Abstract
SARS-CoV-2 has ushered a global pandemic with no effective drug being available at present. Although several FDA-approved drugs are currently under clinical trials for drug repositioning, there is an on-going global effort for new drug identification. In this paper, using multi-omics (interactome, proteome, transcriptome, and bibliome) data and subsequent integrated analysis, we present the biological events associated with SARS-CoV-2 infection and identify several candidate drugs against this viral disease. We found that: (i) Interactome-based infection pathways differ from the other three omics-based profiles. (ii) Viral process, mRNA splicing, cytokine and interferon signaling, and ubiquitin mediated proteolysis are important pathways in SARS-CoV-2 infection. (iii) SARS-CoV-2 infection also shares pathways with Influenza A, Epstein-Barr virus, HTLV-I, Measles, and Hepatitis virus. (iv) Further, bacterial, parasitic, and protozoan infection pathways such as Tuberculosis, Malaria, and Leishmaniasis are also shared by this virus. (v) A total of 50 candidate drugs, including the prophylaxis agents and pathway specific inhibitors are identified against COVID-19. (vi) Betamethasone, Estrogen, Simvastatin, Hydrocortisone, Tositumomab, Cyclosporin A etc. are among the important drugs. (vii) Ozone, Nitric oxide, plasma components, and photosensitizer drugs are also identified as possible therapeutic candidates. (viii) Curcumin, Retinoic acids, Vitamin D, Arsenic, Copper, and Zinc may be the candidate prophylaxis agents. Nearly 70% of our identified agents are previously suggested to have anti-COVID-19 effects or under clinical trials. Among our identified drugs, the ones that are not yet tested, need validation with caution while an appropriate drug combination from these candidate drugs along with a SARS-CoV-2 specific antiviral agent is needed for effective COVID-19 management.
Keywords: COVID-19, SARS-CoV-2, Proteome, Transcriptome, Interactome, Infection pathways, Candidate drugs, Prophylaxis agents
Graphical abstract
Highlights
-
•
SARS-CoV-2 shares Influenza, EBV, HTLV-I, Measles, and Hepatitis virus infection pathways.
-
•
SARS-CoV-2 also shares Tuberculosis, Malaria, and Leishmaniasis infection pathways.
-
•
mRNA splicing, cytokine and IFN signaling, and ubiquitin are important pathways.
-
•
Betamethasone, Estrogen, Statin, Tositumomab, Cyclosporin A are top candidate drugs.
-
•
Ozone, Nitric oxide, plasma components, and photosensitizer drugs are also important against COVID-19.
-
•
Curcumin, Retinoic acids, Vitamin D, Arsenic, Copper, and Zinc are candidate prophylaxis agents.
1. Introduction
The first case of COVID-19 caused by SARS-CoV-2 infection was reported between December 7, 2019 to December 12, 2019 from Huanan, Hubei province, China [[1], [2], [3]] and as of September 28, 2020, the virus has infected 32, 730, 945 people with 9,91,224 deaths globally [4]. Currently, the USA is the most affected country by the COVID-19 pandemic with an estimated 6,960,152 infections and 2,02,478 deaths, followed by India (5,992,532 infected, 94,503 deaths), and Brazil (4,689,613 infected, 1,40,537 deaths) [4]. Although, the “person-to-person transmission” of SARS-CoV-2 was reported on January 24, 2020 [5] and several quarantine and management strategies were adopted in various countries, the infection could still not be controlled and neither the morbidity.
One of the major causes for this uncontrolled number of infection and deaths is the unavailability of SARS-CoV-2 specific vaccines and antiviral drugs. In order to overcome the crisis and to identify effective anti-SARS-CoV-2 drugs, repurposing of the existing FDA approved antiviral drugs are given high priority [6]. Currently, several classes of drugs are under clinical trials to assess their efficacy in COVID-19 treatment. Some of the antiviral regimes that are currently under clinical trials for COVID-19 management can be found at ClinicalTrials.gov with National Clinical Trial (NCT) identifiers such as Remdesivir (NCT04252664, NCT04257656), Favipiravir (NCT04359615), and Lopinavir/Ritonavir (NCT04276688). Similarly, various other therapeutics such as arbidol hydrochloride (Umifenovir) (NCT04260594, NCT04255017), hydroxychloroquine (NCT04342221), recombinant human angiotensin-converting enzyme 2 (APN01) (NCT04287686), convalescent plasma (CCP) transfusion (NCT04412486), anti-inflammatory/antioxidant (NCT04323228), intravenous immunoglobulin (NCT04261426), recombinant interferon (NCT04293887), natural killer cells (NCT04280224), rheumatoid arthritis drug (Kevzara/Sarilumab) (NCT04315298), anti-inflammatory agent (Thalidomide) (NCT04273529, NCT04273581), steroid (Methylprednisolone) (NCT04273321, NCT04263402), immunosuppressor (Tocilizumab) (NCT04317092), antibiotic (Azithromycin) (NCT04359316), and anti-parasitic drug (Ivermectin) (NCT04425707) are under various stages of clinical trials. Likewise, anticancer drugs such as Bevacizumab (NCT04275414), Imatinib (NCT04394416), and Ruxolitinib (NCT04348071) are also going through clinical trials to evaluate their effectiveness in COVID-19 treatment. On the other hand, drug repurposing and/or novel drug development efforts are also underway by identifying and targeting the SARS-CoV-2 infection pathways and biology. Gordon et al., 2020 [7] analysed the SARS-CoV-2-human protein-protein interactions (PPI) map, and proposed a total of 69 drugs, among which 29 are FDA-approved, 12 are under clinical trials, and 28 are at a preclinical stage. Bojkova et al., 2020 [8] analysed the proteome of the SARS-CoV-2 infected human Caco-2 cells to identify SARS-CoV-2 infection pathways in the host and suggested drugs targeting these host pathways. Similar to the interactome and proteome approaches, Blanco-Melo et al., 2020 [9] studied the transcriptome profile of SARS-CoV-2 infected human bronchial epithelial cell and proposed drugs targeting the infection pathways in the host. Like Blanco-Melo et al., 2020 [9]; Xiong et al., 2020 [10] used the transcriptome of COVID-19 patients’ peripheral blood mononuclear cells to identify drugs against SARS-CoV-2 infection.
Although, individual host omics such as interactome [7], proteome [8], and transcriptome [9,10] based pathways of SARS-CoV-2 infected host cell or subject have been described and potential drugs targeting such pathways were reported, so far, no integrative omics based approaches have been employed to study the SARS-CoV-2 infected host biology and pathways in order to predict the potential drugs.
In this paper, we have used an integrative omics approach considering the SARS-CoV-2 infected host interactome, proteome, transcriptome, and bibliome datasets and analysed the COVID-19 associated host genetic information to identify common host pathways that are deregulated during SARS-CoV-2 infection and potential drugs targeting those pathways. We have also reported the SARS-CoV-2 infected host biology/pathways and potential drugs from each omics-based approach separately.
2. Methods
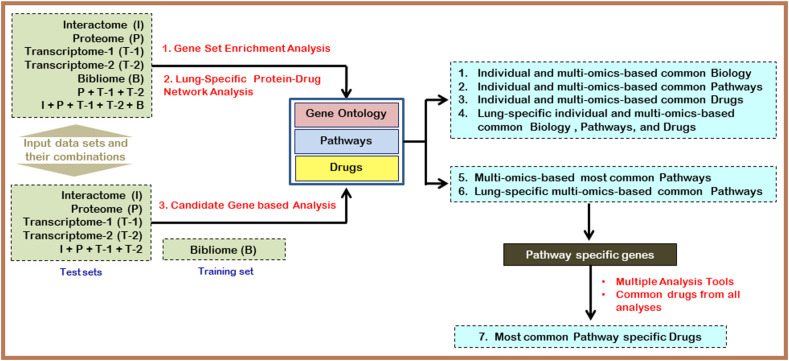
In this work, we analysed five different peer-reviewed published omics data sets to identify the infection biology and candidate drugs against SARS-CoV-2. The overall approach applied in our work is presented in Fig. 1 .
Fig. 1.
The flow diagram of the overall strategy applied in this analysis. For details, see the methods section.
2.1. Datasets
We have used five omics data sets in this analysis. The first data set, designated as the Interactome, outlines SARS-CoV-2 interaction with 332 human (HEK293T/17 cell line) proteins as described by Gordon et al., 2020 [7]. The second data set used in this study is the transcriptome (designated as Transcriptome-1) of lung epithelial cells upon viral infection. We have considered 88 up-regulated genes from this transcriptome profile [9]. The third data set comprise of 1067 up-regulated genes from the peripheral blood of COVID-19 patients (designated as Transcriptome-2) as described by Xiong et al., 2020 [10]. The fourth data set used was the proteome, where 136 up-regulated human proteins of Caco-2 cells upon transfection with SARS-CoV-2 [8] were selected. The final data set considered was the 745 genes associated with COVID-19 as identified from the COVID-19 DisGeNET data collection (https://www.disgenet.org/covid/genes/summary/) [11] and designated as bibliome here. We have considered SARS-CoV-2 infection related omics data published in peer-reviewed publications till May 15, 2020 and only these four data sets [interactome (1 data set), transcriptome (2 data sets), and proteome (1 data set)] were available. Hence, we considered all these four data sets for our analysis along with the bibliome data retrieved from the DisGeNET database.
