Abstract
Background
Patients receiving ABO-incompatible (ABOi) or human leukocyte antigen (HLA)-incompatible (HLAi) kidney transplantation (KT) require potent immunosuppression and are thus at a higher risk of infectious complications. We evaluated the clinical outcomes of KT stratified by ABO and HLA incompatibilities and identified the factors associated with the clinical outcomes.
Material/Methods
Recipients who underwent living-related KT between 2012 and 2017 were included and classified into 4 groups: ABO-compatible and HLA-compatible (ABOc/HLAc), HLA-incompatible (ABOc/HLAi), ABO-incompatible (ABOi/HLAc), and ABO-incompatible and HLA-incompatible (ABOi/HLAi). Cox proportional hazards regression analyses were carried out to evaluate the risk factors of acute rejection. Out of the 1732 patients who underwent KT, 1190, 131, 358, and 53 were in the ABOc/HLAc, ABOi/HLAc, ABOc/HLAi, and ABOi/HLAi groups, respectively.
Results
The ABO/HLAi group showed the lowest 5-year graft survival rate (91.7%). Death-censored graft survival was not significantly different among the groups. The mortality rate from infections was significantly higher in the ABOi/HLAi group (7.5%) than the other groups. Antibody-mediated rejection-free graft survival was the lowest in the ABOi/HLAi group, with significant differences compared with the ABOi/HLAc group (P=0.02) and the ABOc/HLAi group (P=0.03). ABOi/HLAi (hazard ratio [HR], 2.63; 95% confidence interval [CI], 1.04–6.65; P<0.01) and combined infection (HR, 1.91; 95% CI, 1.45–2.51; P<0.01) were significant risk factors for acute rejection.
Conclusions
Patients with both ABO and HLA incompatibilities showed inferior rates of overall patient and graft survival due to infectious complications. Infection was a prominent risk factor of acute rejection following KT after adjusting for possible confounders including ABO and HLA incompatibility.
MeSH Keywords: Blood Grouping and Crossmatching, Infection, Kidney Transplantation
Background
Kidney transplantation (KT) is the best renal replacement therapy in patients with end-stage renal diseases [1]. Advances in immunosuppressants and desensitization have enabled kidney transplantation across immunologic barriers such as blood group A/B or human leukocyte antigen (HLA) incompatibilities. Transplantation from an HLA-incompatible (HLAi) donor has been reported to have survival benefits compared with receiving a KT from a deceased donor or waiting on the transplant list [2,3]. ABO-incompatible (ABOi) or HLAi KT recipients carry distinct immunologic risks that have significant impacts on the postoperative course and graft outcomes. ABOi KT was shown to have comparable outcomes to ABO-compatible (ABOc) KTs, but some larger studies suggested that ABOi KT is associated with early incidences of graft failure or higher posttransplant mortality [4–6]. Studies comparing HLAi KT and HLA-compatible (HLAc) KT continue to show conflicting outcomes, especially in cases with high mean fluorescence intensity levels of donor-specific antibodies (DSA) and in terms of long-term outcomes [7–11]. In addition, recipients receiving ABOi or HLAi KT need potent immunosuppression, including desensitization treatments, and are thus at a higher risk of infection following KT [12–14].
Previously, we reported the outcomes of ABOi and HLAi KT, including positive flow-cytometric crossmatch and complement-dependent cytotoxicity crossmatch KT, and suggested that DSA is a predominant predictor of acute rejection [15–17]. Importantly, whether the combination of ABO and HLA incompatibilities has an additional effect on clinical outcomes compared with either ABO or HLA incompatibility has not been thoroughly investigated. A nationwide cohort study reported the results of ABOi and HLAi KT, but due to the heterogeneity in patient characteristics arising from its design, the study fell short of accurately reflecting the effects of the strength of DSA and infectious complications on the clinical outcomes [18,19]. Here, we evaluated the clinical outcomes of KT stratified by ABO and HLA incompatibilities and identified the factors affecting clinical outcomes of ABOi and HLAi KT.
Material and Methods
Study Population
We included recipients who underwent living-related KT between January 2012 and December 2017 at Asan Medical Center (AMC). To compare the clinical outcomes stratified by ABO and HLA incompatibilities, the patients were categorized into 4 groups: compatible for both ABO and HLA (ABOc/HLAc), HLA-incompatible (ABOc/HLAi), ABO-incompatible (ABOi/HLAc), and incompatible for both ABO and HLA (ABOi/HLAi). The HLAc group included patients with maximal DSA mean fluorescence intensity values below 5000. HLAi KT was defined as a transplant in recipients with positive complement-dependent cytotoxicity crossmatch, flow-cytometric crossmatch, and/or maximal DSA mean fluorescence intensity values above 5000, a cutoff based on previous studies [17]. The Institutional Review Board of AMC approved this study.
Desensitization regimens for ABOi and HLAi kidney transplantation
The pretransplant desensitization regimens of ABO and HLA incompatibilities at our center were described in previous studies [15,20]. Both protocols consisted of anti-CD20 monoclonal antibody (rituximab; Genentech, Inc., South San Francisco, CA, USA) and plasmapheresis (PP). For ABOi recipients, we administered a single dose of rituximab (100–200 mg) at 7 days before the start of PP. Total plasma exchange was performed until the preoperative immunoglobulin M isoagglutinin titer against blood group A or B was reduced to ≤1: 4, and postoperative PP was performed when the rebound isoagglutinin titer was ≥1: 16 (COBE® Spectra; Gambro BCT, Lakewood, CO, USA) [21]. HLAi patients were treated with 200–500 mg of rituximab 1–2 weeks before PP. PP was maintained until the complement-dependent cytotoxicity crossmatch and T-cell flow-cytometric crossmatch became negative.
