Abstract
Autism spectrum disorders (ASDs) are a kind of neurodevelopmental disorder with rapidly increasing morbidity. In recent years, many studies have proposed a possible link between ASD and multiple environmental as well as genetic risk factors; nevertheless, recent studies have still failed to identify the specific pathogenesis. An analysis of the literature showed that oxidative stress and redox imbalance caused by high levels of reactive oxygen species (ROS) are thought to be integral parts of ASD pathophysiology. On the one hand, this review aims to elucidate the communications between oxidative stress, as a risk factor, and ASD. As such, there is also evidence to suggest that early assessment and treatment of antioxidant status are likely to result in improved long-term prognosis by disturbing oxidative stress in the brain to avoid additional irreversible brain damage. Accordingly, we will also discuss the possibility of novel therapies regarding oxidative stress as a target according to recent literature. On the other hand, this review suggests a definite relationship between ASD and an unbalanced gastrointestinal tract (GIT) microbiota (i.e., GIT dysbiosis). A variety of studies have concluded that the intestinal microbiota influences many aspects of human health, including metabolism, the immune and nervous systems, and the mucosal barrier. Additionally, the oxidative stress and GIT dysfunction in autistic children have both been reported to be related to mitochondrial dysfunction. What is the connection between them? Moreover, specific changes in the GIT microbiota are clearly observed in most autistic children, and the related mechanisms and the connection among ASD, the GIT microbiota, and oxidative stress are also discussed, providing a theory and molecular strategies for clinical practice as well as further studies.
1. Introduction
ASD, a loose umbrella term that includes a series of life-long heterogeneous clusters of neurodevelopmental disorders, is characterized by stereotyped behavior and deficits in social communication, interaction, and perception [1]. It is well documented that ASD occurs in all racial, ethnic, and socioeconomic groups. Last updated on July 11, 2016, the US Centers for Disease Control and Prevention (CDC https://www.cdc.gov/) reported an incidence of 1 in 54 children worldwide; in addition, studies in Asia, Europe, and North America have identified individuals with ASD and shown an average prevalence of between 1% and 2% (see Table 1). Additionally, it is conservatively estimated that the prevalence of ASD in China is 1%, among which more than 2 million children aged 0-14 years have the disease [2]. Undoubtedly, ASD, considered a hidden disability, creates an enormous burden on individuals, families, and society [3]. Regrettably, there is no practical and targeted treatment for ASD, which has become a major worldwide health problem [4].
Table 1.
Summary of the Prevalence of ASD in different areas.
| Country | Age range studied | Number of children in population | Criteria used | Methodology used | ASD prevalence (CI) |
|---|---|---|---|---|---|
| USA | 8 | 346,978 | DSM-IV | Case enumeration and record review | 14.6 (8.2-24.6) |
| Faroe Islands | 7 to 16 | 7122 | DSM-IV, ICD-10 | Screening and direct exam | 5.6 |
| Denmark | N/A | 404,816 | DSM-IV | Case enumeration | 6.9 (6.5-7.2) |
| Oman | 0 to 14 | 798,913 | DSM-IV | Case enumeration | 0.1 (0.1-0.2) |
| Taiwan | 0 to 18 | 372,642 | ICD-9 | Case enumeration | 2.9 |
| South Korea | 7 to 12 | 55,266 | DSM-IV | Case enumeration from survey and direct exam | 26.4 (19.1-33.7) |
| Western Australia | N/A | 152,060 | DSM-IV | Case enumeration | 5.1 (4.7-5.5) |
Note: data are from the US Center for Disease Control and Prevention (CDC https://www.cdc.gov/). N/A: not applicable, i.e., the lack of data in a form or table.
Although the causes of ASD remain unclear, many studies have pointed to the possible link between ASD and multiple environmental as well as genetic risk factors [5]. Of note, much evidence indicates that oxidative stress plays a vital role in the pathophysiology of nervous and mental diseases, particularly in ASD [6–9]. Anecdotal reports have suggested that oxidative stress response is crucially important to the neuroinflammatory response, and in a sense, the neuroinflammatory response has always been regarded as one of the pathogenic factors of ASD [10]. A sibling control study by Shannon et al. suggested that autistic children are more susceptible to oxidative stress on account of an imbalance in glutathione levels inside or outside of cells and a decrease in glutathione (GSH) storage capacity [11].
In recent years, as research continues, the research focus of the intestinal microbiota has been rapidly shifting from the abundance and diversity of microbial members to functional aspects. A variety of studies have concluded that the GIT microbiota influences many aspects of human health, including metabolism, the immune and nervous systems, and the mucosal barrier. In addition, the intestinal microbial fermentation of dietary fibres and resistant starch produces short-chain fatty acids (SCFAs), especially butyrate, propionate, and acetate [12]. Notably, propionate can result in GIT metabolic disturbance, reversible behavioral and signalling changes, neuroinflammation, etc. [13]. Therefore, not surprisingly, increasing evidence supports that autistic children are more likely to experience problems related to GIT, including food allergies (FAs), dysbiosis, inflammatory bowel disease (IBD), and indigestion [14, 15]. The GIT dysfunction in autistic children is related to mitochondrial redox imbalance, i.e., mitochondrial dysfunction, and there is an interaction between oxidative stress and mitochondrial dysfunction [16, 17]. Additionally, some connections exist between these factors (see Figure 1). The specific associations are discussed below.
Figure 1.

The connections among oxidative stress, mitochondrial dysfunction, and dysfunction of GIT in autistic children. The dysfunction of GIT in autistic children is related to mitochondrial dysfunction, and there is an interaction between oxidative stress and mitochondrial dysfunction. SCFAs, metabolites of the GIT microbiota, not only participate in the reaction process of oxidative stress but also can result in mitochondrial hyperactivity and further make mitochondria allergic to the oxidative stress.
In this review, many aspects of the role of oxidative stress and GIT microbiota in ASD are described. Furthermore, we will discuss, in the context of the most recent literature, the connections among oxidative stress, ASD, and GIT microbiota. Finally, the possibility of oxidative stress and GIT microbiota as therapeutic targets will be discussed, which will provide theoretical basis and novel strategies for clinical practice and future studies.
