1. Challenges in the treatment of respiratory spreading diseases
The respiratory spreading disease pandemic has had a profound impact on all aspects of society around the world, including mental health and physical health, especially for the outbreak of coronavirus disease 2019 (COVID-19, named by WHO on Feb 11, 2020) [[1], [2], [3], [4], [5]]. Previous coronavirus (CoV) outbreaks, including severe acute respiratory syndrome (SARS)-CoV [6] and Middle East respiratory syndrome (MERS)-CoV [7], also posed a huge threat to public health. Multiple case series and cross-sectional studies found that patients with respiratory infections generally complicate with intestinal symptoms and/or secondary gut dysbiosis [1,8,9], which are related with a more severe clinical course [10]. For these patients with enterogenous infection [11,12], gut microbiota restoration was reported to be an effective treatment. Unfortunately, most physicians currently have limited knowledge on microbiota transplantation [13], which is the most effective way to reconstruct gut microbiota [14], in treating severe respiratory spreading diseases. Currently, an ongoing outbreak of COVID-19 caused by SARS-CoV-2 has prompted an urgent need to provide a specific program to guide physicians on how to make adequate preparations for microbiota transplantation in patients with severe respiratory infections and the related gut dysbiosis.
According to Declaration of Helsinki (Fortaleza, 2013) and International Ethical Guidelines for Health-related Research Involving Humans (Geneva, 2016), the desperately ill patients with respiratory spreading infection such as COVID-19 during disease outbreaks have a moral right to try unvalidated medical interventions (UMIs) and it is therefore unethical to restrict access to UMIs to the clinical trial context [15]. Fecal microbiota transplantation (FMT) has been recommended to rescue refractory or recurrent Clostridium difficile infection in practice and it also shows promising therapeutic role in some other dysbiosis-related diseases in trials [16,17]. The improved methodology of FMT based on the automatic washing process [18] and the related delivering consideration was named as washed microbiota transplantation (WMT) by the consensus statement from the FMT-standardization Study Group in 2019 [19]. Nanjing consensus [19] on methodology of WMT has pushed FMT standardization forward and guided physicians to implement this therapy well in practice and trails. Patients underwent WMT with the decreased adverse events (AEs) and unchanged clinical efficacy in ulcerative colitis (UC) [20,21] and Crohn's disease (CD) [[21], [22], [23]]. Although there is no direct clinical evidence that FMT has the therapeutic role in coronavirus infection up to now, as the leading WMT team for providing national FMT service (www.fmtbank.org) in China and unique experts fighting against respiratory spreading diseases, we are collaborating closely to consider WMT as UMIs which should subsequently be made the object of clinical research, designed to evaluate its rescue value and safety for dysbiosis-related conditions in patients with severe respiratory infection such as COVID-19. We discussed and integrated the following evidences.
2. Feasibility of WMT in the treatment of respiratory spreading diseases
There is a vital link between the intestinal and respiratory tract, which was exemplified by intestinal complications during respiratory disease and vice versa [[24], [25], [26]]. The gut-lung axis plays an important role in respiratory health and disease [27,28]. For example, the first case series study on COVID-19 reported that the common symptoms of patients at onset of illness were fever, cough, diarrhea, which indicated the systematic inflammation involving respiratory, intestinal tract and other organs [1]. Consistently, a recent cross-sectional, multicenter study evaluated the clinical characteristics of COVID-19 patients with digestive symptoms and found that 50.5% (103/204) of patients reported a digestive symptom, including lack of appetite in 81 cases, diarrhea in 35 cases, vomiting in 4 cases, and abdominal pain in 2 cases [8]. Another case-control study from the united states included all variables related with a positive COVID-19 test result into a multivariable model, and reported that the presence of gastrointestinal symptoms was related with a 70% increased risk of testing positive (adjusted odds ratio 1.7, 95% confidence interval 1.1 to 2.5) [9]. Respiratory viral infections predispose patients to secondary bacterial super-infections, and these are related with a more severe clinical course [10]. The antibiotics can cause secondary gut microbiota dysbiosis, such as antibiotic-associated diarrhea (AAD). Li's group reported that microbiota restoration reduced enterogenous secondary infection in critically ill patients with H7N9 infections [11]. A randomized controlled trial recently reported modulating gut microbiota could reduce enteritis and ventilator-associated pneumonia in critically ill patients with sepsis [12]. More importantly, Zuo et al. [29] reported that persistent alterations in the fecal microbiome were observed in patients with COVID-19 during hospitalization, and the gut microbiota alterations were associated with fecal levels of SARS-CoV-2 and COVID-19 severity. Another study [30] found gut microbiota was characterized by losing salutary bacteria and enriching opportunistic pathogens in patients with active SARS-CoV-2 GI infection. Therefore, altering the gut microbiota might be a strategy to reduce disease severity for patients with COVID-19 [31,32]. The success of FMT for treating gastrointestinal conditions shows the promise of ability to respiratory health [27].
