Abstract
Background
In early-stage breast cancer, the cornerstone of treatment is surgery. After breast-conserving surgery, adjuvant radiotherapy has shown to improve locoregional control and overall survival rates. The use of breast radiotherapy in the preoperative (preop) setting is far less common. Nevertheless, it might improve disease-free survival as compared to postoperative radiotherapy. There is also a possibility of downsizing the tumour which might lead to a lower need for mastectomy. There are some obstacles that complicate its introduction into daily practice. It may complicate surgery or lead to an increase in wound complications or delayed wound healing. Another fear of preop radiotherapy is delaying surgery for too long. At Ghent University Hospital, we have experience with a 5-fraction radiotherapy schedule allowing radiotherapy delivery in a very short time span.
Methods
Twenty female breast cancer patients with non-metastatic disease receiving preop chemotherapy will be randomized between preop or postoperative radiotherapy. The feasibility of preop radiotherapy will be evaluated based on overall treatment time. All patients will be treated in 5 fractions of 5.7 Gy to the whole breast with a simultaneous integrated boost to the tumour/tumour bed of 5 × 6.2 Gy. In case of lymph node irradiation, the lymph node regions will receive a dose of 27 Gy in 5 fractions of 5.4 Gy. The total duration of therapy will be 10 to 12 days. In the preop group, overall treatment time is defined as the time between diagnosis and the day of last surgery, in the postop group between diagnosis and last irradiation fraction. Toxicity related to surgery, radio-, and chemotherapy will be evaluated on dedicated case-report forms at predefined time points. Tumour response will be evaluated on the pathology report and on MRI at baseline and in the interval between chemotherapy and surgery.
Discussion
The primary objective of the trial is to investigate the feasibility of preop radiotherapy. Secondary objectives are to search for biomarkers of response and toxicity and identify the involved cell death mechanisms and the effect of preop breast radiotherapy on the in-situ immune micro-environment.
Keywords: Breast cancer, Neo-adjuvant radiotherapy, Neo-adjuvant chemotherapy
Background
In early-stage breast cancer, the cornerstone of treatment is surgery: either mastectomy (ME) or breast-conserving surgery (BCS). After surgery, most patients receive some kind of adjuvant systemic therapy such as hormone therapy, chemotherapy (CT), targeted therapy, or a combination of these. After BCS, adjuvant radiotherapy (RT) has shown to improve locoregional control and overall survival rates. After ME, a benefit of adjuvant RT was observed only in node positive patients [1, 2]. In recent years, adjuvant or postoperative (postop) CT is increasingly replaced by preoperative (preop) or neo-adjuvant CT in patients with larger tumours to avoid mastectomy or tumours with a more aggressive phenotype (triple negative or HER2 amplified cancers) for early response assessment [3, 4]. Several randomized controlled trials demonstrated that there is no difference in overall survival whether CT is given pre- or postoperatively [5, 6].
The use of breast RT in the preop setting is far less common. It has been proposed for patients with inoperable or inflammatory breast cancer, and a recent retrospective study in breast cancer patients showed preop RT might improve disease-free survival as compared to postop RT [7]. In another retrospective study comparing preop versus postop radio- and chemotherapy, a possible benefit of preop treatment was suggested for tumours larger than 2 cm [8]. These benefits have also been observed in other cancer sites. There is evidence from randomized trials that preop RT is more effective than postop RT in patients with rectal carcinoma [9]. For soft tissue sarcoma, better local control rates have been described with preop than with postop RT [10]. From a radiobiological point of view, the benefits of giving RT preoperatively are obvious. In contrast to the postop setting, the vasculature is still intact and less radio-resistant tumour clones are present, both possibly increasing radiosensitivity. But there are other advantages of preop RT treatment such as improved delineation of the tumour and peritumoural bed for RT planning, which is evidently easier with the tumour still in place. In the postop setting, unnecessary larger volumes are delineated [11] and interobserver variability is larger [12] than in the preop setting. In preop RT, regions in need of higher doses can be better targeted. For the latter reason, less acute side effects and a better overall breast cosmesis is expected. There is also a possibility of downsizing the tumour which might lead to a lower need for mastectomy. While preop breast RT clearly has some advantages, there are some obstacles that complicate its introduction into daily practice. Preop RT therapy may complicate surgery or lead to an increase in postop wound complications or delayed wound healing. Whereas delayed wound healing compromises overall treatment time if adjuvant treatment is delivered postoperatively, this is not the case for preop chemo and radiotherapy.