Immunosuppression and postoperative prophylaxis for infection
For induction, 20 mg anti-interleukin-2 receptor antibody (basiliximab) was administered on day 0 and again on day 4. The maintenance immunosuppression regimen consisted of a calcineurin inhibitor (tacrolimus, cyclosporin), a corticosteroid, and mycophenolic acid. The target trough concentrations for tacrolimus ranged from 5 to 8 ng/mL following KT and were gradually decreased to 3–6 ng/mL after 1 year. The target trough concentrations of cyclosporine ranged from 100 to 150 μg/L, which was reduced to 70–100 μg/L. Recipients received 750 mg mycophenolate mofetil twice a day as anti-metabolites starting from 1 week prior to transplantation, and the dosage was reduced to 500 mg twice a day after the fifth postoperative day.
To prevent cytomegalovirus (CMV) and human polyomavirus BK infection, we performed preemptive therapy with monitoring the presence of viremia using polymerase chain reaction (PCR) from the patients’ blood sample. PCR was performed at 1 and 2 weeks; 1, 3, and 6 months; and 1 year after transplantation. Additional PCR for CMV or polyomavirus BK was performed if viremia was detected or clinically indicated. All patients received trimethoprim-sulfamethoxazole (80–400 mg) daily for 6 months as a prophylaxis for Pneumocystis jirovecii pneumonia.
Definitions
The rejection was diagnosed according to the Banff classification [22]. We did not perform protocol biopsies. Rejection-free graft survival (RFGS) was defined as the time between KT to the first incidence of pathologically diagnosed rejection. Graft survival (GS) was defined as the time between transplantation to the return of renal replacement therapy or graft failure. Pneumonia was defined as lung infection caused by viruses, bacteria, or fungi. Combined infection consisted of polyomavirus BK viremia PCR ≥4 logs, CMV viremia PCR ≥4 logs, urinary tract infection, and pneumonia. To evaluate the effect of infection on rejection episodes, an infection that occurred prior to rejection was included in the analysis.
Statistical analysis
Continuous variables underwent analysis of variance (post hoc analysis) for comparisons among multiple groups, and the t-test or Mann-Whitney U test was performed for comparisons between 2 groups as appropriate. Categorical data were analyzed with the chi-squared test or Fisher’s exact test. Survival rates were analyzed with Kaplan-Meier curves (log-rank tests). To investigate the association between risk factors and allograft rejection, univariate and multivariate Cox proportional hazards regression analyses were performed in a backward stepwise fashion. Variables with P values <0.10 on univariate analysis were subjected to multivariable analysis. P values less than 0.05 were considered to indicate statistical significance. We performed statistical analyses using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline demographics
A total of 1732 patients were included in this study, which included 1190 transplants that were compatible for both ABO and HLA and 542 transplants that were incompatible for ABO, HLA, or both. A total of 53 out of the 411 ABOi cases were HLAi, and 131 out of the 1321 ABOc cases were HLAi. The patients were stratified into 4 groups – ABOc/HLAc, ABOc/HLAi, ABOi/HLAc, and ABOi/HLAi – according to their compatibility for ABO and HLA, and these groups included 1190, 131, 358, and 53 patients, respectively.
Table 1 summarizes the baseline demographics and characteristics of the patients. The ABOi/HLAi group had the highest mean age, and the 2 HLAi groups had higher proportions of females. Preemptive KT was the most frequently performed in the ABOc/HLAc group. The ABOi/HLAi group had a significantly higher rate of retransplants (P<0.01). More patients in the ABOc/HLAc group received cyclosporine than in the other groups (P=0.03). Rituximab dosage was significantly higher in the HLAi groups than the ABOi groups (P<0.01). Pretransplant PP number was the highest in the ABOi/HLAi group, followed by the ABOc/HLAi and ABOi/HLAc groups. The ABOc/HLAi group had higher maximal DSA value than did the ABOi/HLAi group (9367±4349 vs. 7292±4193, P<0.01).
Table 1.
Demographics and baseline clinical characteristics.
