlation (n = 102)* | ||||||||||||||
| Main effect CM group† | Main effect CM group† | |||||||||||||||
| CM− group (n = 67) M [SD] | CM+ group (n = 38) M [SD] | F (1, 102) | η2partial | P | PFDR | CM− group (n = 65) M [SD] | CM+ group (n = 39) M [SD] | F (1, 100) | η2partial | P | PFDR | τ | 95% CI (τ) | P | PFDR | |
| Mitochondrial respiration and density | ||||||||||||||||
| Routine respiration‡ | 3.31 [0.62] | 3.62 [0.64] | 7.11 | 0.057 | 0.009 | 0.019 | 3.18 [0.50] | 3.30 [0.60] | 1.04 | 0.012 | 0.309 | 0.412 | 0.16 | [0.01, 0.30] | 0.021 | 0.030 |
| Leak respiration‡ | 0.97 [0.27] | 1.07 [0.31] | 1.03 | 0.010 | 0.314 | 0.428 | 0.86 [0.19] | 0.86 [0.24] | 0.37 | 0.004 | 0.543 | 0.639 | 0.10 | [−0.03, 0.23] | 0.146 | 0.146 |
| ATP-turnover–related respiration‡ | 2.34 [0.47] | 2.55 [0.46] | 6.87 | 0.051 | 0.010 | 0.020 | 2.32 [0.43] | 2.43 [0.46] | 1.84 | 0.018 | 0.178 | 0.274 | 0.20 | [0.06, 0.34] | 0.003 | 0.010 |
| Maximal respiratory capacity‡ | 5.93 [1.33] | 6.24 [1.18] | 1.33 | 0.014 | 0.252 | 0.360 | 5.66 [1.42] | 6.09 [1.60] | 1.53 | 0.021 | 0.219 | 0.313 | 0.16 | [0.01, 0.30] | 0.019 | 0.030 |
| Spare respiratory capacity‡ | 2.62 [1.07] | 2.62 [1.07] | 0.03 | <0.001 | 0.870 | 0.932 | 2.48 [1.21] | 2.80 [1.24] | 1.11 | 0.017 | 0.294 | 0.406 | 0.18 | [0.04, 0.32] | 0.007 | 0.016 |
| Routine control ratio, % | 57 [11] | 59 [12] | 0.57 | 0.006 | 0.450 | 0.587 | 58 [12] | 56 [10] | 0.03 | <0.001 | 0.861 | 0.906 | 0.22 | [0.07, 0.36] | 0.002 | 0.010 |
| Leak control ratio, % | 17 [5] | 18 [5] | 0.17 | 0.002 | 0.682 | 0.787 | 16 [5] | 15 [4] | 1.58 | 0.016 | 0.212 | 0.313 | 0.11 | [−0.02, 0.24] | 0.132 | 0.146 |
| Net routine ratio, % | 41 [8] | 42 [9] | 0.33 | 0.003 | 0.568 | 0.695 | 42 [8] | 41 [8] | 0.01 | <0.001 | 0.903 | 0.926 | 0.29 | [0.16, 0.42] | <0.001 | 0.001 |
| Coupling efficiency, % | 71 [6] | 71 [6] | 0.01 | <0.001 | 0.938 | 0.938 | 73 [5] | 74 [5] | 2.40 | 0.017 | 0.125 | 0.211 | 0.19 | [0.06, 0.32] | 0.008 | 0.016 |
| Citrate synthase activity§ | 0.0038 [0.0007] | 0.0043 [0.0011] | 6.62 | 0.061 | 0.012 | 0.023 | 0.0033 [0.0005] | 0.0034 [0.0005] | 3.35 | 0.039 | 0.070 | 0.133 | 0.13 | [−0.01, 0.26] | 0.072 | 0.090 |
| Blood cell composition¶ | ||||||||||||||||
| Lymphocytes (% in whole blood) | 19.3 [5.2] | 18.8 [4.9] | t(96) = −0.44, d = −0.09# | 0.659 | — | 30.6 [7.9] | 29.8 [6.0] | t(82) = −0.48, d = −0.11# | 0.629 | — | — | — | — | — | ||
| Monocytes (% in whole blood) | 6.1 [1.5] | 6.1 [1.6] | t(96) = −0.002, d < −0.001# | 0.999 | — | 8.3 [2.3] | 9.3 [2.6] | t(82) = 1.85, d = 0.42# | 0.068 | — | — | — | — | — | ||
| Storage time of cryopreserved cells (d) | 1,100 [216] | 1,143 [237] | U = 3,413.50, r = 0.09# | 0.359 | — | 1,101 [218] | 1,140 [237] | U = 3,288.00, r = 0.08# | 0.403 | — | — | — | — | — | ||
All measures are given as mean [SD]. Significant group effects and correlations are given in bold. Parameters for mitochondrial respiration are presented corrected for residual oxygen consumption (ROX). For ROX-uncorrected raw data, please see SI Appendix, Table S2. For the mitochondrial parameters, P values were adjusted for multiple comparisons according to the Benjamini–Hochberg procedure (i.e., false discovery rate, FDR) (52). CM, childhood maltreatment; CM−, women without CM experiences; CM+, women with mild to severe CM experiences.
