Significance
If an individual can anticipate an early death, should they also “live fast”? Fast reproduction is often proposed to be an adaptive response to harsh conditions in early life because early adversity predicts shorter lifespans. Individuals who speed up reproduction after experiencing early adversity might therefore have higher fitness than those who do not. Here, we use extensive data on wild female baboons to test if fast reproduction offers fitness advantages to females who experience nutritional and psychosocial sources of early adversity. Contrary to several influential hypotheses, females who experienced early adversity did not improve their fitness if they sped up reproduction. Our results raise doubts that accelerated reproduction is an adaptive response to early adversity in long-lived, slow-reproducing species.
Keywords: internal predictive adaptive response model, adaptive developmental plasticity, early-life adversity, life history, fitness
Abstract
In humans and other long-lived species, harsh conditions in early life often lead to profound differences in adult life expectancy. In response, natural selection is expected to accelerate the timing and pace of reproduction in individuals who experience some forms of early-life adversity. However, the adaptive benefits of reproductive acceleration following early adversity remain untested. Here, we test a recent version of this theory, the internal predictive adaptive response (iPAR) model, by assessing whether accelerating reproduction following early-life adversity leads to higher lifetime reproductive success. We do so by leveraging 48 y of continuous, individual-based data from wild female baboons in the Amboseli ecosystem in Kenya, including prospective, longitudinal data on multiple sources of nutritional and psychosocial adversity in early life; reproductive pace; and lifetime reproductive success. We find that while early-life adversity led to dramatically shorter lifespans, individuals who experienced early adversity did not accelerate their reproduction compared with those who did not experience early adversity. Further, while accelerated reproduction predicted increased lifetime reproductive success overall, these benefits were not specific to females who experienced early-life adversity. Instead, females only benefited from reproductive acceleration if they also led long lives. Our results call into question the theory that accelerated reproduction is an adaptive response to both nutritional and psychosocial sources of early-life adversity in baboons and other long-lived species.
Exposure to adverse conditions in early life often predicts striking differences in adult health and survival. For example, in humans, harsh early-life circumstances, including famine, neglect, and low socioeconomic status, foreshadow higher risk of cardiovascular disease, type 2 diabetes, and all-cause mortality (1–5). In nonhuman animals, early-life adversity is linked to small adult body size, early reproductive senescence, and short lifespans (6–9). This reduced adult life expectancy has led many researchers to hypothesize that selection favors accelerating the timing and pace of reproduction in response to early-life adversity. Doing so may increase an individual’s lifetime reproductive success (LRS), compared with individuals who experience adversity but do not accelerate reproduction (10–21). The first version of this hypothesis was proposed by Draper and Harpending in 1982 (10) and was later expanded by Belsky and coworkers into the psychosocial acceleration hypothesis (11, 12). More recently, these ideas have found a home in the weathering hypothesis, the parental investment hypothesis, the child development hypothesis, the adaptive calibration model, and the internal predictive adaptive response (iPAR) model (13–21). While all of these hypotheses link early adversity to accelerated reproductive timing, they differ in the types of early-life environments and developmental cues thought to be most salient to triggering a response. For instance, while some ideas have focused on resource limitation (22–26), others have emphasized familial adversity and parental absence (27–30). Additionally, some authors have pointed out that the relevant cues and the magnitude of their effects may depend on the population of interest (e.g., in developed versus developing nations) (31, 32).
Despite this diversity, all of the models cited above are united in proposing that accelerating reproduction is an adaptive developmental response to early-life adversity. However, while a number of studies have documented a correlation between early adversity and reproductive timing (27–30, 33–36), no study in humans or any other long-lived animal has shown that this apparent response actually increases fitness. That is, we do not know if individuals who accelerate reproduction following early-life adversity in fact exhibit higher LRS compared with those who grew up under the same conditions but do not accelerate reproduction and, in particular, if this benefit is a specific response to early-life adversity.
Filling this gap is important because not all theories of adaptive developmental plasticity propose that the accelerated-reproduction phenotype itself is adaptive. For instance, developmental constraints models propose that early-life adversity, especially nutritional adversity, forces organisms to make adaptive tradeoffs in early life that improve juvenile survival but come at the expense of later-life somatic quality (37, 38). Under these models, adversity may lead to delayed maturation and slow reproduction—changes that arise as a byproduct of early-life tradeoffs and are not the immediate target of selection (37–40). Furthermore, early maturation and accelerated reproduction can themselves be costly. These costs include decreased body size and fecundity, increased instantaneous juvenile mortality rates, and lower offspring survival (41). If these costs outweigh the fitness benefits, accelerated reproduction may not be an adaptive response to early-life adversity (41–43). Finally, earlier reproduction could be beneficial to fitness for all individuals, independent of early-life experience. If so, the accelerated-reproduction phenotype should be considered adaptive but should not be considered an example of developmental plasticity.
Here, we test one of the most recent hypotheses to propose accelerated reproduction as a form of adaptive developmental plasticity arising in response to early-life adversity: the iPAR model (19, 20). Predictive adaptive response (PAR) models, including the iPAR model, posit that organisms use their early-life circumstances to predict future conditions. Organisms can maximize their fitness by adjusting their phenotypes in anticipation of those conditions (37, 44, 45). The iPAR model specifically proposes that organisms adapt not in anticipation of the external environment but, instead, to the quality of their expected somatic state in adulthood (19–21). According to this reasoning, harsh conditions in early life lead organisms to develop a soma that is less likely to survive at any age (19–21). This reduced survival may be caused by several processes, including reduced energy available to build somatic tissue during development, reduced somatic maintenance, or higher rates of oxidative stress (20). Organisms then use the quality of their somatic state to predict a shorter lifespan, and accelerate their reproduction accordingly (20). Unlike some other evolutionary models of development (10, 11, 13), nutritional and psychosocial adversity can both be potential cues for reproductive acceleration under the iPAR model. Accordingly, prior research motivated by the iPAR model has investigated multiple types of early-life adversity, including food availability and prenatal maternal stress (46, 47). This flexibility makes the iPAR model one of the most generalizable hypotheses across species and settings. The key quality is that the type of early-life adversity under consideration reduces adult somatic quality and shortens adult lifespans. Thus, individuals with poor somatic states should “live fast” with the expectation that they will “die young” (19–21).
We test the iPAR model using 48 y of detailed, individual-based data on early-life experiences, reproductive timing, and LRS in wild female baboons living in the Amboseli ecosystem in Kenya (48). In this population, we have already identified six sources of early-life adversity (Table 1) whose cumulative effects, and in some cases individual effects, have profound predictive power for adult female lifespan (9). These sources of adversity parallel several major sources of adversity that have been identified in humans (Table 1). Female baboons exposed to three or more of these sources of early-life adversity experience adult lifespans that are more than 10 y shorter, on average, than the adult lifespans of females who experience none of them (9). As such, females who accelerate their reproductive schedules in response to adverse early-life circumstances might recoup some of the reproductive time they are likely to lose as a result of their shorter adult lifespans.
Table 1.
Definitions for the six early-life adversity conditions
| Adverse condition | Criterion | Parallel effects in humans |
| Maternal death | The subject’s mother died before the subject reached 4 y of age | Loss of one or both parents (49–51) |
| Competing younger sibling | The subject’s mother gave birth to another live offspring before the subject reached 1.5 y of age | Close-in-age sibling (52) |
| Drought | During the subject’s first year of life, total rainfall did not exceed 200 mm | Drought, difficult birth season, or famine (53–55) |
| Maternal social isolation | The subject’s mother was socially isolated from other adult females during the first 2 y of the subject’s life (maternal social isolation ≥0.325) | Social isolation (56) |
| Low maternal dominance rank | The subject’s mother had low ordinal dominance rank in the month when the subject was born (maternal dominance rank of ≥12) | Parental socioeconomic status (57, 58) |
| High social density | The subject was born into a large social group; size was defined by the total number of adult group members (social group density of ≥36) | Household crowding (59) |
See SI Appendix, Supplementary Methods for details on how these variables were measured.
We test the iPAR model using a conceptual framework developed by Nettle and Bateson (60), who provide three predictions required to find support for this hypothesis. Nettle and Bateson’s first prediction is that individuals who experience early-life adversity should exhibit poor somatic quality in adulthood (60). This condition is met by the prior observation that, in Amboseli, early-life adversity leads to profoundly shorter female lifespans (9); we replicate this analysis here with a larger, updated dataset. We use adult lifespan as a proxy for somatic quality because it is the most salient measure of adult mortality risk, which is the primary driver of selection for accelerated reproduction under the iPAR model (60). We also note that poor somatic states will often increase vulnerability to both intrinsic and extrinsic causes of death. For instance, individuals who are in poor health (an intrinsic process) may be more susceptible to predation (an extrinsic process).
