Introduction:
This review seeks to consolidate and summarize the existing literature regarding exposure of the parturient to halogen gas or to gases or vapors of halogenated molecules and the consequent maternal and fetal considerations of halogen toxicity. Given the limitations of currently existing peer-reviewed literature regarding halogen exposure in pregnancy, we focus this review primarily on bromine and cardiopulmonary injury. Additionally, we will discuss potential therapeutic targets and considerations for future investigation.
The elemental halogens are oxidizing and electrophile agents comprising Group 17 of the periodic table with a multitude of commercial and industrial uses. While these elements are utilized broadly in a variety of contexts in the modern era, the history of inhaled anesthetics provides an informative context through which to understand the potential benefits, toxic potential, and weaponization of halogen derivatives. The halogenated inhalational anesthetics are a prime example of the medicinal utility of the halogens with halothane being halogenated with fluorine, chlorine, and bromine; isoflurane with fluorine and chlorine; and both sevoflurane and desflurane halogenated only with fluorine(Suckling and Raventos 1958; Sherer and Kuhn 1960; Suckling and Raventos 1960; Rozov et al. 1993; Rozov et al. 1995; Terrell 1999).
Interestingly, the impetus for the development of today’s volatile anesthetic agents was partially due to inadvertent halogen gas injury caused by earlier iterations of inhaled anesthetics. The clinical use of ether and chloroform, two agents employed in the early days of anesthesiology, brought to light significant safety concerns. In particular, the delivery of a chloroform anesthetic posed significant risks to physicians as well as their patients due to the decomposition of the gas to hydrogen chloride, chlorine, and phosgene gases due to exposure of chloroform to oxygen and to the open flames which provided light in the operating rooms of the time. The dangerous combination of open flame and chloroform led to numerous cases of halogenated inhalational injury including incidents reported in a case series from The Lancet in 1894 where the authors wrote, “Under certain circumstances attending its administration, chloroform may act as a severe irritant to the larynx, producing spasmodic cough and dyspnea; secondarily these toxic effects are not exerted upon the patient but upon the operator and his assistants.(Lee 1894; Firth 2016)” Additional reports of upper respiratory symptoms and delayed complications continued to emerge, including several deaths of patients, physicians, and nurses attributed to the combination of chloroform anesthesia, open flames, and poor ventilation. These incidents helped to further encourage development of more stable inhalational anesthetics(Firth 2016). Contemporaneously, diphosgene, a modification of the chloroform degradation product phosgene, was unleashed on the battlefield of Verdun demonstrating the devastating potential of employing halogenated gas as a chemical weapon. Thus, through attempts to avoid inhalational halogen toxicity, the process of halogenation was employed to craft more stable volatile agents suitable for clinical use resulting in the modern class of halogenated volatile anesthetics. In succession, halothane, isoflurane and the sevoflurane were developed with toxicity reduced at each step.
Today the non-medical applications of the halogens are myriad with bromine having continued value in photographic development, dyes, purification agents, disinfectants, and as a flame retardant. Chlorine remains commonly utilized for water sanitation and is employed broadly for numerous industrial and medicinal processes. Several reports indicate that bromine is a more effective disinfectant than chlorine resulting in an increased utilization of bromine for drinking water purification(Floyd et al. 1976; Coulliette et al. 2010; World Health Organization 2018). To facilitate this demand global production of bromine exceeds 500,000 tons annually with the United States, China, and Israel (where the Dead Sea serves as an important source of bromine) responsible for the majority of production(The International Bromine Council). Chlorine is produced on a larger scale with a global estimation of 58 million metric tons of chlorine and 62 million metric tons of sodium hydroxide (a co-product of chlorine) produced annually(World Chlorine Council).
The production process, transport, storage, and utilization of halogens provides numerous potential interfaces for human exposures through accident or deliberate malicious intent. A 2011 railroad accident resulted in release of an estimated 24–50 liters of bromine engulfing a portion of Chelyabinsk, Russia in a cloud of vapor and leading to at least 42 hospitalizations and over 200 patients seeking medical attention(BBC News 2011). Graniteville, South Carolina was the site of another calamitous train accident in 2005 where a collision released 11,500 gallons of chlorine resulting in hundreds of injuries and 9 deaths(Fretwell 2015). A recent incident at a water treatment facility in Birmingham, Alabama resulted in exposure of several individuals to chlorine gas with over 50 patients presenting to local hospitals and some exposed individuals subsequently requiring hospitalization(WVTM 13 2019).