Another fear of preop RT is delaying surgery and/or CT for too long, thus increasing the risk of distant metastases. However, this is not an issue if radiation courses are short. At Ghent University Hospital, we have experience with a 5-fraction RT schedule [13] allowing preop RT delivery in a very short time span. Large randomized trials confirm that moderate hypofractionation schemes in 15 or 16 fractions are at least equivalent in tumour control and toxicity although the biological equivalent total dose is lower than the traditional 50 Gy in 25 fractions [14–16]. Further acceleration to 5 fractions is expected to have an even greater radiobiological advantage concerning tumour control. In the UK FAST randomized trial, a schedule of 5 times 5.7 Gy, once a week, was compared to a normofractionation schedule of 25 times 2 Gy. Tumour control and toxicity were comparable after 3 and 8 years of follow-up [17]. In the UK Fast-Forward trial, a once-weekly 5-fraction schedule is studied. It may be considered for patients in whom a daily visit for 3 or 5 weeks is not acceptable however careful consideration of the dose per fraction is required [18]. At Ghent University Hospital (UZ Gent), a feasibility trial was started using the FAST scheme (5 × 5.7 Gy) over 12 days (instead of 5 weeks) in patients of 65 years or older. Additionally, patients requiring a boost received a simultaneously integrated boost to the tumour bed of 5 × 6.5 Gy. The final analysis on 95 patients shows < 10% grade 2–3 erythema, with only one case of moist desquamation, located at a skin fold [13]. With this RT schedule of 5 fractions in 12 days given preoperatively, we hypothesize that overall treatment time will not be increased.
Since the tumour is still present in preop RT, this presents a unique opportunity to identify the involved cell death mechanisms of breast RT. Classically, RT is considered to mediate its effects via the direct killing of cancer cells. It is now known that RT can induce systemic effects resulting in tumour responses outside the irradiated regions [19]. This phenomenon called the “abscopal”-effect has been reported in breast cancer [20] and several other kinds of malignancies [21] and is nowadays considered to be immune-mediated [22]. The hypothesis is that RT induces immunogenic cell death (ICD) through the release of tumour-associated antigens and damage associated molecular patterns (DAMPs), which leads to antigen uptake and dendritic cell maturation, resulting in the priming and clonal expansion of cytotoxic T-lymphocytes (CTLs) in the lymph nodes [23]. These CTLs then travel back to the tumour, becoming tumour-infiltrating lymphocytes (TILs). Radiation could increase these TILs in a clinical setting [24, 25] and, more importantly, a high level of (post-therapy) TILs is associated with a good prognosis [25–29]. Immunogenic cell death implicates the release of DAMPs and tumoural antigens through a disintegrated cell plasma membrane. The latter correlates with necrosis (regulated or secondary) instead of apoptosis, which was considered to be the principal mechanism of radiation induced cell death for years [30, 31]. Distinct cell death modalities may thus have a different (immunogenic) outcome.
Additional to the immunogenic cell death mechanism, this study will also investigate extracellular vesicles (EVs) as biomarkers for response and toxicity. EVs are nanometer-sized membrane vesicles that contain lipids, proteins, nucleic acids, and metabolites. Different cell types can release EV, including immune cells (monocytes, neutrophils, etc.), tumour cells, fibroblasts, and adipocytes (fat cells) [32, 33]. Extracellular vesicles are promising novel biomarkers because (1) their molecular content is a fingerprint of the releasing cells and their status and consists of proteins, lipids, and nucleic acids, (2) they are released in easily accessible body fluids such as blood, and (3) they are enriched for highly selected biomarkers which otherwise would constitute only a very small proportion (less than 0.01%) of the total molecular content of blood [34]. Analysis of Glypican-1 positive EV in circulation distinguishes with absolute specificity and sensitivity healthy subjects and patients with a benign pancreatic disease from patients with early- and late-stage pancreatic cancer [35, 36] and non-pancreatic cancer [37]. EV transfer from stromal to breast cancer cells regulates therapy resistance pathways [38]. miRNA levels in circulating EV identify remnant vital tumour tissue and are suitable to measure therapy response and relapse monitoring [39]. These pioneering studies suggest that quantification and characterization of EV can be implemented to predict therapy response.
Methods
Objectives
The primary objective of the trial is to investigate the feasibility of preop breast RT. Secondary objectives are to search for biomarkers of response and toxicity and identify the involved cell death mechanisms and the effect of preop breast RT on the in-situ immune micro-environment. The feasibility of preop RT will be assessed in terms of overall treatment time and toxicity related to surgery, CT, and RT. In the preoperative group, overall treatment time is defined as the time between diagnosis and the day of last surgery, in the postoperative group the time between diagnosis and last dose of RT. Secondary endpoints are the tumour response rate, the rate of mastectomy, identification of biomarkers of response and toxicity on EVs from plasma, immunohistochemistry of cell death markers and TILs on pre-treatment, post-RT as well as tumourectomy tissue samples, cardiac toxicity, lung function, and quality of life.
