Significance
The venom of Australian funnel-web spiders contains δ-hexatoxins (δ-HXTXs) that exert fatal neurotoxic effects in humans by inhibiting inactivation of voltage-gated sodium channels, but their precise ecological role remains unclear. Sequencing of venom-gland transcriptomes from 10 funnel-web species uncovered 22 δ-HXTXs. Evolutionary analysis revealed extreme conservation of these toxins, despite their ancient origin. We isolated the lethal δ-HXTX from venom of the Sydney funnel-web spider and showed that it induces pain in mice, suggesting a role in predator deterrence. Although humans are not the target of δ-HXTXs, these toxins likely evolved to deter vertebrate predators commonly encountered by these spiders, such as bandicoots, birds, and lizards. Thus, the lethal potency of δ-HXTXs against humans is an unfortunate evolutionary coincidence.
Keywords: venom, evolution, spider
Abstract
Australian funnel-web spiders are infamous for causing human fatalities, which are induced by venom peptides known as δ-hexatoxins (δ-HXTXs). Humans and other primates did not feature in the prey or predator spectrum during evolution of these spiders, and consequently the primate lethality of δ-HXTXs remains enigmatic. Funnel-web envenomations are mostly inflicted by male spiders that wander from their burrow in search of females during the mating season, which suggests a role for δ-HXTXs in self-defense since male spiders rarely feed during this period. Although 35 species of Australian funnel-web spiders have been described, only nine δ-HXTXs from four species have been characterized, resulting in a lack of understanding of the ecological roles and molecular evolution of δ-HXTXs. Here, by profiling venom-gland transcriptomes of 10 funnel-web species, we report 22 δ-HXTXs. Phylogenetic and evolutionary assessments reveal a remarkable sequence conservation of δ-HXTXs despite their deep evolutionary origin within funnel-web spiders, consistent with a defensive role. We demonstrate that δ-HXTX-Ar1a, the lethal toxin from the Sydney funnel-web spider Atrax robustus, induces pain in mice by inhibiting inactivation of voltage-gated sodium (NaV) channels involved in nociceptive signaling. δ-HXTX-Ar1a also inhibited inactivation of cockroach NaV channels and was insecticidal to sheep blowflies. Considering their algogenic effects in mice, potent insecticidal effects, and high levels of sequence conservation, we propose that the δ-HXTXs were repurposed from an initial insecticidal predatory function to a role in defending against nonhuman vertebrate predators by male spiders, with their lethal effects on humans being an unfortunate evolutionary coincidence.
Despite their fearsome reputation, only a few species of spiders can cause death or serious harm to humans (1). An infamous exception is the Australian funnel-web spider, arguably the world’s deadliest spider (2). These spiders produce extraordinarily complex venoms, with each venom containing up to several thousand peptide toxins (3, 4). Despite this chemical complexity, a single family of toxins known as the δ-hexatoxins (δ-HXTXs) is responsible for the human envenomation syndrome (5). There are currently 35 described species of Australian funnel-web spiders and 38 species of related non-Australian funnel-web spiders in the genus Macrothele, but to date only 12 δ-HXTX sequences have been reported from six species within this broad clade. A homologous δ-actinopoditoxin (δ-AOTX) is present in the venom of the related Australian mouse spider Missulena bradleyi (6), which can cause serious human envenomations with symptoms resembling those from funnel-web spider bites (7).
δ-HXTXs and δ-AOTX comprise 42 to 44 residues and contain four disulfide bonds, three of which are arranged in an inhibitor cystine knot (ICK) motif (8, 9). δ-HXTXs slow the inactivation of vertebrate tetrodotoxin-sensitive voltage-gated sodium (NaV) channels and insect NaV channels by binding to the voltage sensor in channel domain IV (10, 11). In human bite victims, δ-HXTXs cause disturbances in respiration, blood pressure, and heart rate, followed by severe hypotension. Without treatment with commercial antivenom (5), fatalities can occur by respiratory and circulatory failure within a few hours of the bite (12). Interestingly, in striking contrast to humans and other primates, some vertebrates such as dogs and cats are insensitive to funnel-web envenomation (13).
Humans did not feature in the prey or predator spectrum during evolution of funnel-web spiders, as primates were not present 150 to 200 million y ago (MYA) when these spiders originated (14). Thus, the underlying reason for the peculiar susceptibility of humans to δ-HXTXs and the ecological role of these toxins remain enigmatic. The δ-HXTXs are insecticidal (15, 16), which might suggest a role in prey capture. However, in some species, these toxins are secreted in very low abundance in the venoms of female spiders and immature males, consistent with the fact that only sexually mature male spiders cause severe or lethal human envenomations (17). Moreover, it is hard to reconcile a role for these toxins in predation given that sexually mature males, in whose venom the toxins are most abundant, rarely feed during the mating season. Rather, the fact that adult males leave the safety of their burrows to search for female spiders (2), making them more susceptible to predators, suggests a role for the δ-HXTXs in predator deterrence. A well-documented strategy for defensive toxins is to induce pain (18–20), and pain is a common symptom following funnel-web envenomation (2, 17). Consistent with the idea that the δ-HXTXs serve a defensive role by inducing pain in vertebrate predators, Magi 4 from the venom of a Japanese funnel-web spider potentiates the activity of NaV1.1 and NaV1.6 (21), which are involved in pain signaling (19, 22).
In the current study, we identified 22 δ-HXTX sequences from 10 species of Australian funnel-web spiders, and evaluated their molecular evolution, phylogenetic histories, insecticidal activity, and potency against human NaV channels involved in pain signaling. Taken together, our data provide strong evidence that the δ-HXTXs were recruited by funnel-web spiders as a weapon to deter vertebrate predators, and that their lethal effects on humans is an unfortunate evolutionary coincidence.
Results
δ-HXTX Sequences.