2. Oxidative Stress and ASD
2.1. The Definition of Oxidative Stress and the Importance/Role of Reactive Oxygen Species (ROS)
Oxidative stress, considered an out-of-balance state between antioxidants and antioxidants, could lead to the cellular damage caused by reactive ROS or reactive nitrogen species (RNS) [18]. ROS, a kind of signalling molecule, may contribute to cell viability and tissue oxygen metabolism or inhibit the expression of related genes, including antioxidants [19, 20]. When the level of oxidation exceeds the antioxidant defenses, oxidative stress occurs; in addition, different conditions may result in different levels of oxidative stress [21, 22]. On the one hand, when ROS levels are relatively low, cells can appropriately respond to oxidative stress, a condition called “mild oxidative stress” or eustress. On the other hand, the condition that is more reactive to oxidative stress is termed distress, which is reported to be one of the principal causes of neuroinflammation and damage to astrocyte crosstalk resulting in ASD [23, 24].
In light of published references, under normal circumstances, there is a dynamic balance between the production of ROS and the antioxidant capacity of cells [25]. Furthermore, ROS are intermediate products and by-products that are produced in the electron transport in mitochondria [26, 27]. Notably, many enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx), have a high ROS scavenging capacity [8]. However, the specific role of ROS in ASD is still far from understood.
2.2. Blood Oxidative Stress Markers in Autistic Children
An increasing amount of evidence has shown that the pathogenesis of ASD is related to the accumulation of oxidized products and the disorder of antioxidant metabolism [28–30]. The role of oxidative stress in the development of ASD has been studied for decades, and a very large number of markers, as revealed by comparing blood levels of oxidative stress markers in autistic patients to those of healthy individuals, have been the focal point of clinical practice (see Table 2) [31–38]. As shown, the blood levels of oxidative stress markers were all decreased or increased to varying degrees in autistic patients compared with healthy controls, indicating that these markers may be used not only to diagnose ASD but also to provide indicators to monitor and guide individual therapy in the clinic [28]. However, the ability to implement these measures as soon as possible is still far from fully accomplished and requires further insightful evidence, clinical reports, and research studies.
Table 2.
Blood levels of oxidative stress markers in autistic patients.
| Marker/specimen | Units | Values ASD | p value | Reference | |
|---|---|---|---|---|---|
| Autistic children | Controls | ||||
| Lipid hydroperoxide (LOOH) in the temporal cortex | mmol/mg protein | About 21# | About 15# | <0.05 | [31] |
| Plasma malondialdehyde (MDA) | nmol/mL (mean ± SD) | 4.16 ± 1.67 | 1.49 ± 0.58 | <0.001 | [32] |
| Serum malondialdehyde (MDA) | nmol/mL (mean ± SD) | 8.6 ± 0.5 | 1.76 ± 0.33 | ≤0.001 | [33] |
| RBC thiobarbituric acid reactive substances (TBARS) | mmol/g Hb (mean ± SD) | 0.032 ± 0.0077 | 0.015 ± 0.0033 | <0.001 | [32] |
| Plasma protein carbonyl | nmol/mL (mean ± SD) | 4.202 ± 0.3912 | 2.256 ± 0.148 | <0.0001 | [34] |
| Serum 8OHdG | ng/mL (mean ± SD) | 13.134 ± 1.33 | 1.46 ± 0.326 | ≤0.001 | [33] |
| Plasma glutathione peroxidase (GPx) | U/L (mean ± SD) | 40.9 ± 11.3 | 24.2 | <0.0001 | [35] |
| Serum catalase (CAT) | UAE/L (mean ± SD) | 2.836 ± 0.479 | 0.689 ± 0.157 | ≤0.001 | [33] |
| RBC catalase (CAT) | k/g Hb (mean ± SD) | 209.31 ± 61.92 | 515.77 ± 127.9 | <0.001 | [32] |
| RBC superoxide dismutase (SOD) | U/g Hb (mean ± SD) | 2123.59 ± 543.53 | 971.31 ± 239.14 | <0.001 | [32] |
| Plasma reduced glutathione (GSH) | μmol/L (mean ± SD) | 3.1 ± 0.53 | 4.2 ± 0.72 | <0.0001 | [36] |
| Plasma glutathione (GSH) | μmol/L (mean ± SD) | 3.14 ± 0.56 | 4.2 ± 0.72 | <0.0001 | [37] |
| Plasma oxidized glutathione (GSSG) | nmol/L (mean ± SD) | 0.48 ± 0.16 | 0.35 ± 0.05 | <0.001 | [37] |
| MT-1A expression in blood | N/A | Higher (no data available) | N/A | ≤0.001 | [38] |
2.3. Oxidative Stress in the Brain of Autistic Children
It is well documented that oxidative stress is a primary potential cause of neuroinflammatory disorders and damage to the blood-brain barrier (BBB), a highly selective boundary that separates circulating blood from the brain and extracellular fluid from the central nervous system (CNS) [39–41]. In healthy individuals, the BBB is formed by tight junctions between tight junction proteins and the endothelial cells of adjacent brain capillaries; then, the tight junction proteins are fixed in the endothelial cells by scaffolding proteins such as ZO-1 and ZO-2 [42]. When oxidative stress occurs in endothelial cells in autistic children, the BBB may be damaged, resulting in varied diffusion and transport [43]. Under normal conditions, ROS from various sources, including mitochondria, microglia, and astrocytes, accumulate. When excessive ROS are not properly scavenged, tight junctions can be altered, leading to further oxidative stress (see Figure 2) [44]. To combat this condition, some mechanisms are required to detoxify or neutralize the oxygen/nitrogen free radicals in the cell (see Figure 2). Devasagayam et al. found that superoxide (O2−) could be produced as a by-product of normal metabolism; nevertheless, its accumulation may lead to the injury of cell structures and subsequently to oxidative stress [45]. As a result, superoxide enzymes called superoxide dismutase (SODs) immediately convert superoxide to hydrogen peroxide (H2O2). Moreover, H2O2 is likely to be toxic to the cells as it can pass through cell membranes, thus damaging DNA. For this reason, scavenging hydrogen peroxide can be a target to disturb oxidative stress and may be a therapeutic intervention for ASD [46, 47]. According to the study by Popa et al., catalase and GPx are considered the most vital enzymes that have the ability to convert H2O2 to H2O. In addition, the tripeptide glutathione, an important antioxidant, plays a vital role in eliminating ROS [46]. Under a reaction catalysed by glutathione peroxidase (GPx), glutathione provides an electron to H2O2 and is then converted to an oxidized state. By making use of NAD (P) H as the electron donor, glutathione (GSH) could be reproduced again by glutathione reductase. Additionally, glutathione can also obliterate toxic substances from the cells in its role as a cofactor for GSH transferase [46, 48]. In general, the brain is very sensitive to the accumulation of radicals such as ROS on account of the relatively weak protective mechanisms [49, 50].