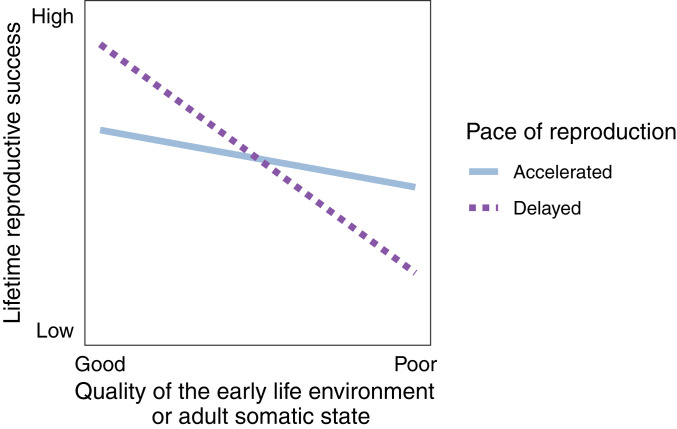
Nettle and Bateson’s second and third predictions both involve interaction effects on fitness that depend on whether individuals accelerate their pace of reproduction and whether they experienced early adversity (60). Specifically, the second prediction posits that individuals who experience early-life adversity and accelerate their reproduction should attain higher LRS than those who experience early-life adversity and exhibit typical or delayed reproductive timing (60). However, typical or delayed reproduction is optimal for individuals who do not experience early-life adversity because it allows them to reach full body size without accelerating growth, creating a “fitness crossover” (Fig. 1). Similarly, the third prediction is a logical extension of the first two predictions. It states that individuals who have poor somatic states and accelerate reproduction will attain higher LRS than those who have poor somatic states and do not accelerate their reproduction (60). In contrast, accelerated reproduction may be neutral or maladaptive for individuals with high-quality somatic states (Fig. 1). Fitness crossovers are crucial to testing for PARs (37) because they capture the requirement that the optimal phenotype—in this case, accelerated versus typical reproductive timing—is state dependent. If the fitness crossover does not exist, then one phenotype is optimal under all conditions, making it adaptive, but not an adaptive response to developmental conditions (37, 60).
Fig. 1.
Visual depiction of the fitness crossovers predicted by the iPAR model. The plot shows the iPAR model’s predicted patterns of LRS (y axis) as a function of the quality of the early-life environment or adult somatic state (x axis: good or poor for each predictor), stratified by the pace of reproduction (accelerated or typical/delayed). Specifically, Nettle and Bateson’s (60) second prediction is that accelerated reproduction (solid blue line) promotes higher LRS when individuals experience poor early-life conditions, compared with those with delayed reproduction (dashed purple line). However, accelerated reproduction results in reduced LRS when early-life conditions are good. Their third prediction is a logical extension of the first two predictions: individuals who exhibit poor somatic states in adulthood will experience higher LRS if they accelerate reproduction, and those with good somatic states will experience higher LRS if they delay reproduction. The fitness crossover is necessary for adaptive developmental plasticity because it requires the optimal phenotype (i.e., the optimal reproductive strategy) to be state dependent. If the fitness crossover does not exist, then one phenotype is optimal under all conditions, making it adaptive, but not an adaptive response to developmental conditions. The figure is adapted from Monaghan (37) and Nettle and Bateson (60).
Both the second and third predictions assume that accelerated reproduction is relevant to fitness. Many previous studies of the iPAR model also focus on linking early adversity to the timing and pace of female reproduction overall, thus identifying a potential response to early adversity (but without explicitly testing its adaptive value) (46, 47). Before testing for fitness crossovers, we therefore also asked whether 1) accelerated reproduction contributes to variation in female LRS and 2) early-life adversity is predictably linked to the timing and pace of female reproduction. Finally, we tested Nettle and Bateson’s second and third predictions (60) in female baboons for whom we have complete information on early-life conditions, reproductive schedules, and lifespans. We specifically asked whether LRS in these females was explained by an interaction between early-life adversity and the pace of reproduction or by an interaction between pace of reproduction and lifespan. Together, these tests capture the idea that, under the iPAR model, the benefits of early maturation should outweigh the costs of early maturation for individuals who both experience early-life adversity and have poor somatic states (41). However, for individuals who do not experience early-life adversity and who have good somatic states, the fecundity and offspring survival costs associated with early maturation may outweigh the benefits, thus giving rise to the fitness crossover depicted in Fig. 1.
Together, our results provide insight into the evolutionary logic underlying developmental plasticity in long-lived species and help reveal how the early environment influences phenotypic variation across the lifespan.
Results
Nettle and Bateson’s First Prediction: Early-Life Adversity Predicts Short Lifespans.
To test Nettle and Bateson’s first prediction (60), we measured six known sources of early-life adversity in 230 adult female baboons (see SI Appendix, Table S1 for sample sizes for all analyses). These six sources lead juveniles to experience nutritional and/or psychosocial adversity in early life and include 1) maternal death before 4 y of age (the approximate age of reproductive maturity for baboon females) (61); 2) the presence of a competing younger sibling, which may divert maternal resources; 3) drought in the first year of life; 4) maternal social isolation in the first 2 y of life; 5) low maternal social status at birth; and 6) high social density at birth (Table 1 and ref. 9). We then summed the presence of these sources of adversity for each female in a cumulative adversity index and tested if cumulative early adversity predicted adult survival in a Cox proportional hazards model. Confirming prior research in our population (9), and in support of the iPAR model’s first prediction, we found that cumulative early-life adversity had profound effects on adult female lifespans. Contingent on reaching 4 y of age, females that experienced zero sources of early-life adversity had median lifespans of 21.7 y of age, while females who experienced three or more sources of adversity typically lived only 14.3 y (r2 = 0.052; Wald test: P = 4.67 × 10−4; n = 230; SI Appendix, Fig. S1). Adding one source of adversity increased the risk of death in any given year of adulthood 1.5-fold (hazard ratio: 1.502; 95% CI: 1.196 to 1.886).
To assess the link between adult survival and the individual sources of adversity, we also fit a multivariate Cox proportional hazards model that included each of the six sources of early adversity as predictor variables (r2 = 0.080; Wald test: P = 2.36 × 10−3; n = 230; SI Appendix, Table S2). The strongest predictors of early mortality were maternal death and having a socially isolated mother. Adult females who experienced maternal death before 4 y of age were almost 2.4 times as likely to die at every adult age (hazard ratio: 2.377; 95% CI: 1.507 to 3.748; P = 1.96 × 10−4), and females with socially isolated mothers were almost 1.5 times as likely to die at every adult age (hazard ratio: 1.459; 95% CI: 1.042 to 2.043; P = 0.028), compared with those who did not experience those sources of early-life adversity. While not statistically significant, having a competing younger sibling also increased mortality risk by 1.7 times at every age (hazard ratio: 1.702; 95% CI: 0.968 to 2.994; P = 0.065). We focused our subsequent analyses on cumulative adversity, maternal death, maternal social isolation, and competing sibling because these variables were most tightly linked to short lifespans (SI Appendix, Table S2).
Initial Analysis 1: Accelerated Reproduction Increases LRS.
Before testing Nettle and Bateson’s (60) second and third predictions, we first tested the underlying assumption that accelerated reproductive schedules improve female LRS overall. For this and all subsequent analyses, we measured LRS as the total number of live offspring born to each female. This measure follows the strict bookkeeping guidelines recommended by quantitative evolutionary biologists, which avoid conflating maternal and offspring phenotypes (offspring survival is a phenotype that combines maternal and offspring characteristics) (62–65). However, because a mother’s ability to raise offspring to independence can be seen as a component of maternal fitness (66), we also repeated our analyses defining LRS as the total number of offspring born to each female that survived to 70 wk (most offspring are weaned by 70 wk) (67). In all cases, this approach produces qualitatively similar results (SI Appendix, Tables S3, S6, and S9).