Correlation between maternal and neonatal mitochondrial respiration and density: Two-tailed Kendall’s tau correlation coefficients (τ) were calculated.
Main effect of the CM group (ANCOVA). CM− group as reference group. For further information, see SI Appendix, Table S5 for the mothers and SI Appendix, Table S8 for the newborns.
Given in pmol O2/s per Mio cells.
Given in µmol/min per Mio cells.
Blood counts available from n = 98 mothers (CM− group: n = 64, CM+ group: n = 34) and n = 84 newborns (CM− group: n = 54, CM+ group: n = 30); Blood cell compositions of postpartum women (53) and umbilical cord blood (54, 55) were comparable to other studies.
Two-tailed Student t tests or Mann–Whitney U tests were calculated where appropriate. CM− as reference group. As effect size measure Cohen’s d or r is reported.
Associations Between Maternal Childhood Maltreatment and Maternal Mitochondrial Bioenergetics.
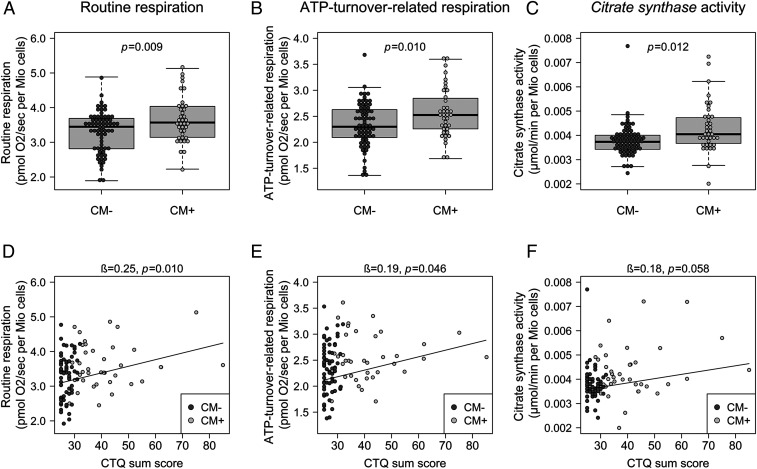
PBMCs of CM+ mothers exhibited significantly higher Routine respiration (η2p = 0.057, P = 0.009, PFDR = 0.019), ATP-turnover–related respiration (η2p = 0.051, P = 0.010, PFDR = 0.020), and CSA (η2p = 0.061, P = 0.012, PFDR = 0.023) than PBMCs of CM− mothers, with medium effect sizes, in analyses of covariance (ANCOVAs) accounting for the storage time of cryopreserved cells as a covariate (Fig. 1, Table 1, and SI Appendix, Table S5). CM+ and CM− mothers did not differ with regard to other mitochondrial respiration parameters or flux control ratios (Table 1 and SI Appendix, Table S5). Mitochondrial parameters that differed significantly between CM− and CM+ mothers were additionally predicted by CM load (CTQ sum score) using linear regression analyses. CM load was linked to significantly higher Routine respiration (β = 0.25, 95% CI [0.06, 0.43], P = 0.010) and significantly higher ATP-turnover–related respiration (β = 0.19, 95% CI [0.004, 0.37], P = 0.046) as well as (nonsignificantly) higher CSA (β = 0.18, 95% CI [−0.01, 0.36], P = 0.058) (Fig. 1 and Table 2), with longer storage time of cryopreserved cells leading to lower mitochondrial respiration. Controlling for possible influences of the PBMC subcell composition (i.e., the percentage of lymphocytes and monocytes in whole blood) in an additional regression model did not change the effect sizes for CM load prediction, but the significance for the given (now smaller) sample size (n = 98; SI Appendix, Table S6). We repeated the analyses with the respiration parameters normalized for CSA. As a result, the associations between CM load and Routine respiration as well as ATP-turnover–related respiration diminished (SI Appendix, Table S7).
Fig. 1.