Study population
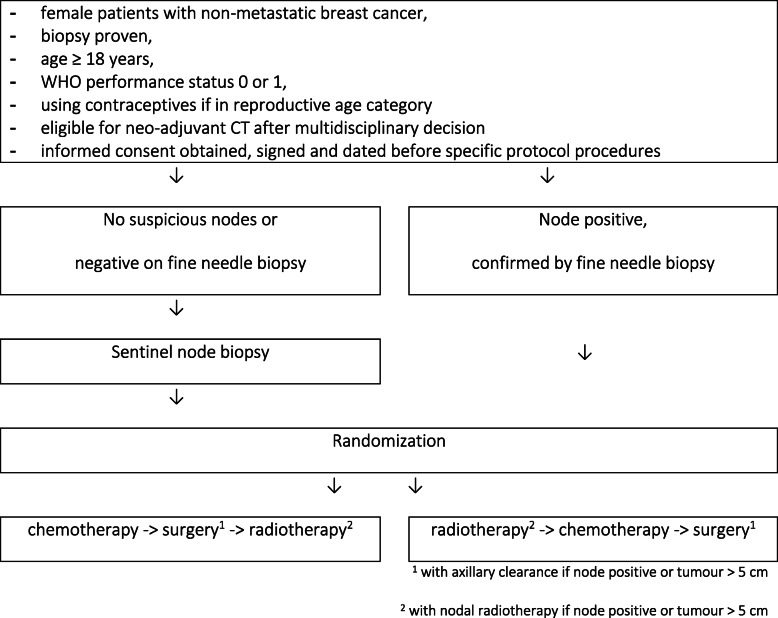
Twenty patients will be randomized between preop or postop RT as illustrated in Fig. 1. Inclusion criteria are female patients with non-metastasized breast cancer, for which a multidisciplinary decision must be made for preop CT, either this is for downsizing locally advanced breast cancer of because of type of tumour, such as triple-negative or HER2-positive early-stage breast cancer. Adjuvant hormone therapy will be administered to eligible women. For each patient, a biopsy with tumour histology, histological grade, ER/PR status, Her2/Neu status (amplification or not), and Ki67 status will be available. Exclusion criteria are distant metastases, inflammatory breast cancer (mastitis carcinomatosa), multifocal tumour, lobular carcinoma, bilateral breast cancer, history of cancer, with the exception of non-melanoma skin cancer, in situ cervix carcinoma, history of chemotherapy, history of radiation treatment, pregnant or breast feeding, or not using contraceptives if in reproductive age category, planned immediate reconstructive surgery, conditions making toxicity evaluation difficult (e.g. skin disorders), and amioderone treatment in the last 6 months. Exceptions to excluded carcinomas are made because these carcinomas occur frequently and result in a better life expectancy, so there is limited interference with the current study. There are also special exclusion criteria in function of chemotherapy, such as less than 2500 leukocytes or less than 1000/μL absolute neutrophil count.
Fig. 1.
Flow chart
In patients with clinically suspicious axillary lymph nodes a fine needle aspiration for cytology (FNAC) will be performed. If lymph node involvement is confirmed by FNAC, they will receive an axillary clearance after neoadjuvant treatment and axillary RT will be performed (either preop or postop). In clinically node-negative patients with a tumour of ≤ 5 cm, a sentinel node biopsy will be performed before the start of RT or CT. Patients with a tumour of > 5 cm, clinically node negative, will receive axillary clearance and axillary RT since a sentinel node biopsy is less reliable in these large tumours. Patients receiving postop RT will receive neoadjuvant CT followed by surgery (21–28 days after CT) and adjuvant RT starting 28–35 days after surgery. Patients receiving preop RT will receive RT first, followed by CT (5–8 days after the end of RT) and surgery (21–28 days after CT). In all patients, a marker clip will be placed in the tumour to determine its location before the start of any treatment. Ethics approval has received (EC2018/0599) and the study is registered at clinicaltrials.gov (NCT03783364).
Treatment
Patients will be treated with 4 cycles of epirubicin and cyclophosphamide either in a dose dense scheme every 2 weeks or in a non-dose dense scheme every 3 weeks, followed by 12 weeks of paclitaxel. The 2 type of durations in chemotherapy makes the comparison of duration of treatment difficult, only the delay in treatment will be measured and not the normal duration of systemic therapy. For Her2 amplified tumours, trastuzumab will be added to the treatment concomitant with paclitaxel, every 3 weeks for a total of 18 cycles.