Animal studies also indicate that targeting gut microbiota might be a new therapeutic option for the treatment of respiratory virus infection. The recent animal study demonstrated that antibiotics could decrease gut microbiota and the lung stromal interferon signature and facilitate early influenza virus replication in lung epithelia [33]. In this study, the above antibiotics caused negative effects can be reversed by FMT which suggested that FMT might be able to induce a significant improvement in the respiratory virus infection. Another evidence is that the microbiota could confer protection against influenza virus and respiratory syncytial virus by priming the immune response to viral evasion [34]. The microbiota could prime the immune response to confer protection against viral infection and some bacterial species could increase the antiviral response [35].
Recent findings partially eliminate the worries from researchers and clinicians, and encourage them to set up new trials to use WMT in critically ill patients. The manual FMT has been replaced by WMT in most centers in China [20,21]. Compared to traditional manual FMT which is used in America and Europe, WMT contributed to the decreased FMT-related AEs from 38.7% to 14.4% in patients with UC [20,21], saves the time exposure to oxygen during manipulating fecal matter, stabilizes the delivery dose based on the enriched bacteria instead of the weight of stool [21], and facilitates delivery of microbiota into intestine. A recent pilot cohort study on using WMT as rescue therapy in critically ill patients with AAD demonstrated the important clinical benefits and safety of WMT [36]. In this study, of the 18 critically ill patients with AAD underwent rescue WMT, 88.9% of patients had respiratory tract infection, 44.4% had sepsis, and 27.8% had multiple organ dysfunction syndrome. After rescue WMT, 72.2% of patients achieved improvement of abdominal symptoms within one week. 44.4% of patients achieved rescue success which referred to recovering from abdominal symptoms without recurrence and surviving for a minimum of 12 weeks after being discharged from intensive care unit. No WMT-related death or infective complications occurred.
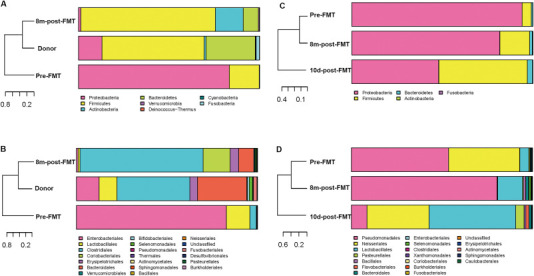
The COVID-19 outbreak and the latest development of FMT remind us to look back a case with respiratory infection, who benefited from the WMT primarily targeting refractory CD in our previous trial 5 years ago (Supplementary file 1). A 75-year-old man with active CD complicated with chronic obstructive pulmonary disease (COPD) was transferred to the Second Affiliated hospital of Nanjing Medical University due to refractory breathing difficulty for three weeks. On admission, his dyspnea was defined as grade 4 according to Modified British Medical Research Council (mMRC). His Harvey-Bradshaw Index (HBI) score was 9, indicating serious active CD. He couldn't even get out of bed for cardiopulmonary complications. He achieved improved life quality and was significantly relieved from the respiratory symptoms within two weeks after microbiota transplantation, including coughing, sputum production, dyspnea, asthma and chest distress. His HBI score decreased to 3 and his mMRC decreased to grade 1. The patient's stool microbial diversity and relative abundance at eight months post-WMT were significantly higher than that before WMT, which was much similar to the healthy donor (Supplementary figure 1). We also observed the obvious changes of respiratory microbial composition at 10 days post-WMT. As we know, this should be the first case report on successful FMT on respiratory disease in practice. Of course, it is not time to draw conclusion based on one case.