| Variables | ABOc & HLAc (N=1190) | ABOc & HLAi* (N = 131) | ABOi & HLAc (N=358) | ABOi & HLAi* (N=53) | P-value |
|---|---|---|---|---|---|
| Age, years | 45.9±12.0 | 48.2±12.2 | 47.3±11.6 | 50.8±11.1 | c0.02 |
| BMI, kg/m2 | 25.0±7.5 | 22.3±3.5 | 23.5±9.1 | 21.5±2.4 | 0.93 |
| Female | 463 (38.9) | 98 (74.8) | 134 (37.4) | 32 (60.4) | <0.01 |
| Cause of ESRD | 0.30 | ||||
| Hypertension | 165 (13.9) | 19 (14.5) | 38 (10.6) | 8 (15.1) | |
| Diabetes mellitus | 282 (23.7) | 27 (20.6) | 106 (29.6) | 8 (15.1) | |
| GN | 146 (12.3) | 16 (12.2) | 38 (10.6) | 7 (13.2) | |
| IgA nephropathy | 160 (13.4) | 15 (11.5) | 58 (16.2) | 10 (18.9) | |
| FSGS | 21 (1.8) | 2 (1.5) | 11 (3.1) | 0 (0.0) | |
| PCKD | 39 (3.3) | 3 (2.3) | 11 (3.1) | 3 (5.7) | |
| Unknown | 267 (22.4) | 33 (25.2) | 65 (18.2) | 14 (26.4) | |
| Others | 110 (9.2) | 16 (12.2) | 31 (8.7) | 3 (5.7) | |
| Dialysis | <0.01 | ||||
| HD | 777 (65.3) | 96 (73.3) | 275 (76.8) | 42 (79.2) | |
| CAPD | 132 (11.1) | 14 (10.7) | 28 (7.8) | 3 (5.7) | |
| Pre-emptive | 280 (23.6) | 21 (16.0) | 55 (15.4) | 8 (15.1) | |
| Duration of dialysis, months | 20.9±37.9 | 23.0±37.2 | 20.2±33.2 | 24.5±36.4 | 0.80 |
| Previous transplant | 58 (4.8) | 21 (16.1) | 22 (6.1) | 13 (24.5) | <0.01 |
| Calcineurin inhibitor | 0.03 | ||||
| Tacrolimus | 815 (68.5) | 104 (79.4) | 260 (72.6) | 41 (77.4) | |
| Cyclosporin | 375 (31.5) | 27 (20.6) | 98 (27.4) | 12 (22.6) | |
| Rituximab dose, mg | – | 390±194 | 196±29 | 392±145 | <0.01d,f |
| Pre-transplant PP, number | – | 5.5±4.6 | 3.0±1.5 | 6.4±5.5 | <0.01d,f 0.04e |
| HLA-incompatible* | 0.18e | ||||
| CDC (+) | – | 15 (11.5) | – | 10 (18.9) | |
| FCXM (+) | – | 97 (74.0) | – | 32 (60.4) | |
| DSA MFI ≥5000 & FCXM (−) | – | 19 (14.5) | – | 11 (20.8) | |
| HLA-A,B,DR mismatch | 3.0±1.6 | 3.4±1.7 | 3.4±1.6 | 3.4±1.4 | 0.03a <0.01b |
| PRA class I | 6.9±18.5 | 45.2±38.6 | 9.2±19.9 | 43.8±35.6 | <0.01a,c,d,f |
| PRA class II | 7.9±19.8 | 35.6±39.4 | 10.0±21.5 | 48.3±38.8 | <0.01a,c,d,e,f |
| Maximal DSA MFI | 346±910 | 9367±4349 | 495±1067 | 7292±4193 | <0.01a,c,d,e,f |
Categorical variables are presented as counts and percentages. Continuous variables are presented as means and standard deviations. ABOc – ABO-compatible; ABOi – ABO-incompatible; BMI – body mass index; ESRD – end-stage renal disease; GN – glomerulonephritis; FSGS – focal segmental glomerulosclerosis; PCKD – polycystic kidney disease; HD – hemodialysis; CAPD – continuous ambulatory peritoneal dialysis; PP – plasmapheresis; CDC – complement-dependent cytotoxicity; FCXM – flow-cytometric crossmatch; MFI – mean fluorescence intensity; HLA – human leukocyte antigen; PRA – panel reactive antibody; DSA – donor-specific antibody.
HLA-incompatible kidney transplantation was defined as CDC XM, FCXM-positive, and/or maximal DSA MFI ≥5000.
P-value between ABOc & HLAc group and ABOc & HLAi group;
P-value between the ABOc & HLAc group and the ABOi & HLAc group;
P-value between the ABOc & HLAc group and the ABOi & HLAi group;
P-value between the ABOc & HLAi group and the ABOi & HLAc group;
P-value between the ABOc & HLAi group and the ABOi & HLAi group;
P-value between the ABOi & HLAc group and the ABOi & HLAi group.
Infectious complications
Infections of BK viremia ≥4 log were significantly different among the 4 groups, with the incidence being more than 2-fold higher in the ABOi groups compared with the ABOc groups (23.6% vs. 8.4%, P<0.01). The incidence of CMV viremia ≥4 log was significantly higher in the HLAi groups than in the HLAc groups (12.0% vs. 8.0%, P<0.01). Urinary tract infection occurred less frequently in the ABOc/HLAc group and the ABOi/HLAc group than in the other groups (P<0.01). The incidence of pneumonia was not significantly different among the study groups. The ABOi/HLAi group had the highest combined infection rate among the study groups, and the ABOc/HLAc group had the lowest combined infection rate (P<0.01). The incidence of mortality due to infectious complications in the ABOi/HLAi group (4 cases, 7.5%) was significantly higher than those in the other groups (Table 2).
Table 2.
Infection prior to rejection.
| Variables | ABOc & HLAc (N=1190) | ABOc & HLAi* (N=131) | ABOi & HLAc (N=358) | ABOi & HLAi* (N=53) | p-Value |
|---|---|---|---|---|---|
| BK viremia positive | 294 (24.7) | 23 (17.6) | 95 (26.5) | 14 (26.4) | 0.23 |
| BK viremia PCR ≥4 logs | 102 (8.6) | 10 (7.6) | 84 (23.5) | 13 (24.5) | <0.01 |
| CMV viremia positive | 443 (37.2) | 67 (51.1) | 129 (36.0) | 27 (50.9) | <0.01 |
| CMV viremia PCR ≥4 logs | 106 (8.9) | 16 (12.2) | 18 (5.0) | 6 (11.3) | 0.03 |
| Urinary tract infection | 133 (11.2) | 25 (19.1) | 29 (8.1) | 11 (20.8) | <0.01 |
| Pneumonia | 65 (5.5) | 9 (6.9) | 20 (5.6) | 4 (7.5) | 0.75 |
| Combined infection** | 318 (26.7) | 43 (32.8) | 127 (35.5) | 25 (47.2) | <0.01 |
| Mortality due to infection | 7 (0.6) | 1 (0.8) | 3 (0.8) | 4 (7.5) | <0.01 |
Categorical variables are presented as counts and percentages. ABOc – ABO-compatible; ABOi – ABO-incompatible; HLA – human leukocyte antigen; CMV – cytomegalovirus.