δ-HXTXs from seven funnel-web spider species (Hadronyche infensa, Hadronyche valida, Hadronyche venenata, Hadronyche versuta, Atrax robustus, Atrax sutherlandi, and Illawarra wisharti) were sequenced via rapid amplification of cDNA (complementary DNA) ends (RACE) (SI Appendix). In addition, we generated cDNA libraries for H. infensa, Hadronyche modesta, Hadronyche cerberea, and Hadronyche formidabilis. Our complete dataset (Dataset S1), based on our RACE and transcriptomic data plus sequences available from the literature, comprised 169 mature peptide sequences and 167 corresponding nucleotide sequences (the latter were only missing for δ-HXTX-Hv1b and δ-AOTX-Mb1a). Our dataset contained 132 δ-HXTXs, 1 δ-AOTX, and 18 homologous U-HXTXs from H. infensa, 17 related barytoxins (U-BATXs) from Trittame loki, and 1 μ-HXTX from Macrothele gigas. Removal of 53 duplicate or incomplete sequences and the two peptide sequences that lacked a corresponding nucleotide sequence yielded a total of 114 nucleotide sequences for phylogenetic analysis. The data revealed 22 mature δ-HXTX sequences to complement the 12 published δ-HXTX sequences.
Phylogenetic History and Molecular Evolution of δ-HXTXs.
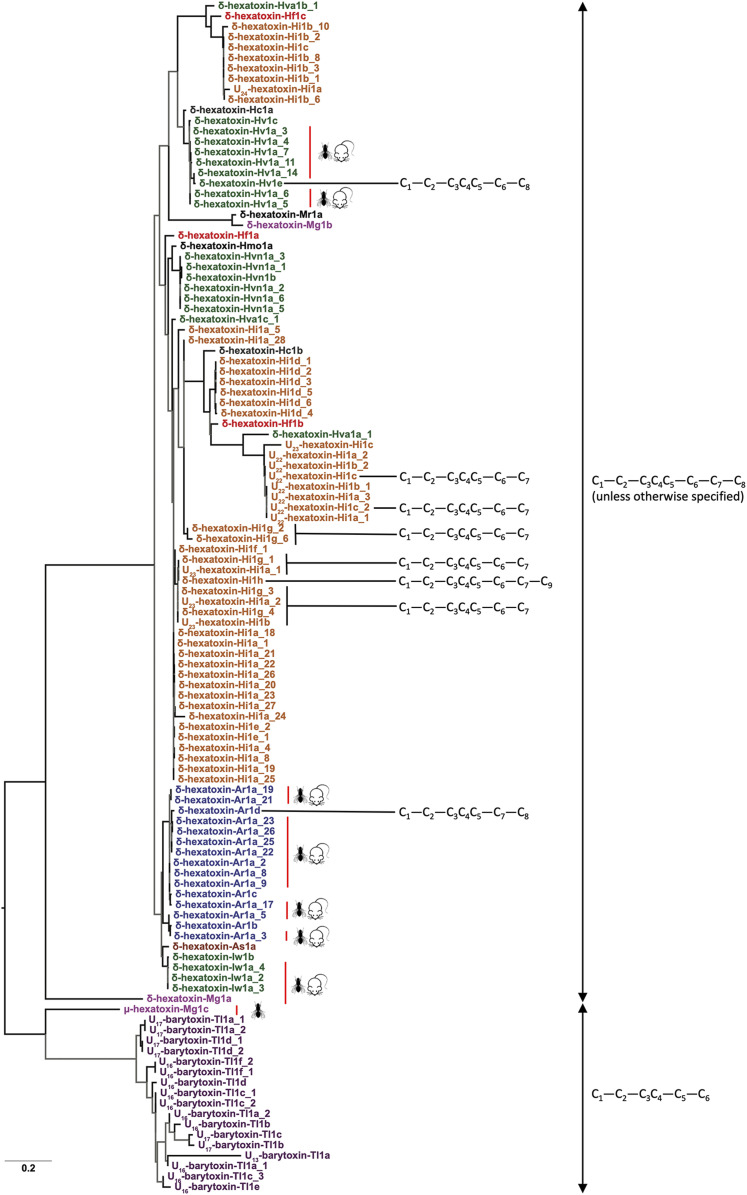
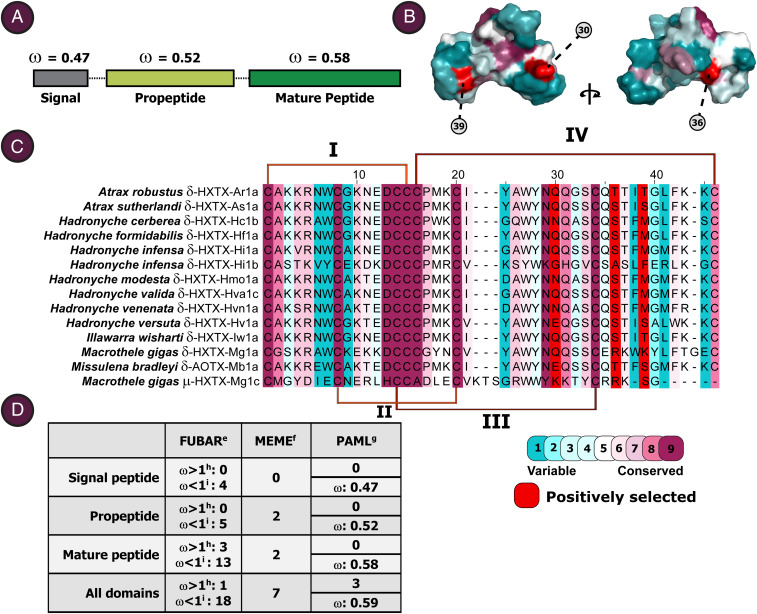
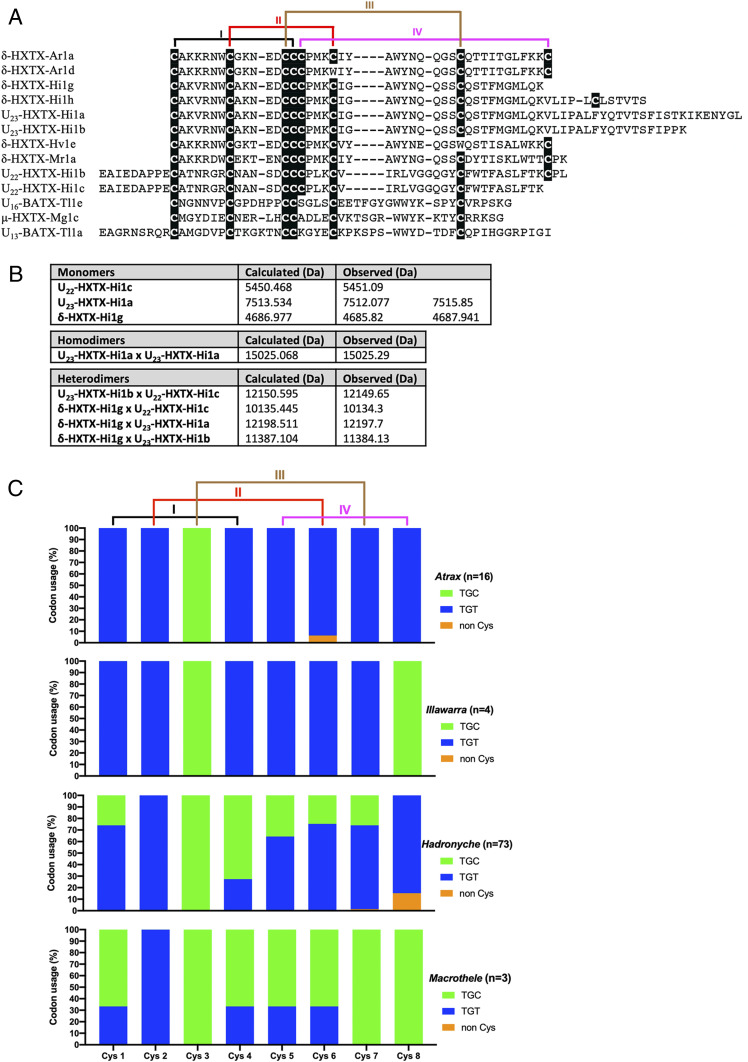
The identification of homologous sequences from the barychelid spider T. loki in this study reveals that these U-BATXs, μ-HXTX-Mg1c, and the δ-HXTXs were probably derived from a common ancestral toxin. However, there are also important differences between the U-BATXs and μ-HXTX-Mg1c in comparison with all δ-HXTXs from atracid and macrothelid funnel-web spiders. First, U-BATXs and μ-HXTX-Mg1c have only six Cys residues that form an ICK motif, whereas the δ-HXTXs usually contain eight Cys residues, with one additional disulfide bond. Second, the δ-HXTXs comprise a rare triplet of Cys residues in positions 14 to 16 (based on δ-HXTX-Ar1a), which is not present in μ-HXTX-Mg1c or any of the U-BATXs. Moreover, in the Bayesian phylogenetic analyses, all funnel-web spider sequences clustered together and were well-separated from the T. loki sequences (Fig. 1). All of these considerations taken together highlight the divergent evolution of δ-HXTXs in atracid and macrothelid funnel-web spiders following their phylogenetic separation from other mygalomorph lineages (Fig. 1).
Fig. 1.