Figure 2.
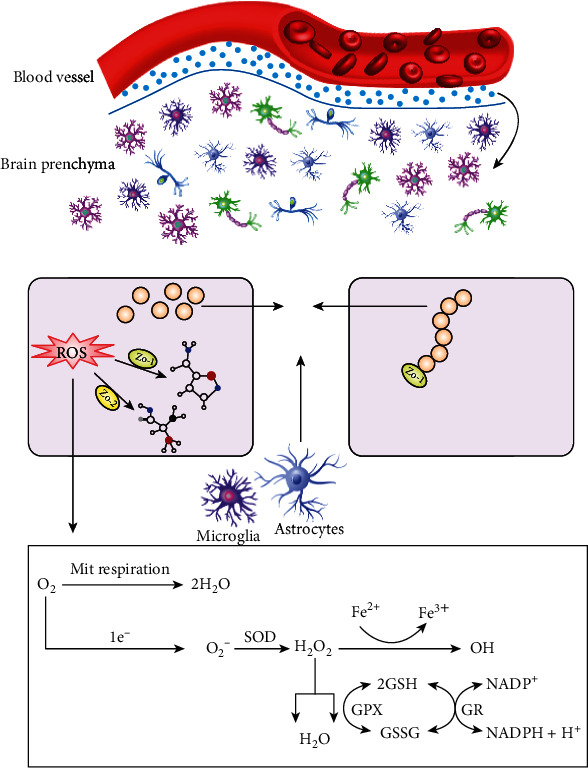
Schematic representation of oxidative stress in the brain.
3. Gastrointestinal Tract (GIT) Microbiota and ASD
3.1. GIT Microbiota and Factors That Affect the GIT Microbiota
In recent years, as research continues, the research focus of the GIT microbiota has been rapidly shifting from the abundance and diversity of the microbial members to functional aspects. A variety of studies have concluded that the intestinal microbiota influences many aspects of human health, including metabolism, the immune and nervous systems, and the mucosal barrier. The intestinal microbial fermentation of dietary fibres and resistant starch produces short-chain fatty acids (SCFAs), especially butyrate, propionate, and acetate [12]. Butyrate is a key energy substrate for colonocytes [51] and can drive the energy metabolism of colonocytes towards β-oxidation by stimulating PPAR-γ signalling and limiting the luminal bioavailability of oxygen, maintaining homeostasis, and preventing gut microbiota dysbiosis [52]. Propionate regulates satiety signalling and gluconeogenesis in the liver, protecting the host from diet-induced obesity and associated glucose intolerance [53]. Other metabolites are produced by intestinal microbiota, and additional clinical evidence is needed to fully investigate their functions in physiology and pathophysiology. Examples include indole propionic acid, which seems to improve mucosal integrity in the gut [54], and ethylphenyl sulfate, which is connected to the exacerbation of autistic behaviour in a mouse model [55]. In addition, the intestinal microbiota has also been shown to have a positive impact on glycaemic control [56], energy homeostasis [57], lipid metabolism [58], and protein metabolism [59]. For the immune and nervous systems, the intestinal microbiota modulates the maturation and function of tissue-resident immune cells in the central nervous system (CNS), including microglia and astrocytes; they also influence the activation of peripheral immune cells, which regulate responses to neuroinflammation, brain injury, autoimmunity, and neurogenesis [60]. In addition, the healthy gut microbiota not only plays a dominant role in reinforcing the immunologic barrier [61] but also maintains the structural integrity of the intestinal mucosal barrier [62]. In summary, the GIT microbiota plays vital roles in human physiology and pathology.
As the GIT microbiota is a popular area in therapeutic research, several factors that contribute to the shaping of the normal GIT microbiota have been demonstrated. The mode of delivery (vaginal or caesarean), the local environment (i.e., mother and hospital), and the type of feeding (breast or formula) are significant factors that impact GIT microbiota composition during the neonatal period, resulting in changes that persist until infancy [63–65]. Age also plays an important role in shaping the gut microbiota. It is widely believed that mammals are first exposed to the microbiota in utero and that the microbiota expands rapidly after birth [66]. Studies have also shown that young children and adolescents have a significantly higher abundance of Clostridium and Bifidobacterium than adults [67, 68]. Diet can also flexibly modulate gut microbiota composition. Just a four-day administration of entirely animal-based or plant-based diet is sufficient to lead to significant shifts in the human gut microbiota [69]. In general, the intake of a diet rich in fruits, vegetables, and fibres is associated with a higher richness and diversity of the gut microbiota [70]. While antibiotics are usually used for saving lives in the fight against infectious disease, many studies have shown their effect on gut bacterial ecology in recent years. The major changes in the GIT microbiota in response to antibiotics include diminished taxonomic diversity and persistence of the changes in a substantial proportion of individuals [71, 72]. In addition to the factors mentioned above, the gut microbiota configuration of individuals is affected by many other factors, including the genotype of the host, ethnicity, and sex [73–75].