HLA-incompatible kidney transplantation was defined as CDC XM, FCXM-positive, and/or maximal DSA MFI ≥500;
Combined infection consisted of BK viremia PCR ≥4 logs, CMV viremia PCR ≥4 logs, urinary tract infection, and pneumonia.
Overall patient survival and graft survival rates
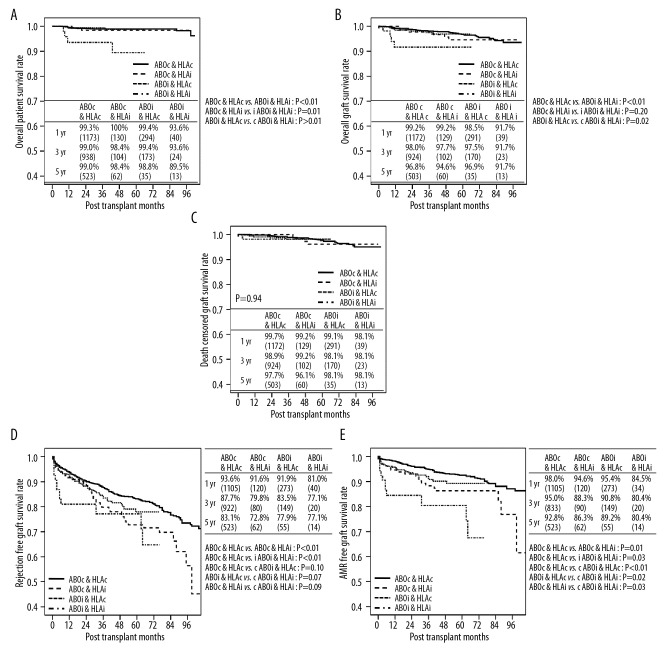
Mortality occurred in a total of 23 cases during the study period. The overall patient survival rates at 1, 3, and 5 years were 99.1%, 98.7%, and 98.4%, respectively. The ABOi/HLAi group showed a significantly lower survival rate compared with the other groups (vs. ABOc/HLAc, P<0.01; vs. ABOc/HLAi, P=0.01; vs. ABOi/HLAc, P<0.01) (Figure 1A). The overall GS rates at 1, 3, and 5 years were 98.8%, 97.7%, and 96.2%, respectively. The 5-year GS rate was the lowest in the ABOi/HLAi group (91.7%), which was significantly inferior compared with the ABOc/HLAc group and the ABOc/HLAi group (P<0.001 and P=0.02, respectively) but not significantly different from the ABOi/HLAc group (P=0.20) (Figure 1B). Death-censored GS did not reveal a significant difference among the 4 groups (Figure 1C).
Figure 1.
Long-term survival after kidney transplantation according to ABO and HLA incompatibilities. (A) Overall patient survival, (B) overall graft survival, (C) death-censored graft survival, (D) rejection-free graft survival, and (E) antibody-mediated rejection-free graft survival.
Comparison of acute rejections and antibody-mediated rejections
The overall rejection rate at 1 year was 6.9%, 15.3%, 8.7%, and 20.8% in the ABOc/HLAc, ABOc/HLAi, ABOi/HLAc, and ABOi/HLAi groups, respectively. The 1-year rejection rates were significantly higher in the ABOc/HLAi group and ABOi/HLAi group than in the HLAc groups (P<0.01) due to the higher rate of antibody-mediated rejection (AMR) (P<0.01). There were no significant differences among study groups in the rate of acute cellular rejection (P=0.70) (Table 3).
Table 3.
Incidence of rejection at 1 year after kidney transplantation.
| Variables | ABOc & HLAc (N=1190) | ABOc & HLAi* (N=131) | ABOi & HLAc (N=358) | ABOi & HLAi* (N=53) | p-Value |
|---|---|---|---|---|---|
| Overall rejection at 1 year | 71 (6.0) | 11 (8.4) | 28 (7.8) | 9 (17.0) | 0.01 |
| ACR only | 46 (3.9) | 3 (2.3) | 12 (3.4) | 1 (1.9) | 0.70 |
| Overall AMR with or without ACR | 25 (2.1) | 8 (6.1) | 16 (4.5) | 8 (15.1) | <0.01 |
Categorical variables are presented as counts and percentages. ACR – acute cellular rejection; AMR – acute antibody-mediated rejection.
HLA-incompatible kidney transplantation was defined as CDC XM, FCXM-positive, and/or maximal DSA MFI ≥5000.
The rate of RFGS was significantly lower in the ABOc/HLAi group and the ABOi/HLAi group compared with the ABOc/HLAc group (P<0.01 in both groups). No significant difference in the RFGS was observed between the ABOi/HLAc group and the ABOc/HLAc group (P=0.10). There was also no significant difference in the RFGS between the ABOi/HLAi group and either the ABOi/HLAc group or the ABOc/HLAi group (P=0.07 and P=0.09, respectively) (Figure 1D). When only the AMR was taken into account, the 3 groups with incompatibilities showed inferior AMR-free GS rates compared with the ABOc/HLAc group (P=0.01 vs. ABOc/HLAi, P=0.03 vs. ABOi/HLAc, P<0.001 vs. ABOi/HLAi). The rate of AMR-free GS was the lowest in the ABOi/HLAi group, which was significantly inferior compared with the ABOi/HLAc group and the ABOc/HLAi group (P=0.02 and P=0.03, respectively) (Figure 1E).