3. Protocol of WMT in treating patients with respiratory spreading diseases
Integrated the progress of the gut-lung axis in recent years, this case inspires us to suggest hypothesis that targeting gut microbiota by WMT should be important for patients with respiratory spreading diseases, including the disease related AAD, potentially preventing the development of infection and enhancing recovery after systematic inflammation. Our recommendations for using rescue WMT in severe respiratory spreading diseases, such as COVID-19 in the specific hospitals focusing disease outbreaks were briefly shown (Box 1 ) as below. During the endoscopic procedure for infected patients, the operators should adopt standard three-level protective measures (Fig. 1 ).
Box 1. General steps of WMT in patients with respiratory spreading diseases.
-
1.Regulation
-
(1)Approval by hospital ethical review board when necessary;
-
(2)Informed consent from patients before WMT;
-
(3)Commercial abuse of WMT must be avoided;
-
(4)The source of WMT should be provided by FMT center in hospital with well-build safety traceability on donors screening, laboratory records and fecal samples.
-
(1)
-
2.Potential indications:
-
(1)Antibiotic-associated refractory diarrhea during hospitalization;
-
(2)Intestinal dysbiosis related symptoms with treatment difficulty or failure by conventional approaches;
-
(3)Long-term antibiotic use before the respiratory infection.
-
(1)
-
3.Contraindications
-
(1)Gastrointestinal perforation;
-
(2)Gastrointestinal obstruction;
-
(3)A recent history of intestinal fistulas;
-
(4)Disturbance of consciousness (risk of aspiration pneumonia).
-
(1)
-
5.Patient preparation
-
(1)Informed of the donor source and the methods of microbiota preparation;
-
(2)Stop antibiotics 12–48 h before WMT;
-
(3)Metoclopramide by im. 30 min before WMT;
-
(4)Proton pump inhibitors by iv. one hour before WMT;
-
(5)Fasting 6 h before WMT if not choosing nasojejunal tube;
-
(6)Undergo blood testing for transmissible infections.
-
(1)
-
6.Delivery ways and frequency
-
(1)Nasogastric tube or oral is acceptable if nasojejunal tube cannot be inserted;
-
(2)At least 10° upright position for 30 min after infusion;
-
(3)Once daily, totally 1–3 times.
-
(1)
-
7.Evaluation of efficacy and safety
-
(1)Efficacy: clinical symptoms and acute inflammatory markers;
-
(2)Adverse events: elevated acute inflammatory markers, worse symptoms;
-
(3)Records: transparent to public at the right time for knowledge spreading.
-
(1)
Alt-text: Box 1
Fig. 1.
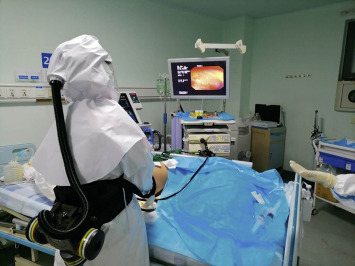
The endoscopist performed mid-gut tube with standard three-level protective equipment in a patient with COVID-19 in China.
Dysfunction and failure of the digestive tract generally existed in critical patients with COVID-19 is, on the one hand, a phenomenon of multiple organ failure, and on the other hand, long-term used many antibiotics leading to serious disturbance of intestinal microecology, thus triggering off impaired gut barrier function, seducing secondary infection, then more antibiotics used, and finally to gastrointestinal functional failure. Intestinal microbiota disorder is found in all critical cases.
In the time of the pandemic, physicians can consider rescue therapy for respiratory spreading diseases based on safety and quality control on WMT. Recently, the worldwide experts have proposed a workflow that FMT centers and stool banks should follow to ensure reliable patient access to FMT while maintaining its safety and quality during the COVID-19 pandemic [37,38]. Chiu et al. [39] proposed that an efficient and stringent protocol should be used to screen FMT donors before a vaccine is available. We have ever registered the pilot trial (NCT04251767) for providing the rescue therapy during the pandemic of SARS-CoV-2. However, because of some uncertain factors, it has not been successfully applied to the clinic. Although we have provided the cutting-edge evidences to support the potential therapeutic of WMT in pneumonia, it might be still difficult for quite a few physicians and researchers to accept this point. During the WMT rescue procedure, the potential challenges were listed in Box 2 . For example, the delivery via enema is not proper because the frequent cough generally causes difficulty to hold the infused fluid within anus. Although there is no specific treatment to control the new respiratory spreading diseases, it might be still difficult for some people to understand the importance of UMIs and the ethical consideration for saving life.