Phylogenetic reconstruction of δ-HXTXs. Tree representing the phylogenetic history of δ-HXTXs as estimated by maximum-likelihood inference. The thickness of branches corresponds to node supports (thick branch, bootstrap ≥ 75; thin branch, bootstrap < 75), and various species are presented in distinct colors. The cysteine pattern (black lines) and activities (red lines) are annotated (Right). Fly and mouse icons indicate insecticidal and vertebrate activity, respectively. The underscore sequence numbers refer to the unique preprotoxin nomenclature used in the ArachnoServer database (23).
In contrast to the massive sequence variations observed in most venom proteins, the δ-HXTXs are surprisingly well-conserved (Fig. 2). The signal peptide, propeptide, and mature peptide coding regions in the δ-HXTXs were characterized by extremely low omega (ω) values (i.e., the ratio of nonsynonymous to synonymous substitutions) (Fig. 2A and Table 1), indicative of strong sequence conservation despite being recruited into the venoms of funnel-web spiders around 150 to 200 MYA (14). Consistent with the findings of the maximum-likelihood method, the Bayesian approaches identified only three positively selected sites (2.6% of sites)—positions that rapidly diversify across time—while many sites (15.6%) were found to be evolving under the pervasive influence of negative selection, which ensures sequence conservation across time. A few amino acid sites (n = 7) were found to be experiencing diversifying selection, albeit in an episodic fashion (i.e., in short bursts across time) (Fig. 2 B–D).
Fig. 2.
Molecular evolution of δ-HXTXs. (A) Toxin precursor domains and their rate of evolution, indicated as ω values (i.e., nonsynonymous-to-synonymous substitution rate ratio). (B) Structure of δ-HXTX-Hv1a (Protein Data Bank ID code 1VTX) (8), depicting the locations of positively selected sites. (C) Sequence alignment of δ-HXTXs from representative species. The positions of the four disulfide bonds are indicated by lines above and below the sequence alignment, while the extent of evolutionary conservation of amino acids (calculated from the complete alignment of 114 sequences) is denoted by the illustrated color code. Note: Loop IV is not present in the plesiotypic T. loki sequence and is instead characteristic of the apotypic δ-HXTX sequences. (D) Statistics associated with selection analyses. eFast unconstrained Bayesian approximation (FUBAR); fsites experiencing episodic diversifying selection (0.05 significance) by the mixed-effects model evolution (MEME); gpositively selected sites detected by the Bayes empirical Bayes approach implemented in M8 of Phylogenetic Analysis by Maximum Likelihood (PAML); hnumber of sites under pervasive diversifying selection at posterior probability ≥0.95 (FUBAR); inumber of sites under pervasive purifying selection at posterior probability ≥0.95 (FUBAR).
Table 1.