Mitochondrial respiration and intracellular mitochondrial density in mothers (n = 105). (A–C) Boxplots for group differences. Routine respiration (A), ATP-turnover–related respiration (B) and citrate synthase activity (C) were significantly higher in PBMCs of mothers with CM (CM+, n = 38) than in PBMCs of CM− mothers (n = 67). (D–F) Scatterplots for the association between maternal CM load and mitochondrial respiration and density. CM load (CTQ sum score) was linked to higher Routine respiration (D), higher ATP-turnover–related respiration (E), as well as (nonsignificantly) higher citrate synthase activity (F) in PBMCs of mothers (n = 105). Please note for D that two women have the same CTQ sum score of 62 and the same Routine respiration (3.55 pmol O2/s per Mio cells). β, standardized regression coefficient; CM, Childhood maltreatment; CM+, women with CM experiences; CM−, women without CM experiences; CTQ, Childhood Trauma Questionnaire.
Table 2.
Results of multiple linear regression analyses concerning associations between maternal CM load and mitochondrial respiration and density in mothers (n = 105) and in their newborns (n = 104)
| Outcome variable | Predictor | B (SE) | β | 95% CI (β) | t | η2partial | P | |
| Mothers (n = 105) | ||||||||
| Routine respiration (pmol O2/s per Mio cells) | Intercept | 3.54 (0.36) | — | — | 9.85 | — | <0.001*** | |
| CTQ sum score | 0.02 (0.01) | 0.25 | [0.06, 0.43] | 2.63 | 0.063 | 0.010* | ||
| Storage time of cryopreserved cells | −5.48×10−4 (2.71×10−4) | −0.19 | [−0.38, −0.004] | −2.02 | 0.039 | 0.046* | ||
| Overall model statistics: F(2,102) = 5.45, P = 0.006**, R2 = 0.097, R2adj = 0.079 | ||||||||
| ATP-turnover–related respiration (pmol O2/s per Mio cells) | Intercept | 2.80 (0.26) | — | — | 10.64 | — | <0.001*** | |
| CTQ sum score | 8.60×10−3 (4.25×10−3) | 0.19 | [0.004, 0.37] | 2.02 | 0.038 | 0.046* | ||
| Storage time of cryopreserved cells | −5.93×10−4 (1.98×10−4) | −0.28 | [−0.46, −0.09] | −2.99 | 0.081 | 0.003** | ||
| Overall model statistics: F(2,102) = 6.48, P = 0.002**, R2 = 0.113, R2adj = 0.095 | ||||||||
| Citrate synthase activity (µmol/min per Mio cells) | Intercept | 2.85×10−3 (4.00×10−4) | — | — | 7.13 | — | <0.001*** | |
| CTQ sum score | 1.48×10−5 (7.72×10−6) | 0.18 | [−0.006, 0.36] | 1.92 | 0.035 | 0.058# | ||
| Storage time of cryopreserved cells | 4.90×10−7 (2.65×10−7) | 0.13 | [−0.009, 0.26] | 1.85 | 0.032 | 0.067# | ||
| Overall model statistics†: F(2,102) = 3.07, P = 0.051#, R2 = 0.097, R2adj = 0.079 | ||||||||
| Newborns (n = 104) | ||||||||
| Routine respiration (pmol O2/sec per Mio cells) | Intercept | 2.90 (0.32) | — | — | 9.13 | — | <0.001*** | |
| CTQ sum score | 3.71×10−3 (5.00×10−3) | 0.07 | [−0.12, 0.26] | 0.74 | 0.008 | 0.460 | ||
| Storage time of cryopreserved cells | 9.49×10−5 (2.35×10−4) | 0.04 | [−0.16, 0.23] | 0.40 | <0.001 | 0.687 | ||
| Female sex | 0.25 (0.11) | 0.24 | [0.04, 0.43] | 2.35 | 0.052 | 0.021* | ||
| Overall model statistics: F(3,100) = 2.10, P = 0.105, R2 = 0.059, R2adj = 0.031 | ||||||||
| ATP-turnover–related respiration (pmol O2/sec per Mio cells) | Intercept | 2.30 (0.25) | — | — | 9.05 | — | <0.001*** | |
| CTQ sum score | 3.85×10−3 (4.01×10−3) | 0.09 | [−0.10, 0.28] | 0.96 | 0.012 | 0.340 | ||
| Storage time of cryopreserved cells | −1.51×10−4 (1.89×10−4) | −0.08 | [−0.27, 0.11] | −0.80 | 0.017 | 0.425 | ||
| Female sex | 0.26 (0.09) | 0.29 | [0.10, 0.49] | 3.00 | 0.083 | 0.003** | ||
| Overall model statistics: F(3,100) = 4.00, P = 0.010*, R2 = 0.107, R2adj = 0.080 | ||||||||
| Citrate synthase activity (µmol/min per Mio cells) | Intercept | 2.77×10−3 (2.72×10−4) | — | — | 10.18 | — | <0.001*** | |
| CTQ sum score | 1.60×10−6 (4.29×10−6) | 0.04 | [−0.16, 0.23] | 0.37 | 0.001 | 0.709 | ||
| Storage time of cryopreserved cells | 4.66×10−7 (2.02×10−7) | 0.23 | [0.03, 0.42] | 2.31 | 0.054 | 0.023* | ||
| Female sex | −2.79×10−5 (9.25×10−5) | −0.03 | [−0.23, 0.17] | −0.30 | 0.001 | 0.764 | ||
| Overall model statistics: F(3,100) = 1.99, P = 0.120, R2 = 0.056, R2adj = 0.028 | ||||||||
#P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001. Multiple linear regression or robust multiple regression (†) where appropriate. B, unstandardized regression coefficient; β, standardized regression coefficient; CTQ, Childhood Trauma Questionnaire; SE, standard error.