We used three measures of female reproductive timing: 1) the start of reproduction as age at first live birth, defined as the age at which the female produced her first live offspring (range: 4.75 to 8.35 y; mean ± SD: 6.19 ± 0.65 y); 2) the rate of reproduction as surviving interbirth interval (IBI), defined as the number of days between consecutive live births, where the first offspring survived for at least 70 wk (range: 410 to 1,223 d; mean ± SD: 667 ± 140 d); and 3) combined reproductive pace as an integrative measure of both the start and rate of reproduction. We included this third metric to account for the fact that females may achieve the greatest reproductive acceleration by combining both phenotypes: an early start and a short turn-around time. Indeed, this metric predicts LRS with a slightly higher R2 than the first two metrics combined (combined reproductive pace: 9.3% of variance in LRS; age at first birth: 5.0%; average IBI: 2.8%; Table 2). However, to avoid biasing this metric toward young adults or females with short lifespans, we only calculated it for females with completed lifespans and for whom all members of the same birth year cohort were dead (this concern specifically relates to combined reproductive pace because both age at first live birth and each IBI are single events in time, whereas combined reproductive pace is a measure that spans adulthood). These constraints make the sample sizes for this metric substantially smaller than the other pace of reproduction measures (SI Appendix, Table S1). Combined reproductive pace was calculated as the mean of each female’s centered, standardized age at first live birth and average surviving IBIs. Negative values represent females with early ages at first live birth and short IBIs, and positive values represent individuals with late ages at first live birth and long IBIs (range: −2.09 to 2.58; mean ± SD: −0.03 ± 0.82).
Table 2.
Effects of lifespan and pace of reproduction on female LRS
| Predictor variable* | Coefficient | SE | z | P | Variance explained, % |
| Model 1: Do lifespan, age at first birth, and average IBI predict LRS? | |||||
| Lifespan | 0.516 | 0.018 | 29.351 | 1.04 × 10−52 | 82.8 |
| Age at first birth | −0.591 | 0.146 | −4.036 | 1.03 × 10−4 | 5.0 |
| Average IBI | −3.286 | 0.591 | −5.561 | 2.02 × 10−7 | 2.8 |
| Model 2: Do lifespan and combined reproductive pace predict LRS? | |||||
| Lifespan | 0.515 | 0.021 | 24.656 | 1.44 × 10−38 | 80.0 |
| Combined reproductive pace | −1.043 | 0.126 | −8.267 | 2.86 × 10−12 | 9.3 |
Results using the alternate definition of LRS (which includes offspring survival to weaning) are found in SI Appendix, Table S3.
Lifespan and age at first birth are measured in years, while average IBI is the natural log-transformed length of the mean IBI measured in days.
Unsurprisingly for a long-lived, slow-reproducing animal, we found that longevity was the dominant driver of female fitness. Long-lived females had higher LRS than short-lived females, and longevity explained between 80.0 and 82.8% of the variance in LRS (see Table 2 and SI Appendix, Table S3 for results based on offspring that survive to weaning). However, in support of the idea that variation in reproductive rates contributes to differences in female fitness, all three measures of the pace of female reproduction made small but significant contributions to female LRS. Specifically, age at first birth explained 5% (P = 1.0 × 10−4), average IBI explained 2.8% (P = 2.0 × 10−7), and combined reproductive pace explained 9.3% (P = 2.9 × 10−12) of the overall variance in female LRS (Table 2). In all cases, earlier or faster reproductive timing predicted higher LRS.
Initial Analysis 2: Early-Life Adversity Does Not Reliably Predict Accelerated Reproduction.
We also tested the common assumption that early-life adversity is predictably linked to the timing and pace of female reproduction. While not a prerequisite of the iPAR model, this test is commonly used to infer this form of adaptive developmental plasticity (e.g., refs. 33, 46, 47). For each of the four sources of adversity that best predicted lifespan—the cumulative adversity index, maternal death, maternal social isolation, and the presence of a competing sibling—we fit three linear mixed models, one for each reproductive measure, using the lmekin function in the coxme package, which allowed us to control for genetic relatedness between females. Our other covariates were several environmental and behavioral covariates known to predict resource access during adulthood that do not covary with our sources of early-life adversity and that also explain variation in reproductive timing and pace in our population: the number of mature females in the group, ordinal dominance rank, age, age squared, and a variable coding for primiparity (68, 69) (see Materials and Methods for details of covariates used in each model). Controlling for these covariates allows us to isolate the effects of early-life adversity from the largest known sources of variance in resource access.
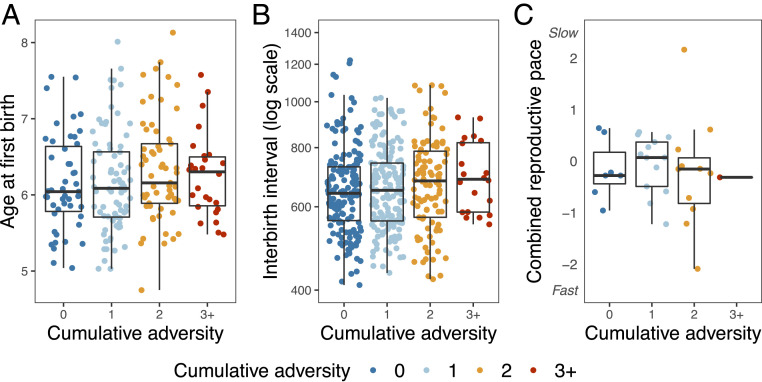
Despite the fact that accelerated reproduction contributed to female LRS (Table 2 and SI Appendix, Table S3), we found no evidence that females accelerated reproduction in response to early-life adversity. Overall, neither cumulative early-life adversity, maternal death, maternal social isolation, nor the presence of a competing sibling significantly predicted age at first live birth, surviving IBI, or combined reproductive pace (Fig. 2 and SI Appendix, Fig. S2 and Table S4). The only possible exception was the presence of a competing younger sibling, which exhibited a nonsignificant trend for a faster combined reproductive pace (P = 0.07; n = 80; SI Appendix, Fig. S2 and Table S4). However, none of our tests produced P values even close to canonical significance thresholds after multiple hypothesis testing correction (all adjusted P > 0.5) (70).
Fig. 2.
Cumulative early-life adversity is not linked to the timing or pace of reproduction in female baboons (full model results are in SI Appendix, Table S4). Plots depict the relationship between cumulative early-life adversity and age at first live birth (n = 211; P = 0.66) (A), duration of surviving IBIs (plotted on a log scale; n = 452; P = 0.69) (B), and the combined reproductive pace (n = 32; P = 0.73) (C). Colors indicate the number of adverse conditions occurring in early life. All points are jittered along the x axis to increase readability.
Nettle and Bateson’s Second Prediction: Accelerated Reproduction Is Not an Adaptive Response to Early-Life Adversity.
Nettle and Bateson’s (60) second prediction, as applied to the iPAR model, is that the fitness benefits of reproductive acceleration should change depending on exposure to early adversity (i.e., it should exhibit evidence of the fitness crossover in Fig. 1). We tested this idea using an information theoretic approach that assessed whether female LRS was better fit by a linear model that included an interaction effect between early-life adversity and the pace of reproduction versus a model without this interaction effect. We tested this prediction 12 times, including all pairwise combinations of the three measures of reproductive pace and the four measures of early-life adversity that most strongly predicted lifespan: the cumulative adversity index, maternal death, competing sibling, and maternal social isolation.