Collection sites and species used for venom-gland library construction
| Species | Collection site | Number and sex of specimens | Collector |
| Atrax robustus | Sydney, NSW | 1 M/1 F | Graham Nicholson |
| Atrax sutherlandi | Gerringong, NSW | 1 M | D.T.R.W. |
| Hadronyche infensa | Fraser Island and Toowoomba, QLD | 5 F (RACE) | D.T.R.W. |
| Hadronyche infensa | Fraser Island and Toowoomba, QLD | 3 F (454) | S.S.P. |
| Hadronyche modesta | Kalorama, Dandenong Ranges, VIC | 4 F | B.G.F. |
| Hadronyche valida | Binna Burra, QLD | 1 juvenile M | D.T.R.W. |
| Hadronyche venenata | Tooms Lake, TAS | 1 F | Glenn Gregg |
| Hadronyche versuta | Blue Mountains, NSW | 1 F | Graham Nicholson |
| Hadronyche formidabilis | South Tamborine, QLD | 1 F/4 juveniles | S.D. |
| Hadronyche cerberea | Blue Mountains, NSW | 2 F | S.D. |
| Illawara wisharti | Gerringong, NSW | 1 M | D.T.R.W. |
F, female; M, male.
Cys Derivations and Codon Usage.
The three-dimensional structure of many venom peptides, including the δ-HXTXs, relies heavily on the formation of disulfide bonds. Analysis of the arrangement of eight Cys residues in the δ-HXTXs (Fig. 3A) indicates that Cys residues 1 to 5 are extremely well conserved in all funnel-web spiders. However, many subtypes were discovered with either missing or novel cysteines, indicating the probable evolution of novel forms and functions. For instance, while one derivation each was found with a missing cysteine at positions 6 and 7, δ-HXTXs lacking Cys-8 were more common with 11 derivations in δ-HXTXs from H. infensa, with one of them even possessing a novel cysteine residue (which we refer to as position 9). The precursor peptides with the eighth Cys residue missing correspond to four mature toxin sequences, and comparison with combined matrix-assisted laser desorption/ionization (MALDI) and Orbitrap mass spectrometry (MS) data for H. infensa venom (4) reveals closely matching molecular masses for three of the monomers, one homodimer, and four heterodimers (Fig. 3B). Analysis of the codons used for Cys residues also revealed some genus-specific variations (Fig. 3C). In all funnel-web δ-HXTXs, Cys-2 is encoded by TGT, whereas Cys-3 is encoded by TGC. In Atrax and Illawarra, all other Cys residues are encoded only by TGT (except for Cys-8 in Illawarra being solely encoded by TGC). In Hadronyche, Cys-1, 5, 6, 7, and 8 are dominated by TGT and Cys-4 is preferentially encoded by TGC. In contrast to all other funnel-web spider genera, in Macrothele all Cys residues (except for Cys-2) are dominantly encoded by TGC. The observed codon bias could be important for the rapid expression of toxins in the venom gland.
Fig. 3.
Cys derivations and codon usage in δ-HXTXs. (A) Alignment of δ-HXTXs representing major changes in the Cys framework. (B) Expected and experimentally determined molecular masses for the seven Cys residue derivations of δ-HXTX homologs from H. infensa. Several masses closely matching either monomers or homo/heterodimers were observed using MALDI or Orbitrap mass spectrometry. (C) Codon usage for Cys residues in δ-HXTXs from different funnel-web genera. Lines at the top represent the four disulfide bonds in the prototypical Ar1a toxin.
NaV Channel Subtype Selectivity of δ-HXTX-Ar1a.
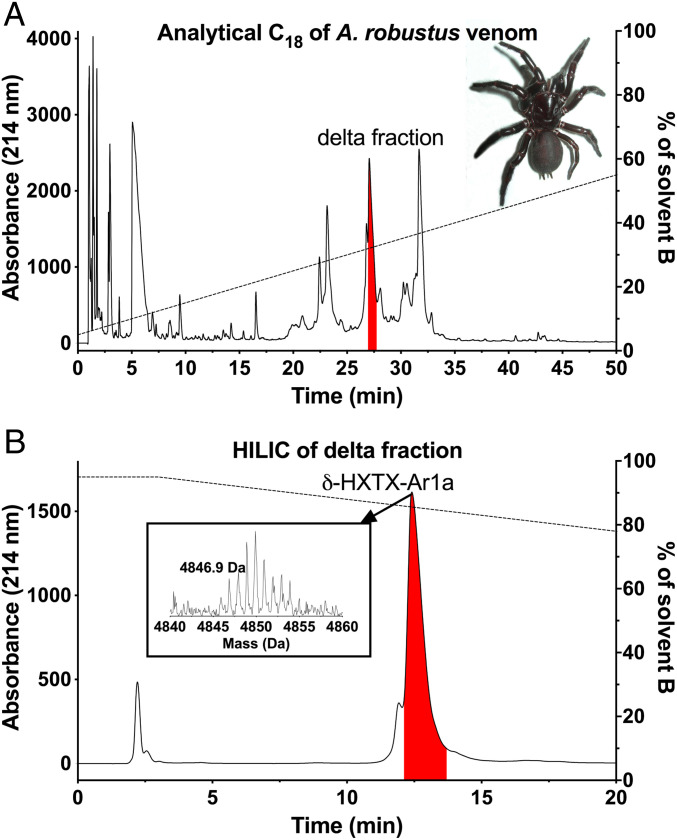
In order to examine the biological role of the δ-HXTXs, we used reversed-phase high-performance liquid chromatography (RP-HPLC) to isolate δ-HXTX-Ar1a (hereafter Ar1a) from the venom of A. robustus (Fig. 4). We then used a Fluorescent Imaging Plate Reader (FLIPR)-based fluorescence assay to assess the ability of Ar1a to potentiate currents from human NaV1.1 to NaV1.8 channels stably expressed in HEK293 cells. This assay was previously used to determine the pharmacological activity of the scorpion venom peptide OD1. OD1 activates several NaV channel subtypes and the potency and subtype selectivity of OD1 as determined by FLIPR was found to be comparable to data obtained using electrophysiological assays (24).
Fig. 4.