3.2. Relationship between the GIT Microbiota and ASD
The study by Wang et al. showed that GIT symptoms, such as constipation (20%) and diarrhoea (19%), are more common in autistic children than in healthy children [76], similar to the results of meta-analyses by Coury et al. and McElhanon et al. [77, 78]. In addition, Buie et al. found that autistic children with GIT symptoms may display more apparent behavioral manifestations including anxiety, automatization, and aggression [79]. An increasing amount of evidence has shown that the GIT microbiota is directly or indirectly related to the symptoms of autistic children, mostly by affecting the mucosal immune system and human metabolism [3, 80]. According to a study on laboratory animals published online by the journal Cell, GIT barrier defects and GIT microbial disorder occur in mouse models of ASD; additionally, the abundance of Porphyromonadaceae, Prevotellaceae, Bacteroidales, and Lachnospiraceae in the offspring of mothers with maternal immune activation (MIA) was greater than that of the controls. Notably, the abundance of Ruminococcaceae, Erysipelotrichaceae, and Alcaligenaceae was greater in the controls [81]. The evidence shown by Finegold et al. demonstrates that the GIT microbiota of autistic children has a greater abundance of Lactobacillus, Clostridium, Bacteroidetes Desulfovibrio, Caloramator, and Sarcina as well as lower levels of Bifidobacterium and Firmicutes than the GIT microbiota of nonautistic children [82]. Furthermore, autistic children with GIT symptoms show lower levels of Prevotella, Coprococcus, and Veillonellaceae than autistic children without the abovementioned symptoms [83]. As stated, numerous studies have demonstrated the changes in the GIT microbiota of autistic children, but the relationship between GIT microbiota and ASD is relatively unexplored.
In recent years, as study continues, the microbiota-gut-brain-axis has been considered a bidirectional physiological communication between the brain, the GIT microbiota, and the GIT; not surprisingly, accumulating evidence demonstrates that this axis is related to the aetiology and pathogenesis of ASD [84–86]. As shown in Figures 3(a) and 3(b), the metabolites produced by the GIT microbiota, especially SCFAs, can pass through enterocytes (ECs) to have an impact on the function of the brain. In addition, some kinds of GIT microbiota can generate neuroactive substances such as 5-HT and GABA, which can also pass through the EC, affect the function of the brain, and further lead to unexpected behaviors [87]. On the one hand, neuroactive substances, some GIT microbiota, and metabolic products could activate neurons in the brain and act on the function of the brain via vagus nerves. On the other hand, among the abovementioned substances, the neuroactive substances that directly affect the hypothalamic-pituitary-adrenal (HPA) axis can ultimately increase circulating levels of cortisol. Additionally, some GIT microbiota and metabolic products could also activate and induce GIT-immunizing cells to liberate cytokines (CKs) to play a corresponding role in the body's circulation [88, 89].
Figure 3.

Relationships between the GIT microbiota and ASD (the microbiota-gut-brain-axis). Note: BBB: blood-brain barrier; ENS: enteric nervous system; GABA: γ-aminobutyric acid; HPA: hypothalamic-pituitary-adrenal; SCFAs: short-chain fatty acids.
An increasing amount of evidence suggests that modulation of the GIT microbiota is a potential therapy in autistic children by elucidating the relationship between the GIT microbiota and ASD, including faecal microbiota transplantation (FMT), probiotics, and dietotherapy. The literature on ASD treatments linked to the GIT microbiota in the past five years is summarized and analysed in Table 3 [90–95]. First, probiotics are considered to prevent intestinal diseases through functions such as regulating and controlling the blood-brain barrier (BBB) and gap-associated proteins [96]. The recently developed approach FMT is an intervention in which the faecal microorganisms from healthy persons are delivered to patients with bad GIT microbiota [97]. However, although many scholars and experts have speculated about the safety of FMT, but no final conclusions have been reached on this matter. Of course, the other abovementioned therapies also have their own limitations (see Table 3). In my opinion, additional well-designed studies with a larger sample size are needed to offer further evidence supporting the feasibility of these treatments.
Table 3.
Literatures on the treatments of ASD linked to GIT microbiota.
| Model | Behavior tests | Treatments | Dosages | Time | Effects | Limitations | Year | References |
|---|---|---|---|---|---|---|---|---|
| 10 autistic children, 9 nonautistic siblings, 10 control | CARS and ADI | Probiotic including Lactobacillus, Bifidobacteria and Streptococci | One pill three times a day | 4 months | Increased abundance of the Bacteroidetes/Firmicutes, normalized the amount of Bifidobacterium and Lactobacillus can decrease the TNFα levels in the autistic children. | No follow-up was performed after treatment | 2015 | [90] |
| A 12-years-old boy with ASD, severe cognitive disability | ADOS-2 | Probiotic (lyophilized bifidobacteria, lactobacilli, and Streptococci) | 9 − 20 × 1010 | 4 weeks | Reduced GIT symptoms and improved in dominating autistic symptoms | More well-designed studies with a larger sample size are needed to offer more proofs supporting the feasibility of it. | 2016 | [91] |
| 3 autistic child, 3 nonautistic children | N/A | Prebiotic: galactooligosaccharide and B-GOS | 2 g | Everyday | Increased abundance of Bifidobacterium spp, acetate, and butyrate | It is in an in vitro gut model system | 2017 | [92] |
| 18 autistic children | PGI-III and CARS | Microbiota transfer therapy (MTT) | Vancomycin (40 mg/kg per day) | 2 weeks | Improved both GIT and ASD-related symptoms; normalized the microbiota of autistic children | No placebo controlled, blinded or randomized | 2017 | [93] |
| ASD animal model | Self-grooming evaluation, three chambers social test | Ketogenic diet | N/A | 2 weeks | Prevention of autism symptoms | The ketosis and glucose levels were not measured | 2016 | [94] |
| C57BL/6 and BTBR mice | Three-chamber sociability test et al. | Ketogenic diet | N/A | 2 weeks | Decreased all host bacterial abundance in cecal and fecal matter | N/A | 2016 | [95] |
4. The Relationship among GIT Microbiota, Oxidative Stress, and ASD
As discussed above, the relationship between GIT symptoms and ASD via mitochondrial dysfunction is quite convincing and worthy of study, as it is well documented that ASD is linked to GIT symptoms and mitochondrial dysfunction; additionally, the latter two are strongly related [98]. According to authoritative reports, the GIT, as an available site, can induce the production of SCFAs such as PPA and BUT [99]. Additionally, GIT dysfunction is observed in autistic children, such as the increased abundance of Clostridia spp., which produce PPA and BUT. There is no doubt that both PPA and BUT can regulate metabolism, including acting as mitochondrial fuels [100], despite entering into the mitochondrial energy pathways at slightly different sites (see Figure 4) [101]. In addition, SCFAs impair the physiological function of cells, resulting in further GIT symptoms related to ASD, including nonspecific inflammation [79, 93, 102].
Figure 4.