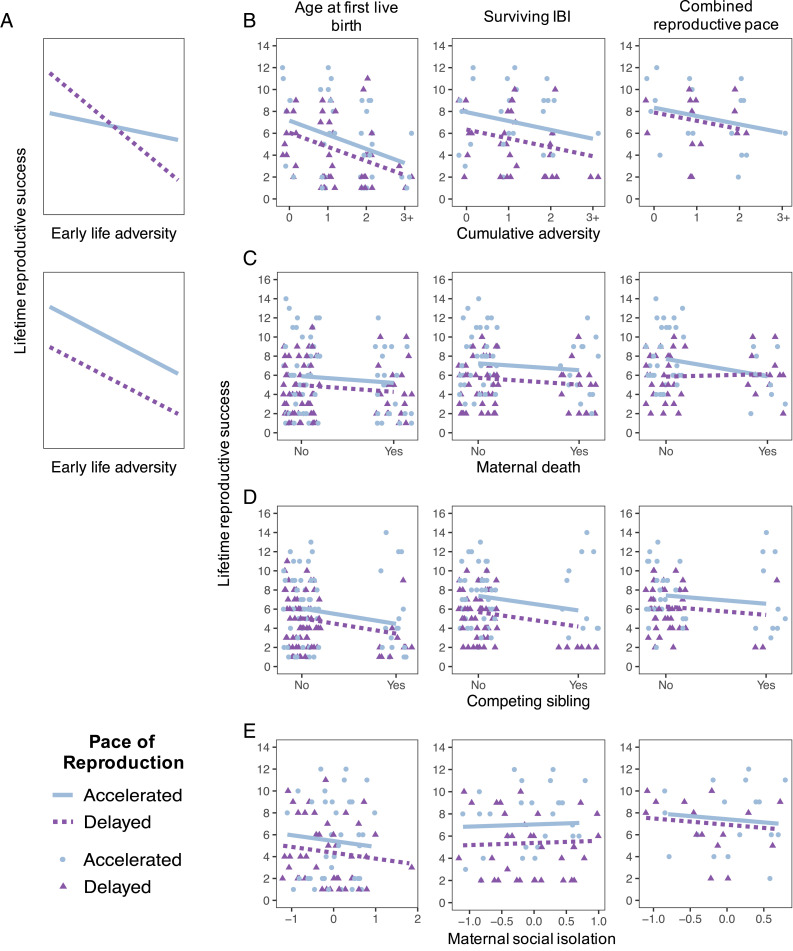
Contrary to the predictions of the iPAR model, we found no evidence that accelerated reproduction led to consistent fitness advantages specific to females who experienced cumulative early-life adversity. Instead, accelerated reproduction predicted higher fitness, independent of the presence or absence of cumulative early-life adversity. Hence, when modeling female LRS, we never observed a significant interaction effect (i.e., fitness crossover in the expected direction) between cumulative adversity and any measure of reproductive timing (Fig. 3B and SI Appendix, Table S5). While we found some evidence for an interaction between cumulative early adversity and combined reproductive pace (difference in Akaike information criterion [ΔAIC]: 1.999; n = 32), this interaction was in the opposite direction relative to the iPAR model’s prediction: accelerated reproduction only improved fitness for females who did not experience early-life adversity (SI Appendix, Fig. S3 and Table S5; see also SI Appendix, Table S6).
Fig. 3.
Accelerated reproduction does not result in higher LRS for individuals that experienced early-life adversity. (A) Predicted relationships between early-life adversity and LRS under the iPAR model (Top) and an alternative in which accelerated reproduction is advantageous, independent of early-life experience (Bottom). (B–E) The relationship between LRS and measures of early-life adversity, stratified by pace of reproduction. The four rows show the results for each of the four measures of early-life adversity: cumulative early-life adversity (B), maternal death (C), competing sibling (D), and maternal social isolation (E). The three columns show the results for each of the three measures of reproductive pace: age at first birth (Left), average surviving IBI (Middle), and combined reproductive pace (Right). The points represent the raw data, based on whether the pace of reproduction value was above (accelerated: blue circles) or below (delayed: purple triangles) the median value. Lines show predicted values from the linear model that best fit the data when holding pace of reproduction at the bottom 25th percentile (delayed: purple dashed) or the top 25th percentile (accelerated: blue solid). All results were more similar to the alternative prediction (A, Bottom) than the iPAR model prediction (A, Top). The only case of a statistically supported interaction (C, Right) was in the opposite direction predicted by the iPAR model, such that accelerated reproduction only predicted higher fitness in the absence of early-life adversity. Data points in B–D are jittered along the x axis to increase readability.
We repeated this analysis for the three individual sources of adversity that were the strongest predictors of female lifespan. For all of these analyses, the model with the interaction effect was never a significantly better fit for the data (Fig. 3 C–E), except for measures of combined reproductive pace for females who experienced maternal death (ΔAIC = 4.001; n = 81; Fig. 3C, Right; SI Appendix, Table S5). Again, for this model, the interaction effect (P = 0.017) was in the opposite direction with respect to the iPAR model: accelerated combined reproductive pace only predicted higher fitness for females that did not lose their mother (Fig. 3C, Right; SI Appendix, Table S5). When we repeated these analyses with the definition of LRS that considered offspring survival, all analyses but one yielded the same results. The one exception was for the analysis of the effects of maternal death on IBIs, where we again detected a significant interaction but in the opposite direction predicted by the iPAR model: short IBIs only improved fitness for females who did not experience maternal death (SI Appendix, Table S6).
The observation that females who experienced maternal death—one of the strongest predictors of short life expectancy—did not experience any fitness benefits from accelerated reproduction suggests that acceleration could sometimes be costly and, possibly, more so to females that experienced early-life adversity. To test this idea, we ran three post hoc Cox proportional hazards models of maternal death and our three measures of reproductive pace on female survival. In support of the idea that accelerated reproduction is sometimes costly, we found a significant interaction effect between maternal death and average surviving IBIs (P = 0.035; n = 110) but no significant result for age at first birth or combined reproductive pace. Females who lost their mother and exhibited shorter IBIs between surviving offspring had shorter lifespans than females who lost their mothers and did not accelerate their IBIs (SI Appendix, Fig. S4 and Table S7).
Nettle and Bateson’s Third Prediction: Accelerated Reproduction Is Not an Adaptive Response to a Short Expected Lifespan.
Nettle and Bateson’s (60) third prediction states that the fitness benefits of reproductive acceleration should differ depending on the quality of a female’s somatic state (Fig. 1). We again used an information theoretic approach to test whether a model with an interaction between pace of reproduction and lifespan, as a proxy for somatic state, was a better predictor of female LRS than a model without this interaction effect. This method allows us to test for the presence of this key fitness crossover (Fig. 1).
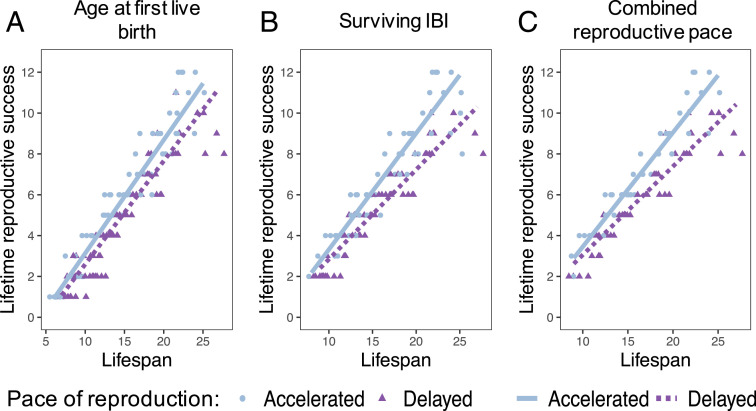
We found no evidence that females with short lifespans benefitted from accelerated reproduction. While an interaction between lifespan and pace of reproduction always improved model fit (age at first birth: ΔAIC = 4.064 [n = 145]; average IBI: ΔAIC = 23.553 [n = 110]; combined reproductive pace: ΔAIC = 17.381 [n = 81]), this interaction was always in the opposite direction predicted by the iPAR model (Fig. 4 and SI Appendix, Table S8). Specifically, the advantages of accelerated reproduction only paid off if females led long lives. Females who led short lives and accelerated reproduction did not experience higher LRS than females who led short lives and delayed reproduction. These analyses were repeated using the alternative definition of LRS based on offspring that survived to weaning, with concordant results (SI Appendix, Table S9).
Fig. 4.
Accelerated reproduction is not linked to higher LRS for females with short lifespans. Plots depict the relationship between female lifespan, as a measure of her somatic quality, and her LRS, partitioned by whether the female’s pace of reproduction measure was above (accelerated: blue circles) or below (delayed: purple triangles) the median value of the dataset. A is partitioned by age at first live birth, B is partitioned by mean IBI, and C is partitioned by the combined reproductive pace measure. On each plot, the points represent the raw data, and the lines represent the predicted values from the linear model that best fit the data when holding pace of reproduction at the bottom 25th percentile (delayed: purple dashed) or the top 25th percentile (accelerated: blue solid). For all of these analyses, the model with the interaction was a better fit for the data (age at first birth: ΔAIC = 4.064 [n = 145]; IBI: ΔAIC = 23.553 [n = 110]; combined reproductive pace: ΔAIC = 17.381 [n = 81]). However, the interaction was in the direction opposite the iPAR model’s prediction: females only accrued higher LRS by accelerating reproduction if they also led long lives.