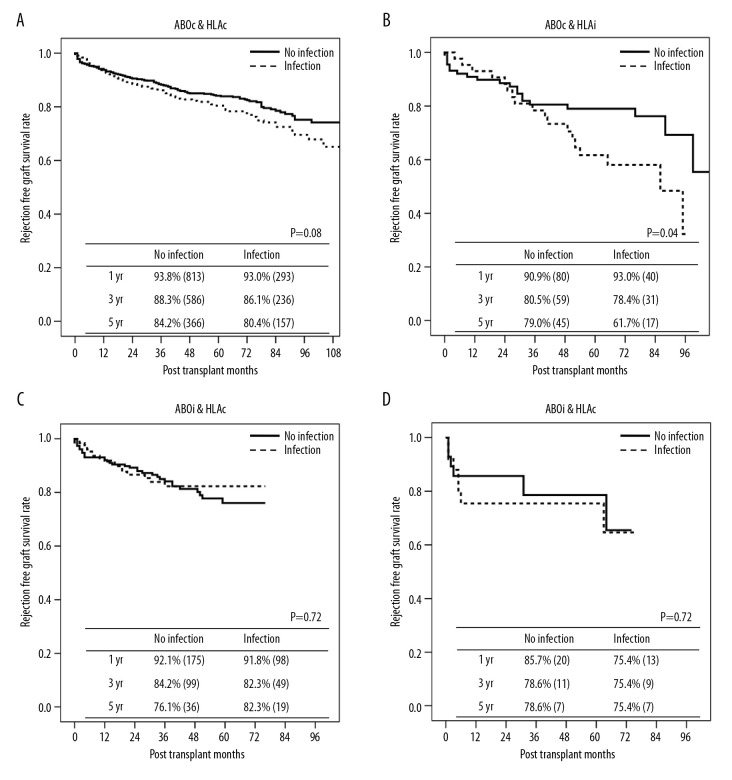
We also evaluated the long-term rejection rate of each group according to the presence of infection before rejection (Figure 2). Recipients in the ABOc/HLAc group with infectious complications tended to have a higher rate of rejection, albeit without statistical significance (P=0.08). Within the ABOc/HLAi group, the rate of RFGS was significantly lower in patients with infection compared with those without infection (P=0.04). There were no significant differences in the RFGS within the ABOi/HLAc and ABOi/HLAi groups based on the history of infection (P=0.72 and P=0.62, respectively). The median time to rejection after infection was 13 months (interquartile range, 4.0–37.0 months)
Figure 2.
Rejection-free graft survival stratified by infection history. (A) ABO- and HLA-compatible group, (B) ABO-compatible and HLA-incompatible group, (C) ABO-incompatible and HLA-compatible group, and (D) ABO- and HLA-incompatible group.
Univariate and multivariate Cox proportional hazards regression analyses were carried out to identify the risk factors for acute rejection (Table 4). After adjustment for possible confounding factors, multivariable analysis showed that ABOi/HLAi (hazard ratio [HR], 2.63; 95% confidence interval [CI], 1.04–6.65; P<0.01) and combined infection (HR, 1.91; 95% CI, 1.45–2.51; P<0.01) were independent risk factors for acute rejection.
Table 4.
Factors associated with the occurrence of acute rejection.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Female (vs. Male) | 1.05 (0.76–1.45) | 0.76 | NA | NA |
| Cyclosporin (vs. tacrolimus) | 1.22 (0.87–1.70) | 0.25 | NA | NA |
| PP number | 1.09 (1.04–1.14) | <0.01 | 1.01 (0.94–1.08) | 0.83 |
| Rituximab dose=0 mg | Reference | |||
| 100–299 mg | 1.22 (0.91–1.65) | 0.19 | NA | NA |
| 300–500 mg | 1.36 (0.93–1.98) | 0.11 | NA | NA |
| BK viremia PCR ≥4 logs | 1.54 (1.23–1.94) | <0.01 | NA | NA |
| CMV viremia PCR ≥4 logs | 1.81 (1.46–2.24) | <0.01 | NA | NA |
| Urinary tract infection | 1.25 (0.92–1.69) | 0.16 | NA | NA |
| Pneumonia | 2.00 (1.41–2.86) | <0.01 | NA | NA |
| Combined infection* | 1.89 (1.47–2.43) | <0.01 | 1.91 (1.45–2.51) | <0.01 |
| ABOc & HLAc | Reference | |||
| ABOc & HLAi | 1.29 (0.87–1.90) | 0.20 | 1.90 (0.79–4.57) | 0.15 |
| ABOi & HLAc | 1.22 (0.89–1.66) | 0.22 | 1.22 (0.83–1.78) | 1.22 |
| ABOi & HLAi | 1.93 (1.05–3.55) | 0.34 | 2.63 (1.04–6.65) | 0.04 |
| HLA-compatible | Reference | |||
| CDC-positive | 3.13 (1.61–6.09) | <0.01 | 1.25 (0.39–4.04) | 0.71 |
| FCXM-positive | 1.08 (0.72–1.63) | 0.72 | 0.52 (0.21–1.34) | 0.52 |
PP – plasmapheresis; CMV – cytomegalovirus; ABOc – ABO-compatible; ABOi – ABO-incompatible; HLA – human leukocyte antigen; CDC – complement-dependent cytotoxicity; FCXM – flow-cytometric crossmatch; DSA – donor-specific antibody.