Associations Between Maternal Childhood Maltreatment and Neonatal Mitochondrial Respiration and Density.
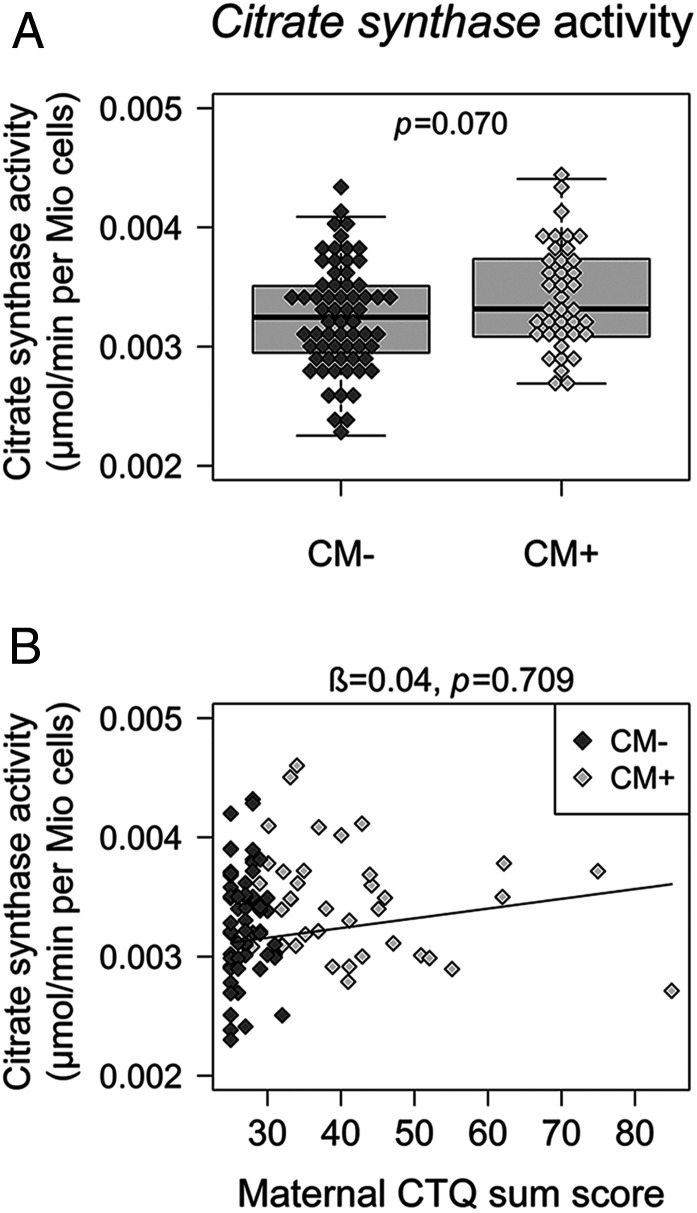
Mitochondrial respiration parameters did not differ between newborns of CM+ and CM− mothers (Table 1). Newborns of CM+ mothers had higher CSA values than newborns of CM− mothers; however, the effect was small (η2p = 0.039) and not significant for the given sample size (P = 0.070, PFDR = 0.133) when newborns’ biological sex and the storage time of cryopreserved cells were considered as covariates (Fig. 2, Table 1, and SI Appendix, Table S8). In linear regression analyses, maternal CM load predicted neither neonatal Routine respiration, nor ATP-turnover–related respiration, nor CSA, when infants’ biological sex and storage time of cryopreserved cells were considered as covariates (Fig. 2 and Table 2). Including UBMC subcell composition (SI Appendix, Table S9) or neonatal gestational age and birth weight (SI Appendix, Table S10) as further covariates or normalizing the mitochondrial respiration parameters for CSA did not change the results (SI Appendix, Table S11).
Fig. 2.
Intracellular mitochondrial density in newborns (n = 104). (A) Boxplot for group difference. Citrate synthase activity (A) was slightly higher in newborns of CM+ women (n = 39) than in newborns of CM− women (n = 65) but did not reach significance for the given sample size. (B) Scatterplot for the association between maternal CM load and neonatal mitochondrial density. Higher maternal CM load (CTQ sum score) was not linked to citrate synthase activity in UBMCs of newborns (n = 104). β, standardized regression coefficient; CM, childhood maltreatment; CM+, women with CM experiences; CM−, women without CM experiences; CTQ, Childhood Trauma Questionnaire.