2.2. Data analysis
2.2.1. Gene set enrichment-based functional annotation
We have used the ToppFun module (https://toppgene.cchmc.org/enrichment.jsp) of the ToppGene suite [12] for functional enrichment of gene sets from each data set, and based on the gene set enrichment analysis, the top ten Biological Processes (BP), Molecular Functions (MF), Cellular Localizations (CC), Pathways, Diseases, and Drugs associated with each gene set are identified. The ToppGene module (https://toppgene.cchmc.org/prioritization.jsp) of ToppGene suite [12] was used for candidate gene prioritization and subsequent analysis. In this analysis, the 745 COVID-19 related genes identified from bibliome data was used as a training set and the other omics data was used as test set to identify the candidate genes and functional annotation. The top ten BP, MF, CC, Pathways, Disease, and Drugs were considered for the results. In all the analysis with ToppGene, default parameters were used and a p-value <0.05 was considered for final results.
2.2.2. Gene set enrichment-based drug identification
For identification of potential drugs from various gene sets, we used two additional tools Enrichr (https://amp.pharm.mssm.edu/Enrichr/) [13] and WEB-based GEne SeT AnaLysis Toolkit (WebGestalt, www.webgestalt.org) [13] apart from the ToppFun module of ToppGene suite [12]. Enrichr uses DSigDB (a drug signatures database) [14] and DrugMatrix (a Comprehensive Toxicogenomic Database) for gene set analysis based candidate drug enrichment. All the default parameters were used in Enrichr and ToppFun. In WebGestalt [15], the over-representation analysis was performed using human genome as reference set and DrugBank [16] as the functional database. Default parameters for all these tools were used and a p-value <0.05 was considered significant and used to generate the results.
2.2.3. Network-based functional annotation and pathway-based candidate drug identification
To understand the network-based biological processes, pathways, and protein-drug interactions for each data set and also the combined data set, NetworkAnalyst 3.0 (www.networkanalyst.ca) [17] was used. Since SARS-COV-2 predominantly infects the lung cells, in our network analysis the human species was used, and to generate the lung tissue-specific Protein- Protein Interaction (PPI) network, from the tissue-specific PPI module of NetworkAnalyst, the lung tissue type was selected. For generic PPI, the IMEx Interactome database (InnateDB) [18] was used. The top ten genes, based on their degree and Betweenness, were considered. For network-based pathways, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [19] and for Biological Processes, the PANTHER:BP [20] modules that were integrated with NetworkAnalyst was used. The common pathways and BPs under top ten hits were selected for further analysis. The genes associated with the selected common BPs and Pathways were collected and analysed using ToppFun [12] to identify drugs for these gene sets. Additionally, the Protein-Drug Interactions module of NetworkAnalyst 3.0 [17] was also used to identify drugs for each omics sample-based network using the DrugBank database [16] and filtered with minimum-network.
2.2.4. Consolidating pathways and drugs from all the analysis
In this approach, we combined the top ten pathways from each of our analysis (individual and combined omics analysis). Based on their cumulative number of appearances, the common pathways were ranked. Pathways enriched with at least 3 omics-based analyses are presented in the results. We followed a similar approach for selecting BPs also. To identify the most frequent drugs enriched across all our analysis, we also adopted a similar strategy. Additionally, in this case, we classified all the drugs following the drug categories as suggested by the Drug Information Portal of the U.S. National Library of Medicine (https://druginfo.nlm.nih.gov/drugportal/) and listed the most common drug categories across all the analysis. Further, we also presented the most frequent drugs under each category for better identification of the important drugs. This drug based enrichment was performed for both omics-based and pathway-based drugs that were identified in our analysis.
3. Results
In our analysis, several common and unique biological processes and drugs are enriched depending on the omic data sets or the analysis tools used. Here, we report the important pathways, biological process, and drugs. The detailed outcomes of each analysis can be found in the supplementary materials.
3.1. Candidate gene-based SARS-CoV-2 infection biology and candidate drugs
First, we used the ToppGene suite [12] for candidate gene prioritization. As the bibliome based gene set is a combination of all kinds of SARS-CoV-2 infection associated human genetic data, we used the bibliome based gene set collected through DisGeNET [11] as the training set for this ToppGene based analysis.
The identified candidate top ten genes are as follows: from the interactome: RHOA, PRKACA, ITGB1, OS9, SCARB1, SLC9A3R1, RIPK1, AKAP8L, RAB7A, and GNB1; from upregulated proteome: ACTB, RHOA, IGF1R, YWHAE, ANXA2, HSPD1, MYH9, ITGA6, MIF, and XIAP; from in vitro transcriptome: STAT1, PLEKHA4, FGG, EIF2AK2, BST2, CFB, SERPINB3, IRF7, HSD11B1, and KCNJ2; from patient's blood transcriptome: INSR, CD36, LRP1, FN1, LDLR, NRG1, IGHG4, CDK5, CTSH, and CHRNA7; and the integrative omics (all the data sets) based top ten genes are INSR, CD36, LRP1, ACTB, RHOA, IGF1R, OS9, PRKACA, ITGB1, and LDLR. The analysis revealed that the Cytokine signaling, Signaling by interleukins, Toll-like receptor signaling pathway, and Rheumatoid arthritis are the common deregulated pathways in SARS-CoV-2 infection. Further, it is also observed that the Influenza A, Tuberculosis, and Leishmaniasis disease pathways are shared by the SARS-CoV-2 infection.
Adrenal cortex hormone (Betamethasone), antiasthmatic agent (Nitric oxide), anti-infective agent (Acetylcysteine), Antineoplastic antibiotics (Streptozocin, Doxorubicin), and anticholesteremic agent (Simvastatin) are the common drugs that were found to be enriched across all the omics data sets (individual or combinations) (Table S1).
However, while we combined the top 10 genes from all the candidate gene-based analysis and performed the gene set enrichment-based drug identification, we found several new therapeutic agents such as antineoplastic agents (Brigatinib, Imatinib, Etoposide), hypoglycemic agents (Rosiglitazone, Insulin), adrenal cortex hormone (Betamethasone), Estrogen (Genistein), tissue plasminogen activator (Lanoteplase), cardiovascular agent (Tenecteplase), anticonvulsant (Valproic Acid), anti-infective agent (Cyclosporin A), antioxidant (Tocopherol), analgesics (Resveratrol), and metal/growth substance (Arsenic) (Table S1).
3.2. Interactome-based SARS-CoV-2 infected host biology and candidate drugs
Gordon et al., 2020 [7] in their PPI study showed that 40% of the interactome are related to endomembrane and ER/Golgi/mitochondrial trafficking. SARS-CoV-2 predominantly interacts with innate immune pathways, host translation machinery, and Cullin ubiquitin ligase complex. In our interactome set enrichment, we also observed that, the interactome is mostly associated with organelle membranes and involved in intracellular protein targeting or transport. Additionally, we observed that the SARS-CoV-2 interacting human proteins are mostly involved in cell cycle, nuclear pore complex (NPC) disassembly, mitochondrial protein import, and regulation of glucokinase (Table S2 (I)). In our lung-specific interactome analysis, this interactome was also found to be involved in viral carcinogenesis, mRNA splicing, negative regulation of apoptosis, ubiquitin mediated proteolysis, protein processing in endoplasmic reticulum, and spliceosome pathways (Table S3 (I)).
In their analysis, Gordon et al., 2020 [7] suggested that protein biogenesis inhibitors, Sigma1 and Sigma2 receptor inhibitors, eIF4A inhibitors, Sec61 inhibitors, anti-cancer compounds, and antipsychotic antihistamines could be potential drugs against SARS-CoV-2. In our analysis, we also observed enrichment of anticancer or antipsychotic drugs. Our identified potential drugs are Antineoplastic antibiotics (Idarubicin, Romidepsin, Daunorubicin, Epirubicin), antineoplastic agent (Irinotecan), anti-depressive and anti-psychotic agents (Fluoxetine, Fluphenazine), Estrogens (Coumestrol, conjugated estrogen), anti-bacterial agents (Tunicamycin, Latamoxef), enzyme inhibitor (Thapsigargin), central nervous system agent (Chlorzoxazone), dermatologic agent (Verteporfin), adrenergic agent (Dobutamine), and metal/growth substance (Polaprezinc). Apart from these, Flavin adenine dinucleotide, Guanosine-5′-Diphosphate, Myristic acid, and Kappadione are also enriched in our analysis (Table S2 (I), Table S3 (I)).
3.3. Proteome-based SARS-CoV-2 infected host biology and candidate drugs
Bojkova et al., 2020 [8] based on their in vitro SARS-CoV-2 infected host cell proteome profile showed that the spliceosomal and carbon metabolism related proteins are highly up-regulated after 12 h of infection with SARS-CoV-2. Further, translation inhibitors such as Cycloheximide and Emetine and splicing factor SF3B117 targeting spliceosome inhibitor Pladienolide-B inhibited SARS-CoV-2 replication. The authors also showed that NMS873, which inhibits Influenza A virus, is also effective against SARS-CoV-2. In our analysis, we also observed that the up-regulated host proteome is mainly involved in RNA splicing, spliceosome, negative regulation of apoptotic process, focal adhesion, viral carcinogenesis, and viral infection pathways such as Epstein-Barr virus, Hepatitis B, and HTLV-I infections (Table S2 (P)) and (Table S3 (P)).
The identified drugs using the up-regulated proteome are antineoplastic agents (Vorinostat, Phenethyl Isothiocyanate, Bromovanin), antineoplastic antibiotic (Doxorubicin), anti-bacterial agents (Clindamycin, Amikacin, Roxithromycin), anti-infective agents (Puromycin, Cytarabine), anticoagulants (Bromfenacoum, Tenecteplase), antihemophilic factor (Lonoctocog alfa), tissue plasminogen activator (Lanoteplase), expectorants (Ambroxol), angiotensin-converting enzyme inhibitor (Captopril), adrenergic agent (Racepinephrine), cathartics (Bisacodyl), and analgesic (Sulindac). Apart from these drugs, Antimalarial agent (Artenimol), muscular mystrophy drug (Laevadosin), metal/growth substance (Copper), and anticoagulants (Citric acid) are also found to be good candidates (Table S2 (P), Table S3 (P)).