All patients will be treated according to routine practice at Ghent University Hospital. If lymph node irradiation is not indicated (i.e. patients with a negative sentinel node procedure), patients will be treated in the prone position if possible [40–42]. All other patients will receive treatment in the supine position. Left-sided breast cancer patients treated in the prone position will undergo two simulation CT’s: shallow breathing and deep inspirational breath hold (DIBH). This is a technique used to reduce heart dose by increasing the distance between the treated breast and the heart. Patients are asked to take a deep breath and block inspiration for a limited time span during which radiation is delivered. Only when the mean heart dose exceeds 0.73 Gy will the technique be used for radiation delivery. In all other cases, treatment will be delivered during shallow breathing [43]. All patients will be treated in 5 fractions of 5.7 Gy to the whole breast with a simultaneous integrated boost (SIB) to the tumour/tumour bed of 5 × 6.2 Gy. In case of lymph node irradiation, the lymph node regions will receive a dose of 27 Gy in 5 fractions of 5.4 Gy. Radiotherapy will be performed every other day, thus permitting cell repair in between fractions. The total duration of therapy will be 10–12 days. The whole breast and lymph node regions (in case of lymph node irradiation) are delineated based on the ESTRO/PROCAB guidelines [44]. The heart is delineated based on the guidelines provided by Feng et al. [45]. In the preop radiotherapy group, gross tumour volume (GTV) is delineated on the CT-simulation scan with guidance of MRI. The clinical target volume for boost irradiation (CTV_boost) includes the GTV with a margin of 5 mm around the GTV. Around the CTV, a planning target volume for the SIB (PTV_boost) is created by adding a margin of 5 mm. A median dose of 31 Gy (5 × 6.2 Gy) is prescribed to the PTV_boost with a dose fall off region of 1.5 cm around this PTV_boost, not extending outside the breast. The dose fall off region receives a minimum dose of 27.08 Gy with 95% receiving at least 27.9 Gy. In the postop radiotherapy group, CTV_boost will be delineated based on the surgical clips, the histology report, and all available pre-operative information (clinical investigation, imaging). Around the CTV_boost, a dose fall off region of 2 cm is defined. The dose fall off region receives a minimum dose of 27.08 Gy with 95% receiving at least 27.9 Gy.
Evaluation of endpoints
The feasibility of preop RT will be evaluated based on overall treatment time. From a clinical point of view, it is not warranted that preop RT leads to an increase in the overall treatment time, since this may compromise locoregional control and survival. However, it is assumed that preop RT will shorten the overall treatment time by about 14 days (SD 9days) since the interval between RT and CT is shortened considerably. A difference of less than 14 days is not considered clinically relevant.
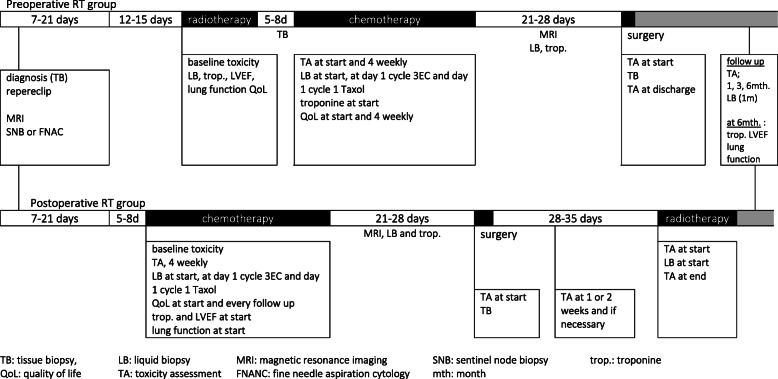
As a start point for measuring the overall treatment time, diagnosis of breast cancer by biopsy is taken, while delays in treatment by decision-making can be taken in account. In the preop group, overall treatment time is defined as the time between diagnosis (biopsy) and the day of last surgery. In the postop group, overall treatment time is defined as the time between diagnosis (biopsy) and the last day of RT. Toxicity related to surgery, RT, and CT will be evaluated on dedicated case-report forms (in Appendix) at predefined time points as illustrated in Fig. 2. Tumour response will be evaluated on the pathology report (complete response rate and Pinder regression score) and on MRI at baseline and in the interval between CT and surgery.
Fig. 2.
Study time table
To determine the mode of cell death evoked by pre-operative RT and its effect on the in situ immune micro-environment measurement (by immunohistochemistry (IHC)) of cell death markers and TILs will be performed on pre-treatment, post-RT (only in case of pre-operative RT), as well as tumourectomy tissue samples. The results of the IHC stainings for cell death markers will be correlated with the presence (or increase) of TILs in the same tissue samples and response to treatment.