Discussion
Over the last few decades, numerous hypotheses—including the iPAR model—have proposed that, because early-life adversity predictably leads to poor health and short life expectancy, individuals who experience early adversity should accelerate their life histories to maximize fitness (10–21). Despite the popularity of this hypothesis, no studies in humans or other long-lived animals have yet tested whether variation in reproductive schedules associated with early-life adversity is itself adaptive. This study does so using a recent framework developed to test the iPAR model (19–21, 60). We find no evidence that accelerated reproductive schedules are an adaptive response to early-life adversity. Despite the fact that adversity in early life led to dramatically shorter lifespans in female baboons, females who experienced early-life adversity did not accelerate their reproduction compared with those who did not experience early-life adversity. Further, females who accelerated their reproduction had higher LRS than females with normal or slow reproductive schedules—including for females who experienced early-life adversity. However, these fitness benefits were not unique to individuals who experienced early-life adversity. In other words, we find no evidence for the required interaction effect between early adversity and pace of reproduction. In fact, females only reaped measurable benefits of reproductive acceleration if they led long lives. Thus, even if accelerated reproduction is adaptive overall in baboons and other long-lived species, our results suggest that accelerated reproduction is not an adaptive response to particular early environmental conditions.
PAR models are commonly cited to provide an adaptive explanation for the developmental plasticity observed in a wide range of species. Classic, external PAR (ePAR) models posit that organisms use early-life environments to predict the quality of their adult environment (37, 44, 45). These ePAR models have not been well supported in long-lived species, perhaps because early-life environments rarely predict adult environments over long time scales (20, 71–75). iPAR models attempt to solve this problem by proposing that organisms adapt, not in anticipation of the external environment but to the quality of their expected somatic state in adulthood, which in turn predicts lifespan (19–21). Specifically, under the iPAR model, harsh early environments lead organisms to exhibit poor somatic states, which presage shorter life expectancies; in turn, organisms accelerate their reproduction (20).
There are several reasons why accelerated reproductive schedules may not be an adaptive response to early-life adversity in our population. First, compared with the fitness effects of variation in lifespan, the effects of changes in maturation timing and reproductive pace may be too small in long-lived species to elicit a response to selection. For example, in the Amboseli baboon population, lifespan is the dominant driver of female LRS, explaining between 80 and 83% of the overall variation; in contrast, age at first birth only explains 5% of the variation in fitness (Table 2). Furthermore, female baboons gain relatively small benefits by maturing a few months early: in order to gain one additional offspring, female baboons must accelerate their age at first birth by ∼1.5 y—the average surviving IBI in this population. Such a change would represent an extreme acceleration of 25% relative to average age at first birth in this population (∼6 y). Therefore, because of the drastic acceleration needed to improve fitness, it is unsurprising that accelerated maturation has not evolved in response to early-life adversity. Accelerated reproduction might offer greater fitness benefits for species in which more of the variation in fitness is determined by an individual’s pace of reproduction. However, the idea that the pace of reproduction must make a substantial contribution to female fitness for accelerated reproduction to be favored by natural selection has been largely ignored in the literature.
Second, other lines of evidence argue that reproductive pace is most often explained by immediate, not past, environmental conditions. In baboons and humans, the physiological “decision” to reproduce is most sensitive to energy balance (energy consumed minus energy expended), as opposed to energy status (the amount of energy stored in the body due to long-term energetic state, as reflected, for example, in body mass index; refs. 69, 76, 77). Even if early-life experiences shape energy status or overall somatic quality, a female’s energy balance is most reflective of her immediate energetic state as a result of current resource availability—and current resource availability is, in turn, most strongly affected by dominance rank, social density, and recent rainfall (69). Further, dominance rank in baboons and other cercopithecine primates is highly nepotistic: a female’s adult dominance rank is very strongly determined by the ranks of her mother and older sisters, such that dominance rank is “inherited” from one generation to the next in female cercopithecines with very high fidelity (78–81). Consequently, we expect dominance rank—and by extension energy balance and reproductive rate—to be relatively insensitive to early-life adversity. Indeed, the only source of early-life adversity likely to affect dominance rank, and hence reproductive rate, in our study is maternal loss and only indirectly through maternal support for daughters during adult rank attainment (82). Thus, the effects of other, more proximate environmental factors are likely to swamp any effects of early-life adversity on adult female energy balance, potentially explaining the disconnect we observe between early-life adversity and reproductive rate.
Third, the types of adversity in our population may not provide salient early-life environments for reproductive acceleration. A growing body of evidence indicates that sources of psychosocial adversity most often lead to accelerated reproduction (29, 33, 35), while nutritional adversity is more often linked to delayed reproduction (32, 83, 84). However, other authors have pointed out that there is no formal life history theory that outlines the effects of psychosocial stress on the timing and pace of reproduction (43). In Amboseli, the sources of adversity the baboons experienced likely led them to experience both nutritional and psychosocial stress. For instance, maternal loss during the juvenile period may prevent juveniles from foraging efficiently, increase the aggression they receive from conspecifics, and create challenges in forming social bonds in adulthood (85, 86). The resulting effects on reproductive timing may be complex. Resource scarcity, which is characteristic of our population, may also affect whether accelerated reproduction is adaptive. In humans, support for accelerated reproduction in response to adversity is strongest in developed, Western societies with abundant resources but uncommon in non-Western, developing societies (31, 32). Hence, early-life adversity may only lead to reproductive acceleration when psychosocial adversity is paired with nutritional excess.
Fourth, early maturation is associated with several fitness costs that the iPAR model and similar hypotheses do not consider. These costs can include decreased adult body size, increased instantaneous juvenile mortality, decreased initial and later fecundity, and decreased offspring survival (41). In light of these costs, Stearns and Koella (42) developed a set of models to identify the appropriate response, in terms of age at maturation, to slower-than-normal growth rates, a common outcome of nutritional limitation or intense competition in early life (8, 87–89). Importantly, Stearns and Koella’s (42) models failed to find any circumstances under which accelerated reproduction is a favored response to slow growth, because the costs of early maturation outweigh the benefits. Hence, within species, individuals who grow up under resource limitation are not expected to accelerate their reproduction (42)—consistent with our finding that female baboons that experienced early maternal loss and exhibited fast combined reproductive pace pay a cost in overall LRS.
We note some potential caveats to our study. Although we statistically controlled for all socioenvironmental effects known to affect female reproductive schedules in Amboseli (68, 69), as well as key indicators of resource access at the population level (e.g., population growth rate), residual correlations between early-life adversity and resource access in adulthood may still be present. Such correlations could confound our findings if early adversity and resource access are associated because they are affected by other, unspecified variables. For example, in human populations, low socioeconomic status early in life predicts both exposure to other forms of early adversity and low socioeconomic status in adulthood (90). Alternatively, early adversity could predict reproductive pace via a mediating path through reduced resource access. We believe our results do not suggest a strong contribution of either confounding or mediation: early-life adversity does not reliably predict reproductive pace in either direction, and even among the females who experienced early adversity, accelerated reproduction does not predict increased fitness. However, residual correlation with resource access could influence our findings for maternal loss. Under a mediation scenario, the costs of accelerated reproduction in females who experienced early maternal loss could be due to mediation through reduced lifelong resource access. Under a confounding scenario, a true fitness-increasing effect of accelerated reproduction in females who experienced maternal loss could be masked by reduction in resource access. If so, our observation that the benefits of accelerated reproduction are only detectable in females who did not lose their mothers could be incorrect. Such a confound, however, would likely only change our observation of an interaction that opposes the iPAR model’s predictions to a case in which no interaction (and no support for the iPAR model) existed.
Finally, we caution that viability selection may sometimes confound studies that find evidence for accelerated reproduction. If harsh forms of early-life adversity, such as famine, war, or drought, lead to higher juvenile mortality, these types of adversity may selectively remove low-quality individuals who would otherwise exhibit delayed reproduction, while retaining high-quality, fast-reproducing individuals. Future tests of the iPAR model—especially those using sources of adversity that are strongly linked to juvenile mortality—should consider such selection biases to determine if the observed patterns are due to unexplained differences between subpopulations that did/did not experience adversity and did/did not exhibit accelerated reproduction.
In conclusion, despite the attention given to the iPAR model and related hypotheses, our results suggest that accelerated reproduction is not an adaptation to harsh early-life conditions in wild baboons. For long-lived species, where the majority of LRS is explained by lifespan, accelerating the timing or pace of reproduction will have comparatively small benefits for fitness, especially if it is also associated with fitness costs.
Materials and Methods
Study Population.