Box 2. Potential challenges of WMT in patients with respiratory spreading diseases.
-
1.Risk of human-to-human transmission
-
(1)Via personal contact with a sick person or their belongings;
-
(2)Via respiratory transmission through droplets and aerosol.
-
(1)
-
2.Different cognition on WMT affected clinical decision-making
-
(1)Limited physician's knowledge on WMT in those without specialty of microbial therapy;
-
(2)Limited understanding on WMT in hospital managers;
-
(3)Failure to obtain patient's informed consent.
-
(1)
-
3.Difficulties in selecting delivering ways
-
(1)Oral or enema may decrease the efficacy;
-
(2)Not each patient has a nasojejunal tube in advance;
-
(3)Endoscopy or colonic TET [40] required further endoscopic procedure.
-
(1)
TET, transendoscopic enteral tubing.
Alt-text: Box 2
4. Conclusion
In general, we report expert recommendations of the novel rescue WMT for patients with severe respiratory spreading diseases, such as COVID-19 in order to set clinical work-flow during disease outbreaks. We believe the expert opinion would encourage more researchers to focus on microbiome-virome interactions and more physicians to attempt the new treatment targeting microbiota in controlling diseases. However, we must point out that the motion of this study may become the evidence of commercial profit driven FMT abuse, which is a problem we must be aware of in the face of disaster.
Author contributions
Ting Zhang, Data collection, Methodology, Software, Writing - original draft. Xiao Ding, Data curation, Visualization, Writing - original draft. Min Dai, Investigation, Data curation, Writing - original draft preparation. Huijie Zhang, Investigation, Supervision. Fang Xiao, Validation, Writing- Reviewing and Editing. Xingxiang He, Validation, Writing-Reviewing and Editing. Faming Zhang, Conceptualization, Writing-Reviewing and Editing, Supervision. Xiaoyin Zhang, Conceptualization, Writing-Reviewing and Editing, Supervision.
Ethical approval
This work was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). This work was reviewed and approved by the Second Affiliated Hospital of Nanjing Medical University Institutional Review Board.
Patient consent
The patient provided written informed consent prior to participate in this study.
Funding
This work was supported by Jiangsu Provincial Medical Innovation Team (Zhang F), and China National Center for Clinical Research of Digestive Diseases (201502026).
Declaration of competing interest
Faming Zhang conceived the concept of GenFMTer and the related devices. All authors declare no competing interests.
Acknowledgments
Most of the expert opinions in this article were from Nanjing consensus on methodology of washed microbiota transplantation, which was released by a panel of experts in December 12, 2019, Nanjing, China. We appreciate the contribution from team members including Pan Li, Zhaoyang Zhao, Gaochen Lu, Xinhui Ji, and Miao Wang during disease outbreaks.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.medmic.2020.100024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren L.L., Wang Y.M., Wu Z.Q. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020 doi: 10.1097/cm9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, february 12-march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. Jama. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Minjin Wang Y.Z., Zong Zhiyong, Liang Zongan, Cao Yu, Tang Hong, Song Bin, Huang Zixing, Kang Yan, Feng Ping, Ying Binwu, Li Weimin. A precision medicine approach to managing 2019 novel coronavirus pneumonia. Precision Clinical Medicine. 2020;3(1):14–21. doi: 10.1093/pcmedi/pbaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X.Y., Guo L.Z. [A novel coronavirus---SARS virus. Zhonghua Yufang Yixue Zazhi. 2003;37(4):281–283. [PubMed] [Google Scholar]
- 7.Cunha C.B., Opal S.M. Middle East respiratory syndrome (MERS): a new zoonotic viral pneumonia. Virulence. 2014;5(6):650–654. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobel Y.R., Phipps M., Zucker J. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanada S., Pirzadeh M., Carver K.Y. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin N., Zheng B., Yao J. Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci Rep. 2015;5:14771. doi: 10.1038/srep14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu K., Yamada T., Ogura H. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):239. doi: 10.1186/s13054-018-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X., Dai M., Buch H. The recognition and attitudes of postgraduate medical students toward fecal microbiota transplantation: a questionnaire study. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819869144. 1756284819869144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F., Zhang T., Zhu H. Evolution of fecal microbiota transplantation in methodology and ethical issues. Curr Opin Pharmacol. 2019;49:11–16. doi: 10.1016/j.coph.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 15.London A.J. Social value, clinical equipoise, and research in a public health emergency. Bioethics. 2019;33(3):326–334. doi: 10.1111/bioe.12467. [DOI] [PubMed] [Google Scholar]