All IHC stainings (cell death markers and TILs) will be performed on consecutive 3.5 μm slides of a representative formalin-fixed paraffin-embedded tissue block. A representative tissue block will be selected, taking into account the cellularity of the remaining tumour after RT (tissue biopsy after pre-operative RT), CT (tumourectomy post-op RT arm), or the combination of both (tumourectomy specimen pre-operative RT-arm). If residual tumour cells are absent, a tissue block with reactive changes (fibrosis, infiltration by foamy macrophages) will be selected. The following cell death markers will be examined: calreticulin (CRT), mobility group box 1 protein (HMGB-1), and Heat-Shock-Protein 70 (HSP70) for ICD; Cytokeratine 18 and caspase-3 for apoptosis; Senescense-associated β-galactosidase for senescence; phosphorylated mixed lineage kinase domain-like protein (pMLKL), and receptor-interacting protein kinase 3 (RIP3) for necroptosis and gluthatione peroxidase-4 (GPX-4) for ferroptosis.
HRQoL will be collected prospectively using different HRQoL instruments. For our analyses, only the items likely to be influenced by breast RT will be analysed. The European Organisation for Research and Treatment of Cancer (EORTC) 30-item Quality of Life Questionnaire (QLQ-C30) will be used, complemented by the breast cancer-specific module (QLQ-BR23). The EORTC QLC-C30 is a cancer-specific measuring instrument that describes five functional scales, three symptoms scales, six single-items scales, and a global health scale. Of these, we selected 2 functional scales (physical and social functioning), 2 symptoms scales (fatigue and pain) and the global health scale [46]. The EORTC QLQ-BR23, consists of 23 items, of which we included 2 symptom scales (i.e. arm symptoms and breast symptoms) and one functional scale (i.e. future perspective )[47]. The third questionnaire, the BREAST-Q questionnaire, was designed to evaluate outcome among women undergoing different types of breast surgery [48]. Breast satisfaction and physical well-being of the breast will be measured with respectively 6 and 7 questions. All questions of the BREAST-Q questionnaire will be used in this analysis. For all three questionnaires, a higher score indicates a better functioning for functional scales, while a higher score for symptom scales indicates a higher level of symptoms. The 3 questionnaires will be completed by the patient at 3 time points: before start of RT, 2 to 4 weeks after RT and 1 year after RT.
Sample size and statistical analysis
While reduction of overall treatment time is the primary end point, sample size is made for this item. With 20 patients (10 patients in every treatment arm), a 14-day difference in overall treatment time can be detected with a power of > 90% (2-sided t test, α = 0.05).
The statistical package SPSS version 26 will be used to analyse the data. RT-related toxicity will be defined as any baseline toxicity that deteriorated during or after RT and any toxicity that arose during or after RT and was not present at baseline. A clinically relevant deterioration of HRQoL will be defined as a difference in score between baseline and 2–4 weeks after RT of 10 points or more. Differences in RT-related toxicity and clinically relevant deterioration of HRQoL between groups will be analysed by performing a chi-square test with a significance level of p < 0.05. For HRQoL, statistical differences between baseline scores and scores after 2 to 4 weeks will be evaluated with the Mann–Whitney U test. Due to the multiple tests for HRQoL, the Bonferroni correction will be used to avoid type I errors which leads to an adjusted p value of p < 0.005. 95% confidence intervals will be calculated. Ethics approval is received by the ethical board of University Hospital Ghent.
Analysis of tissue and liquid biopsies
The results of the IHC stainings for these cell death markers will be correlated with profiles of CD8, CD4, CD3, CD68, and FOXP3 TILs (and the change in their presence after RT, CT or the combination of both). Patients will be divided into a high- and low-TIL group according to international guidelines.
Clinical (MRI) and pathological response assessed according to the Pinder regression grade (microscopic versus macroscopic disease)
Liquid biopsies (plasma samples) will be collected at consecutive time points (cfr. Fig. 2) to search for EV-associated biomarkers of response and toxicity. Blood will be collected in citrate tubes and platelet free plasma (PFP) will be prepared (2 × 2500×g centrifugation for 15 ) within 1 h after blood collection and stored at – 80 °C. EV will be isolated and characterized in compliance with MISEV2018, EV-TRACK, and Coumans et al. [49]. Chromatographic approaches will be combined with density gradient centrifugation to separate EV in two dimensions, size, and density, from contaminants such as lipoproteins, Argonaute-2 protein-miRNA complexes, and protein aggregates [50, 51]. Standard operating procedures have been optimized to enrich plasma EVs, to extract proteins/RNA, and to perform proteomics/small RNA sequencing. Currently, EVs from 6 ml of plasma allows to perform essential quality control experiments combined with proteomics and RNA sequencing [52]. Blood will be collected in citrate tubes and platelet free plasma (PFP) will be prepared (2 × 2500×g centrifugation for 15 min) within 1 h after blood collection and stored at – 80 °C. EVs will be isolated following SOPs, EV will be quantified by nanoparticle tracking analysis (NTA) and analysed by label-free mass spectrometry and RNA sequencing. We will implement receiver operating characteristics (ROC) to illustrate the performance of a biomarker, the sensitivity versus specificity, and will allow the selection of possible optimal EV biomarkers (number of EV and/or protein content and/or RNA content of EV). Advanced data analysis methods such as Perseus software [53] will further be used to enable comparison of expression levels within treatment groups and between treatment groups.