The Amboseli Baboon Research Project has collected continuous, individual-based data on wild baboons in the Amboseli ecosystem since 1971. Baboons in Amboseli are primarily yellow baboons (Papio cynocephalus) with some natural admixture from neighboring anubis baboon (Papio anubis) populations (91, 92). Over the course of the 48-y study, we followed female subjects distributed over 18 social groups that persisted for varying lengths of time. All 18 groups were derived from 2 original study groups, following natural patterns of social group fission and fusion. We focused on females because male postnatal dispersal typically prevents us from measuring male lifespans and LRS. All baboons are visually identifiable by trained observers who monitor each group two to four times per week throughout the year. During each monitoring visit, the observers conduct group censuses and capture all demographic and life-history events (births, maturation events, immigrations, emigrations, and deaths), allowing us to calculate age at maturity and lifespan with precision. In addition, observers record agonistic interactions and affiliative interactions, allowing us to calculate social dominance ranks and estimates of individual social connectedness (SI Appendix, Supplementary Methods). This research was approved by the Institutional Animal Care and Use Committees at Duke University, University of Notre Dame, and Princeton University and adhered to all the laws and guidelines of Kenya.
Measuring Early-Life Adversity.
We measured the same six sources of early-life adversity used by Tung et al. (9) (Table 1; see SI Appendix, Supplementary Methods for methods on each source of adversity). Following Tung et al. (9), we used these data to calculate a cumulative adversity index in which continuous variables (maternal social isolation, maternal dominance rank, and social density at birth) were converted to binary variables and scored as present if the female’s experience fell in the highest adversity quartile of the population distribution (variables were always coded so that higher values reflected more adverse environments). The cumulative adversity index was calculated as the total number of adverse conditions each female experienced. The final index could theoretically range from zero to six, but in practice, no subject experienced more than four sources of early-life adversity (21% of the population experienced zero sources of adversity; 37% experienced one; 30% experienced two; 9% experienced three; and 3% experienced four sources of adversity). As in the study by Tung et al. (9), individuals that experienced three or more sources of adversity were grouped into one category because of the small number of females who experienced more than three exposures.
Measuring the Pace of Reproduction.
We used three measures of female pace of reproduction: 1) age at first live birth, 2) surviving IBI, and 3) a metric of combined reproductive pace, which considered both the advent and rate of reproduction.
Age at first live birth.
Age at first live birth was defined as a female’s age in years when she gave birth to her first live offspring. For this measure, we excluded all females born after 2011, which represents the most recent year when all living individuals born in that year have already achieved their first live birth. Using this criterion prevented us from biasing our dataset toward early-maturing individuals.
Surviving IBI.
Surviving IBI was defined as the number of days between consecutive live births, where the first offspring in the series survived at least 70 wk. Seventy weeks represents the approximate age at weaning in our population (67); in baboons, as in most mammals, females rapidly return to ovarian cycling if offspring die before weaning. When this happens, IBIs are unusually short and not informative about the rate of offspring production when offspring survive. For all analyses except initial analysis 2, we averaged the duration of surviving IBIs for each female (or used the duration of a single IBI if that was all that was available); for initial analysis 2, we modeled multiple surviving IBIs for each female, when these were available (see Statistical Analyses).
Combined reproductive pace.
Combined reproductive pace was calculated for each individual by centering and standardizing the age at first live birth and the average IBI and then taking the mean of these two values. Negative values represent individuals with early ages at first live birth and short IBIs, while positive values represent individuals with late ages at first live birth and long IBIs. For this measure, we excluded all females born after 1996, which represents the most recent year when all individuals born in that year are no longer living. Incorporating this criterion into our analyses prevented us from biasing our dataset toward short-lived individuals but decreased our sample size relative to the two other measures of reproductive pace (IBI and age at first live birth).
For all three measures of reproductive pace, we excluded females whose measure was more than three SDs from the mean because these females may have pathological or fundamentally different reproductive physiologies compared with the rest of the population. These exclusions included 5 of 284 measures of age at first live birth, 5 of 648 IBIs, and 1 of 33 measures of combined reproductive pace (see SI Appendix, Table S1 for final sample sizes for all analyses).
Statistical Analyses.
All statistical analyses were conducted in R, version 3.4.0 (93, 94). Sample sizes varied across analyses because of differences in our inclusion criteria for measures of female reproduction and because some subjects lacked information on maternal social isolation (SI Appendix, Table S1).
To confirm Nettle and Bateson’s (60) first prediction that early-life adversity is linked to shorter expected lifespan, we replicated Tung et al.’s (9) analysis linking early adversity to female survival in an updated, larger dataset: n = 230 females in this analysis versus n = 196 in that by Tung et al. (9), a 17.3% increase. Briefly, we fit both a univariate Cox proportional hazards model—with our cumulative adversity index as the single predictor variable—and a multivariate model, which included each of the six individual sources of adversity as predictor variables. In the multivariate model, maternal death, competing sibling, and drought were binary variables representing either the presence or absence of the adverse event, while maternal social isolation, maternal dominance rank, and social density were represented as continuous variables. Both models were fit using the function coxph, from the R package survival (95).
Before testing Nettle and Bateson’s (60) second and third predictions, we conducted two initial analyses to understand whether 1) accelerated reproduction contributes to variation in female LRS and 2) early-life adversity is predictably linked to the timing and pace of female reproduction (i.e., the three measures of pace of reproduction described in Measuring the Pace of Reproduction). For the first analysis, we constructed two linear models of LRS, defined as the total number of live offspring born to each female over the course of her life. Only females with completed lifespans were included in this analysis. Both models included female lifespan as a predictor variable. The first model additionally tested the predictive power of age at first live birth and the natural log-transformed average surviving IBI, while the second model tested the predictive power of our combined reproductive pace metric that integrates both the timing and pace of reproduction. We did not model all three predictors in a single model because of the strong correlation between combined reproductive pace and both age at first live birth and mean surviving IBI (as the combined pace variable is a composite of the other two). We also replicated these analyses with a second measure of LRS, defined as the total number of offspring born to each female who survived to the typical age of weaning (70 wk).
In the second of our initial analyses, we tested whether early-life adversity leads to predictable differences in our three measures of the timing and pace of female reproduction. For each of the four sources of adversity that most strongly predicted lifespan—the cumulative adversity index, maternal death, maternal social isolation, and the presence of a competing sibling—we fit three linear mixed models, one for each reproductive measure. Here, surviving IBIs were modeled individually and natural log-transformed. All models were fit using the lmekin function from the coxme package in R (96), which allowed us to account for genetic contributions to the timing of reproduction by modeling a random effect that incorporates multigenerational pedigree data constructed using the pedantics package (97). We modeled two other random effects to account for cohort effects: birth year and population growth rate from 6 mo before birth to 1.5 y after birth (the period of maternal dependence). The latter measure captures information about the level of resources available to the population, which could in turn affect reproductive timing. For each model, fixed-effect predictors were the adversity measure and other variables previously shown to influence each pace of reproduction measure (and that were not colinear with the early-life adversity measures). Specifically, for the age at first live birth models, we included the number of mature females in the group at the time of the first birth (68). For the surviving IBI models, we included a variable coding whether the female was primiparous at the birth of the first infant in the IBI series, as well as the female’s ordinal dominance rank, age, and age squared at the birth of the first infant in the IBI series (69). For the combined reproductive pace models, we included the number of mature females in the group at the time of the first birth and the female’s average ordinal rank at the birth of the first infant in each IBI series. Including these variables as fixed effects allowed us to control for the current environment and factors besides early-life experiences that may have had an influence on the timing and pace of reproduction.
After completing these initial analyses, we tested Nettle and Bateson’s (60) second and third predictions for the iPAR model. The second prediction posits a crossover for the fitness benefits of accelerated reproduction in the presence and absence of early-life adversity (Fig. 1). We tested this prediction using both of our LRS definitions. For each definition of LRS, we used an information theoretic approach that identified whether female LRS was best fit by a linear model that included an interaction effect between early-life adversity and the pace of reproduction or a model without this interaction effect. All models were fit using the lm function in R. The best-fitting model was determined via a ΔAIC greater than 2 (98). This prediction was tested 12 different times, including all pairwise combinations of the three measures of reproductive pace and the four measures of early-life adversity that most strongly predicted lifespan: the cumulative adversity index, maternal death, competing sibling, and maternal social isolation. For the surviving IBI models, the IBIs were log-transformed and then averaged for each female.
Nettle and Bateson’s (60) third prediction posits that accelerated reproduction should be especially advantageous for females that exhibit poor somatic quality in adulthood but detrimental for females with good somatic quality and long lifespans (Fig. 1). To test this prediction, we used lifespan as a measure of somatic quality and again used an information theoretic approach to test whether a model with an interaction between pace of reproduction and lifespan was a better predictor of female LRS than a model without this interaction effect. This test was repeated for each of the three pace of reproduction measures (age at first birth, average IBI, and combined reproductive pace). Again, for the surviving IBI models, we used the natural log-transformed average IBI for each female.