Isolation and purification of Ar1a from A. robustus venom. (A) Chromatogram from C18 RP-HPLC fractionation of A. robustus venom with the peak containing Ar1a highlighted in red. (A, Inset) Photo of a female A. robustus. (B) Chromatogram from hydrophobic interaction liquid chromatography (HILIC) of the Ar1a-containing peak from A. The dashed line indicates the solvent gradient, with the percentage of solvent B (90% acetonitrile/0.045% trifluoroacetic acid) indicated on the right ordinate axis. (B, Inset) MALDI-MS spectrum of purified Ar1a.
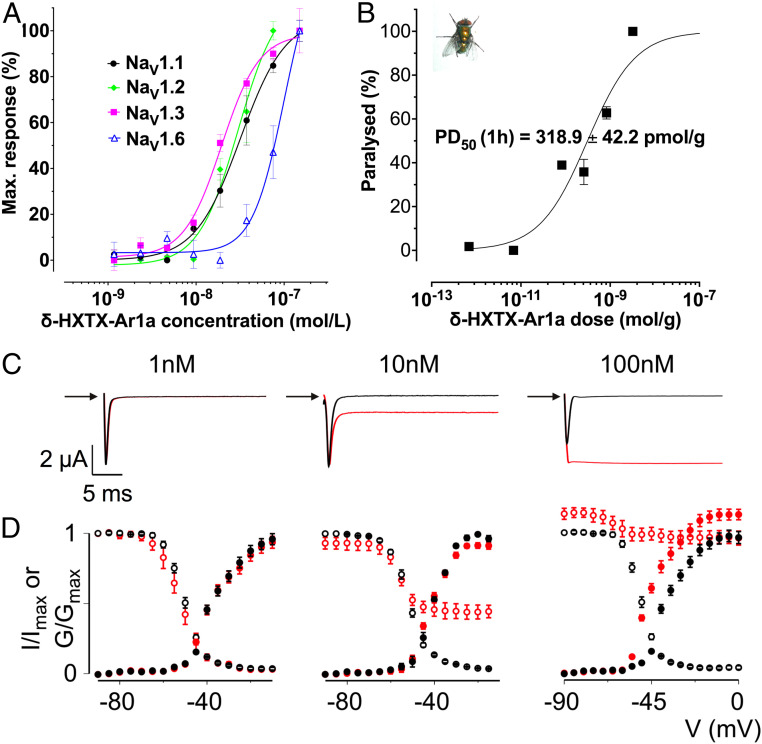
Ar1a had no effect on NaV1.4, NaV1.5, NaV1.7, and NaV1.8 at concentrations up to 150 nM, whereas a concentration-dependent potentiation of veratridine-evoked responses was observed in cells expressing NaV1.1, NaV1.2, NaV1.3, and NaV1.6 (Fig. 5A). Ar1a was an equipotent potentiator of NaV1.1 (half-maximum effective concentration (EC50) of 30.2 nM), NaV1.2 (EC50 38.9 nM), NaV1.3 (EC50 38.9 nM), and NaV1.6 (EC50 91.2 nM) with comparable mean pEC50 (i.e., negative logarithm of the EC50) values of 7.52 ± 0.24, 7.41 ± 0.09, 7.41 ± 0.15, and 7.04 ± 0.08, respectively.
Fig. 5.
Biological characterization of Ar1a. (A) NaV subtype selectivity of Ar1a. The effect of Ar1a on NaV1.1 to NaV1.8 heterologously expressed in HEK293 cells in combination with the human b1 subunit was assessed using a membrane potential assay. Ar1a elicited a concentration-dependent increase in membrane potential in the presence of veratridine (5 µM) in cells expressing NaV1.1 (pEC50 7.52 ± 0.24), NaV1.2 (pEC50 7.41 ± 0.09), NaV1.3 (pEC50 7.41 ± 0.15), and NaV1.6 (pEC50 7.04 ± 0.08). Data are presented as mean ± SEM (n = 3). (B) Dose–response curve for paralysis of L. cuprina blowflies (shown in Inset) injected with Ar1a. Paralysis was assessed at 1 h postinjection. Error bars indicate SEM. The PD50 was determined as the mean ± SEM of three independent experiments. All paralytic effects were reversible within 24 h and no lethal effects were observed. (C) Dose-dependent inhibition of BgNaV1 fast inactivation by Ar1a. Representative sodium currents were elicited by a depolarization to −20 mV before (black) and after (red) addition of toxin from a holding potential of −90 mV. (D) Normalized conductance–voltage relationships (G/Gmax; black filled circles) and steady-state inactivation relationships (I/Imax; black open circles) of BgNaV1 before (black circles) and after (red circles) Ar1a application. Channel-expressing oocytes were depolarized in 5-mV steps from a holding potential of −90 mV. Error bars represent SEM; n = 3 to 5.
Mammalian Nocifensive Responses.
Administration of Ar1a (100 nM, 20 µL) by shallow subcutaneous (intraplantar) injection into the foot pad of male C57BL/6 mice elicited mild, transient nocifensive responses consisting of flinching, lifting, licking, and shaking of the hind paw. This nocifensive behavior was characterized by relatively slow onset (5 min postinjection) to a peak at 15 min postinjection with 10.5 ± 1.5 flinches per 5 min. No systemic effects, such as the muscle fasciculations, salivation, or other effects associated with the human envenomation syndrome, were observed at this dose.
Toxicity of Ar1a in Blowflies.
Injection of Ar1a into sheep blowflies (Lucilia cuprina) caused contractile paralysis with a median paralytic dose (PD50) of 319 ± 42 pmol/g at 1 h postinjection (Fig. 5B). However, even at the highest dose tested (which was limited by the amount of native δ-HXTX-Ar1a available), all flies fully recovered within 24 h, indicating that the toxin’s insecticidal effects in blowflies are reversible.
Effect of Ar1a on BgNaV1.