The unpredictable nature of a small-scale or mass casualty incident necessitates that clinicians and researchers assess for threats to numerous unique and potentially vulnerable demographic groups including infants, children, elderly individuals, and pregnant women. At any given time pregnant women are estimated to account for 1–2% of the general population in the United States and up to 5% of women of reproductive age are pregnant or up to 6 weeks postpartum(Mosher et al. 2004; Jamieson et al. 2009; Centers for Disease Control and Prevention 2019). The Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (H.R. 307) identifies women within the peripartum period as a population with special clinical needs, recognizing the unique pathophysiological milieu as potentially increasing risk in the event of a natural or man-made disaster or pandemic(Centers for Disease Control and Prevention 2019). The normal maternal physiological adaptations to pregnancy that allow for fetal growth and development along with maternal hemostasis in the peripartum period have significant implications for cardiorespiratory function and consequently create a unique risk profile for exposure to cardiopulmonary toxins.
Bromine gas exposure:
Bromine exists as a corrosive, volatile liquid at room temperature and violation of a container or lead-lined tank will result in release of an orange-tinted malodorous vapor. Occupational exposures may occur in facilities utilizing bromine for industrial purposes or during routine maintenance of pools or hot tubs. Transportation mishaps also may result in individual or large-scale exposures as occurred in Russia and South Carolina. Bromine storage facilities are potential targets for terrorist attacks as demonstrated by a suicide attack in Ashdod, Israel in 2004 where the explosive detonation at a port fortunately did not destroy the nearby bromine tanks thought to be the target of the attack(Makarovsky et al. 2007). Bromine and chlorine content of tobacco smoke is largely overlooked, but well-documented(Häsänen et al. 1990; Müller et al. 2011; Müller et al. 2012; Azman et al. 2016). Finally, direct employment of bromine gas as a weapon by regimes or terrorist factions remains a possibility.
Direct pulmonary toxicity of halogen exposure:
Bromine gas exposure can acutely lead to bronchospasm, lung edema, respiratory distress, and alveolar hemorrhage with the eventual development of chronic fibrosis or peribronchiolar abscesses noted in survivors(Lam et al. 2016). The US Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) lists an Immediately Dangerous to Life or Health Concentration (IDLH) of 3 ppm for bromine based on acute toxicity data in humans. The NIOSH recommended exposure limit is 0.1 ppm and the US Department of Labor Occupational Safety and Health Administration (OSHA) takes a similar stance and lists a permissible exposure limit of 0.1 ppm(Centers for Disease Control and Prevention National Institute for Occupational Safety and Health 2014). The National Advisory Committee for Acute Exposure Guideline Levels (AEGL) for Hazardous Substances are based on small human studies where eye irritation manifested at lower concentrations (basis for AEGL-1 [non-disabling] of 0.033 ppm for any exposure duration) followed by conjunctival, nose, and throat irritation (basis for AEGL-2 [disabling] of 0.095 – 0.55 contingent upon exposure duration). Lethal doses (AEGL-3) were derived from animal experiments and influenced by noted lethal dose of chlorine gas and range from 19 ppm for a 10m exposure to 3.3 ppm for 8 hours(National Research Council Committee on Acute Exposure Guideline Levels 2010). Of note, our data indicate a higher lethal dose for mice. Data on large animals is lacking.
In mice post-exposure to Br2, elevated levels of heme were found in lung tissue, bronchoalveolar lavage (BAL) fluid, and plasma. Increased measured heme levels correlated with lung injury, inflammation, and oxidative stress. Facilitation of heme degradation by hemopexin administered post-exposure improved survival of the mice and reduced lung injury and inflammation(Lam et al. 2016). Additionally, in mice exposed to Br2 a biphasic pattern of morbidity and mortality is observed with a high immediate mortality followed by a 4–5 day plateau leading into a subsequent second spike in mortality(Aggarwal et al. 2016; Lam et al. 2016). This second, chronic phase of injury is characterized by peribronchial fibrosis and emphysema-like enlargement of alveoli(Aggarwal et al. 2018). The developing lung appears to be especially sensitive to the toxic effects of bromine. Exposure of neonatal mice to bromine resulted in impaired alveolar development, inflammation, and altered gene expression indicative of severe derangements in lung development(Jilling et al. 2018).