Supplementary Material
Acknowledgments
We thank the editor and two anonymous reviewers for comments that greatly improved the quality of the manuscript. We thank Jeanne Altmann for her essential role in stewarding the Amboseli Baboon Project. We acknowledge the support of the NIH and the NSF for the majority of the data represented here. Our work is currently supported through NIH Grants R01AG053330, R01AG053308, R01HD088558, and P01AG031719 and NSF Grant IOS 1456832. We also thank Duke University, Princeton University, and the University of Notre Dame for financial and logistical support. In Kenya, we thank the Kenya Wildlife Service, University of Nairobi, Institute of Primate Research, National Museums of Kenya, and the National Council for Science, Technology, and Innovation. We also thank the members of the Amboseli-Longido pastoralist communities and the Enduimet Wildlife Management Area for their cooperation and assistance in the field. Particular thanks go to the Amboseli Baboon Project long-term field team (R. S. Mututua, J. K. Warutere, and L. Siodi) and to T. Wango and V. Oudu for their untiring assistance in Nairobi. The baboon project database, Babase, is expertly managed by N. Learn and J. Gordon. Database design and programming are provided by K. Pinc. For a complete set of acknowledgments of funding sources, logistical assistance, and data collection and management, please visit amboselibaboons.nd.edu/acknowledgements/.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004018117/-/DCSupplemental.
Data Availability.
All data reported in this paper have been deposited in the Dryad Digital Repository (https://doi.org/10.5061/dryad.cjsxksn3n).
References
- 1.Felitti V. J. et al., Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 14, 245–258 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Lynch J., Smith G. D., A life course approach to chronic disease epidemiology. Annu. Rev. Public Health 26, 1–35 (2005). [DOI] [PubMed] [Google Scholar]
- 3.O’Rand A. M., Hamil-Luker J., Processes of cumulative adversity: Childhood disadvantage and increased risk of heart attack across the life course. J. Gerontol. B Psychol. Sci. Soc. Sci. 60, 117–124 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Roseboom T., de Rooij S., Painter R., The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 82, 485–491 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Miller G. E., Chen E., Parker K. J., Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 137, 959–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Descamps S., Boutin S., Berteaux D., McAdam A. G., Gaillard J.-M., Cohort effects in red squirrels: The influence of density, food abundance and temperature on future survival and reproductive success. J. Anim. Ecol. 77, 305–314 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Douhard M. et al., Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. Biol. Sci. 281, 20140276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pigeon G., Pelletier F., Direct and indirect effects of early-life environment on lifetime fitness of bighorn ewes. Proc. Biol. Sci. 285, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung J., Archie E. A., Altmann J., Alberts S. C., Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper P., Harpending H., Father absence and reproductive strategy: An evolutionary perspective. J. Anthropol. Res. 38, 255–273 (1982). [Google Scholar]
- 11.Belsky J., Steinberg L., Draper P., Childhood experience, interpersonal development, and reproductive strategy: And evolutionary theory of socialization. Child Dev. 62, 647–670 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Belsky J., The development of human reproductive strategies: Progress and prospects. Curr. Dir. Psychol. Sci. 21, 310–316 (2012). [Google Scholar]
- 13.Chisholm J. S. et al., Death, hope, and sex: Life-history theory and the development of reproductive strategies [and Comments and Reply]. Curr. Anthropol. 34, 1–24 (1993). [Google Scholar]
- 14.Ellis B. J., Timing of pubertal maturation in girls: An integrated life history approach. Psychol. Bull. 130, 920–958 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Gluckman P. D., Hanson M. A., Evolution, development and timing of puberty. Trends Endocrinol. Metab. 17, 7–12 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Geronimus A. T., The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn. Dis. 2, 207–221 (1992). [PubMed] [Google Scholar]
- 17.Boyce W. T., Ellis B. J., Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 17, 271–301 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Del Giudice M., Ellis B. J., Shirtcliff E. A., The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 35, 1562–1592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells J. C., Obesity as malnutrition: The role of capitalism in the obesity global epidemic. Am. J. Hum. Biol. 24, 261–276 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Nettle D., Frankenhuis W. E., Rickard I. J., The evolution of predictive adaptive responses in human life history. Proc. Biol. Sci. 280, 20131343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickard I. J., Frankenhuis W. E., Nettle D., Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspect. Psychol. Sci. 9, 3–15 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Cooper C., Kuh D., Egger P., Wadsworth M., Barker D., Childhood growth and age at menarche. Br. J. Obstet. Gynaecol. 103, 814–817 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Adair L. S., Size at birth predicts age at menarche. Pediatrics 107, E59 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Sloboda D. M., Hart R., Doherty D. A., Pennell C. E., Hickey M., Age at menarche: Influences of prenatal and postnatal growth. J. Clin. Endocrinol. Metab. 92, 46–50 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Painter R. C. et al., Increased reproductive success of women after prenatal undernutrition. Hum. Reprod. 23, 2591–2595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nettle D., Coall D. A., Dickins T. E., Birthweight and paternal involvement predict early reproduction in British women: Evidence from the National Child Development Study. Am. J. Hum. Biol. 22, 172–179 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Tither J. M., Ellis B. J., Impact of fathers on daughters’ age at menarche: A genetically and environmentally controlled sibling study. Dev. Psychol. 44, 1409–1420 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Pesonen A.-K. et al., Reproductive traits following a parent-child separation trauma during childhood: A natural experiment during World war II. Am. J. Hum. Biol. 20, 345–351 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Belsky J., Steinberg L., Houts R. M., Halpern-Felsher B. L.; NICHD Early Child Care Research Network , The development of reproductive strategy in females: Early maternal harshness– earlier menarche– increased sexual risk taking. Dev. Psychol. 46, 120–128 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Webster G. D., Graber J. A., Gesselman A. N., Crosier B. S., Schember T. O., A life history theory of father absence and menarche: A meta-analysis. Evol. Psychol. 12, 273–294 (2014). [PubMed] [Google Scholar]
- 31.Sear R., Sheppard P., Coall D. A., Cross-cultural evidence does not support universal acceleration of puberty in father-absent households. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyweluk M. A., Georgiev A. V., Borja J. B., Gettler L. T., Kuzawa C. W., Menarcheal timing is accelerated by favorable nutrition but unrelated to developmental cues of mortality or familial instability in Cebu, Philippines. Evol. Hum. Behav. 39, 76–81 (2018). [Google Scholar]
- 33.Sloboda D. M., Howie G. J., Pleasants A., Gluckman P. D., Vickers M. H., Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One 4, e6744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumby H. S. et al., Elephants born in the high stress season have faster reproductive ageing. Sci. Rep. 5, 13946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berghänel A., Heistermann M., Schülke O., Ostner J., Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl. Acad. Sci. U.S.A. 114, E10658–E10666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mell H., Safra L., Algan Y., Baumard N., Chevallier C., Childhood environmental harshness predicts coordinated health and reproductive strategies: A cross-sectional study of a nationally representative sample from France. Evol. Hum. Behav. 39, 1–8 (2018). [Google Scholar]
- 37.Monaghan P., Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1635–1645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grafen A., “On the uses of data on lifetime reproductive success” in Reproductive Success, Clutton-Brock T. H., Ed. (University of Chicago Press, 1988), pp. 454–471. [Google Scholar]
- 39.Hales C. N., Barker D. J. P., The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Lindström J., Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Stearns S. C., The Evolution of Life Histories, (Oxford University Press, ed. 1, 1992). [Google Scholar]
- 42.Stearns S. C., Koella J. C., The evolution of phenotypic plasticity in life-history traits: Predictions of reaction norms for age and size at maturity. Evolution 40, 893–913 (1986). [DOI] [PubMed] [Google Scholar]
- 43.Stearns S. C., Rodrigues A. M. M., On the use of “life history theory” in evolutionary psychology. Evol. Hum. Behav., https://www.sciencedirect.com/science/article/pii/S109051382030026X?casa_token=IuvsHFEQ4bEAAAAA:BTT8wE5OWhwtkwKDD_PdkQex4mGwtKWI2EtO8bX21d_u0W-8Z4azXsWr_Y85jsZ1MlH8zkjv6Q.