Mitochondrial pathways involved in SCFAs as substrates. There are two different starting points in the electron cycle chain, i.e., Complex I and Complex II, which have their exclusive fuel sources. Notedly, Complexes III, IV, and IV are all involved in the abovementioned reactions; furthermore, butyrate and propionic acid enter into mitochondria to participate in related reaction via two crossed and overlapped pathways. Butyrate which resembles the glucose commonly enters into the citric acid (TCA) cycle via Acetyl-CoA, a key reaction substance. The TCA cycle mainly generates a kind of substrate of Complex I called Nicotinamide adenine dinucleotide (NADH). FADH2, as the substrate of Complex II, can be massively produced in two varied metabolic pathways which propionic acid participates in. Equally, propionic acid can produce some substrates of oxidative stress such as SCFAs et al. to be involved in related responses.
To verify whether a connection among the GIT microbiota, oxidative stress, and ASD truly exists, Shannon et al. performed a blinded case-control study and unsurprisingly found differences in the function of mitochondria during several enzymatic reactions in autistic children compared to control children, indicating that differences actually exist in mitochondria rather than in certain enzymes. These researchers also found that the mitochondrial physiology of the GIT in autistic children differs from that in healthy children; notably, the discrepancies are particularly prominent in the caecum [17]. We speculate that autistic children might have different mitochondrial parameters, particularly in the caecum.
Mitochondria are characterized by energy generation; in addition, approximately 5% of autistic children exhibited impaired energy generation to make energy, and more than 30% of autistic children exhibit elevated biological markers. Therefore, we hypothesized that autistic children may have nontraditional mitochondrial diseases [103]. Similarly, a sibling control study found that lymphoblastoid cell lines (LCLs) from autistic children are active in the mitochondrial respiration process, which further leads to a greater sensitivity to oxidative stress [11]. Indeed, PPA and BUT are also abundant in the caecum, which indicates a role for the GIT microbiota in mitochondrial dysfunction in autistic children [104]. In general, the abovementioned correlated responses could result in a clearer understanding of the pathological relationship linked to GIT dysfunction and oxidative stress in ASD, which provides a theoretical basis as well as molecular strategies for new treatment paradigms.
5. Novel Therapeutic Approaches for Oxidative Stress in ASD
Based on the relationship discussed above, we will next discuss, in the context of the most recent literature, the possibility of novel therapies regarding oxidative stress as a target (Table 4) [105–108]. It is well documented that COX-2, as a vital enzyme that is overexpressed in tissues under oxidative stress, affects the metabolism of polyunsaturated acid (PUFA). ω-3 is also considered to be connected with the high expression of COX-2. Leukotrienes are reported to decrease the expression or activity of an iron-containing dioxygenase named 5-LOX, ameliorating neuroinflammation, restoring normal synaptic plasticity, and improving learning ability and memory [11]. Additionally, docosahexaenoic acid (DHA) and ω-3 are both needed for the appropriate growth and development of the brain, for proper synapse formation and to improve cognitive function [104, 105]. Notably, the use of vitamin B12 as a novel therapeutic approach that has recently aroused public attention has been shown to be used for the treatment of ASD; however, its efficacy is unclear [105].
Table 4.
The novel therapies of ASD regarding the oxidative stress as a target.
| Drugs | Pesticide effect | References |
|---|---|---|
| Leukotrienes | Inhibition of the expression or activity of 5-LOX; ameliorate neuroinflammation; restore normal synaptic plasticity; Improve learning and memory function in depressed rats |
[105] |
| Docosahexaenoic acid (DHA) | Be good for the growth and development of the brain and effective at improving cognitive function | [106] |
| ω-3 | Be needed for the appropriate growth and development of the brain, proper synapse formation, and to improve cognitive function | [107] |
| Vit. B12 | Normalization of the Hcy level and amelioration of impaired lipid metabolism | [108] |
6. Concluding Remarks
Many reports have highlighted the relationship among the GIT microbiota, oxidative stress, and ASD. A number of studies emphasize the important role of oxidative stress, which is connected to mitochondrial dysfunction, GIT microbiota disturbance, and thus the production of various metabolites. This review has summarized the latest research on the related mechanisms, but most of the authors have only concentrated on individual pathogenic mechanisms or metabolites; thus, there is an urgent need for studies considering broader biochemical pathways. Additionally, among all the novel ASD therapies mentioned above, the potential usefulness of most have been investigated. Although the results are promising, these therapies are still thought to have limitations and lack safety testing. In general, the author thinks that additional well-designed studies with a larger sample size are needed to offer further evidence supporting the feasibility of these treatments. In addition, the appropriate dose and timing of therapy also require further study.
Acknowledgments
This study was supported by grants from the Hunan Innovative Provincial Construction Project (2019SK2211) and Changsha Science and Technology Plan Project (kq2001044).
Contributor Information
Mingyi Zhao, Email: 36163773@qq.com.
Qingnan He, Email: heqn2629@163.com.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
TH drafted the manuscript, YD generated the figure, CH and YD performed the background research, and MZ and QH edited the manuscript. All authors have read and approved the content of the manuscript. Tingting Hu and Yinmiao Dong contributed equally to this study.