Systemic absorption and distribution:
While pulmonary injury is perhaps the most dramatic and immediately apparent sequelae of Br2 exposure, broader systemic involvement was observed in both humans and in animal models post bromine exposure. Table 1 summarizes the acute and chronic effects of bromine exposure by organ system. The systemic distribution of symptoms distant from the site of acute exposure (ie. skin, mucous membranes, and the lungs) may reflect downstream effects of mediators generated by bromine exposure, such as brominated lipids disseminating throughout the body, free heme generated during the course of the injury, and the secondary effects of inflammatory and hormonally-mediated stress responses(Aggarwal et al. 2016; Lam et al. 2016; Aggarwal et al. 2018; Duerr et al. 2018; Ahmad et al. 2019).
Table 1.
Systemic clinical manifestations and associations of acute and chronic bromine exposure
| Organ System | Clinical and systemic manifestations or associations | |
|---|---|---|
| Integumentary | Irritation, vesicles, pustules, blistering | Contact dermatitis |
| Ulcerating chemical burns | Halogen acne* | |
| Tissue necrosis | Bromoderma tuberosum* | |
| Endocrine | Hypothyroidism* | Goiter* |
| Neurological | Headache† | Memory deficits† |
| Ataxia | Chronic fatigue* | |
| Nausea/vomiting | Psychosis | |
| Cognitive impairment† | Schizophrenia* | |
| Cardiovascular | Cardiac arrhythmias | Myocardial degeneration* |
| Circulatory arrest | ||
| Renal | Nephrotoxicity | |
| Pulmonary | Bronchospasm | Alveolar rupture |
| Pulmonary edema | Pneumomediastinum | |
| Airway hyperreactivity† | Chemical pneumonitis | |
| Rhinorrhea | Pulmonary fibrosis* | |
| Acute respiratory failure | Emphysematous changes* | |
| ARDS | ||
| Gastrointestinal | Oropharyngeal chemical burns | Gastroenteritis |
| Mucous membrane damage | Ulceration or gastric perforation | |
| Other | Possible carcinogen | |
Chronic;
Acute and chronic;
ARDS: acute respiratory distress syndrome
Sources: – Makarovsky, Markel, et al.; Lam, Vetal, et al.
Chlorine, bromine, and their hydrolysis products hypochlorous and hypobromous acid, respectively, are oxidants and as such interact with the antioxidants ascorbate, urate, and glutathione contained within the epithelial lung fluid(Squadrito et al. 2010; Yadav et al. 2010; Lam et al. 2016). Upon exposure to chlorine antioxidant stores are quickly depleted and chlorine and hypochlorous acid are free to interact directly with lung epithelial plasma membrane components(Leustik et al. 2008; Squadrito et al. 2010; Yadav et al. 2010). Bromine exposure likely progresses through an analogous process with bromine and hypobromous acid leading to formation of brominated fatty acids and aldehydes(Lam et al. 2016). Evidence of this was demonstrated in rats exposed to bromine where 2-Bromohexadecanal (a brominated fatty acid) was found in both the plasma and left ventricles of the animals(Ahmad et al. 2019). Brominated fatty acids have been shown to injure the vasculature and the heart. Additionally, exposure of red blood cells to brominated fatty acids or to brominated fatty aldehydes increases their fragility, likely contributing to increased plasma heme levels measured post-exposure to Br2(Aggarwal et al. 2016). Increased heme levels in the systemic circulation have been shown to injure the vasculature, heart, kidney, and other organs(Ryter and Tyrrell 2000; Wagener et al. 2001; Vermeulen Windsant et al. 2010; Kubota et al. 2017).
Halogen exposure in pregnancy or infancy:
Data regarding pregnancy and bromine or other halogen exposures are scarce, with the preponderance of evidence stemming from mouse models. Environmental outcomes data support a potential linkage between elevated relative levels of bromine disinfection by-products detected in water supplies and both preterm delivery and birth defects(Chisholm et al. 2008; Horton et al. 2011). Experiments in pregnant rats to determine the influence of brominated flame retardants on mammary gland development have demonstrated that dietary administration of brominated flame retardants in analogous concentrations to those seen in household dust reduce phosphor-ser675 β-catenin (p-βcatSer675), reducing its interaction with E-cadherin. Preservation of intracellular junctions is important for mammary gland function and abnormalities in junctional proteins (including E-cadherin) are linked to breast cancer. However, in these experiments the structure and function of the mammary gland was not appreciably altered and interestingly the most pronounced reduction in p-βcatSer675 occurred in the lowest dose treatment groups thus having more limited relevance to the question of severe, acute bromine gas exposure (Dianati et al. 2017).