3.4. Transcriptome-based SARS-CoV-2 infected host biology and candidate drugs
Blanco-Melo et al., 2020 [9] in their in vitro transcriptome (Transcriptome-1) study using normal human bronchial epithelial (NHBE) cells have shown that SARS-CoV-2 induces a low IFN-I and –III and high chemokine signalling. This unique and inflammatory response was not observed in SARS-CoV-1, MERS-CoV, IAV, and RSV [9]. In our analysis, we also found that this up-regulated transcriptome is associated with interferon and cytokine signaling pathways. Further, the Transcriptome-1 is also enriched for Influenza A, Herpes, and Hepatitis C infections (Table S2 (T-1)). The network-based analysis enriched negative regulation of apoptotic process, mRNA splicing, viral carcinogenesis, Hepatitis B, Epstein-Barr virus infection, NOD-like receptor signaling pathway, ubiquitin mediated proteolysis, IL-17 signaling pathway, Measles, Hepatitis C, and Influenza A (Table S3 (T-1)). The results are mostly common to the cellular proteome-based analysis, except the ubiquitin mediated proteolysis, which is not found in case of the proteome data set (Table S3 (P)).
For the up-regulated cellular transcriptome (Transcriptome-1), we identified several potential drugs. These drugs belongs to several drug categories such as-adrenal cortex hormones (Methylprednisolone Prenylamine, Prednisone, Betamethasone), anti-inflammatory agents (Fludrocortisone Acetate, Hydrocortisone), adrenergic agent (Alfuzosin), cardiovascular agent (Suloctidil), anti-arrhythmia agents (Flecainide, Dofetilide, Ibutilide), antihistamine agent (Terfenadine), analgesic (Niflumic acid), antineoplastic agent (Amsacrine), anti-bacterial agent (Erythromycin), and anti-infective agent (Halofantrine). Additionally, calcium channel blocker (Prenylamine lactate) and vitamin D/bone density conservation agents (Alendronic acid) were also identified (Table S2 (T-1), Table S3 (T-1)).
Xiong et al., 2020 [10] reported that, upon infection with SARS-COV-2, the patient's blood transcriptome (Transcriptome-2) profile shows increased complement activation, humoral immune response, immunoglobulin mediated response, acute inflammatory response, and lymphocyte apoptosis. We also observed a similar profile for Transcriptome-2, based on our gene set enrichment analysis. However, in addition to these biological processes and pathways, we found that the Malaria pathway is also shared by SARS-CoV-2 (Table S2 (T-2)). In the network-based lung-specific PPI analysis, we found a profile that is similar to the bronchial epithelial cell specific transcriptome-based (Transcriptome-1) findings by Blanco-Melo et al., 2020 [9]. We found viral carcinogenesis, negative regulation of apoptotic process, endocytosis, mRNA splicing, Epstein-Barr virus infection, Hepatitis B, HTLV-I infection, Measles, and ubiquitin mediated proteolysis pathways in our analysis (Table S3 (T-2)).
The candidate drugs for this up-regulated patient's blood transcriptome are identified as: antineoplastic agents (Palbociclib, Dasatinib, Etoposide, Gemtuzumab ozogamicin, Trastuzumab, Cetuximab), antineoplastic antibiotic (Idarubicin), adrenal cortex hormones (Betamethasone, Hydrocortisone), antimetabolites (Azathioprine, Capecitabine), anticonvulsants (Acetazolamide, Zonisamide), anticoagulant/cardiovascular agents (Tenecteplase, Citric Acid), immunologic factors (immune globulin, anti-thymocyte globulin), tissue plasminogen activator (Lanoteplase), anti-infective agent (Ciclopirox), Phytoestrogen, abortifacient agent (Methotrexate), alkylating agent (Chlorambucil), antihypertensive agent (Hydrochlorothiazide), antirheumatic agent (Leflunomide), anti-asthmatic agent (Theophylline), and anesthetic (Procaine). We also found that anthelmintic drug Lucanthone is enriched in our analysis (Table S2 (T-2), Table S3 (T-2)).
3.5. Bibliome -based SARS-CoV-2 infected host biology and candidate drugs
The bibliome-based gene set analysis showed mostly similar biological events that we had observed in the transcriptome based analysis. The identified key BPs affected upon SARS-CoV-2 infection are inflammatory and cytokine responses. The identified pathways are Influenza A, Tuberculosis, Leishmaniasis, cytokine and interleukins signaling, and Toll-like receptor signaling pathways (Table S2 (B)). The lung-specific network-based analysis identified a similar profile as observed in transcriptome-based analysis. Epstein-Barr virus infection, viral carcinogenesis, neurotrophin signaling, HTLV-I infection, Hepatitis B, T-cell receptor signaling, and Measles pathways were enriched in our analysis. We also observed negative regulation of apoptotic process, rhythmic process, and RNA splicing like BPs are among the top 10 BPs (Table S3 (B)).
Antineoplastic antibiotics (Streptozocin, Doxorubicin. Epirubicin), antineoplastic agents (Tretinoin, Irinotecan, Sorafenib), adrenal cortex hormones (Betamethasone, Dexamethasone, Hydrocortisone), analgesics (Loxoprofen. Acetaminophen, Resveratrol, Acetylsalicylic acid), anti-infective agents (Acetylcysteine, Drotrecogin alfa), anti-asthmatic agents (Nitric oxide, Pseudoephedrine), metal/growth substances (Polaprezinc, Arsenic trioxide), anticholesteremic agent (Simvastatin), anti-bacterial agent (Gentamicin), enzyme inhibitor (Mycophenolate mofetil), adrenergic agent (Carvedilol), antihemophilic factor, and coagulation factor IX are identified as potential candidate drugs from this bibliome based gene analysis (Table S2 (B), Table S3 (B)).
3.6. Integrative omics-based SARS-CoV-2 infected host biology and candidate drugs
Our combined omics associated gene set enrichment analysis (proteome and transcriptomes) revealed a similar biology and pathways of SARS-CoV-2 as we found in each individual transcriptome analysis. The key BPs and pathways involved are complement activation, humoral immune response, and cytokine and interferon signaling (Table S2 (P + T)) as observed by Xiong et al., 2020 [10] and Blanco-Melo et al., 2020 [9] as well as our bibliome-based analysis (Table S2 (B)). When we used all the five omics data sets, additional processes such as immune effector process, leukocyte mediated immunity, and neutrophil degranulation are enriched (Table S2 (P + T + I + B)). Furthermore, SARS-CoV-2 infection was also found to share Influenza A, Tuberculosis, Rheumatoid arthritis, Malaria, and Leishmaniasis pathways (Table S2 (P + T + I + B)). The network-based analysis also shows similar findings. The important BPs and pathways involved are: cell cycle, negative regulation of apoptotic process, mRNA splicing, endocytosis, ubiquitin mediated proteolysis, and neurotrophin signaling pathway. The viral pathways that are shared by SARS-CoV-2 are identified as viral carcinogenesis, Hepatitis B, HTLV-I infection, Epstein-Barr virus infection, and Measles (Table S3 (P + T), (P + T + I + B)).
For the integrated omics (proteome + transcriptomes) data sets, the important drugs identified are: antineoplastic agents (Palbociclib, Etoposide, Gemtuzumab ozogamicin, Rituximab), cardiovascular agents (Suloctidil, Tenecteplase), analgesics (Sulindac, Etanercept), adrenal cortex hormone (Betamethasone), antimetabolite (Azathioprine), hypoglycemic agent (Acetohexamide), antirheumatic agent (Leflunomide), anti-infective agent (Drotrecogin alfa), anticholesteremic agent (Ezetimibe), adrenergic agent (Icatibant), anthelmintic agent (Lucanthone), anticoagulants (Abciximab), tissue plasminogen activator (Lanoteplase) and antihemophilic factor. Further, Ozone, Phytoestrogens, and Copper are also identified from this analysis (Table S1 (P + T), Table S3 (P + T)).
For the combination of all the five omics data sets, we identified antineoplastic agents (Trastuzumab, Gemtuzumab ozogamicin, Rituximab), antineoplastic antibiotics (Doxorubicin, Tositumomab), Analgesics (Curcumin, Resveratrol, Loxoprofen, Sulindac, Acetylsalicylic acid), anti-infective agents (Acetylcysteine, Oxiconazole, Cyclosporin A), anti-infective agent (Drotrecogin alfa), alkylating agent (Ifosfamide), anti-asthmatic agent (Nitric oxide), Estrogen (Genistein), anticholesteremic agent (Simvastatin), cardiovascular agent (Pseudoephedrine), Anticoagulants (Citric acid, Abciximab), antihemophilic factor, and coagulation factor IX. Additionally, Arsenenous acid, Copper, Polaprezinc, and l-Glutamic Acid are also found to be enriched in this analysis (Table S1 (P + T + I + B), Table S3 (P + T + I + B)).