- 44.Gluckman P. D., Hanson M. A., Spencer H. G., Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Gluckman P. D., Hanson M. A., Spencer H. G., Bateson P., Environmental influences during development and their later consequences for health and disease: Implications for the interpretation of empirical studies. Proc. Biol. Sci. 272, 671–677 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berghänel A., Heistermann M., Schülke O., Ostner J., Prenatal stress effects in a wild, long-lived primate: Predictive adaptive responses in an unpredictable environment. Proc. Biol. Sci. 283, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douhard M., et al. , The influence of weather conditions during gestation on life histories in a wild Arctic ungulate. Proc. Biol. Sci. 283, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberts S. C., Altmann J., . “The Amboseli baboon research project: 40 years of continuity and change” in Long-Term Field Studies of Primates, Kappeler P. M., Watts D. P., Eds. (Springer-Verlag, 2012), pp. 261–287. [Google Scholar]
- 49.Winking J., Gurven M., Kaplan H., The impact of parents and self-selection on child survival among the Tsimane of Bolivia. Curr. Anthropol. 52, 277–284 (2011). [Google Scholar]
- 50.Sear R., Mace R., Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 (2008). [Google Scholar]
- 51.Virk J. et al., Prenatal exposure to bereavement and type-2 diabetes: A Danish longitudinal population based study. PLoS One 7, e43508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heaton J., Noyes J., Sloper P., Shah R., Families’ experiences of caring for technology-dependent children: A temporal perspective. Health Soc. Care Community 13, 441–450 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Moore S. E. et al., Season of birth predicts mortality in rural Gambia. Nature 388, 434 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Schulz L. C., The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. U.S.A. 107, 16757–16758 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumey L. H., Stein A. D., Susser E., Prenatal famine and adult health. Annu. Rev. Public Health 32, 237–262 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspi A., Harrington H., Moffitt T. E., Milne B. J., Poulton R., Socially isolated children 20 years later: Risk of cardiovascular disease. Arch. Pediatr. Adolesc. Med. 160, 805–811 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Bradley R. H., Corwyn R. F., Socioeconomic status and child development. Annu. Rev. Psychol. 53, 371–399 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Chen E., Martin A. D., Matthews K. A., Understanding health disparities: The role of race and socioeconomic status in children’s health. Am. J. Public Health 96, 702–708 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans G. W., Lepore S. J., Shejwal B. R., Palsane M. N., Chronic residential crowding and children’s well-being: An ecological perspective. Child Dev. 69, 1514–1523 (1998). [PubMed] [Google Scholar]
- 60.Nettle D., Bateson M., Adaptive developmental plasticity: What is it, how can we recognize it and when can it evolve? Proc. Biol. Sci. 282, 20151005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onyango P. O., Gesquiere L. R., Altmann J., Alberts S. C., Puberty and dispersal in a wild primate population. Horm. Behav. 64, 240–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold S., . “Sexual selection: The interface of theory and empiricism” in Mate Choice, Bateson P., Ed. (Cambridge University Press, 1983), pp. 67–107. [Google Scholar]
- 63.Lande R., Arnold S. J., The measurement of selection on correlated characters. Evolution 37, 1210–1226 (1983). [DOI] [PubMed] [Google Scholar]
- 64.Cheverud J. M., Evolution by kin selection: A quantitative genetic model illustrated by maternal performance in mice. Evolution 38, 766–777 (1984). [DOI] [PubMed] [Google Scholar]
- 65.Wolf J. B., Wade M. J., On the assignment of fitness to parents and offspring: Whose fitness is it and when does it matter? J. Evol. Biol. 14, 347–356 (2001). [Google Scholar]
- 66.Clutton-Brock T. H., Ed., Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems, (The University of Chicago Press, 1988). [Google Scholar]
- 67.Altmann S. A., Foraging for Survival: Yearling Baboons in Africa, (University of Chicago Press, 1998). [Google Scholar]
- 68.Charpentier M. J., Tung J., Altmann J., Alberts S. C., Age at maturity in wild baboons: Genetic, environmental and demographic influences. Mol. Ecol. 17, 2026–2040 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Gesquiere L. R., Altmann J., Archie E. A., Alberts S. C., Interbirth intervals in wild baboons: Environmental predictors and hormonal correlates. Am. J. Phys. Anthropol. 166, 107–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995). [Google Scholar]
- 71.Botero C. A., Weissing F. J., Wright J., Rubenstein D. R., Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl. Acad. Sci. U.S.A. 112, 184–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayward A. D., Rickard I. J., Lummaa V., Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc. Natl. Acad. Sci. U.S.A. 110, 13886–13891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lea A. J., Altmann J., Alberts S. C., Tung J., Developmental constraints in a wild primate. Am. Nat. 185, 809–821 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nettle D., What the future held: Childhood psychosocial adversity is associated with health deterioration through adulthood in a cohort of British women. Evol. Hum. Behav. 35, 519–525 (2014). [Google Scholar]
- 75.Nettle D., Frankenhuis W. E., Rickard I. J., The evolution of predictive adaptive responses in humans: Response. Proc. Biol. Sci. 281, 20132822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellison P. T., Energetics and reproductive effort. Am. J. Hum. Biol. 15, 342–351 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Ellison P. T., Endocrinology, energetics, and human life history: A synthetic model. Horm. Behav. 91, 97–106 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Hrdy S. B., Hrdy D. B., Hierarchical relations among female Hanuman Langurs (Primates: Colobinae, Presbytis entellus). Science 193, 913–915 (1976). [DOI] [PubMed] [Google Scholar]
- 79.Hausfater G., Altmann J., Altmann S., Long-term consistency of dominance relations among female baboons (Papio cynocephalus). Science 217, 752–755 (1982). [DOI] [PubMed] [Google Scholar]
- 80.Horrocks J. A., Hunte W., Rank relations in vervet sisters: A critique of the role of reproductive value. Am. Nat. 122, 417–421 (1983). [Google Scholar]
- 81.Kawai M., On the system of social ranks in a natural troop of Japanese monkeys: Basic and dependent rank. Primates 1, 111–130 (1958). [Google Scholar]
- 82.Lea A. J., Learn N. H., Theus M. J., Altmann J., Alberts S. C., Complex sources of variance in female dominance rank in a nepotistic society. Anim. Behav. 94, 87–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osteria T. S., Nutritional status and menarche in a rural community in the Philippines. Philipp. J. Nutr. 36, 150–156 (1983). [PubMed] [Google Scholar]
- 84.Riley A. P., Determinants of adolescent fertility and its consequences for maternal health, with special reference to rural Bangladesh. Ann. N. Y. Acad. Sci. 709, 86–100 (1994). [DOI] [PubMed] [Google Scholar]
- 85.Silk J. B., Altmann J., Alberts S. C., Social relationships among adult female baboons (papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 61, 183–195 (2006). [Google Scholar]
- 86.Silk J. B., Alberts S. C., Altmann J., Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 61, 197–204 (2006). [Google Scholar]
- 87.Hewison A. J. M., Gaillard J. M., Angibault J. M., Laere G. V., Vincent J. P., The influence of density on post-weaning growth in roe deer Capreolus capreolus fawns. J. Zool. 257, 303–309 (2002). [Google Scholar]
- 88.Madsen T., Shine R., Silver spoons and snake body sizes: Prey availability early in life influences long-term growth rates of free-ranging pythons. J. Anim. Ecol. 69, 952–958 (2000). [Google Scholar]
- 89.Vincenzi S., Hatch S., Mangel M., Kitaysky A., Food availability affects onset of reproduction in a long-lived seabird. Proc. Biol. Sci. 280, 20130554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Rand A. M., The precious and the precocious: Understanding cumulative disadvantage and cumulative advantage over the life course. Gerontologist 36, 230–238 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Alberts S. C., Altmann J., Immigration and hybridization patterns of yellow and anubis baboons in and around Amboseli, Kenya. Am. J. Primatol. 53, 139–154 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Tung J., Charpentier M. J., Garfield D. A., Altmann J., Alberts S. C., Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Mol. Ecol. 17, 1998–2011 (2008). [DOI] [PubMed] [Google Scholar]
- 93.R Core Team , R: A Language and Environment for Statistical Computing, (R Foundation for Statistical Computing, 2017). [Google Scholar]
- 94.RStudio Team , RStudio: Integrated Development for R (RStudio, Inc., 2016). [Google Scholar]
- 95.Therneau T. M., A Package for Survival Analysis in S. R package version 3.1-8 (2019).
- 96.Therneau T. M., coxme: Mixed Effects Cox Models. R package version 2.2-16 (2019).
- 97.Morrissey M., pedantics: Functions to Facilitate Power and Sensitivity Analyses for Genetic Studies of Natural Populations. R package version 1.7 (2018).
- 98.Burnham K. P., Anderson D. R., Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper have been deposited in the Dryad Digital Repository (https://doi.org/10.5061/dryad.cjsxksn3n).