The scientific literature is replete with basic, translational, and clinical investigations regarding the safety of modern halogenated volatile anesthetics particularly in relation to early childhood neurodevelopment. There is much interest in this topic globally given the potential clinical implications of anesthetic-induced neurotoxicity, however there are significant limitations to translating the negative neurodevelopmental effects noted in animal models (alterations in cellular organization, neuronal apoptosis, and abnormal synaptogenesis for example) to the clinical arena(Clausen et al. 2019). Fortunately, the clinical evidence appears to reinforce the relative safety of modern anesthetic techniques with the largest and most robust trials to date not findings a significant deleterious effect after early childhood administration of halogenated anesthestics(Sun et al. 2016; Warner et al. 2018; McCann M. E. et al. 2019; McCann Mary Ellen and Soriano 2019; Vutskits and Culley 2019). There is considerably less evidence available regarding maternal anesthetic exposure and future neurodevelopmental outcomes in offspring.
At present, clinically relevant doses of halogenated anesthetics do not seem to present a significant risk to human neurodevelopment. However, it is important to differentiate the relatively small doses of short-term volatile anesthetics administered in the highly-controlled environment of the operating room and the chronic, trace environmental exposure possible from water purification from the starkly contrasting acute, high-dose exposures seen in recent industrial accidents. High-level, acute exposures to halogen vapors as would be seen in an industrial accident or terrorist incident are fortunately not documented in parturients.
Physiology of pregnancy and considerations for toxic halogen gas exposure:
Due to the increased metabolic demands of pregnancy the parturient both consumes more oxygen as well as produces more carbon dioxide with a concurrent 45–50% increase in minute ventilation (MV) by the second trimester to meet the increased demand. This increase is driven by a rise in tidal volume with a minimal change noted in respiratory rate and a reduction in alveolar dead space(Bedson and Riccoboni 2013). Thus, pregnancy induces a state of relative arterial hypocarbia with a compensatory renal excretion of bicarbonate resulting in a mild, compensated respiratory alkalosis (normal range: pH 7.40–7.47, HCO3− 20 mEq/L, PaCO2 30 mmHg)(Chestnut 2019). Additionally, the gravid uterus displaces the diaphragm cephalad resulting in a significant decrease in functional residual capacity (FRC). This constellation of changes predisposes the parturient to rapid hypoxemia during apnea as well as to an increased uptake of inhalational halogenated anesthetic agents. Subsequent to toxic exposure to a halogenated agent the clinician should anticipate a parturient to demonstrate an increased susceptibility and more rapid progression to arterial hypoxemia both acutely during exposure and post-exposure if acute lung injury is incurred. In addition, the parturient is at risk for increased alveolar exposure and uptake of halogenated agents, potentially increasing the toxic exposure at a given concentration. Indeed the equilibration of alveolar and blood concentrations of halogenated volatile anesthetic agents is accelerated in pregnancy due to the combination of alveolar hyperventilation (increased MV) and decreased FRC despite the concurrent increase in cardiac output. In clinical practice this effect combines with the reduced minimum alveolar concentration (MAC) observed in pregnancy to speed the induction of general anesthesia in the parturient. While human data for accidental halogen exposure is lacking, these insights from halogenated anesthetics in the clinical arena suggest that a combination of increased biological sensitivity (as evidenced by decreased MAC) as well as a higher dose exposure (increased halogen uptake into the bloodstream) could synergize to increase the risk to pregnant women after halogen exposure. Conversely, other physiological adaptations including an increase in plasma volume along with an increase in cardiac output may alter the response of a parturient to a halogenated gas and could be protective to some degree.
Finally, pregnancy presents the clinician with two patients to address. The impact of halogen-containing toxins on fetal development, growth, and outcomes must be considered, particularly since halogenated compounds could catabolize to form halogenated molecules with toxic fetal effects.