References
- 1.Baio J., Wiggins L., Christensen D. L., et al. Prevalence of autism Spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C. Autism Research Institute. Report on the Development of China’s Autism Education and Rehabilitation Industry (in Chinese). II. Beijing: Huaxia Publishing House; 2017. [Google Scholar]
- 3.De Angelis M., Francavilla R., Piccolo M., De Giacomo A., Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015;6(3):207–213. doi: 10.1080/19490976.2015.1035855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenfield L. F., Hanifin J. M., Beck L. A., et al. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics. 2003;111(3):608–616. doi: 10.1542/peds.111.3.608. [DOI] [PubMed] [Google Scholar]
- 5.Saad K., Abdel-rahman A. A., Elserogy Y. M., et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutritional Neuroscience. 2015;19(8):346–351. doi: 10.1179/1476830515Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 6.Nadeem A., Ahmad S. F., Attia S. M., al-Ayadhi L. Y., al-Harbi N. O., Bakheet S. A. Dysregulated enzymatic antioxidant network in peripheral neutrophils and monocytes in children with autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2019;88:352–359. doi: 10.1016/j.pnpbp.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Messina A., Monda V., Sessa F., et al. Sympathetic, metabolic adaptations, and oxidative stress in autism Spectrum disorders: how far from physiology? Frontiers in Physiology. 2018;9:261–277. doi: 10.3389/fphys.2018.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochimica et Biophysica Acta. 2012;1822(9):1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla C. M., Lodhi I. J. Peroxisomal dysfunction in age-related diseases. Trends in Endocrinology and Metabolism. 2017;28(4):297–308. doi: 10.1016/j.tem.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjørklund G., Meguid N. A., El-Bana M. A., et al. Oxidative stress in autism spectrum disorder. Molecular Neurobiology. 2020;57(5):2314–2332. doi: 10.1007/s12035-019-01742-2. [DOI] [PubMed] [Google Scholar]
- 11.Rose S., Bennuri S. C., Wynne R., Melnyk S., James S. J., Frye R. E. Mitochondrial and redox abnormalities in autism lymphoblastoid cells: a sibling control study. The FASEB Journal. 2017;31(3):904–909. doi: 10.1096/fj.201601004R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J., Lin S., Zheng B., Cheung P. C. K. Short-chain fatty acids in control of energy metabolism. Critical Reviews in Food Science and Nutrition. 2018;58(8):1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 13.MacFabe D. F. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microbial Ecology in Health and Disease. 2015;26:p. 28177. doi: 10.3402/mehd.v26.28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codina-Solà M., Rodríguez-Santiago B., Homs A., et al. Integrated analysis of whole-exome sequencing and transcriptome profiling in males with autism spectrum disorders. Molecular Autism. 2015;6(1):21–27. doi: 10.1186/s13229-015-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazaro C. P., Ponde M. P., Rodrigues L. E. Opioid peptides and gastrointestinal symptoms in autism spectrum disorders. Revista Brasileira de Psiquiatria. 2016;38(3):243–246. doi: 10.1590/1516-4446-2015-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang D. W., Ilhan Z. E., Isern N. G., et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018;49:121–131. doi: 10.1016/j.anaerobe.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Rose S., Bennuri S. C., Murray K. F., Buie T., Winter H., Frye R. E. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: a blinded case-control study. PLoS One. 2017;12(10, article e0186377) doi: 10.1371/journal.pone.0186377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirumbolo S., Bjorklund G. PERM hypothesis: the fundamental machinery able to elucidate the role of xenobiotics and hormesis in cell survival and homeostasis. International Journal of Molecular Sciences. 2017;18(1):p165–p171. doi: 10.3390/ijms18010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Q., Li F., Duan Y., et al. Oxidative stress, nutritional antioxidants and beyond. Science China. Life Sciences. 2020;63(6):866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- 20.Chevallier V., Andersen M. R., Malphettes L. Oxidative stress-alleviating strategies to improve recombinant protein production in CHO cells. Biotechnology and Bioengineering. 2020;117(4):1172–1186. doi: 10.1002/bit.27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costanzo M., Boschi F., Carton F., et al. Low ozone concentrations promote adipogenesis in human adipose-derived adult stem cells. European Journal of Histochemistry. 2018;62(3):p2969–p2975. doi: 10.4081/ejh.2018.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam M. T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurological Research. 2017;39(1):73–82. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 23.El-Ansary A., Bjørklund G., Khemakhem A. M., Al-Ayadhi L., Chirumbolo S., Bacha A. B. Metabolism-associated markers and childhood autism rating scales (CARS) as a measure of autism severity. Journal of Molecular Neuroscience. 2018;65(3):265–276. doi: 10.1007/s12031-018-1091-5. [DOI] [PubMed] [Google Scholar]
- 24.Russo F. B., Freitas B. C., Pignatari G. C., et al. Modeling the interplay between neurons and astrocytes in autism using human induced pluripotent stem cells. Biological Psychiatry. 2018;83(7):569–578. doi: 10.1016/j.biopsych.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Newsholme P., Cruzat V. F., Keane K. N., Carlessi R., de Bittencourt P. I. H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. The Biochemical Journal. 2016;473(24):4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 26.Hameister R., Kaur C., Dheen S. T., Lohmann C. H., Singh G. Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. Journal of Biomedical Materials Research. Part B, Applied Biomaterials. 2020;108(5):2073–2087. doi: 10.1002/jbm.b.34546. [DOI] [PubMed] [Google Scholar]
- 27.Kim C. ROS-driven oxidative modification: its impact on chloroplasts-nucleus communication. Frontiers in Plant Science. 2019;10:1729–1735. doi: 10.3389/fpls.2019.01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frustaci A., Neri M., Cesario A., et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radical Biology & Medicine. 2012;52(10):2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Raymond L. J., Deth R. C., Ralston N. V. Potential role of selenoenzymes and antioxidant metabolism in relation to autism etiology and pathology. Autism Research and Treatment. 2014;2014:15. doi: 10.1155/2014/164938.164938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjørklund G., Meguid N. A., El-Ansary A., et al. Diagnostic and severity-tracking biomarkers for autism spectrum disorder. Journal of Molecular Neuroscience. 2018;66(4):492–511. doi: 10.1007/s12031-018-1192-1. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan A., Gu F., Essa M. M., et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. Journal of Neurochemistry. 2011;117(2):209–220. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoroglu S. S., Armutcu F., Ozen S., et al. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. European Archives of Psychiatry and Clinical Neuroscience. 2004;254(3):143–147. doi: 10.1007/s00406-004-0456-7. [DOI] [PubMed] [Google Scholar]