3.7. SARS-CoV-2 infection associated key host pathways and pathway-based candidate drugs
In our analysis, we observed SARS-CoV-2 infection shares other viral pathways such as Influenza A, Epstein-Barr virus infection, HTLV-I infection, Measles, and Hepatitis B infection. Further, in our analysis, viral carcinogenesis was also found to be an important pathway for this virus. Transcriptome and Bibilome-based gene set profiles also show that SARS-CoV-2 infection shares Tuberculosis, Leishmaniasis, Malaria like bacterial, parasitic, and protozoan disease pathways. Other important common SARS-CoV-2 infection pathways enriched from various omics data are mRNA splicing, ubiquitin mediated proteolysis, cytokine signaling in immune system, and protein processing in endoplasmic reticulum.
To identify pathway specific drugs, we used the genes involved in the five most important common pathways (viral processes including all the individual virus pathways, mRNA splicing, ubiquitin mediated proteolysis, cytokine signaling in immune system, and protein processing in endoplasmic reticulum). For each pathway, genes from all the five omics data sets (identified from our network based analysis and ToppFun) were combined and ToppFun [12], WebGestalt [15], and Enrichr [13] were used to identify gene set specific drugs.
Arsenenous acid/Arsenic trioxide, Resveratrol, and Curcumin are found as candidate agents to block most of the viral infection pathways. Copper is found to inhibit three important pathways: mRNA splicing, ubiquitin mediated proteolysis, and protein processing in endoplasmic reticulum pathways. Clindamycin (antibiotic) and Danazol (antigonadotropic and anti-estrogen) are found to be effective to hinder mRNA splicing and ubiquitin mediated proteolysis pathways. Arsenic could be effective against viral, cytokine signaling, and ubiquitin mediated proteolysis pathways. For ubiquitin mediated proteolysis and protein processing in endoplasmic reticulum pathways, Sertraline (Serotonin (5-HT) inhibitor) may be effective. Vitamin D analogs (Ergocalciferol or Alfacalcidol) and anti-infective immunosuppressant Cyclosporin A could be useful against ubiquitin mediated proteolysis and cytokine signaling pathways. Zinc/Polaprezinc is found to be enriched for both protein processing in endoplasmic reticulum and cytokine signaling pathways (Table S4).
Additionally, several candidate drugs are also identified for each pathway. Glibenclamide (hypoglycemic agent/ATP-sensitive potassium channels inhibitor), Artenimol (antimalarial), Chlortetracycline, Amikacin (anti-bacterial), Mitomycin (antineoplastic antibiotic), Cisplatin and Noscapine (antineoplastic), and Arabinosyluracil are identified for mRNA splicing pathway. Irinotecan (antineoplastic, Topoisomerase I inhibitor) and Indomethacin (Cyclooxygenase inhibitor, analgesic) are identified against the ubiquitin mediated proteolysis pathway. Thapsigargin (sarco/endoplasmic reticulum Ca2⁺ ATPase inhibitor, enzyme inhibitor), Tunicamycin (anti-bacterial), Geldanamycin (inhibitor of heat shock protein 90 (HSP90), anti-infective), Tenecteplase (cardiovascular agent)), and Lanoteplase (plasminogen activator) are found to target protein processing in the endoplasmic reticulum pathway. For cytokine signaling pathway the enriched drugs are Simvastatin (anticholesteremic agent), Leflunomide (antirheumatic agent), Sulindac (Cyclooxygenase-2 inhibitor, analgesic), Andrographolide (cytokine inhibitor, analgesic), Apremilast (PDE4 inhibitor, analgesic), Tofacitinib (JAK inhibitor, anti-rheumatoid arthritis), Brigatinib (antineoplastic agent), and Seliciclib (anti-infective agent) (Table S4).
3.8. Consolidated biological process, pathways and drugs from all the analysis
Since we identified several pathways, biological process, and drugs in our various analyses, to identify the most important ones, we combined all the results and then ranked them according to their number of appearances. We selected the pathways, biological process, and drugs that are commonly enriched in at least 3 of our omics-based analysis.
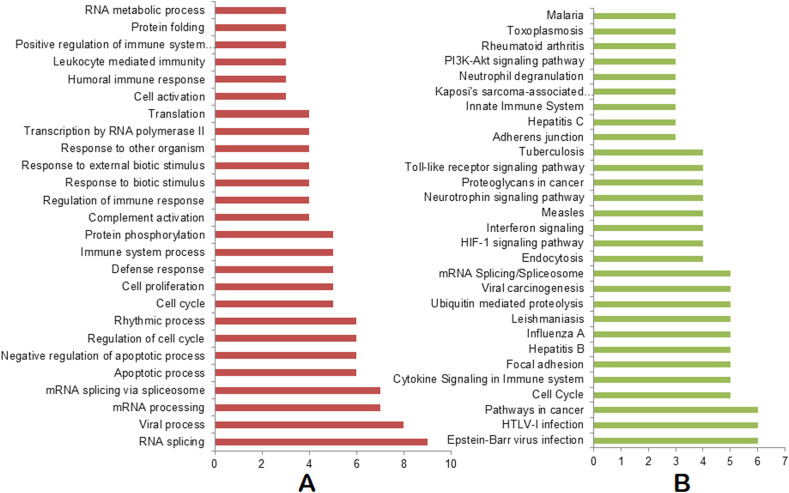
While we combined biological processes from all our analysis and performed the ranking, we found that RNA splicing, viral process, mRNA processing, mRNA splicing via spliceosome, negative regulation of apoptotic process, regulation of cell cycle, rhythmic process, cell proliferation, defense response, immune system process, protein phosphorylation, and complement activation are the top 15 pathways involved in the SARS-CoV-2 infection process (Fig. 2 A, Table S5). Similarly, Epstein-Barr virus infection, HTLV-I infection, pathways in cancer, cell cycle, cytokine signaling in immune system, focal adhesion, Hepatitis B, Influenza A, Leishmaniasis, ubiquitin mediated proteolysis, viral carcinogenesis, and mRNA splicing/spliceosome are found as top pathways (Fig. 2B, Table S5). Apart from these pathways, Measles, Tuberculosis, Toxoplasmosis, and Malaria pathways are also found to be common in at least three of our analyses (Fig. 2B, Table S5).
Fig. 2.
Consolidated biological processes and pathways. (A) Biological processes enriched in at least 3 omics-based analyses. (B) Pathways enriched in at least 3 omics-based analyses. The X axis represents the number of appearances of the biological processes/pathways combining all of our omics-based analyses.
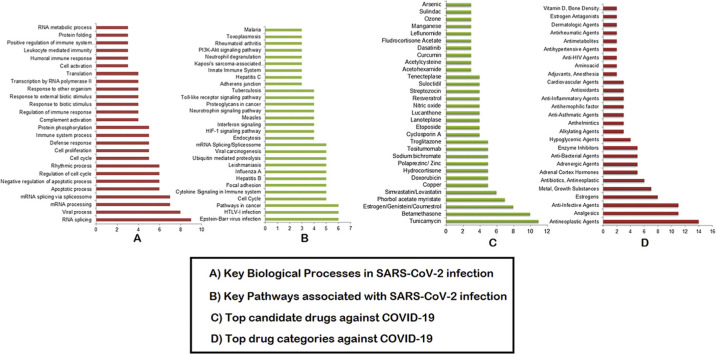
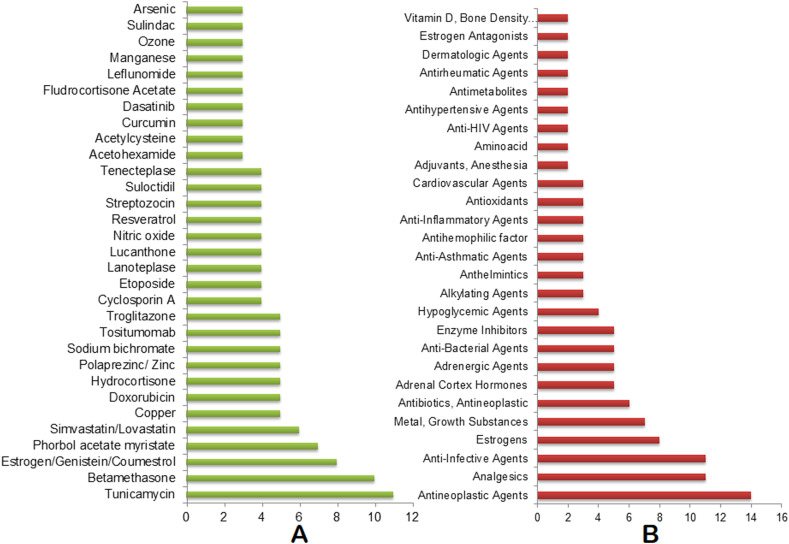
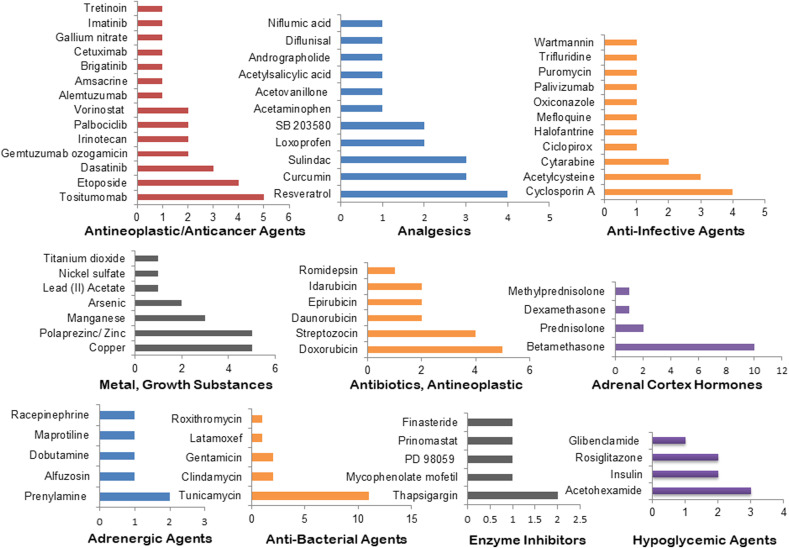
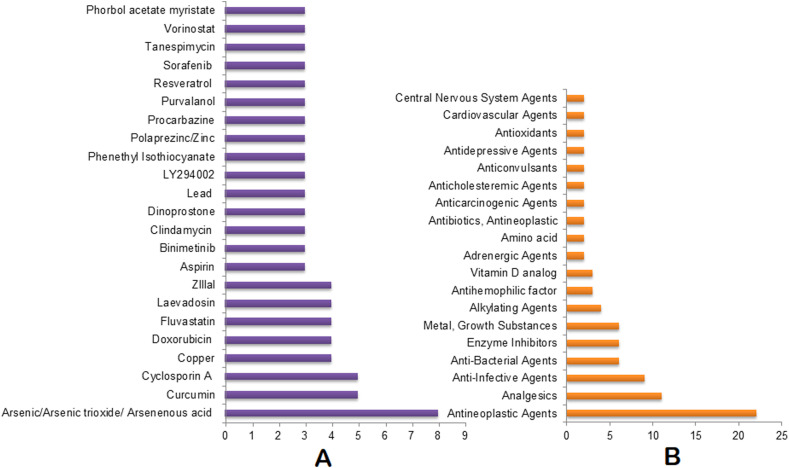
The top 30 drugs that are identified in at least 3 of our omics-based drugs analyses are: Tunicamycin, Betamethasone, Estrogen/Genistein/Coumestrol, Phorbol acetate myristate, Simvastatin/Lovastatin, Copper, Doxorubicin, Hydrocortisone, Zinc, Sodium bichromate, Tositumomab, Cyclosporin A, Etoposide, Lanoteplase, Lucanthone, Nitric oxide, Resveratrol, Streptozocin, Suloctidil, Tenecteplase, Acetohexamide, Acetylcysteine, Curcumin, Dasatinib, Fludrocortisone Acetate, Leflunomide, Manganese, Ozone, Sulindac, and Arsenic (Fig. 3 A, Table S6). The top drug categories include: antineoplastic agents, analgesics, anti-infective agents, Estrogens, metal/growth substances, antineoplastic antibiotics, adrenal cortex hormones, adrenergic agents, enzyme inhibitors, and hypoglycemic agents. These drug categories are found to have at least 4 drugs under each class (Fig. 3B, Table S6). Tositumomab, Cyclosporin A, Resveratrol, Copper, Zinc, Doxorubicin, Streptozocin, Betamethasone, Tunicamycin, Thapsigargin, and Acetohexamide are identified as the top candidate drugs/agents under the top 10 drug categories (Fig. 3, Fig. 4 ).