Halogen exposure in pregnant mice:
Acute exposure:
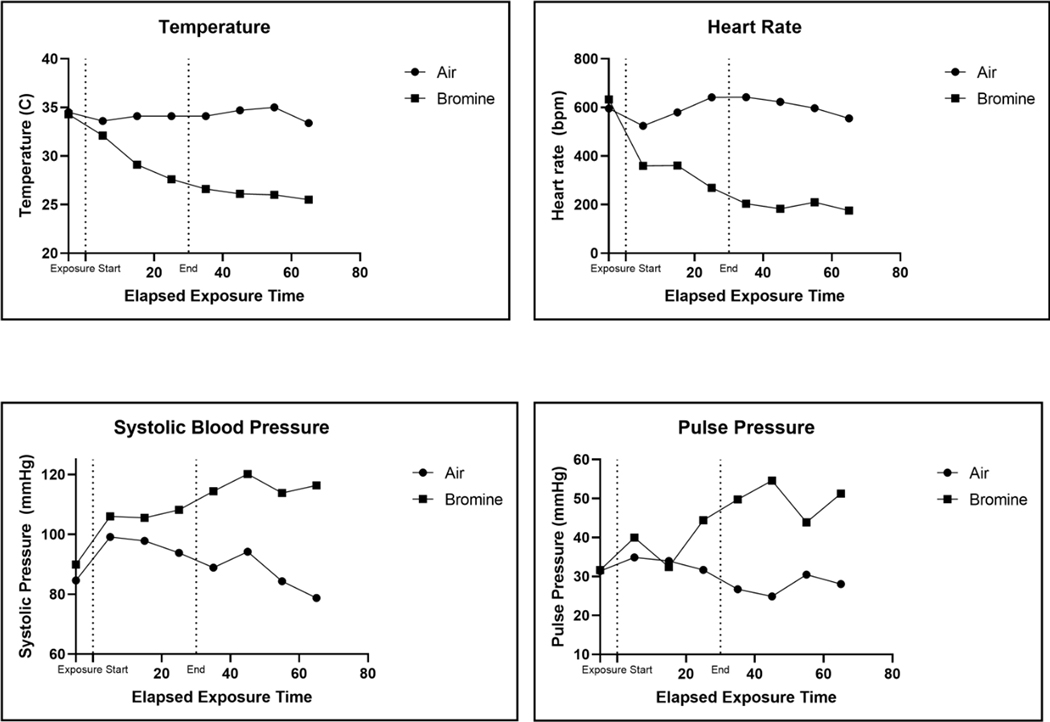
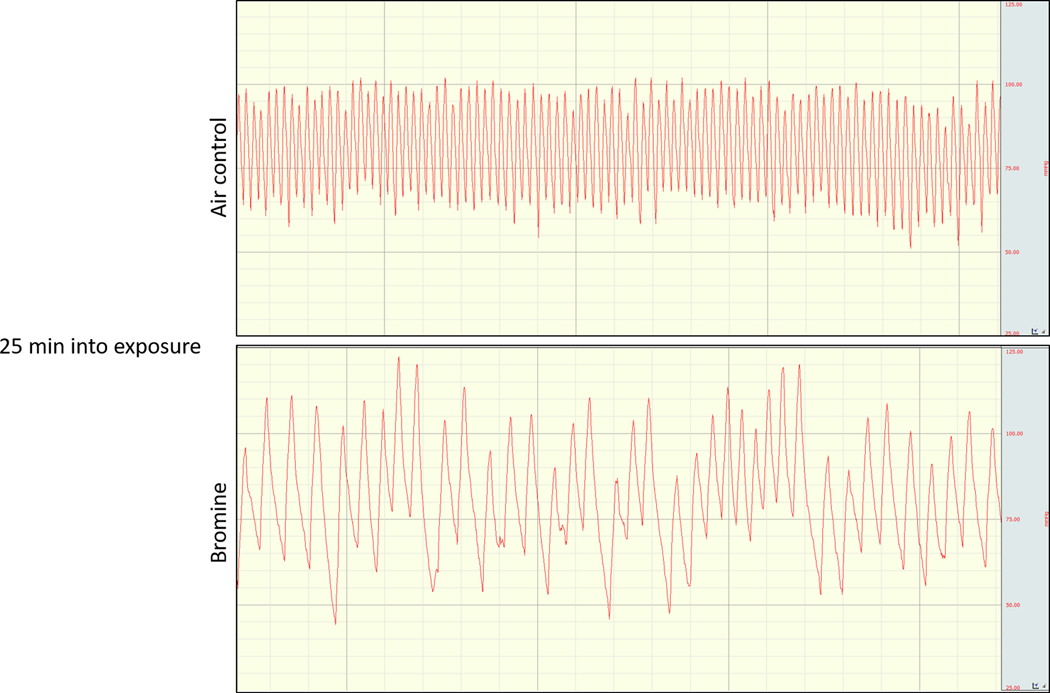
Pregnant and non-pregnant mice demonstrated profound hemodynamic changes during 30 minute exposures to 600 ppm bromine. To assess hemodynamic changes during and after bromine exposure the pressure transducer of a telemetry implant was advanced to the aortic arch via access through the left carotid artery and a telemetry device was implanted subcutaneously in pregnant and non-pregnant mice. Blood pressure in the aortic arch was continuously recorded at a 20 MHz sampling frequency, and temperature at the site of implant was recorded in 5 min intervals. Both pregnant and non-pregnant mice demonstrated profound bradycardia during Br2 exposure (Figures 1 & 2). Concurrently the animals exhibited an increase in systemic blood pressure with a widening of the pulse pressure. Finally, significant hypothermia developed in mice (both pregnant and non-pregnant) exposed to bromine (Figure 1). These hemodynamic changes persisted during the course of exposure and for 60 minutes post-exposure. Bromine exposure causes respiratory depression in both pregnant and non-pregnant mice analogous to the respiratory depressant effects of chlorine exposure. Chlorine is known to activate the chemosensory transient receptor potential cation channel, subfamily A, member 1 (TRPA1) triggering a respiratory depressant response in mice(Bessac and Jordt 2008). It is plausible that bromine causes hypopnea by the same mechanism. TRPA1-induced hypopnea may reduce respiratory rate in pregnant mice such that differences in minute ventilation between pregnant and non-pregnant animals are negated. To-date, no studies including tidal volume measurements during acute bromine exposure have been performed limiting our ability to compare minute ventilation between groups.