- 33.Faber S., Zinn G. M., Boggess A., Fahrenholz T., Kern J. C., II, Kingston H. M. S. A cleanroom sleeping environment's impact on markers of oxidative stress, immune dysregulation, and behavior in children with autism spectrum disorders. BMC Complementary and Alternative Medicine. 2015;15(1):71–77. doi: 10.1186/s12906-015-0564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essa M. M., Guillemin G. J., Waly M. I., et al. Increased markers of oxidative stress in autistic children of the Sultanate of Oman. Biological Trace Element Research. 2012;147(1-3):25–27. doi: 10.1007/s12011-011-9280-x. [DOI] [PubMed] [Google Scholar]
- 35.Söğüt S., Zoroğlu S. S., Özyurt H., et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clinica Chimica Acta. 2003;331(1-2):111–117. doi: 10.1016/S0009-8981(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 36.Geier D. A., Kern J. K., Garver C. R., et al. Biomarkers of environmental toxicity and susceptibility in autism. Journal of the Neurological Sciences. 2009;280(1-2):101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Geier D. A., Kern J. K., Garver C. R., Adams J. B., Audhya T., Geier M. R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochemical Research. 2009;34(2):386–393. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- 38.Pintaudi M., Veneselli E., Voci A., et al. Blood oxidative stress and metallothionein expression in Rett syndrome: probing for markers. The World Journal of Biological Psychiatry. 2015;17(3):198–209. doi: 10.3109/15622975.2015.1077990. [DOI] [PubMed] [Google Scholar]
- 39.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harbor Perspectives in Biology. 2015;7(1):p. a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serlin Y., Shelef I., Knyazer B., Friedman A. Anatomy and physiology of the blood-brain barrier. Seminars in Cell & Developmental Biology. 2015;38:2–6. doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patching S. G. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Molecular Neurobiology. 2017;54(2):1046–1077. doi: 10.1007/s12035-015-9672-6. [DOI] [PubMed] [Google Scholar]
- 42.Jyonouchi H., Geng L., Rose S., Bennuri S. C., Frye R. E. Variations in mitochondrial respiration differ in IL-1ss/IL-10 ratio based subgroups in autism spectrum disorders. Frontiers in Psychiatry. 2019;10:71–75. doi: 10.3389/fpsyt.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellwanger J. H., Franke S. I. R., Bordin D. L., Prá D., Henriques J. A. P. Biological functions of selenium and its potential influence on Parkinson’s disease. Anais da Academia Brasileira de Ciências. 2016;88(Supplement 3):1655–1674. doi: 10.1590/0001-3765201620150595. [DOI] [PubMed] [Google Scholar]
- 44.Georgieva E., Ivanova D., Zhelev Z., Bakalova R., Gulubova M., Aoki I. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of “free radical diseases”. Anticancer Research. 2017;37(10):5373–5381. doi: 10.21873/anticanres.11963. [DOI] [PubMed] [Google Scholar]
- 45.Devasagayam T. P. A., Tilak J. C., Boloor K. K., Sane K. S., Ghaskadbi S. S., Lele R. D. Free radicals and antioxidants in human health: current status and future prospects. The Journal of the Association of Physicians of India. 2004;52:794–804. [PubMed] [Google Scholar]
- 46.Popa-Wagner A., Mitran S., Sivanesan S., Chang E., Buga A.-M. ROS and brain diseases: the good, the bad, and the ugly. Oxidative Medicine and Cellular Longevity. 2013;2013:14. doi: 10.1155/2013/963520.963520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Medicine Reviews. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Ohja K., Gozal E., Fahnestock M., et al. Neuroimmunologic and neurotrophic interactions in autism spectrum disorders: relationship to neuroinflammation. Neuromolecular Medicine. 2018;20(2):161–173. doi: 10.1007/s12017-018-8488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pangrazzi L., Balasco L., Bozzi Y. Oxidative stress and immune system dysfunction in autism spectrum disorders. International Journal of Molecular Sciences. 2020;21(9):3293–3307. doi: 10.3390/ijms21093293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ormstad H., Bryn V., Saugstad O. D., Skjeldal O., Maes M. Role of the immune system in autism spectrum disorders (ASD) CNS & Neurological Disorders Drug Targets. 2018;17(7):489–495. doi: 10.2174/1871527317666180706123229. [DOI] [PubMed] [Google Scholar]
- 51.Donohoe D. R., Garge N., Zhang X., et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabolism. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byndloss M. X., Olsan E. E., Rivera-Chávez F., et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vadder F., Kovatcheva-Datchary P., Goncalves D., et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1-2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Long S. L., Gahan C., Joyce S. A. Interactions between gut bacteria and bile in health and disease. Molecular Aspects of Medicine. 2017;56:54–65. doi: 10.1016/j.mam.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Li T., Chiang J. Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacological Reviews. 2014;66(4):948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunkwall L., Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60(6):943–951. doi: 10.1007/s00125-017-4278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenbaum M., Knight R., Leibel R. L. The gut microbiota in human energy homeostasis and obesity. Trends in Endocrinology and Metabolism. 2015;26(9):493–501. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Reviews in Endocrine & Metabolic Disorders. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madsen L., Myrmel L. S., Fjære E., Liaset B., Kristiansen K. Links between dietary protein sources, the gut microbiota, and obesity. Frontiers in Physiology. 2017;8:1047–1061. doi: 10.3389/fphys.2017.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fung T. C., Olson C. A., Hsiao E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nature Neuroscience. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belkaid Y., Hand T. W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellegrini C., Antonioli L., Colucci R., Blandizzi C., Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathologica. 2018;136(3):345–361. doi: 10.1007/s00401-018-1856-5. [DOI] [PubMed] [Google Scholar]
- 63.Shao Y., Forster S. C., Tsaliki E., et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laursen M. F., Bahl M. I., Michaelsen K. F., Licht T. R. First foods and gut microbes. Frontiers in Microbiology. 2017;8:356–371. doi: 10.3389/fmicb.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fattorusso A., di Genova L., Dell’Isola G., Mencaroni E., Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. 2019;11(3):521–529. doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gensollen T., Iyer S. S., Kasper D. L., Blumberg R. S. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agans R., Rigsbee L., Kenche H., Michail S., Khamis H. J., Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiology Ecology. 2011;77(2):404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ringel-Kulka T., Cheng J., Ringel Y., et al. Intestinal microbiota in healthy U.S. young children and adults--a high throughput microarray analysis. PLoS One. 2013;8(5, article e64315) doi: 10.1371/journal.pone.0064315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.David L. A., Maurice C. F., Carmody R. N., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jandhyala S. M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D. N. Role of the normal gut microbiota. World Journal of Gastroenterology. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S., Covington A., Pamer E. G. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews. 2017;279(1):90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panda S., el khader I., Casellas F., et al. Short-term effect of antibiotics on human gut microbiota. PLoS One. 2014;9(4, article e95476) doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurilshikov A., Wijmenga C., Fu J., Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends in Immunology. 2017;38(9):633–647. doi: 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Brooks A. W., Priya S., Blekhman R., Bordenstein S. R. Gut microbiota diversity across ethnicities in the United States. PLoS Biology. 2018;16(12, article e2006842) doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Insenser M., Murri M., del Campo R., Martínez-García M. Á., Fernández-Durán E., Escobar-Morreale H. F. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. The Journal of Clinical Endocrinology and Metabolism. 2018;103(7):2552–2562. doi: 10.1210/jc.2017-02799. [DOI] [PubMed] [Google Scholar]