Fig. 3.
Consolidated drugs and drug categories. (A) Top drugs enriched in at least 3 omics-based analyses. (B) Top drug categories with at least 2 drugs. The X axis represents the number of appearances of the drugs/drug categories combining all of our omics-based analyses.
Fig. 4.
Consolidated drugs under the top ten drug categories. The X axis represents the number of appearances of the drugs under each category as per our combined all omics-based analyses.
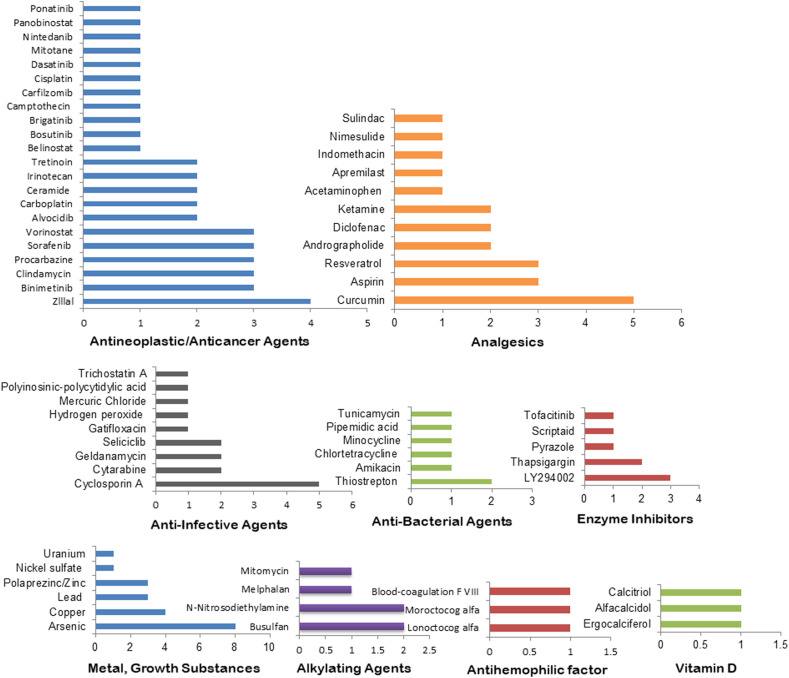
The five pathways (various virus and viral processes, mRNA splicing, ubiquitin mediated proteolysis, cytokine signaling in immune system, and protein processing in endoplasmic reticulum) based analysis identified the most frequent 20 therapeutic agents as Arsenic, Curcumin, Cyclosporin A, Copper, Doxorubicin, Fluvastatin, Laevadosin, Zlllal, Aspirin, Binimetinib, Clindamycin, Lead, LY294002, Phenethyl Isothiocyanate, Zinc, Procarbazine, Purvalanol, Resveratrol, Sorafenib, Tanespimycin, and Vorinostat (Fig- 5A, Table S7). The identified top drug categories are antineoplastic agents, analgesics, anti-infective agents, anti-bacterial agents, enzyme inhibitors, metal/growth substances, alkylating agents, antihemophilic factors, and Vitamin D that have at least 3 drugs in each category (Fig- 5B, Table S7). Further, Zlllal, Curcumin, Cyclosporin A, Thiostrepton, LY294002, Thapsigargin, Arsenic, Copper, and Zinc are found as the most frequently enriched agents under the top nine drug categories (Fig. 5, Fig. 6 ).
Fig. 5.
Consolidated drugs and drug categories by combining the five pathways. (A) Top drugs enriched in at least 3 omics-based analyses. (B) Top drug categories with at least 2 drugs. The X axis represents the number of appearances of the drugs/drug categories combining all of our omics-based analyses.
Fig. 6.
Consolidated drugs under the top nine drug categories. The X axis represents the number of appearances of the drugs under each category as per our combined all omics-based analyses.
The common top drug categories for both the omics-based and pathway-based analyses are antineoplastic antibiotics, antineoplastic agents, anti-bacterial agents, anti-infective agents, enzyme inhibitors, analgesics, Estrogens, vitamin D, antioxidants, adrenergic agents, alkylating agents, and amino acid. Similarly, Arsenic, Copper, Curcumin, Cyclosporin A, Doxorubicin, Phorbol acetate myristate, Resveratrol, and Zinc are found to be common in our pathways as well as in the multi-omics based analyses (Table S6 and S7).
4. Discussion
Previous report based on host-pathogen PPIs analyses suggests that the translation machinery, Cullin ubiquitin ligase complex, and immune pathways are involved in SARS-CoV-2 infection [7]. The proteome based understanding of the SARS-CoV-2 infection is mostly through spliceosomal and carbon metabolism related pathways [8]. Similarly, transcriptome based reports indicate that the chemokine signalling [9], complement activation, humoral immune response, immunoglobulin mediated response, acute inflammatory response, and lymphocyte apoptosis [10] are involved in SARS-CoV-2 pathogenesis. In our integrated multi-omics-based analysis, we found that RNA splicing/spliceosome, viral process/viral carcinogenesis, cytokine signaling in immune system, ubiquitin mediated proteolysis, and protein processing in endoplasmic reticulum are important pathways along with the previously identified pathways identified by other groups. Furthermore, in our analysis, SARS-CoV-2 is also found to share pathways associated with Epstein-Barr virus, HTLV-I, Hepatitis B, Influenza A, Herpes, Measles, Tuberculosis, Toxoplasmosis, Leishmaniasis, and Malaria infection pathways.
Based on host-pathogen protein-protein interactions, Gordon et al., 2020 [7] have proposed anti-cancer compounds, antipsychotic drugs, antihistamines, protein biogenesis inhibitors, and inhibitors of eIF4A, Sec61, and Sigma1 and Sigma2 receptors as good candidates against SARS-CoV-2. On the other hand, transcriptome based analysis had suggested Cycloheximide and Emetine (translation inhibitors), Pladienolide B (spliceosome inhibitor), and NMS873 (anti- Influenza A) as candidate drugs [8]. In our analysis, we identified several drugs based on the individual omics or combined-omics or pathway-based analyses. Our identified drugs belong to anticancer, antidepressant, antipsychotic, anti-inflammatory, channel blockers, TPA and other plasma components, Estrogen, steroid drugs, antibiotics, antimalarial, immunosuppressants, anti-cholesteremic agents, photosensitizer drugs, and several other drug categories. We also found some important candidate prophylaxis agents. Among our identified drugs, ~70% of them have previously been predicted or tested on SARS-CoV-2 with positive outcomes and several drugs are under various phases of clinical trials.