Figure 1.
Hemodynamic data obtained from telemetry recordings in two mice exposed to either air or Br2 (600 ppm for 30 min). Dashed lines demarcate the beginning and end of Br2 exposure. The telemetry pressure transducer was located in the aortic arch. Br2: Bromine
Figure 2.
Representative telemetry aortic arch pressure tracings obtained during exposure to bromine (600 ppm) or air at 25 minutes into a 30 minute exposure. Bradycardia, an increased systolic blood pressure, and a widened pulse pressure are evident.
Maternal Survival:
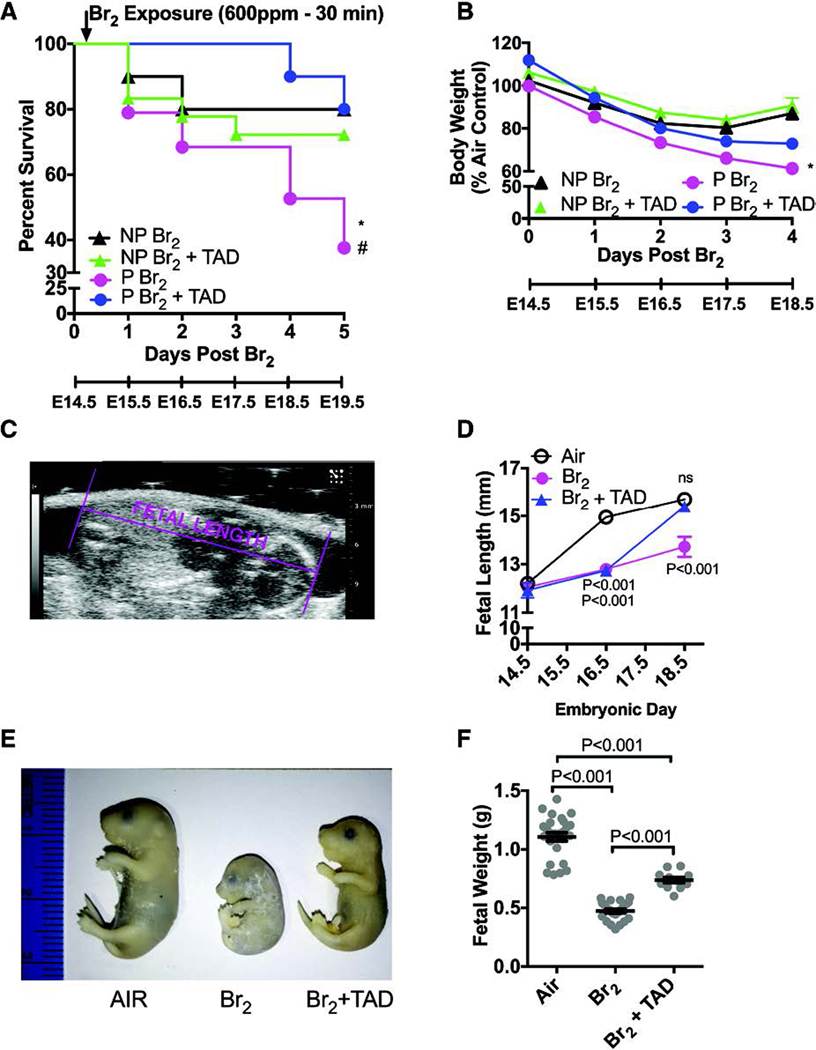
The pregnant state appears to confer physiologic changes that reduce the resilience of the parturient during an exposure to bromine. This increased susceptibility is illustrated by a significant decrease in survival post-exposure. Interestingly, the outcomes of pregnant compared to non-pregnant mice diverge at 24 hours post-exposure indicating that pregnancy-specific mechanisms of toxicity take time to develop and manifest. Figure 3 depicts the survival curves of pregnant and non-pregnant air control mice over the five days subsequent to exposure corresponding to gestational day (E) 14.5 to E 19.5. Pregnant mice exposed to 600ppm bromine for 30 minutes demonstrated a 36% mean survival at five days compared with non-pregnant control mice (80%) for a mean a difference of 44%(Lambert et al. 2017). A subsequent investigation similarly found a 48% mean survival in pregnant mice with the same exposure parameters(Addis et al. 2020). The precise underlying etiology of death in these mice remains unclear but is likely multifactorial.
Figure 3. Pregnant mice exhibit increased body weight loss and mortality and fetal growth restriction.