- 76.Wang L. W., Tancredi D. J., Thomas D. W. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. Journal of Developmental and Behavioral Pediatrics. 2011;32(5):351–360. doi: 10.1097/DBP.0b013e31821bd06a. [DOI] [PubMed] [Google Scholar]
- 77.Connolly N., Anixt J., Manning P., Ping-I Lin D., Marsolo K. A., Bowers K. Maternal metabolic risk factors for autism spectrum disorder-an analysis of electronic medical records and linked birth data. Autism Research. 2016;9(8):829–837. doi: 10.1002/aur.1586. [DOI] [PubMed] [Google Scholar]
- 78.McElhanon B. O., McCracken C., Karpen S., Sharp W. G. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133(5):872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 79.Buie T., Fuchs G. J., III, Furuta G. T., et al. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics. 2010;125(Supplement 1):S19–S29. doi: 10.1542/peds.2009-1878D. [DOI] [PubMed] [Google Scholar]
- 80.Shmarina G. V., Ershova E. S., Simashkova N. V., et al. Oxidized cell-free DNA as a stress-signaling factor activating the chronic inflammatory process in patients with autism spectrum disorders. Journal of Neuroinflammation. 2020;17(1):212–219. doi: 10.1186/s12974-020-01881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsiao E. Y., McBride S. W., Hsien S., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Magistris L., Familiari V., Pascotto A., et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of Pediatric Gastroenterology and Nutrition. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 83.Kang D. W., Park J. G., Ilhan Z. E., et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7, article e68322) doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srikantha P., Mohajeri M. H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. International Journal of Molecular Sciences. 2019;20(9):2115–2129. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santocchi E., Guiducci L., Fulceri F., et al. Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry. 2016;16(1):183–189. doi: 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinan T. G., Cryan J. F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. The Journal of Physiology. 2017;595(2):489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Q., Han Y., Dy A. B. C., Hagerman R. J. The gut microbiota and autism spectrum disorders. Frontiers in Cellular Neuroscience. 2017;11:120–136. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Theije C. G. M., Wopereis H., Ramadan M., et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain, Behavior, and Immunity. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Buffington S. A., di Prisco G. V., Auchtung T. A., Ajami N. J., Petrosino J. F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomova A., Husarova V., Lakatosova S., et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 91.Grossi E., Melli S., Dunca D., Terruzzi V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Medical Case Reports. 2016;4 doi: 10.1177/2050313X16666231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grimaldi R., Cela D., Swann J. R., et al. In vitro fermentation of B-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiology Ecology. 2017;93(2, article fiw233) doi: 10.1093/femsec/fiw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang D. W., Adams J. B., Gregory A. C., et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10–24. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro K., Baronio D., Perry I. S., dos Santos Riesgo R., Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutritional Neuroscience. 2017;20(6):343–350. doi: 10.1080/1028415X.2015.1133029. [DOI] [PubMed] [Google Scholar]
- 95.Newell C., Bomhof M. R., Reimer R. A., Hittel D. S., Rho J. M., Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Molecular Autism. 2016;7(1):37–46. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yatsunenko T., Rey F. E., Manary M. J., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L., Christophersen C. T., Sorich M. J., Gerber J. P., Angley M. T., Conlon M. A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Molecular Autism. 2013;4(1):42–51. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frye R. E., Rose S., Slattery J., MacFabe D. F. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microbial Ecology in Health and Disease. 2015;26:p. 27458. doi: 10.3402/mehd.v26.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nankova B. B., Agarwal R., MacFabe D. F., la Gamma E. F. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells--possible relevance to autism spectrum disorders. PLoS One. 2014;9(8, article e103740) doi: 10.1371/journal.pone.0103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keşli R., Gökçen C., Buluğ U., Terzi Y. Investigation of the relation between anaerobic bacteria genus clostridium and late-onset autism etiology in children. Journal of Immunoassay & Immunochemistry. 2013;35(1):101–109. doi: 10.1080/15321819.2013.792834. [DOI] [PubMed] [Google Scholar]
- 101.Finegold S. M. Therapy and epidemiology of autism--clostridial spores as key elements. Medical Hypotheses. 2008;70(3):508–511. doi: 10.1016/j.mehy.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Buie T., Campbell D. B., Fuchs G. J., III, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Supplement 1):S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 103.Rossignol D. A., Frye R. E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Molecular Psychiatry. 2012;17(3):290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bresnahan M., Hornig M., Schultz A. F., et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72(5):466–474. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo Y., Kuang S., Xue L., Yang J. The mechanism of 5-lipoxygenase in the impairment of learning and memory in rats subjected to chronic unpredictable mild stress. Physiology & Behavior. 2016;167:145–153. doi: 10.1016/j.physbeh.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 106.Das U. N., Ramos E. J., Meguid M. M. Metabolic alterations during inflammation and its modulation by central actions of omega-3 fatty acids. Current Opinion in Clinical Nutrition and Metabolic Care. 2003;6(4):413–419. doi: 10.1097/01.mco.0000078981.18774.5e. [DOI] [PubMed] [Google Scholar]
- 107.Jamali N., Sorenson C. M., Sheibani N. Vitamin D and regulation of vascular cell function. American Journal of Physiology. Heart and Circulatory Physiology. 2018;314(4):H753–H765. doi: 10.1152/ajpheart.00319.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alfawaz H., Al-Onazi M., Bukhari S. I., et al. The independent and combined effects of omega-3 and vitamin B12 in ameliorating propionic acid induced biochemical features in juvenile rats as rodent model of autism. Journal of Molecular Neuroscience. 2018;66(3):403–413. doi: 10.1007/s12031-018-1186-z. [DOI] [PubMed] [Google Scholar]