4.1. Anticancer drugs
Among the anticancer drugs identified in our analysis, a combination of topoisomerase inhibitors (Irinotecan and Etoposide) is reported to be an effective treatment strategy to counter cytokine storms in critically ill COVID-19 patients [21]. Our identified Doxorubicin was previously suggested for repurposing to treat COVID-19 patients [22]. Another identified anticancer drug Dasatinib is found to be safer to treat Chronic Myeloid Leukemia (CML) patients infected with SARS-CoV-2 [23]. Binimetinib, Brigatinib, Seliciclib, Sorafenib, and Vorinostat were also previously predicted to be effective against SARS-CoV-2 infection [[24], [25], [26], [27], [28]]. Therefore, the additional anticancer drugs identified by our analyses, such as Phenethyl isothiocyanate, Abciximab, Lucanthone, Cisplatin, Tositumomab, Procarbazine, and Noscapine may also be tested against SARS-CoV-2.
4.2. Antidepressant and antipsychotic drugs
Our identified antidepressant drug Fluoxetine (Prozac) was previously reported to inhibit the replication of SARS-CoV-2 [29] and therefore, may be tested for potential COVID-19 therapy. The antipsychotic medicine Fluphenazine is also enriched in our analysis that has previously been tested on SARS-CoV-2 [30]. Similarly, Sertraline (a Serotonin (5-HT) inhibitor), that is identified in our study, has recently been found to block SARS‐CoV‐2 endocytosis and suggested for drug repurposing against COVID-19 [31]. Thus, the additional antidepressant/antipsychotic drugs identified by our analyses, such as Droperidol (antidopaminergic), may also be tested to understand their effectiveness against SARS-CoV-2.
4.3. Anti-inflammatory drugs and channel blockers
In our analysis, several Cyclooxygenase (COX) inhibitors and anti-histamine compounds found potential against COVID-19. Among these drugs, Indomethacin (COX inhibitor) is shown to exert its anti-SARS-COV-2 activity in canine coronavirus model [32]. Further, Indomethacin in combination with Resveratrol (also identified in our analysis) has been proposed to be used for treating COVID‐19 [33]. Two other COX inhibitors (Loxoprofen and Acetaminophen) identified in our analysis were also previously tested in silico for their potential antiviral activity against SARS-CoV-2 [34]. Niflumic acid which is also a COX inhibitor as well as a Ca2+-activated Cl− channel blocker is also enriched in our analysis and also been previously identified as a potential drug against SARS-CoV-2 [35]. Other anti-inflammatory drugs such as Andrographolide, Apremilast (phosphodiesterase type 4 inhibitor) that are identified in our analysis were also predicted by previous studies as potential COVID-19 therapeutics [36,37]. In our analysis, we also found the rheumatoid arthritis drug Tofacitinib as a good candidate for COVID-19 management. Tofacitinib is a JAK inhibitor and reduces immune system inflammation and is currently under clinical trials for COVID-19 treatment (NCT04415151, NCT04412252), Therefore, the additional anti-allergic and anti-inflammatory drugs identified by our analyses including Sulindac (COX inhibitor) and Terfenadine (H1-receptor binding anti-histamine drug) need attention to be tested against SARS-CoV-2. Similarly, other channel inhibitors enriched in our analysis such as Acetohexamide (ATP-dependent K+ channel inhibitor), Suloctidil (Calcium antagonist), Glibenclamide (ATP-sensitive potassium channels inhibitor), and Thapsigargin (a sarco/endoplasmic reticulum Ca2⁺ ATPase inhibitor) may also be tested.
4.4. TPA and other plasma components
Tissue plasminogen activators (TPA), antihemophilic factors, immune globulin, and anti-thymocyte globulin are found to be good candidates in COVID-19 treatment as per our various omics-based analyses. Reports suggest that, TPA with targeted anti-inflammatory treatment may be a potential therapy against COVID-19 [38]. Tenecteplase, a TPA, that is identified in our analysis, is considered in managing acute coronary syndromes associated with COVID-19 [39] and is currently under clinical trial to manage COVID-19 patients (NCT04505592). Lanoteplase, a third generation recombinant plasminogen derived from Alteplase, is also identified in our analysis. Alteplase is currently under clinical trial to evaluate its efficacy against COVID-19 (NCT04357730). We also found Antihemophilic factors could be also effective against COVID-19. The Factor VIII which is an Antihemophilic factor is elevated in COVID-19 patients [40]. Therefore, Factor VIII inhibitors may be a potential therapeutic strategy. Immune globulin is also enriched in our analysis. Immune globulin is present in plasma and convalescent plasma therapy is effective in treating COVID-19 patients [41]. Our identified human serum albumin may be a good delivery vehicle to maximize the cellular internalization and enhancement of other drugs used in COVID-19 treatment [42]. Since most of our identified blood based components are suggested to be effective in COVID-19, the Anti-thymocyte globulin which is also identified in our analysis may also be tested for its efficacy in the management of SARS-CoV-2 infection.
4.5. Estrogen and steroids
Estrogen or Estrogen analogs are enriched in almost all of our omics data set-based analyses. Dimorphism of COVID-19 infection has been recently reported. Men are found to be more prone to SARS-CoV-2 infection or COVID-19 associated deaths as compared to women [43]. The disease severity of COVID-19 is also found to be dependent on ERα:ERβ expression ratio and E2 level [43]. Reports also suggest that the expression of human ACE2, which is the receptor for SARS-CoV-2, is regulated by Estrogen [44]. Therefore, Estradiol agonist may have a protective role against COVID-19 [45]. Recently, Estrogen patch is under clinical trial to evaluate its efficacy against COVID-19 (NCT04359329). In our analysis, we observed that Phytoestrogens including Coumestrol could also be a potential therapeutic against COVID-19.
We observed that, steroids could play an important role in fighting against COVID-19. The corticosteroid Dexamethasone and Methylprednisolone which we identified in our analysis, were previously found to significantly reduce mortality rates from COVID-19 [46,47] and currently under clinical trials (NCT04327401, NCT04323592). The other corticosteroid that is predominantly enriched in our integrative omics approach is Betamethasone. Betamethasone is found to be safe for pregnant subjects infected with SARS-CoV-2 [48] and is currently also under clinical trial at sites where Dexamethasone is not easily available (NCT04509973). Therefore, other corticosteroids identified in our analysis such as Fludrocortisone acetate may also be tested against COVID-19. Among our identified glucocorticoids, Hydrocortisone has been tested on COVID-19 patients [49] and Prednisone and Procaine may be tested in the coming days.
4.6. Antibiotics
No known targeted antiviral drug is enriched in our analysis. However, we identified several antibiotics. Among these antibiotics, Clindamycin was previously suggested by a computational approach [50] and is recommended to treat SARS-CoV-2 infection [51]. We identified two additional antitumor antibiotics, Geldanamycin and Tanespimycin, which are also heat shock protein 90 (HSP90) inhibitors. While Geldanamycin is suggested for COVID-19 treatment [52], Tanespimycin was previously identified by an in silico method [52]. Erythromycin is enriched in our analysis and Azithromycin which is similar to Erythromycin is under clinical trial for COVID-19 treatment (NCT04381962). Therefore, other antibiotics identified in our analysis such as Chlortetracycline, Thiostrepton (cyclic oligopeptide antibiotic), Tunicamycin (a nucleoside antibiotic that blocks protein folding), and Mitomycin and Streptozocin (antineoplastic antibiotic) or their derivatives also warrant evaluation for their efficacy against SARS-CoV-2 infection.
4.7. Prostaglandins and anti-cholesteremic agents
In our pathway based drug enrichment, Aspirin, Dinoprostone (prostaglandin E2), and three lipid-lowering drugs including Simvastatin are enriched. Recently, it is hypothesised that selective inhibition of prostaglandin E synthase-1 (mPGES-1) could be a potential therapeutic for COVID-19 [53] and Aspirin (a cyclooxygenase inhibitor) inhibits prostaglandin E2 (PGE2). Therefore, Aspirin can inhibit SARS-CoV-2 replication and currently Aspirin is under clinical trial for COVID-19 (NCT04365309). Hence, Dinoprostone should be neglected. The lipid-lowering drug Simvastatin is currently under several clinical trials (NCT04407273/NCT04348695) and other lipid-lowering drugs identified in our analysis, such as Fluvastatin and Lovastatin, may therefore need attention for testing against SARS-CoV-2 infection.
4.8. Antimalarial drug
Apart from sharing various viral pathways, we also found that SARS-CoV-2 shares pathways associated with Tuberculosis, Leishmaniasis, and Malaria. The antimalarial drug Hydroxychloroquine alone or in combination with Remdesivir effectively inhibits SARS-CoV-2 infection [54,55]. Hydroxychloroquine is under clinical trial (NCT04345692). In our analysis, we found that Artenimol or Artesunate, an alternative of Chloroquine could also be effective in treating COVID-19. Moreover, Artesunate was previously predicted to be a good candidate against SARS-CoV-2 [56,57] and is currently under clinical trial (NCT04387240).
4.9. Immunosuppressants
In our analysis, we found immunosuppressant drugs may also be effective against COVID-19. Among the identified immunosuppressants, Cyclosporin A is enriched in our multiple analyses. A recent in vitro study indicates that Cyclosporin A is effective against SARS-CoV-2 [58] and therefore, can be a potential drug to be further tested in COVID-19 patients [59]. Cyclosporin A is currently under clinical trials (NCT04412785, NCT04451239). Similarly, the other immunosuppressant Leflunomide identified in our analyses is also under clinical trial (NCT04361214).