Non-pregnant (NP) and pregnant (P) (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 min and returned to room air; they received tadalafil (TAD; 2 mg/kg BW in 0.1 ml of sterile saline) or vehicle via oral gavage at 1 h post-exposure and every 24 h thereafter. Body weights and survival times were recorded daily. A) Kaplan-Meyer curves of pregnant and non-pregnant mice with tadalafil or vehicle, post Br2 exposure. Non-pregnant mice exposed to Br2 and returned to room air lived longer than similarly exposed pregnant mice (* = P<0.05). Tadalafil improved survival times of pregnant mice post Br2 (# = P<0.05) but not of non-pregnant mice; n=10–19; Log-Rank Test. B) Body weights normalized to weights of air-exposed mice. Pregnant mice exposed to Br2 and returned to room air exhibit more severe weight loss compared to similarly exposed non-pregnant mice (* = P<0.05). Tadalafil administration mitigated weight loss in pregnant mice at four days post-exposure, but has no effect in non-pregnant mice; n=6–8; ANOVA. C) Representative ultrasound of a fetus showing how fetal length was measured. D) Summary data of fetal length measurements at E14.5, E16.5, & E18.5. Exposure to Br2 resulted in decreased fetal lengths which were restored to their air control values at E18.5 in the tadalafil group; n= 6–10 pups (2 pups per litter) for each condition; ANOVA; p values as compared to the corresponding air controls for the indicated gestational age. E) Representative photograph of paraformaldehyde-fixed fetuses at E18.5 for the indicated conditions. Fetuses of Br2-exposed pregnant mice exhibit severe fetal growth restriction, and tadalafil improves fetal growth. F) Fetal weights were recorded after extraction of fetuses at E18.5. Fetal weights of fetuses from Br2-exposed pregnant mice weighed considerably less and were partially rescued by tadalafil (TAD); n=pups (11–25) (2 pups per litter); ANOVA. All data are means±S.E.M.