Given its activity on human NaV channels, we decided to examine if the insecticidal effects of Ar1a are due to potentiation of the activity of insect NaV channels. For this purpose, we examined the effects of Ar1a on the BgNaV1 channel from the German cockroach Blattella germanica expressed in Xenopus oocytes. At 1 nM, Ar1a had no effect on BgNaV1 currents whereas 10 nM Ar1a induced a substantial persistent current (Fig. 5C). BgNaV1 fast inactivation was completely inhibited by 100 nM Ar1a. Boltzmann fits of the normalized conductance–voltage relationships and steady-state inactivation relationships revealed no significant difference in V1/2 before and after addition of 1 or 10 nM Ar1a (t test, P ≥ 0.01) but 100 nM Ar1a caused substantial hyperpolarizing shifts in the V1/2 of both channel activation (from −37.2 ± 0.6 to −44.9 ± 0.7 mV) and steady-state inactivation (from −50.3 ± 0.1 to −63.3 ± 0.7 mV) (Fig. 5D).
Discussion
In the present study, we employed a multipronged approach involving venom-gland transcriptomics, molecular and phylogenetic analyses, and functional assays to determine the role of the lethal δ-HXTXs in the ecology of funnel-web spiders.
Enigmatic Evolutionary Conservation of δ-HXTXs.
Molecular evolutionary assessments revealed that the genes encoding for δ-HXTXs have remained nearly unchanged despite originating in the common ancestor of atracid and macrothelid funnel-web spiders 150 to 200 MYA (14). Together with our phylogenetic analysis, this indicates that despite the single early origin of δ-HXTXs in funnel-web spiders, they have diversified at a much slower evolutionary rate than many other spider toxins (25–29). The increased level of sequence conservation is consistent with a role for the δ-HXTXs in defense. Due to their relatively limited use and consequent exclusion from the typical Red Queen mode of competitive evolution, defensive toxins are theorized to evolve slower than their predatory counterparts (30).
Our phylogenetic analysis demonstrates that all δ-HXTX sequences evolved from a common ancestral toxin scaffold, with early gene duplications and diversification present before the recently proposed split into the families Macrothelidae and Atracidae (31). Further support of a split between these families is provided by the nucleotide sequences used to encode the Cys residues, with Atracidae being dominated by TGT and Macrothelidae being dominated by TGC. Our phylogeny also provides evidence for at least two neofunctionalization events. The first event occurred during the early evolution of δ-HXTXs. μ-HXTX-Mg1c, the most basal of the funnel-web spider sequences included in our analysis, has an ICK motif but lacks the two Cys residues involved in the formation of the fourth disulfide bond (32). μ-HXTX-Mg1c is a homolog of μ-HXTX-Mg1a and μ-HXTX-Mg1b, which are both known to be insecticidal but not active against vertebrates (33). On the other hand, δ-HXTX-Mg1a, which is sister to the remaining δ-HXTX sequences, has both vertebrate and insecticidal activities (33). These activities would be consistent with a repurposing of δ-HXTXs from the purely insecticidal activity of their ancestral plesiotypic form to dual activity against both mammals and insects, which occurred around 150 to 200 MYA (14). Given the absence of primates in Australia at the time when δ-HXTXs originated [humans first populated Australia 65,000 y ago (34), and Australia lacks indigenous nonhuman primates], the primate toxicity of δ-HXTXs can only be regarded as coincidental. The second neofunctionalization event is apparent in the genus Hadronyche with multiple convergent losses of the last Cys residue. Such convergence points toward a strong selection pressure, possibly resulting in a change in selectivity or potency. The fact that an odd number of Cys residues is energetically unfavored led us to investigate whether these seven Cys residue derivations of δ-HXTXs form dimers. Mass spectrometry analysis of H. infensa venom (4) revealed the presence of masses corresponding to monomers and homo- and heterodimers. Unfortunately, nothing is yet known about the activities of these δ-HXTX derivations or the dimers that are formed, which provides an exciting area for future investigations.
Clues from the Activity of Ar1a.
δ-HXTXs were previously demonstrated to inflict potent but reversible paralysis in blowfly larvae and crickets (16). We found that Ar1a potently inhibits fast inactivation of the cockroach BgNaV channel, which is consistent with the contractile paralysis induced in blowflies and reminiscent of the effects of other toxins that target insect NaV channels (reviewed in ref. 35).
Employing venom components to cause pain is a common evolutionary strategy for self-defense in venomous animals (19, 30, 36–38). Strong support for a defensive role of δ-HXTXs is therefore provided by the nocifensive response that Ar1a induced following intraplantar injections in mice. Although the lethal dose of δ-HXTXs varies considerably within vertebrates (13), we presume that high local tissue concentrations of δ-HXTXs resulting from a funnel-web spider bite will induce algogenic effects in a much wider range of vertebrates than half-maximum lethal dose (LD50) experiments might indicate.
With regard to the subtype selectivity of δ-HXTXs, δ-HXTX-Mg1a (Magi 4) from the Japanese funnel-web spider preferentially activated rat NaV1.1 and NaV1.3 and mouse NaV1.6 while also showing weak activity on rat NaV1.2 channels (3). For Ar1a, we observed equipotent activity across NaV1.1, NaV1.2, NaV1.3, and NaV1.6. Overall, this is consistent with a defensive role, as both NaV1.1 and NaV1.6 are known to be involved in pain signaling (19, 22, 39). The activity of Ar1a at NaV1.6 is further consistent with the observed effects of Australian funnel-web venoms in the chick biventer assay (SI Appendix, Fig. S1) (40), as NaV1.6 is the predominant isoform at the nodes of Ranvier in motor neurons. Thus, inhibition of the inactivation of this NaV channel isoform could contribute to both sensory and motor effects in envenomed individuals, making δ-HXTXs a powerful weapon to deter predators.
Differential Expression of δ-HXTXs.
The clinical syndrome resulting from funnel-web spider envenomation of vertebrates is driven by the δ-HXTXs (5). Male A. robustus venom was reported to be at least six times more potent than the female venom (41). In addition, the venoms of six male funnel-web species were found to be more potent than females’ in inducing toxic effects in the chick biventer nerve-muscle preparation (40), consistent with increased expression of δ-HXTXs in male venoms. Male funnel-web spiders are more exposed to vertebrate predation once they leave the safety of their burrows to search for female mates, so increased expression of a defensive toxin would make ecological sense to allow adult males to defend against these predators. Moreover, since adult male mygalomorph spiders consume less food than females (42), the increased expression of δ-HXTXs in mature male spiders is inconsistent with a role for these toxins in prey capture.