Discussion
All types of treatment have an influence on general health-related quality of life [54]. A larger decrease in HRQoL due to RT is seen if patients started chemotherapy before or during RT [55–57]. As well as length of illness and treatment duration affect HRQoL negatively [58]. By integrating the boost and an accelerated RT in 5 fractions, overall treatment time can be reduced, with less acute [59] and 2 years toxicity [60] and better health-related quality of life [61]. Preop RT may reduce the overall treatment time with 2 weeks, which can lead to better quality of life for patients.
Supplementary information
Additional file 1. Pre or postoperative accelerated radiotherapy (POP-ART)
Acknowledgments
Ackowledegements
Liv Veldeman is recipient of a Clinical Mandate of Stand up to Cancer (Flemish Cancer Society).
Katrien Vandecasteele holds a postdoctoral mandate for fundamental, translational, and clinical research funded by the Foundation against Cancer.I declare that the study status is ongoing: 18 of the 20 patients are included. No publications containing the results of this study have already been published to any journal. First, it has been submitted to BMC Cancer.
Study status
The protocol is ongoing. Eighteen of the 20 patients are included.
Related articles
We clarify that no publications containing the results of this study have already been published or submitted to any journal.
Abbreviations
- BCS
Breast-conserving surgery
- CRT
Calreticulin
- CT
Chemotherapy
- CTV
Clinical target volume
- HMGB-1
Mobility group box 1 protein
- HSP70
Heat-Shock-Protein 70
- IHC
Immunohistochemistry
- DIBH
Deep inspirational breath hold
- EV
Extracellular vesicles
- FNAC
Fine needle aspiration for cytology
- GPX-4
Gluthatione peroxidase-4
- GTV
Gross tumour volume
- LB
Liquid biopsy
- ME
Mastectomy
- MRI
Magnetic resonance imaging
- NTA
Nanoparticle tracking analysis
- pMLKL
Phosphorylated mixed lineage kinase domain-like protein
- preop
Preoperative
- PTV
Planning target volume
- QoL
Quality of life
- RIP3
Receptor-interacting protein kinase 3
- ROC
Receiver operating characteristics
- RT
Radiotherapy
- SIB
Simultaneous integrated boost
- SNB
Sentinel node biopsy
- TA
Toxicity assessment
- TB
Tissue biopsy
- TIL
Tumour-infiltrating lymphocytes
- trop.
Troponine
Authors’ contributions
HVH: conception and design, writing of manuscript. VV: conception and design, writing of manuscript. GP: patient follow-up, data collection. AVG: patient follow-up, data collection. CM: review of manuscript. AH:co-writer, review of manuscript. KVDV: co-writer, review of manuscript. JV: review of manuscript. PDV: co-writer, review of manuscript. GB: co-writer, review of manuscript. KV: co-writer, review of manuscript. HD: co-writer, review of manuscript. WDN: co-writer, review of manuscript. LV: study coordination, writing of manuscript. All authors have read and approved the manuscript.
Funding
The study is sponsored by ‘Kom op tegen Kanker’.
Availability of data and materials
We do not wish to share our data. Our study protocol has undergone peer-review by the funding body.
Ethics approval and consent to participate
Ethics approval is received by the ethical board of University Hospital Ghent.
Ethical and Funding Approval Documentation is sent to BMCSeriesEditorial@biomedcentral.com.
Consent for publication
Not applicable
Competing interests
This work is funded by Stand up to Cancer (Flemish Cancer Society).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hans Van Hulle, Email: hans.vanhulle@ugent.be.
Vincent Vakaet, Email: vincent.vakaet@ugent.be.
Giselle Post, Email: giselle.post@ugent.be.
Annick Van Greveling, Email: annick.vangreveling@uzgent.be.
Chris Monten, Email: chris.monten@uzgent.be.
An Hendrix, Email: an.hendrix@ugent.be.
Koen Van de Vijver, Email: koen.vandevijver@uzgent.be.