Combined infection consisted of BK viremia PCR ≥4 logs, CMV viremia PCR ≥4 logs, urinary tract infection, and pneumonia.
Discussion
In this study, we found that infection is a significant risk factor for acute rejection in ABOi or HLAi KT. Desensitization and depletion of antibodies using PP, antigen-specific immunoadsorption, and B-cell suppression with rituximab have enabled transplants across immunologic barriers. However, infectious complications resulting from these immunologic treatments increase the risks of rejection and reduce the overall GS. Notably, the high mortality rate in the ABOi/HLAi group due to lethal infection suggests that reaching the goal of desensitization is more challenging in patients who have both ABO and HLA antibodies. We used a higher dose of rituximab in HLAi KT than in ABOi KT. In addition, pretransplant PP was the highest in the ABOi/HLAi group. Padmanabhan et al. [23] reported similar results, which showed that patients who had both immunologic barriers needed more treatments during the perioperative period. There is also a possibility of immunologic vulnerability against pathogens in the ABOi/HLAi group. Although we are not able to suggest the exact mechanism at this point, the notably higher rate of mortality due to infection in the ABOi/HLAi group compared with the ABOc/HLAi group seems to support this hypothesis.
Among the HLAc groups in our study, ABOi KT showed a similar overall GS with ABOc KT. A registry-based analysis comparing living-donor ABOi and ABOc KTs revealed a higher early graft failure rate following ABOi KT. However, there was no significant difference in long-term patient survival [6]. Recent studies also reported that the intermediate-term survival rates of ABOi KT were comparable to those of ABOc KT [24–26]. However, the rates of early mortality related to infectious complications were reported to be significantly higher among the ABOi recipients [4]. We also observed early lethal infectious complications in 89 cases of ABOi KT in which patients were administered 500 mg rituximab for desensitization [16,27]. Seven deaths occurred during the study period, 6 of which were caused by infection. After reducing the rituximab dose from 500 to 200 mg and the mycophenolate mofetil dose from 1.5 to 1.0 g/d, we achieved successful GS in ABOi KT [16,27].
RFGS was somewhat inferior in the ABOi/HLAc group compared with the ABOc/HLAc group (P=0.10). Among the patients in the HLAc groups, ABOi KT showed a significantly lower long-term AMR-free GS than ABOc KT (P=0.03). Considering that only 2 cases of early clinical rejection due to ABO antibodies occurred and that grafts usually achieved accommodation after critical periods, especially the first 2 weeks after transplantation, we assume that the higher acute rejection rate of ABOi KT was likely due to infection [28,29]. Although subgroup analysis showed similar RFGS rates among the patients in the ABOi/HLAc group according to combined infection episodes prior to rejection, BK viremia ≥4 log were more frequently observed in the ABOi/HLAc group; therefore, the high rate of BK viremia (23.5%) might be the cause of the high rejection rate in this group. Our center previously reported that the immunosuppression reduction for the control of BK viremia was associated with acute rejection [30]. Even with a lower dose of rituximab, the ABOi/HLAc group showed a greater tendency to have high-titer BK virus infection than the ABOc/HLAi group, which is consistent with the recent study by Sharif et al. [31].
The overall patient survival rate was significantly lower in the ABOi/HLAi group than the other 3 groups. Recent studies reported that the mortality rate caused by infectious complications was high in ABOi KT, and that tailored desensitization treatment may reduce posttransplant infections [4,24]. These findings suggest that the degree of desensitization is a more potent risk factor than the status of ABOi or HLAi for infection-related death [18]. The GS was inferior in the ABOi/HLAi group compared with the ABOc/HLAc and ABOi/HLAc groups, and DCGS did not significantly differ across the 4 groups. These findings collectively suggest that the inferior GS rate of the ABOi/HLAi group can be attributed to the difference in patient survival. We administered trimethoprim-sulfamethoxazole (80–400 mg) daily as prophylaxis for P. jirovecii pneumonia during the 6 months after KT. Among the 542 patients who received rituximab, there were 4 cases of mortality resulting from P. jirovecii pneumonia. We published an article recommending the extension of the prophylaxis duration to more than 6 months in patients who receive rituximab [32]. To improve the GS of immunologically high-risk patients, an attempt to apply minimal immunosuppression that can sustain graft function without rejection is required. Close monitoring and proper prophylaxis for preventable infectious complications are also needed. Meanwhile, the efficacy of rituximab induction in preventing transplant rejection is controversial [33]. A randomized controlled study evaluating the effect of single-dose (375 mg/m2) rituximab induction did not demonstrate a significant difference in the incidence of infection between the rituximab and control groups with immunological benefits [34]. Kamar et al. [35] suggested that the combined use of rituximab with anti-thymocyte-globulin induction significantly increased the risk of infection-related death [35]. We used basiliximab as an induction agent to avoid infectious complications in recipients treated with rituximab. Further studies are required to determine the proper dose of rituximab, the combination of desensitization treatment, and the type of induction drug.