4.10. Ozone, Nitric oxide, and photosensitizer drugs
In our integrative omics approach, we found that Ozone could be a potential therapy against SARS-CoV-2. Ozone therapy is beneficial against COVID-19 [60] and currently several Ozone-based management for early control of COVID-19 are under various stages of clinical trials (NCT04366089, NCT04370223). Nitric oxide, which is known to provide innate antiviral protection [61], is also enriched in our analysis. Nitric oxide inhalation is recently reported to be beneficial to manage the acute respiratory distress syndrome due to SARS-CoV-2 infection [62] and currently Nitric oxide inhalation therapy is under clinical trials to understand its efficacy in managing COVID-19 patients (NCT04383002, NCT04421508, NCT04306393). Verteporfin, a photosensitizer drug for photodynamic therapy is found to be an effective drug in our analysis. Verteporfin is also previously reported to be a potential antiviral therapy against SARS-CoV-2 [63].
4.11. Other drugs
In our multi-omics-based analysis, Acetylcysteine is enriched. Acetylcysteine is a mucolytic agent that can prevent the severity of SARS-CoV-2 infection. At present the drug is under clinical trial for COVID-19 management (NCT04419025, NCT04374461). Another drug Laevadosin is found to be frequently enriched in our pathway based analysis. Laevadosin is used to treat the Duchenne type of muscular dystrophy and may be tested for COVID-19 management.
4.12. Prophylaxis candidates
4.12.1. Curcumin, retinoic acids, vitamin D
We identified Curcumin and Resveratrol as potentially effective molecules in blocking viral pathways in SARS-CoV-2 infection. Recently, Curcumin was shown to interact with S protein of SARS-CoV-2 and its human receptor ACE2 [64]. Further, Curcumin in combination with Vitamin C and Glycyrrhizic acid may boost immunity and modulate inflammatory response associated with SARS-CoV-2 infection [65]. Currently a combination of Artemisinin and Curcumin is under clinical trial (NCT04382040). Resveratrol, an all-trans retinoic acid has shown antiviral effects on Epstein-Barr virus [66], Herpes simplex virus [67], and MERS-CoV [68]. Further, in silico analysis demonstrates that Resveratrol can potentially inhibit the main protease of SARS-CoV-2 [69]. Resveratrol is also currently under clinical trial to test its efficacy against COVID-19 (NCT04400890). In our analysis, another all-trans retinoic acid, Tretinoin, is also identified from protein processing in endoplasmic reticulum pathway. Isotretinoin (a 13- cis-retinoic acid) is currently under assessment for its therapeutic activity against COVID-19 (NCT04353180). We found Ergocalciferol (a Vitamin D2 analog) may target the ubiquitin mediated proteolysis pathway. Reports suggest that, Vitamin D deficiency increases the risk of COVID-19 severity [70] and Vitamin D prophylaxis may reduce the severity from SARS‐CoV‐2 infection [71]. Currently there are several clinical trials on Vitamin D and COVID-19 management in progress (NCT04407286, NCT04385940). Therefore, our results suggest that our analytic approach in this study is highly accurate and Curcumin, Retinoic Acids, and Ergocalciferol could be good prophylaxis candidates against COVID-19.
4.12.2. Arsenic, Copper, zinc
Arsenenous acid and Arsenic trioxide are identified both in our candidate gene-based integrative omics analysis as well as viral pathway-based analysis. Recent in silico analysis suggests that Arsenic based drug Darinaparsin may inhibit SARS-CoV-2 RNA polymerase and Proteases, and therefore, may inhibit viral replication [72]. In homeopathy medicine Arsenicum album 30, prepared from Arsenic trioxide, is recommended as a prophylactic medicine against SARS-CoV-2 infection [73]. In our analysis, Copper is found to inhibit all three important pathways: mRNA splicing, Ubiquitin mediated proteolysis, and protein processing in endoplasmic reticulum pathways. Copper irreversibly disintegrates Coronavirus genomes, envelope, and spike [74]. Report also suggests that enrichment of copper levels in plasma could boost both the innate and adaptive immunity [75] and therefore, Copper could also be a good prophylactic agent against SARS-CoV-2 infection. Similarly, Polaprezinc is identified as a potential molecule to block protein processing in the endoplasmic reticulum pathway. Zinc supplementation is recommended as a good prophylaxis and therapeutic strategy against COVID-19 [76]. Currently, several clinical trials are on-going to evaluate the anti-COVID-19 efficacy of Zinc in combination with other drugs (NCT04351490, NCT04447534).
5. Conclusion
In this work, we have used four different host specific omics data sets that were recently published along with a bibliome based gene set to identify the SARS-CoV-2 infection biology, potential drugs, and prophylaxis agents against this virus. Based on our individual omics-based or their combination-based analysis, it is evident that the SARS-CoV-2 infection shares various virus, bacteria, and protozoon infection pathways. Although, we identified several candidate drugs and prophylaxis candidates, we were not able to identify any SARS-CoV-2 specific antiviral agent. Nearly ~70% of our identified agents were previously reported to have anti-COVID-19 activity, and a number of these agents are currently under various stages of clinical trials. Since the most observed effective drugs/agents are among those that were used (or introduced) previously, our used integrative omics-based methodology is highly credible and warrants its wide-spread adoption and application. This method would also be highly applicable for drug repositioning research in future waves of COVID-19 infection and also other possible pandemics. However, as SARS-CoV-2 shares multiple pathogens’ infection pathways, individual drugs targeting a single pathway may not be effective and therefore, a combination of drugs needs to be formulated to block the multiple infection pathways of this virus. Further, the prophylaxis agents identified here also need an effective combination. All these identified drugs need multiple validations, large scale clinical trials, and caution before their use in COVID-19 management.
Author contribution
DB: conceived and designed the experiment, data collection and analysis, result interpretation, and wrote the paper; ST: preformed re-analysis; PG: data interpretation and edited the article, MEI, AGN, MMG and VA: provided technical inputs.
Funding
None.
Declaration of competing interest
Authors declare no competing interest.
Acknowledgement
We acknowledge Dr. Ranga Sai N. V. S. and Dr. Hemu Narayanam for their critical inputs in analyzing the prophylaxis agents.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.compbiomed.2020.104051.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Candidate gene based analysis of multi-omics data.
Gene set enrichment based analysis of multi-omics data.
Network analysis based lung-specific multi-omics data.
Lung specific pathway based drugs.
Consolidated Biological Processes and Pathways from all the analysis.
Consolidated drugs from all the omics based analysis.
Consolidated drugs from selected important pathways.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . 2019. Weekly epidemiological update, coronavirus disease. (COVID-19), 28 September 2020. [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu Y.F., Chien C.S., Yarmishyn A.A., Lin Y.Y., Luo Y.H., Lin Y.T., Lai W.Y., Yang D.M., Chou S.J., Yang Y.P., Wang M.L., Chiou S.H. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Munch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583(7816):469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045 e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinero J., Ramirez-Anguita J.M., Sauch-Pitarch J., Ronzano F., Centeno E., Sanz F., Furlong L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Bardes E.E., Aronow B.J., Jegga A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A., McDermott M.G., Monteiro C.D., Gundersen G.W., Ma'ayan A., Enrichr A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo M., Shin J., Kim J., Ryall K.A., Lee K., Lee S., Jeon M., Kang J., Tan A.C. DSigDB: drug signatures database for gene set analysis. Bioinformatics. 2015;31:3069–3071. doi: 10.1093/bioinformatics/btv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Y., Wang J., Jaehnig E.J., Shi Z., Zhang B., WebGestalt Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47(2019):W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou G., Soufan O., Ewald J., Hancock R.E.W., Basu N., Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuer K., Foroushani A.K., Laird M.R., Chen C., Sribnaia A., Lo R., Winsor G.L., Hancock R.E., Brinkman F.S., Lynn D.J. InnateDB: systems biology of innate immunity and beyond--recent updates and continuing curation. Nucleic Acids Res. 2013;41:D1228–D1233. doi: 10.1093/nar/gks1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi H., Muruganujan A., Ebert D., Huang X., Thomas P.D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovetrue B. 2020. The AI-Discovered Aetiology of COVID-19 and Rationale of the Irinotecan+Etoposide Combination Therapy for Critically Ill COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Motawa M.A., Wijten Hafsa, Patrick de la Fuente, Xue Alberto, Rabbani Mingzhan, Thornalley Naila, Paul J. bioRxiv; 2020. Vulnerabilities of the SARS-CoV-2 Virus to Proteotoxicity – Opportunity for Repurposed Chemotherapy of COVID-19 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abruzzese E., Luciano L., D'Agostino F., Trawinska M.M., Pane F., De Fabritiis P. SARS-CoV-2 (COVID-19) and chronic myeloid leukemia (CML): a case report and review of ABL kinase involvement in viral infection. Mediterr. J. Hematol. Infect. Dis. 2020;12 doi: 10.4084/MJHID.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirabelli C., Wotring J.W., Zhang C.J., McCarty S.M., Fursmidt R., Frum T., Kadambi N.S., Amin A.T., O'Meara T.R., Pretto C.D., Spence J.R., Huang J., Alysandratos K.D., Kotton D.N., Handelman S.K., Wobus C.E., Weatherwax K.J., Mashour G.A., O'Meara M.J., Sexton J.Z. bioRxiv; 2020. Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cava C., Bertoli G., Castiglioni I. Silico discovery of candidate drugs against covid-19. Viruses. 2020;12(4):404. doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B., Garmire L. ArXiv; 2020. Prediction of Repurposed Drugs for Treating Lung Injury in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke Y.-Y., Peng T.-T., Yeh T.-K., Huang W.-Z., Chang S.-E., Wu S.-H., Hung H.-C., Hsu T.-A., Lee S.-J., Song J.-S., Lin W.-H., Chiang T.-J., Lin J.-H., Sytwu H.-K., Chen C.-T. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed. J. 2020:S2319–S4170. doi: 10.1016/j.bj.2020.05.001. 20)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M.Á., Urquiza J., Ramírez D., Alonso C., Campillo N.E., Martinez A. COVID-19: drug targets and potential treatments. J. Med. Chem. 2020 doi: 10.1021/acs.jmedchem.0c00606. acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 29.Zimniak M.K. bioRxiv; 2020. Luisa Hilpert, Helen Seibel, Jürgen Bodem, Jochen, the Serotonin Reuptake Inhibitor Fluoxetine Inhibits SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weston S.C., Haupt Christopher M. bioRxiv; 2020. Rob Logue, James Matthews, Krystal Frieman, Matthew B., Broad Anti-coronaviral Activity of FDA Approved Drugs against SARS-CoV-2 in Vitro and SARS-CoV in Vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glebov O.O. Understanding SARS‐CoV‐2 endocytosis for COVID‐19 drug repurposing. FEBS J. 2020 doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu T.G., Wu Xuejuan, Selinger Zengbin, Zhou Douglas W. bioRxiv; 2020. Zichen, Indomethacin Has a Potent Antiviral Activity against SARS CoV-2 in Vitro and Canine Coronavirus in Vivo. [Google Scholar]