Jo Van Dorpe, Email: jo.vandorpe@uzgent.be.
Pieter De Visschere, Email: pieter.devisschere@uzgent.be.
Geert Braems, Email: geert.braems@uzgent.be.
Katrien Vandecasteele, Email: katrien.vandecasteele@uzgent.be.
Hannelore Denys, Email: hannelore.denys@uzgent.be.
Wilfried De Neve, Email: wilfried.deneve@uzgent.be.
Liv Veldeman, Email: liv.veldeman@uzgent.be.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40814-020-00693-z.
References
- 1.Early Breast Cancer Trialists' Collaborative, Group, et al., Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (London, England), 2011. 378(9804): p. 1707-16. [DOI] [PMC free article] [PubMed]
- 2.Castaneda SA, Strasser J. Updates in the Treatment of Breast Cancer with Radiotherapy. Surgical oncology clinics of North America. 2017;26(3):371–382. doi: 10.1016/j.soc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi P, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 4.van der Hage JA, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(22):4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 5.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. Journal of the National Cancer Institute. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 6.Riet, F.G., et al., Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. European journal of cancer (Oxford, England : 1990), 2017. 76: p. 45-51. [DOI] [PubMed]
- 7.Poleszczuk J, et al. Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival. Breast cancer research : BCR. 2017;19(1):75. doi: 10.1186/s13058-017-0870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth, S.L., et al., Retrospective study of neoadjuvant versus adjuvant radiochemotherapy in locally advanced noninflammatory breast cancer : survival advantage in cT2 category by neoadjuvant radiochemotherapy. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al], 2010. 186(6): p. 299-306. [DOI] [PubMed]
- 9.Glimelius, B., et al., A systematic overview of radiation therapy effects in rectal cancer. Acta oncologica (Stockholm, Sweden), 2003. 42(5-6): p. 476-92. [DOI] [PubMed]
- 10.Pollack A, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. International journal of radiation oncology, biology, physics. 1998;42(3):563–572. doi: 10.1016/S0360-3016(98)00277-6. [DOI] [PubMed] [Google Scholar]
- 11.Nichols EM, et al. Comparative analysis of the post-lumpectomy target volume versus the use of pre-lumpectomy tumor volume for early-stage breast cancer: implications for the future. International journal of radiation oncology, biology, physics. 2010;77(1):197–202. doi: 10.1016/j.ijrobp.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 12.van der Leij F, et al. Target volume delineation in external beam partial breast irradiation: less inter-observer variation with preoperative- compared to postoperative delineation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2014;110(3):467–470. doi: 10.1016/j.radonc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Monten C, et al. Highly Accelerated Irradiation in 5 Fractions (HAI-5): Feasibility in Elderly Women With Early or Locally Advanced Breast Cancer. International journal of radiation oncology, biology, physics. 2017;98(4):922–930. doi: 10.1016/j.ijrobp.2017.01.229. [DOI] [PubMed] [Google Scholar]
- 14.Haviland JS, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The Lancet. Oncology. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 15.Whelan TJ, et al. Long-term results of hypofractionated radiation therapy for breast cancer. The New England journal of medicine. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 16.Mulliez T, et al. Hypofractionated whole breast irradiation for patients with large breasts: a randomized trial comparing prone and supine positions. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;108(2):203–208. doi: 10.1016/j.radonc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 17.group, F.T., et al., First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology, 2011. 100(1): p. 93-100. [DOI] [PubMed]
- 18.Brunt AM, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2016;120(1):114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Formenti SC, Demaria S. Systemic effects of local radiotherapy. The Lancet. Oncology. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Current breast cancer reports. 2017;9(1):45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Current problems in cancer. 2016;40(1):25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. International journal of radiation oncology, biology, physics. 2012;84(4):879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamrava M, et al. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Molecular bioSystems. 2009;5(11):1262–1270. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng F, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. American journal of cancer research. 2015;5(6):2064–2074. [PMC free article] [PubMed] [Google Scholar]
- 25.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for immunotherapy of cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyashita M, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast cancer research : BCR. 2015;17:124. doi: 10.1186/s13058-015-0632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladoire S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. The Journal of pathology. 2011;224(3):389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 28.Tao H, et al. Density of tumor-infiltrating FOXP3+ T cells as a response marker for induction chemoradiotherapy and a potential prognostic factor in patients treated with trimodality therapy for locally advanced non-small cell lung cancer. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2014;20(6):980–6. doi: 10.5761/atcs.oa.13-00237. [DOI] [PubMed] [Google Scholar]