In our study, the RFGS tended to be lower in the ABOi/HLAi group compared with the ABOc/HLAi group. Moreover, the AMR-free survival rate was the lowest in the ABOi/HLAi group among the study groups. Subgroup analysis showed no significant difference in the rejection rate among patients in the ABOi/HLAi group when stratified by infection episodes. Multivariate analysis also showed that ABOi/HLAi was a significant risk factor for rejection. Therefore, we can infer that there is a possibility of synergistic effects between anti-HLA and anti-blood group A/B antibodies. In our recent study using combined data from a nationwide cohort registry, we suggested that the anti-HLA and anti-blood group antibodies may have a synergistic effect [32]; however, this cohort study had several limitations because several variables that were needed for proper interpretation of the results were missing, including specified data of infectious complications and desensitization treatment. Although the present study is from a single center, we have analyzed a large number of HLAi/ABOi patients with unified protocolized desensitization treatment and monitoring schedules. Therefore, we believe that our study potentially excluded the possibility of heterogeneity that limits multicenter cohort studies and fortified the previously established hypothesis on the synergistic effects in recipients with double immunologic barriers.
Infections are well-known risk factors for adverse outcomes in graft and patient survival. The consequences of infectious complications following KT vary from direct injury of the graft to associated inflammation resulting from indirect pathways, including rejection due to the activation of the alloimmune reaction that has cross-reactivity to graft antigens [32]. Many previous studies demonstrated the cross-reactivity of CMV-specific T cells to HLA [36,37]. It is unclear whether the patients who experienced infection were more likely to develop rejection due to the direct effects of the infection or the immunologic reactions from cross-reactive antibodies. Gut microbial dysbiosis by infection and antiviral or microbial therapy may be a possible explanation for the increased risk of rejection after infection [38]. The novel finding of our research is that infection is an independent risk factor even when taking the immunologic risk factors into account.
Our study has some limitations. First, there may have been selection biases due to its retrospective design. Second, the smaller number of recipients in the ABOi/HLAi group compared with the other groups may have reduced the statistical power. However, as far as we know, our study analyzed the largest number of patients stratified by ABO and HLA incompatibility to date. Third, our study results may have racial differences from non-Asian populations in terms of genetic or medical circumstances. In addition, the patients in our study were relatively young and had a lower rate of comorbidities than Western patients. Therefore, the 5-year overall PS (98%) and GS (92%) rates in this study showed much better outcomes than those in European and United Network for Organ Sharing data [39,40]. However, recent studies involving Asian or Korean patients have reported similar clinical outcomes as those in our study [41,42]. Consideration of these factors are needed to interpret the results.
Conclusions
Patients who had both ABO and HLA incompatibilities showed inferior rates of overall GS, which was mainly due to infectious complications. Infection was a prominent risk factor for acute rejection following KT after adjusting for possible confounders including ABO and HLA incompatibility. Our results suggest that a balance between immunosuppression and the prevention of infectious complications is needed to improve clinical outcomes in immunologically high-risk patients. Further research is required to reveal the mechanism for the immunologic vulnerability against pathogens and synergistic effects between anti-HLA antibodies and anti-blood group A/B antibodies.
Acknowledgments
Hyunwook Kwon would like to express his sincere appreciation to his principal supervisor, Professor Yong-Pil Cho.
Footnotes
Conflict of interest
None.
Source of support: Departmnetal sources
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 3.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940–50. doi: 10.1056/NEJMoa1508380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opelz G, Morath C, Susal C, et al. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99:400–4. doi: 10.1097/TP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe K, Ishida H, Inui M, et al. ABO-incompatible kidney transplantation: Long-term outcomes. Clin Transpl. 2013:307–12. [PubMed] [Google Scholar]
- 6.Montgomery JR, Berger JC, Warren DS, et al. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–9. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins R, Lowe D, Hathaway M, et al. Human leukocyte antigen antibody-incompatible renal transplantation: Excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900–6. doi: 10.1097/TP.0b013e31822dc38d. [DOI] [PubMed] [Google Scholar]
- 8.Willicombe M, Brookes P, Santos-Nunez E, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011;11:470–77. doi: 10.1111/j.1600-6143.2010.03421.x. [DOI] [PubMed] [Google Scholar]
- 9.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76–85. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo AA, Sinha A, Haas M, et al. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation. 2015;99:1423–30. doi: 10.1097/TP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 12.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am J Transplant. 2014;14:1573–80. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 13.de Weerd AE, Betjes MGH. ABO-incompatible kidney transplant outcomes: A meta-analysis. Clin J Am Soc Nephrol. 2018;13:1234–43. doi: 10.2215/CJN.00540118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustian MN, Cannon RM, MacLennan PA, et al. Landscape of ABO-incompatible live donor kidney transplantation in the US. J Am Coll Surg. 2018;226:615–21. doi: 10.1016/j.jamcollsurg.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon H, Kim YH, Kim JY, et al. The results of HLA-incompatible kidney transplantation according to pre-transplant crossmatch tests: Donor-specific antibody as a prominent predictor of acute rejection. Clin Transplant. 2019;33:e13533. doi: 10.1111/ctr.13533. [DOI] [PubMed] [Google Scholar]