Conclusion
In summary, our data suggest that the δ-HXTXs likely evolved from having an ancestral role in predation to a primary role in defense against ecologically important vertebrate predators, with their lethal potency against humans being an unfortunate evolutionary coincidence.
Materials and Methods
Australian funnel-web spiders were collected from various locations and states across Australia, as summarized in Table 1. The spiders were individually housed at ∼23 to 25 °C in dark cabinets until venom and venom glands were dissected.
Nomenclature.
Toxins were named according to the rational nomenclature described previously (43). Spider taxonomy was taken from World Spider Catalog version 21.0 (44).
Messenger RNA Isolation and cDNA Library Construction.
Messenger RNA and cDNA libraries were isolated and constructed using the protocols summarized in SI Appendix. For details of RACE, Sanger, and next-generation sequencing, see SI Appendix.
Phylogenetics and Selection Analyses.
Reconstruction of the phylogenetic history and molecular evolution of δ-HXTXs was performed as detailed in SI Appendix.
RP-HPLC Purification of Ar1a.
Milked lyophilized venom from male A. robustus specimens was supplied by the Australian Reptile Park. The venom was reconstituted in MilliQ water to a concentration of ∼5 mg/mL and Ar1a was purified using RP-HPLC as outlined in SI Appendix.
Determination of the NaV Subtype Selectivity of Ar1a.
The activity of Ar1a on human NaV channels stably expressed in HEK293 cells was determined using a FLIPRTetra assay (24) as described in SI Appendix.
Algogenic Effects of Ar1a.
Ethical approval for in vivo experiments was obtained from The University of Queensland Animal Ethics Committee (PHARM/512/12/RAMACIOTTI) and they were conducted in accordance with the Queensland Animal Care and Protection Act (2002), the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (eighth edition, 2013), and the International Association for the Study of Pain Guidelines for the Use of Animals in Research. Details of intraplantar administration of Ar1a and assessment of induced nocifensive behavior are detailed in SI Appendix.
Insecticidal Effects of Ar1a.
Native Ar1a was tested for insecticidal toxicity by injection into sheep blowflies (45) as described in SI Appendix.
Activity of Ar1a on BgNaV1.
Two-electrode voltage-clamp electrophysiology was used to determine the activity of Ar1a on BgNaV1 heterologously expressed in Xenopus oocytes, as outlined in SI Appendix.
Supplementary Material
Acknowledgments
We acknowledge support from the Australian National Health & Medical Research Council (Principal Research Fellowship APP1136889 and Program Grant APP1072113 to G.F.K.; Career Development Fellowship APP1162503 to I.V.), the Australian Research Council (Discovery Grant DP190100304 to B.G.F.; Future Fellowship FT190100482 to V.H.). K.S. was supported by the Department of Science and Technology (DST) INSPIRE Faculty Award (DST/INSPIRE/04/2017/000071), DST - Fund for Improvement of S&T Infrastructure in Higher Educational Institutions (DST-FIST) (SR/FST/LS-II/2018/233), and the Department of Biotechnology-Indian Institute of Science (DBT-IISc) Partnership Program. I.V. was supported by an Early Career Researcher (ECR) grant from The Clive & Vera Ramaciotti Foundation. We thank Dr. Roger Drinkwater for assistance with sequencing, Dr. Robert Raven (Queensland Museum) and Mr. Graham Wishart for specimen collection and identification, Mr. Glenn Gregg and Prof. Graham Nicholson for providing spiders, the Australian Reptile Park for provision of A. robustus venom, Geoff Brown (Department of Agriculture and Fisheries, Queensland) for blowflies, and Ke Dong (Michigan State University) for sharing BgNaV1/TipE clones.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004516117/-/DCSupplemental.
Data Availability.
Metadata and annotated nucleotide sequences generated in this paper were deposited in the European Nucleotide Archive under project accession nos. PRJEB6062 for H. infensa, PRJEB14734 for H. cerberea, and PRJEB14965 for H. formidabilis. All UniProt and ArachnoServer accessions for sequences used for phylogenetic analysis are listed in SI Appendix. Raw data have also been deposited in Figshare, 10.6084/m9.figshare.12798617.
All study data are included in the article and SI Appendix.
References
- 1.Hauke T. J., Herzig V., Dangerous arachnids—Fake news or reality? Toxicon 138, 173–183 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Isbister G. K., Fan H. W., Spider bite. Lancet 378, 2039–2047 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Escoubas P., Sollod B., King G. F., Venom landscapes: Mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 47, 650–663 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Pineda S. S. et al., Structural venomics reveals evolution of a complex venom by duplication and diversification of an ancient peptide-encoding gene. Proc. Natl. Acad. Sci. U.S.A. 117, 11399–11408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson G. M., Little M. J., Tyler M., Narahashi T., Selective alteration of sodium channel gating by Australian funnel-web spider toxins. Toxicon 34, 1443–1453 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Bond J. E., Hendrixson B. E., Hamilton C. A., Hedin M., A reconsideration of the classification of the spider infraorder Mygalomorphae (Arachnida: Araneae) based on three nuclear genes and morphology. PLoS One 7, e38753 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbister G. K., Mouse spider bites (Missulena spp.) and their medical importance. A systematic review. Med. J. Aust. 180, 225–227 (2004). [PubMed] [Google Scholar]
- 8.Fletcher J. I., Chapman B. E., Mackay J. P., Howden M. E., King G. F., The structure of versutoxin (δ-atracotoxin-Hv1) provides insights into the binding of site 3 neurotoxins to the voltage-gated sodium channel. Structure 5, 1525–1535 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Pallaghy P. K., Nielsen K. J., Craik D. J., Norton R. S., A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides. Protein Sci. 3, 1833–1839 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunning S. J. et al., Isolation of delta-missulenatoxin-Mb1a, the major vertebrate-active spider delta-toxin from the venom of Missulena bradleyi (Actinopodidae). FEBS Lett. 554, 211–218 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Nicholson G. M., Little M. J., Birinyi-Strachan L. C., Structure and function of δ-atracotoxins: Lethal neurotoxins targeting the voltage-gated sodium channel. Toxicon 43, 587–599 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Mylecharane E. J., Spence I., Sheumack D. D., Claassens R., Howden M. E., Actions of robustoxin, a neurotoxic polypeptide from the venom of the male funnel-web spider (Atrax robustus), in anaesthetized monkeys. Toxicon 27, 481–492 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Nicholson G. M., Graudins A., Spiders of medical importance in the Asia-Pacific: Atracotoxin, latrotoxin and related spider neurotoxins. Clin. Exp. Pharmacol. Physiol. 29, 785–794 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Ayoub N. A., Hayashi C. Y., “Spiders (Araneae)” in The Timetree of Life, Hedges S. B., Kumar S., Eds. (Oxford University Press, Oxford, UK, 2009), pp. 255–259. [Google Scholar]