- 29.Dieci MV, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Frontiers in oncology. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauber K, et al. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Frontiers in oncology. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanez-Mo M, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhondt B, et al. Function of extracellular vesicle-associated miRNAs in metastasis. Cell and tissue research. 2016;365(3):621–641. doi: 10.1007/s00441-016-2430-x. [DOI] [PubMed] [Google Scholar]
- 34.Hendrix A, et al. An ex(o)citing machinery for invasive tumor growth. Cancer research. 2010;70(23):9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- 35.Melo SA, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, K.S., et al., Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Science translational medicine, 2017. 9(391). [DOI] [PMC free article] [PubMed]
- 37.Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boelens MC, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eijndhoven MA, et al. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI insight. 2016;1(19):e89631. doi: 10.1172/jci.insight.89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deseyne, P., et al., Whole breast and regional nodal irradiation in prone versus supine position in left sided breast cancer. Radiation oncology (London, England), 2017. 12(1): p. 89. [DOI] [PMC free article] [PubMed]
- 41.Boute B, et al. Potential benefits of crawl position for prone radiation therapy in breast cancer. Journal of applied clinical medical physics. 2017;18(4):200–205. doi: 10.1002/acm2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veldeman L, et al. The 2-Year Cosmetic Outcome of a Randomized Trial Comparing Prone and Supine Whole-Breast Irradiation in Large-Breasted Women. International journal of radiation oncology, biology, physics. 2016;95(4):1210–1217. doi: 10.1016/j.ijrobp.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Mulliez T, et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;114(1):79–84. doi: 10.1016/j.radonc.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 44.Verhoeven K, et al. Vessel based delineation guidelines for the elective lymph node regions in breast cancer radiation therapy - PROCAB guidelines. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;114(1):11–16. doi: 10.1016/j.radonc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Feng M, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. International journal of radiation oncology, biology, physics. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen J, et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. Journal of comparative effectiveness research. 2015;4(2):157–166. doi: 10.2217/cer.14.76. [DOI] [PubMed] [Google Scholar]
- 47.Xia J, et al. Use of item response theory to develop a shortened version of the EORTC QLQ-BR23 scales. Scientific Reports. 2019;9. [DOI] [PMC free article] [PubMed]
- 48.Pusic AL, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 49.Coumans FAW, et al. Methodological Guidelines to Study Extracellular Vesicles. Circulation research. 2017;120(10):1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 50.Van Deun J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. Journal of extracellular vesicles. 2014;3. [DOI] [PMC free article] [PubMed]
- 51.Tulkens J, et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2018. [DOI] [PMC free article] [PubMed]
- 52.Consortium E-T, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nature methods. 2017;14(3):228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 53.Tyanova S, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nature methods. 2016;13(9):731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 54.Hamer J, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(2):409–419. doi: 10.1007/s00520-016-3417-6. [DOI] [PubMed] [Google Scholar]
- 55.Ho PJ, et al. Health-related quality of life in Asian patients with breast cancer: a systematic review. BMJ open. 2018;8(4):e020512. doi: 10.1136/bmjopen-2017-020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mortada EM, et al. Comparing Health-Related Quality of Life among Breast Cancer Patients Receiving Different Plans of Treatment, Egypt. Journal of community health. 2018;43(6):1183–1191. doi: 10.1007/s10900-018-0538-5. [DOI] [PubMed] [Google Scholar]
- 57.Xiao C, et al. A prospective study of quality of life in breast cancer patients undergoing radiation therapy. Advances in radiation oncology. 2016;1(1):10–16. doi: 10.1016/j.adro.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang HY, et al. Quality of life of breast and cervical cancer survivors. BMC women's health. 2017;17(1):30. doi: 10.1186/s12905-017-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Hulle H, et al. Accelerating adjuvant breast irradiation in women over 65 years: Matched case analysis comparing a 5-fractions schedule with 15 fractions in early and locally advanced breast cancer. J Geriatr Oncol. 2019;10(6):987–989. doi: 10.1016/j.jgo.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Van Hulle H, et al. Two-year toxicity of hypofractionated breast cancer radiotherapy in five fractions. Acta Oncol. 2020;59(7):872–875. doi: 10.1080/0284186X.2020.1747638. [DOI] [PubMed] [Google Scholar]
- 61.Van Hulle, H., Vakaet, V., Bultijnck, R., Deseyne, P., Schoepen, M., Van Greveling, A., Post, G., De Neve, W., Monten, C., Lievens, Y., Veldeman, L., Health-related quality of life after accelerated breast irradiation in five fractions: a comparison with fifteen fractions,. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology, 2020. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Pre or postoperative accelerated radiotherapy (POP-ART)
Data Availability Statement
We do not wish to share our data. Our study protocol has undergone peer-review by the funding body.