Discussion
Investigating healthy mother-newborn dyads, we traced whether maternal CM-related alterations in mitochondrial bioenergetics also occur in the next generation. Our results show that mitochondrial bioenergetic parameters had a similar physiological range in mothers and their newborns, exhibiting small- to medium-sized positive correlations. These results are in line with the fact that mitochondria as well as mtDNA are predominantly maternally inherited (32).
In women with CM, we found increased mitochondrial bioenergetic function and density. In detail, higher Routine respiration (i.e., a higher basal physiological activity), higher ATP-turnover–related respiration (i.e., an augmented ATP production), and higher CSA (i.e., a higher mitochondrial density per cell) were observed in PBMCs of CM+ women. Supporting these results, we further found mitochondrial alterations associated with maternal CM load in a dose–response relationship, such that women with a more severe history of CM exhibited higher Routine respiration and ATP-turnover–related respiration as well as higher mitochondrial density (CSA) in PBMCs. These observations suggest that the total energy demand of immune cells is elevated in individuals with higher CM exposure. Correspondingly, we previously reported elevated Routine respiration and ATP-turnover–related respiration linked to more severe CM exposure among women 3 mo postpartum, however only in interaction with increasing serum cortisol levels (20).
In the present study, there have been no significant CM-related alterations in mitochondrial flux control ratios or respiration parameters normalized for CSA. Thus, CM exposure per se might not be associated with an elevated respiratory activity or increased averaged energy production of single mitochondria. Rather, CM exposure appears to be linked to an elevated mitochondrial density per cell, with the higher number of mitochondria per cell probably resulting in an increased overall cellular respiratory activity. However, this interpretation needs further clarification and support through future studies, which are designed to assess mitochondrial density with different markers (e.g., CSA, mtDNAcn) and with a specific focus on postpartum women.
So far, studies assessing the association between CM and mitochondrial density (measured as mtDNAcn or CSA) in humans revealed mixed results: In our previous study, mitochondrial density was not altered in postpartum women with CM (16). In contrast, higher leukocyte mtDNAcn was found in nonpostpartum adults with CM (21). A significantly higher mtDNAcn in saliva was reported only in adult women with a history of childhood sexual abuse along with current major depression; however, this was not found in healthy controls with a history of childhood sexual abuse (22).
As we have investigated women shortly after giving birth, their bioenergetic status probably also reflects the elevated energy demand of pregnancy (36), parturition itself, or the inflammatory processes related to wound healing in the early postpartum period (37). Importantly, chronic and traumatic stress is associated with slower wound healing, probably due to an impaired immune regulation (19). Thus, the elevated mitochondrial bioenergetic function and density in immune cells of mothers with CM could reflect the increased energy demand due to slower wound healing. According to a previous study of our group, the CM-related alterations in mitochondrial density were no longer present among women 3 mo after birth (16), indicating that the CM-related alterations may disappear later after birth, i.e., when the immune reaction eventually weakens and the acute energy requirement decreases.
Taken together, our results suggest that CM-related physiological alterations, e.g., in the immunocellular energy production, might only be seen in stressful phases. According to the mitochondrial allostatic load model (30), mitochondrial energy production might determine the limits of an organism’s capacity to adapt to external and internal stressors. An up-regulation of mitochondrial density and function could thus indicate an increased adaptability and biological resilience, whereas a reduction of both parameters limits the organism’s adaptive capacity (30, 38). However, as a consequence, higher mitochondrial function might increase inflammation and the production of ROS, which was already observed in individuals with CM (16–18, 39).
Regarding the intergenerational perspective, we found maternal and neonatal bioenergetics significantly positively correlated. However, maternal CM exposure did not account for differences in newborns’ mitochondrial respiration. CM history (CM group) of the mother showed a small positive effect on neonatal CSA. However, the effect was not significant for the given sample size when newborns’ biological sex and the storage time of cryopreserved cells were considered as covariates. This result may be seen as a hint for a possible intergenerational transmission of CM-related alterations in terms of increased mitochondrial density in newborns of CM+ mothers. There was further no association between the maternal CM load and mitochondrial density of the newborns. Future studies with larger cohorts are needed to replicate the small effect of maternal CM exposure on newborns’ mitochondrial density and to investigate the biological relevance of such small effects. Moreover, our finding underlines the importance to control for the intracellular mitochondrial density in future studies.