- 16.Kwon H, Kim YH, Choi JY, et al. Analysis of 4000 kidney transplantations in a single center: Across immunological barriers. Medicine (Baltimore) 2016;95:e4249. doi: 10.1097/MD.0000000000004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon H, Kim YH, Choi JY, et al. Impact of pretransplant donor-specific antibodies on kidney allograft recipients with negative flow cytometry cross-matches. Clin Transplant. 2018;32:e13266. doi: 10.1111/ctr.13266. [DOI] [PubMed] [Google Scholar]
- 18.Ko EJ, Yu JH, Yang CW, Chung BH. Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: A nationwide cohort study. Transpl Int. 2017;30:1215–25. doi: 10.1111/tri.12979. [DOI] [PubMed] [Google Scholar]
- 19.Kwon H, Kim JY, Kim DH, et al. Effect of simultaneous presence of anti-blood group A/B and -HLA antibodies on clinical outcomes in kidney transplantation across positive crossmatch: A nationwide cohort study. Sci Rep. 2019;9:18229. doi: 10.1038/s41598-019-54397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek CH, Kim H, Yang WS, et al. Clinical significance of isoagglutinin titre with the current desensitization protocol in ABO-incompatible kidney transplantation. Nephrology (Carlton) 2019;24:654–60. doi: 10.1111/nep.13412. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Choe W, Shin S, et al. ABO-incompatible kidney transplantation can be successfully conducted by monitoring IgM isoagglutinin titers during desensitization. Transfusion. 2020;60:598–606. doi: 10.1111/trf.15672. [DOI] [PubMed] [Google Scholar]
- 22.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–83. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 23.Padmanabhan A, Ratner LE, Jhang JS, et al. Comparative outcome analysis of ABO-incompatible and positive crossmatch renal transplantation: A single-center experience. Transplantation. 2009;87:1889–96. doi: 10.1097/TP.0b013e3181a76ae1. [DOI] [PubMed] [Google Scholar]
- 24.Barnett AN, Manook M, Nagendran M, et al. Tailored desensitization strategies in ABO blood group antibody incompatible renal transplantation. Transpl Int. 2014;27:187–96. doi: 10.1111/tri.12234. [DOI] [PubMed] [Google Scholar]
- 25.Wilpert J, Fischer KG, Pisarski P, et al. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778–86. doi: 10.1093/ndt/gfq229. [DOI] [PubMed] [Google Scholar]
- 26.Habicht A, Broker V, Blume C, et al. Increase of infectious complications in ABO-incompatible kidney transplant recipients – a single centre experience. Nephrol Dial Transplant. 2011;26:4124–31. doi: 10.1093/ndt/gfr215. [DOI] [PubMed] [Google Scholar]
- 27.Choi BH, Cho HK, Jung JH, et al. How to reduce lethal infectious complications in ABO-incompatible kidney transplantation. Transplant Proc. 2015;47:653–59. doi: 10.1016/j.transproceed.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11:128–41. doi: 10.1007/s10157-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 29.Tasaki M, Saito K, Nakagawa Y, et al. Acquired downregulation of donor-specific antibody production after ABO-incompatible kidney transplantation. Am J Transplant. 2017;17:115–28. doi: 10.1111/ajt.13937. [DOI] [PubMed] [Google Scholar]
- 30.Baek CH, Kim H, Yu H, et al. Risk factors of acute rejection in patients with BK nephropathy after reduction of immunosuppression. Ann Transplant. 2018;23:704–12. doi: 10.12659/AOT.910483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:1320–27. doi: 10.2215/CJN.00770112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YH, Kim JY, Kim DH, et al. Pneumocystis pneumonia occurrence and prophylaxis duration in kidney transplant recipients according to perioperative treatment with rituximab. BMC Nephrol. 2020;21:93. doi: 10.1186/s12882-020-01750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohmig GA, Farkas AM, Eskandary F, Wekerle T. Strategies to overcome the ABO barrier in kidney transplantation. Nat Rev Nephrol. 2015;11:732–47. doi: 10.1038/nrneph.2015.144. [DOI] [PubMed] [Google Scholar]
- 34.van den Hoogen MW, Kamburova EG, Baas MC, et al. Rituximab as induction therapy after renal transplantation: A randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. 2015;15:407–16. doi: 10.1111/ajt.13052. [DOI] [PubMed] [Google Scholar]
- 35.Kamar N, Milioto O, Puissant-Lubrano B, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10:89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- 36.Ishibashi K, Tokumoto T, Shirakawa H, et al. The presence of antibodies against the AD2 epitope of cytomegalovirus glycoprotein B is associated with acute rejection after renal transplantation. Microbiol Immunol. 2014;58:72–75. doi: 10.1111/1348-0421.12112. [DOI] [PubMed] [Google Scholar]
- 37.ten Berge I, Heutinck K, Yapici U, et al. The interplay between antiviral immunity and allo-immune reactivity after renal transplantation: Consortium between the Centres Amsterdam, Leiden and Nijmegen (ALLOVIR) Transpl Immunol. 2014;31:191–94. doi: 10.1016/j.trim.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad S, Bromberg JS. Current status of the microbiome in renal transplantation. Curr Opin Nephrol Hypertens. 2016;25:570–76. doi: 10.1097/MNH.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oettl T, Halter J, Bachmann A, et al. ABO blood group-incompatible living donor kidney transplantation: A prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24:298–303. doi: 10.1093/ndt/gfn478. [DOI] [PubMed] [Google Scholar]
- 40.Opelz G, Döhler B, Ruhenstroth A, et al. The collaborative transplant study registry. Transplant Rev (Orlando) 2013;27:43–45. doi: 10.1016/j.trre.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Lim JH, Cho JH, Jung HY, et al. Excellent outcome after desensitization in high immunologic risk kidney transplantation. PLoS One. 2019;14:e0222537. doi: 10.1371/journal.pone.0222537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiyama N, Satoh S, Saito M, et al. Impact of persistent preformed and de novo donor-specific antibodies detected at 1 year after kidney transplantation on long-term graft survival in Japan: A retrospective study. Clin Exp Nephrol. 2019;23:1398–406. doi: 10.1007/s10157-019-01780-z. [DOI] [PubMed] [Google Scholar]