- 16.Ng S.C., Kamm M.A., Yeoh Y.K. Scientific frontiers in faecal microbiota transplantation: joint document of asia-pacific association of gastroenterology (APAGE) and asia-pacific society for digestive endoscopy (APSDE) Gut. 2020;69(1):83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allegretti J.R., Mullish B.H., Kelly C. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394(10196):420–431. doi: 10.1016/s0140-6736(19)31266-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T., Lu G., Zhao Z. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11(4):251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fecal Microbiota Transplantation-standardization Study Group Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J (Engl) 2020;133(19):2330–2332. doi: 10.1097/cm9.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X., Li Q., Li P. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42(7):869–880. doi: 10.1007/s40264-019-00809-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T., Lu G., Zhao Z. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020 doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Cui B., Li Q. The safety of fecal microbiota transplantation for crohn's disease: findings from A long-term study. Adv Ther. 2018;35(11):1935–1944. doi: 10.1007/s12325-018-0800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P., Zhang T., Xiao Y. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn's disease. Appl Microbiol Biotechnol. 2019;103(1):349–360. doi: 10.1007/s00253-018-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsland B.J., Trompette A., Gollwitzer E.S. The gut-lung Axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 25.Yazar A., Atis S., Konca K. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(5):1511–1516. doi: 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 26.Ekbom A., Brandt L., Granath F. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186(3):167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 27.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20(10):1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 28.Schuijt T.J., Lankelma J.M., Scicluna B.P. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo T., Zhang F., Lui G.C.Y. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo T., Liu Q., Zhang F. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu S., Chen Y., Wu Z. Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley K.C., Finsterbusch K., Schnepf D. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28(1):245–256. doi: 10.1016/j.celrep.2019.05.105. e244. [DOI] [PubMed] [Google Scholar]
- 34.Dominguez-Diaz C., Garcia-Orozco A., Riera-Leal A. Microbiota and its role on viral evasion: is it with us or against us? Front Cell Infect Microbiol. 2019;9:256. doi: 10.3389/fcimb.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domínguez-Díaz C., García-Orozco A., Riera-Leal A. Microbiota and its role on viral evasion: is it with us or against us? Front Cell Infect Microbiol. 2019;9:256. doi: 10.3389/fcimb.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai M., Liu Y., Chen W. Rescue fecal microbiota transplantation for antibiotic-associated diarrhea in critically ill patients. Crit Care. 2019;23(1):324. doi: 10.1186/s13054-019-2604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ianiro G., Mullish B.H., Kelly C.R. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020 doi: 10.1136/gutjnl-2020-321829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng S.C., Chan F.K.L., Chan P.K.S. Screening FMT donors during the COVID-19 pandemic: a protocol for stool SARS-CoV-2 viral quantification. Lancet Gastroenterol Hepatol. 2020;5(7):642–643. doi: 10.1016/s2468-1253(20)30124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu C.H., Tsai M.C., Cheng H.T. Fecal microbiota transplantation and donor screening for Clostridioides difficile infection during COVID-19 pandemic. J Formos Med Assoc. 2020 doi: 10.1016/j.jfma.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Long C., Cui B. Colonic transendoscopic tube-delivered enteral therapy (with video): a prospective study. BMC Gastroenterol. 2020;20(1):135. doi: 10.1186/s12876-020-01285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