According to the mitochondrial allostatic load model (30), a higher mitochondrial density in UBMCs might be related to increased adaptability and biological resilience of the newborns. More mitochondria per cell might provide the newborns of CM+ mothers with higher energy production and, thus, prepare them for stressful phases they may face later in life. Longitudinal research is needed to gain comprehensive knowledge on the intergenerational effects of CM-related mitochondrial alterations, to investigate whether the observed small effect of maternal CM on higher neonatal mitochondrial density persists, and to trace its possible consequences for child development. We additionally performed a power analysis to estimate required sample sizes for future studies (SI Appendix). To our knowledge, no animal studies have yet investigated the intergenerational transmission of mitochondrial alterations related to early life stress.
Contrary to our observation, previous studies reported lower placental mtDNAcn as a marker of lower mitochondrial density among pregnant mothers with higher lifetime trauma exposure (34, 35). As the placenta is an important regulator of maternal-fetal communication throughout pregnancy (40), trauma-related alterations in placental mitochondrial biogenesis might indirectly affect the fetal physiology. However, those findings were based on parameters measured in placental blood, which is part of the maternal blood circulation. Instead, to test a direct intergenerational transmission, we used cells of the umbilical cord blood, which is physiologically and genetically part of the fetus. We demonstrated the methodological possibility to measure mitochondrial respiration in newborns’ UBMCs. Moreover, our study design allowed the provision of intergenerational data that (largely) excludes the influences of psychosocial factors. By investigating the epigenetic signature of genes involved in the physiological stress response again in UBMCs, we further found no evidence for a direct intergenerational transmission of maternal CM on newborns’ epigenetic signature in a previous study of the “My Childhood—Your Childhood” project (41). In other studies on intergenerational transmission of stress- and trauma-related biological alterations, descendants were already grown up (42, 43) and, thus, exposed to psychosocial factors during their life (e.g., parenting behavior, lifestyle factors like nutrition or psychosocial stress), whereby possible intergenerational transmission effects were confounded.
Of additional interest, our study indicated possible sex differences in mitochondrial respiration in newborns’ UBMCs. Independent of maternal CM exposure, UBMCs of female newborns exhibited higher mitochondrial respiration than those of male newborns, although the absolute sex differences were relatively small with accounting for less than 10% of variance. A previous study investigated sex differences in mitochondrial function in adults and found higher mitochondrial respiration in women (44), which is consistent with our findings. Furthermore, CSA was also significantly higher in women compared to men (44). Sex hormones influence mitochondrial respiration and density (45), which might explain the higher mitochondrial bioenergetics in females. Potentially, sex differences in mitochondrial biology could contribute to sex differences in stress physiology and disease risk throughout life. Future research is needed on the mechanisms underlying sex differences in mitochondrial functioning as well as their long-term consequences for stress physiology and health.
Limitations.
Future research is required to generalize our findings and to further investigate whether CM-related alterations in mitochondrial bioenergetics become evident specifically in stressful situations. For the same, studies should include severely stressed women in different phases of pregnancy as well as in post pregnancy phase. Similarly, for an intergenerational perspective, severely stressed individuals descending from CM-affected mothers should be investigated. A further limitation of the study is the assessment of mitochondrial bioenergetics in cryopreserved cells. Although storage time of cryopreserved cells was statistically considered, we cannot exclude that freezing and thawing procedures might have differentially affected the immune cells of individuals with and without CM. Furthermore, the used cells included different proportions of immune cell subsets (i.e., lymphocytes, monocytes, and dendritic cells), which differ in mitochondrial respiration and density (46). However, CM was not associated with the proportion of monocytes and lymphocytes neither in fresh whole blood in this study, nor within thawed PBMCs of women 3 mo postpartum (47). Moreover, including lymphocyte and monocyte proportions as covariates in additional regression analyses did not change the results. Building on our previous studies (47, 48), further studies should examine mitochondrial bioenergetics in immune cell subsets separately as well as across different cell types to more broadly investigate possible CM-related mitochondrial alterations. As the body mass index (BMI) is an important confounding variable for biological analyses, future studies should monitor the maternal BMI over the course of the pregnancy to analyze its influence on mitochondrial bioenergetics in postpartum women. Moreover, the retrospective cross-sectional design of the study does not allow causal conclusions.
Conclusion. By analyzing the mitochondrial bioenergetics in peripheral immune cells in healthy mother-newborn dyads, we investigated a possible intergenerational transmission of CM-related alterations in mitochondrial bioenergetics from mothers to their newborns. Maternal CM exposure was linked to a higher mitochondrial density in mothers, probably leading to the higher mitochondrial respiration per cell. Although maternal and neonatal mitochondrial parameters were positively correlated, maternal CM exposure did not predict mitochondrial respiration in newborns. Immune cells of newborns with CM-exposed mothers showed slightly higher CSA but no association with maternal CM load. The biological relevance of this finding needs further investigation in larger and longitudinal study cohorts. Increased mitochondrial density in UBMCs might, however, contribute to higher resilience toward bioenergetic demands, including more immunological, endocrine, or central nervous system-related challenges due to early environmental stressors. Future longitudinal research needs to investigate the long-term consequences of maternal CM for the behavioral, temperamental, and immunological development of the upcoming generation.