- 15.Grolleau F. et al., Electrophysiological analysis of the neurotoxic action of a funnel-web spider toxin, δ-atracotoxin-HV1a, on insect voltage-gated Na+ channels. J. Exp. Biol. 204, 711–721 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Little M. J. et al., δ-Atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding on both rat brain and insect sodium channels. FEBS Lett. 439, 246–252 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Isbister G. K. et al., Funnel-web spider bite: A systematic review of recorded clinical cases. Med. J. Aust. 182, 407–411 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Bohlen C. J., Julius D., Receptor-targeting mechanisms of pain-causing toxins: How ow? Toxicon 60, 254–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osteen J. D. et al., Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 534, 494–499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin King J. V. et al., A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell 178, 1362–1374.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaji N. et al., Synthesis, solution structure, and phylum selectivity of a spider delta-toxin that slows inactivation of specific voltage-gated sodium channel subtypes. J. Biol. Chem. 284, 24568–24582 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israel M. R. et al., NaV 1.6 regulates excitability of mechanosensitive sensory neurons. J. Physiol. 597, 3751–3768 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Pineda S. S. et al., ArachnoServer 3.0: An online resource for automated discovery, analysis and annotation of spider toxins. Bioinformatics 34, 1074–1076 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Durek T. et al., Chemical engineering and structural and pharmacological characterization of the α-scorpion toxin OD1. ACS Chem. Biol. 8, 1215–1222 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Garb J. E., Hayashi C. Y., Molecular evolution of α-latrotoxin, the exceptionally potent vertebrate neurotoxin in black widow spider venom. Mol. Biol. Evol. 30, 999–1014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haney R. A. et al., Effects of gene duplication, positive selection, and shifts in gene expression on the evolution of the venom gland transcriptome in widow spiders. Genome Biol. Evol. 8, 228–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCowan C., Garb J. E., Recruitment and diversification of an ecdysozoan family of neuropeptide hormones for black widow spider venom expression. Gene 536, 366–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineda S. S. et al., Diversification of a single ancestral gene into a successful toxin superfamily in highly venomous Australian funnel-web spiders. BMC Genomics 15, 177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Undheim E. A. B. et al., A proteomics and transcriptomics investigation of the venom from the barychelid spider Trittame loki (brush-foot trapdoor). Toxins (Basel) 5, 2488–2503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casewell N. R., Wüster W., Vonk F. J., Harrison R. A., Fry B. G., Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 28, 219–229 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Hedin M., Derkarabetian S., Ramírez M. J., Vink C., Bond J. E., Phylogenomic reclassification of the world’s most venomous spiders (Mygalomorphae, Atracinae), with implications for venom evolution. Sci. Rep. 8, 1636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craik D. J., Daly N. L., Waine C., The cystine knot motif in toxins and implications for drug design. Toxicon 39, 43–60 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Corzo G. et al., Distinct primary structures of the major peptide toxins from the venom of the spider Macrothele gigas that bind to sites 3 and 4 in the sodium channel. FEBS Lett. 547, 43–50 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Clarkson C. et al., Human occupation of northern Australia by 65,000 years ago. Nature 547, 306–310 (2017). [DOI] [PubMed] [Google Scholar]
- 35.King G. F., Escoubas P., Nicholson G. M., Peptide toxins that selectively target insect NaV and CaV channels. Channels (Austin) 2, 100–116 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Bohlen C. J. et al., A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141, 834–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohlen C. J. et al., A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 479, 410–414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson S. D. et al., A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 4, eaau4640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deuis J. R. et al., An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain 154, 1749–1757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graudins A., Wilson D., Alewood P. F., Broady K. W., Nicholson G. M., Cross-reactivity of Sydney funnel-web spider antivenom: Neutralization of the in vitro toxicity of other Australian funnel-web (Atrax and Hadronyche) spider venoms. Toxicon 40, 259–266 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Sutherland S. K., Tibballs J., The Genera Atrax and Hadronyche, the Funnel-Web Spiders. Australian Animal Toxins, (Oxford University Press, Melbourne, 2001). [Google Scholar]
- 42.Herzig V., Ontogenesis, gender, and molting influence the venom yield in the spider Coremiocnemis tropix (Araneae, Theraphosidae). J. Venom Res. 1, 76–83 (2010). [PMC free article] [PubMed] [Google Scholar]
- 43.King G. F., Gentz M. C., Escoubas P., Nicholson G. M., A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon 52, 264–276 (2008). [DOI] [PubMed] [Google Scholar]
- 44.World Spider Catalog, version number 21.0 https://wsc.nmbe.ch, (Natural History Museum Bern, 2020). [Google Scholar]
- 45.Bende N. S. et al., The insecticidal neurotoxin Aps III is an atypical knottin peptide that potently blocks insect voltage-gated sodium channels. Biochem. Pharmacol. 85, 1542–1554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metadata and annotated nucleotide sequences generated in this paper were deposited in the European Nucleotide Archive under project accession nos. PRJEB6062 for H. infensa, PRJEB14734 for H. cerberea, and PRJEB14965 for H. formidabilis. All UniProt and ArachnoServer accessions for sequences used for phylogenetic analysis are listed in SI Appendix. Raw data have also been deposited in Figshare, 10.6084/m9.figshare.12798617.
All study data are included in the article and SI Appendix.