- 33.Marinella M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS‐CoV‐2/COVID‐19. Int. J. Clin. Pract. 2020;74(9) doi: 10.1111/ijcp.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadioglu O.S., Greten Mohamed, Henry Johannes Efferth Thomas. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Bull. World Health Organ. 2020 doi: 10.2471/BLT.20.255943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuhai Li A.P.M., Foraker Randi, Zhan Ming, Payne Philip R.O. arXiv; 2020. Repurposing Drugs for COVID-19 Based on Transcriptional Response of Host Cells to SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020:1–7. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olisova O.Y., Anpilogova E.M., Svistunova D.A. Dermatologic Therapy; 2020. Apremilast as a potential treatment option for COVID‐19: No symptoms of infection in a psoriatic patient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papamichalis P., Papadogoulas A., Katsiafylloudis P., Skoura A.L., Papamichalis M., Neou E., Papadopoulos D., Karagiannis S., Zafeiridis T., Babalis D., Komnos A. Combination of thrombolytic and immunosuppressive therapy for coronavirus disease 2019: a case report. Int. J. Infect. Dis. 2020;97:90–93. doi: 10.1016/j.ijid.2020.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briedis K., Aldujeli A., Aldujeili M., Briede K., Zaliunas R., Hamadeh A., Stoler R.C., McCullough P.A. Considerations for management of acute coronary syndromes during the SARS-CoV-2 (COVID-19) pandemic. Am. J. Cardiol. 2020;131:115–119. doi: 10.1016/j.amjcard.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabatabai A., Rabin J., Menaker J., Madathil R., Galvagno S., Menne A., Chow J.H., Grazioli A., Herr D., Tanaka K., Scalea T., Mazzeffi M. Factor VIII and functional protein C activity in critically ill patients with coronavirus disease 2019: a case series. In Pract. 2020;14 doi: 10.1213/XAA.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in korea. J. Kor. Med. Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mani Mishra P., Uversky V.N., Nandi C.K. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Med. Hypotheses. 2020;140:109790. doi: 10.1016/j.mehy.2020.109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulhaq Z.S., Soraya G.V. Estrogen and the disease severity of SARS-CoV-2 infection. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3606018. [DOI] [Google Scholar]
- 44.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L1280–L1281. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breithaupt-Faloppa A.C., Correia C.J., Prado C.M., Stilhano R.S., Ureshino R.P., Moreira L.F.P. 17 beta-Estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics. 2020;75 doi: 10.6061/clinics/2020/e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. medRxiv; 2020. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. [Google Scholar]
- 47.Veronese N., Demurtas J., Yang L., Tonelli R., Barbagallo M., Lopalco P., Lagolio E., Celotto S., Pizzol D., Zou L., Tully M.A., Ilie P.C., Trott M., Lopez-Sanchez G.F., Smith L. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front. Med. 2020;7:170. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Favilli A., Mattei Gentili M., Raspa F., Giardina I., Parazzini F., Vitagliano A., Borisova A.V., Gerli S. Effectiveness and safety of available treatments for COVID-19 during pregnancy: a critical review. J. Matern. Fetal Neonatal Med. 2020:1–14. doi: 10.1080/14767058.2020.1774875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W., Wong V.W., Chan P.K., Wong K.T., Wong E., Cockram C.S., Tam J.S., Sung J.J., Lo Y.M. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alia Nazarullah C.L., Villarreal Andrew, Higgins Russell A., Mais Daniel D. Peripheral blood examination findings in SARS-CoV-2 infection. Am. J. Clin. Pathol. 2020;154(3):319–329. doi: 10.1093/ajcp/aqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sultan I., Howard S., Tbakhi A. Research Square; 2020. Drug Repositioning Suggests a Role for the Heat Shock Protein 90 Inhibitor Geldanamycin in Treating COVID-19 Infection. [Google Scholar]
- 53.Smeitink J., Jiang X., Pecheritsyna S., Renkema H., van Maanen R., Beyrath J. 2020. Hypothesis: mPGES-1-derived prostaglandin E2, a so far missing link in COVID-19 pathophysiology?, preprints.org. [Google Scholar]
- 54.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uzun T., Toptas O. Artesunate: could be an alternative drug to chloroquine in COVID-19 treatment? Chin. Med. 2020;15:54. doi: 10.1186/s13020-020-00336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.chemat S., Sehailia M. chemrxiv; 2020. In-silico Studies of Antimalarial-Agent Artemisinin and Derivatives Portray More Potent Binding to Lys 353 and Lys31-Binding Hotspots of SARS-CoV-2 Spike Protein than Hydroxychloroquine: Potential Repurposing of Artenimol for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dittmar M., Lee J.S., Whig K., Segrist E., Li M., Jurado K., Samby K., Ramage H., Schultz D., Cherry S. bioRxiv; 2020. Drug Repurposing Screens Reveal FDA Approved Drugs Active against SARS-Cov-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cour M., Ovize M., Argaud L. Cyclosporine A: a valid candidate to treat COVID-19 patients with acute respiratory failure? Crit. Care. 2020;24:276. doi: 10.1186/s13054-020-03014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Sanchez G., Schwartz A., Donna V.D. Potential cytoprotective activity of Ozone therapy in SARS-CoV-2/COVID-19. Antioxidants. 2020;9:389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta D.R., Ashkar A.A., Mossman K.L. The nitric oxide pathway provides innate antiviral protection in conjunction with the type I interferon pathway in fibroblasts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi J., Murata I. Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann. Intensive Care. 2020;10 doi: 10.1186/s13613-020-00681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu C., Wu Y., Guo H., Zhu Y., Xu W., Wang Y., Sun Z., Cai X., Li Y., Liu J., Yuan Z., Zhang R., Deng Q., Qu D., Xie Y. bioRxiv; 2020. Potent Antiviral Effect of Protoporphyrin IX and Verteporfin on SARS-CoV-2 Infection. [Google Scholar]
- 64.Jena A.B., Kanungo N., Nayak V., Chainy G.B.N., Dandapat J. Research Square; 2020. Catechin and Curcumin Interact with Corona (2019-nCoV/SARS-CoV2) Viral S Protein and ACE2 of Human Cell Membrane: Insights from Computational Study and Implication for Intervention. [Google Scholar]
- 65.Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. A novel combination of vitamin C, Curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12:1193. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yiu C.-Y., Chen S.-Y., Chang L.-K., Chiu Y.-F., Lin T.-P. Inhibitory effects of resveratrol on the epstein-barr virus lytic cycle. Molecules. 2010;15:7115–7124. doi: 10.3390/molecules15107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Annunziata G., Maisto M., Schisano C., Ciampaglia R., Narciso V., Tenore G., Novellino E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: an overview. Viruses. 2018;10:473. doi: 10.3390/v10090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin S.-C., Ho C.-T., Chuo W.-H., Li S., Wang T.T., Lin C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishra A., Pathak Y., Tripathi V. Research Square; 2020. Natural Compounds as Potential Inhibitors of Novel Coronavirus (COVID-19) Main Protease: an in Silico Study. [Google Scholar]
- 70.Biesalski H.K. Vitamin D deficiency and co-morbidities in COVID-19 patients – a fatal relationship? NFS J. 2020;20:10–21. [Google Scholar]
- 71.Panarese A., Shahini E. Letter: covid-19, and vitamin D. Aliment. Pharmacol. Ther. 2020;51:993–995. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chowdhury T R.G., Mandal S.M. ChemRxiv; 2020. Silico Identification of a Potent Arsenic Based Approved Drug Darinaparsin against SARS-CoV-2: Inhibitor of RNA Dependent RNA Polymerase (RdRp) and Necessary Proteases. [DOI] [PubMed] [Google Scholar]
- 73.India A.M.G.o. 2020. Advisory for Corona Virus Homoeopathy for Prevention of Corona Virus Infections Unani Medicines Useful in Symptomatic Management of Corona Virus Infection. [Google Scholar]
- 74.Warnes S.L., Little Z.R., Keevil C.W., Colwell R. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6 doi: 10.1128/mBio.01697-15. e01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144:109848. doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Candidate gene based analysis of multi-omics data.
Gene set enrichment based analysis of multi-omics data.
Network analysis based lung-specific multi-omics data.
Lung specific pathway based drugs.
Consolidated Biological Processes and Pathways from all the analysis.
Consolidated drugs from all the omics based analysis.
Consolidated drugs from selected important pathways.