Materials and Methods
Detailed description of blood sampling, mitochondrial respiration, assessment of the intracellular mitochondrial density, and statistical methods is available in SI Appendix, SI Materials and Methods.
Study Participants.
In accordance with the Declaration of Helsinki (49), all participants declared their written informed consent and all study procedures were approved by the ethics committee of Ulm University. Mothers were recruited within one week after parturition within the study “My Childhood—Your Childhood” Mothers with CM (CM+) and their newborns did not differ from mothers without CM (CM−) and their newborns with regard to descriptive characteristics, except for CM load (CTQ sum score) (SI Appendix, Table S1).
Assessing Mitochondrial Bioenergetics (Mitochondrial Respiration and Mitochondrial Density).
In n = 105 mothers and n = 104 newborns, mitochondrial respiration in intact PBMCs and UBMCs was measured with high-resolution respirometry using an O2k Oxygraph (Oroboros Instruments, Innsbruck, Austria) as previously described in detail (16, 20, 50). For quantifying the density of the mitochondrial network (51), CSA was determined spectrophotometrically in freshly thawed samples at 30 °C as previously described (50, 51).
Supplementary Material
Acknowledgments
Data acquisition was funded by the German Federal Ministry of Education and Research Grant 01KR1304A. Biological analyses and data analyses were funded by university resources of I.-T.K. A.M.G., and A.M.B. were supported by a PhD scholarship of the Konrad Adenauer Foundation; C.B. by a PhD scholarship of the Carl Zeiss Foundation; and A.B. by a PhD scholarship from the German Academic Scholarship Foundation (Studienstiftung des deutschen Volkes). We thank Traudl Hiller for her tremendous contribution to blood processing and PBMCs/UBMCs isolation. We thank Suchithra Varadarajan for language correction. We further acknowledge Prof. Peter Radermacher and his team (University Hospital Ulm) for supporting and providing access to technical resources required for measuring citrate synthase activity. We acknowledge the general support of Dr. Frank Reister and the whole maternity-ward staff at Ulm University Hospital. Finally, we would like to thank the complete team of the “My Childhood—Your Childhood” project.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. C.A.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005885117/-/DCSupplemental.
Data Availability.
The dataset of this manuscript is not publicly available because the data may not be passed on or published to third parties outside the research project. We do not have the consent of the ethics committee or the participants to grant access to the collected data. Upon reasonable request, the corresponding authors will consult the ethics committee to decide on the admissibility to share the data in the specific case.
References
- 1.WHO , WHO fact sheet: Child maltreatment. https://www.who.int/en/news-room/fact-sheets/detail/child-maltreatment. Accessed 22 November 2019.
- 2.Nemeroff C. B., Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron 89, 892–909 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Nelson C. A., Biological embedding of early life adversity. JAMA Pediatr. 167, 1098–1100 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Tarullo A. R., Gunnar M. R., Child maltreatment and the developing HPA axis. Horm. Behav. 50, 632–639 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Smith M. V., Gotman N., Yonkers K. A., Early childhood adversity and pregnancy outcomes. Matern. Child Health J. 20, 790–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomfohr-Madsen L. M., Bayrampour H., Tough S., Maternal history of childhood abuse and risk of asthma and allergy in 2-year-old children. Psychosom. Med. 78, 1031–1042 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Bosquet Enlow M., Englund M. M., Egeland B., Maternal childhood maltreatment history and child mental health: Mechanisms in intergenerational effects. J. Clin. Child Adolesc. Psychol. 47 (suppl. 1), S47–S62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts A. L., Liew Z., Lyall K., Ascherio A., Weisskopf M. G., Association of maternal exposure to childhood abuse with elevated risk for attention deficit hyperactivity disorder in offspring. Am. J. Epidemiol. 187, 1896–1906 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts A. L., Lyall K., Rich-Edwards J. W., Ascherio A., Weisskopf M. G., Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry 70, 508–515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collishaw S. et al., Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse Negl. 31, 211–229 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Fuchs A., Möhler E., Resch F., Kaess M., Impact of a maternal history of childhood abuse on the development of mother-infant interaction during the first year of life. Child Abuse Negl. 48, 179–189 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Koenig A. M. et al., Intergenerational gene × environment interaction of FKBP5 and childhood maltreatment on hair steroids. Psychoneuroendocrinology 92, 103–112 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Bowers M. E., Yehuda R., Intergenerational transmission of stress in humans. Neuropsychopharmacology 41, 232–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bellis M. D., Zisk A., The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 23, 185–222, vii (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schury K., Kolassa I.-T., Biological memory of childhood maltreatment: Current knowledge and recommendations for future research. Ann. N. Y. Acad. Sci. 1262, 93–100 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Boeck C. et al., Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 30, 197–207 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Coelho R., Viola T. W., Walss-Bass C., Brietzke E., Grassi-Oliveira R., Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatr. Scand. 129, 180–192 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Danese A., Pariante C. M., Caspi A., Taylor A., Poulton R., Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U.S.A. 104, 1319–1324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser J. K., Marucha P. T., Malarkey W. B., Mercado A. M., Glaser R., Slowing of wound healing by psychological stress. Lancet 346, 1194–1196 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Boeck C. et al., The association between cortisol, oxytocin, and immune cell mitochondrial oxygen consumption in postpartum women with childhood maltreatment. Psychoneuroendocrinology 96, 69–77 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Tyrka A. R. et al., Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol. Psychiatry 79, 78–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai N. et al., Molecular signatures of major depression. Curr. Biol. 25, 1146–1156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard M. et al., A mitochondrial health index sensitive to mood and caregiving stress. Biol. Psychiatry 84, 9–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzo P., Drago I., Filadi R., Pozzan T., Mitochondrial Ca2+ homeostasis: Mechanism, role, and tissue specificities. Pflugers Arch. 464, 3–17 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Schulz E., Wenzel P., Münzel T., Daiber A., Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 20, 308–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newmeyer D. D., Ferguson-Miller S., Mitochondria: Releasing power for life and unleashing the machineries of death. Cell 112, 481–490 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Miller W. L., Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 379, 62–73 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Mills E. L., Kelly B., O’Neill L. A. J., Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Manoli I. et al., Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 18, 190–198 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Picard M., Juster R.-P., McEwen B. S., Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Picard M., McEwen B. S., Epel E. S., Sandi C., An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 49, 72–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyle A. et al., Extreme-depth Re-sequencing of mitochondrial DNA finds No evidence of paternal transmission in humans. PLoS Genet. 11, e1005040 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo S. et al., Biparental inheritance of mitochondrial DNA in humans. Proc. Natl. Acad. Sci. U.S.A. 115, 13039–13044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunst K. J. et al., Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. Am. J. Epidemiol. 186, 1227–1236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunst K. J. et al., Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ. Int. 112, 49–58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King J. C., Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 71 (suppl. 5), 1218S–1225S (2000). [DOI] [PubMed] [Google Scholar]
- 37.Maes M., Ombelet W., De Jongh R., Kenis G., Bosmans E., The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J. Affect. Disord. 63, 85–92 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Morava E., Kozicz T., Mitochondria and the economy of stress (mal)adaptation. Neurosci. Biobehav. Rev. 37, 668–680 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Boeck C. et al., The association of childhood maltreatment with lipid peroxidation and DNA damage in postpartum women. Front. Psychiatry 10, 23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrera E. A. et al., The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front. Pharmacol. 5, 149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramo-Fernández L. et al., The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: An intergenerational approach. Sci. Rep. 9, 983 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yehuda R. et al., Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 171, 872–880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radtke K. M. et al., Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 1, e21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silaidos C. et al., Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 9, 34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaignard P. et al., Role of sex hormones on brain mitochondrial function, with special reference to aging and neurodegenerative diseases. Front. Aging Neurosci. 9, 406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chacko B. K. et al., Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. 93, 690–700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeck C. et al., History of child maltreatment and telomere length in immune cell subsets: Associations with stress- and attachment-related hormones. Dev. Psychopathol. 30, 539–551 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Karabatsiakis A., Kolassa I.-T., Kolassa S., Rudolph K. L., Dietrich D. E., Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry 14, 192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Medical Association , World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Karabatsiakis A. et al., Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl. Psychiatry 4, e397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eigentler A. et al., Laboratory protocol: Citrate synthase a mitochondrial marker enzyme. Mitochondrial Physiol. Netw. 17, 1–11 (2012). [Google Scholar]
- 52.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995). [Google Scholar]
- 53.Gennaro S. et al., Lymphocyte, monocyte, and natural killer cell reference ranges in postpartal women. Clin. Diagn. Lab. Immunol. 4, 195–201 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pranke P. et al., Hematologic and immunophenotypic characterization of human umbilical cord blood. Acta Haematol. 105, 71–76 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Chang Y.-H. et al., Complete blood count reference values of cord blood in Taiwan and the influence of gender and delivery route on them. Pediatr. Neonatol. 52, 155–160 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset of this manuscript is not publicly available because the data may not be passed on or published to third parties outside the research project. We do not have the consent of the ethics committee or the participants to grant access to the collected data. Upon reasonable request, the corresponding authors will consult the ethics committee to decide on the admissibility to share the data in the specific case.