Lung Injury:
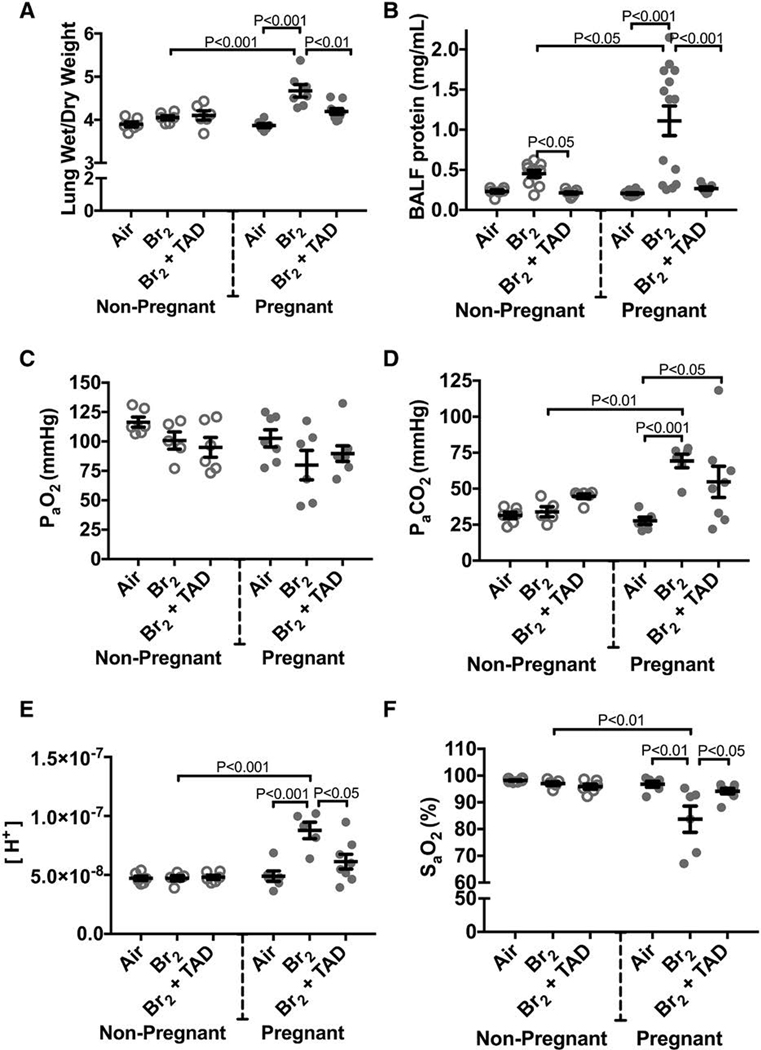
Within 24 hours of exposure to bromine both pregnant and non-pregnant mice demonstrate elevated broncheoalveolar lavage fluid protein content and increased wet/dry lung weight ratios (Figure 4)(Aggarwal et al. 2016; Lambert et al. 2017; Addis et al. 2020). In the surviving non-pregnant exposed animals this acute injury pattern demonstrates progression to a chronic injury pattern around 3 days post-exposure. This chronic phase of injury is characterized by developing peribronchial fibrosis and emphysema-like dilation and destruction of alveoli(Aggarwal et al. 2018). In contrast, pregnant animals demonstrated a pattern consistent with ongoing and progressive acute injury past post-exposure day 3. The ratio between wet and dry weight of lungs procured at E 18.5 were significantly increased in pregnant mice exposed to bromine indicating an increased degree of pulmonary edema (Figure 4). This was in contrast to non-pregnant mice which exhibited normal lung wet:dry weight ratios at three days post exposure. Pregnant animals had significantly increased levels of BAL fluid protein as compared to air-exposed control pregnant mice at E18.5, and bromine-exposed non-pregnant mice did not exhibit elevated BAL fluid protein at the same time point (Figure 4). SaO2 was also decreased in pregnant mice exposed to bromine and arterial blood gas measurements indicated a respiratory acidosis (mean pH 7.05, mean PaCO2 69.2)(Figure 4)(Lambert et al. 2017). These findings were demonstrated in subsequent experiments again noting significantly higher lung wet:dry ratios, and BAL fluid protein(Addis et al. 2020). Additional investigation into BAL fluid content was performed and pregnant Br2 exposed mice demonstrated increases in total cell count, macrophages, and neutrophils(Addis et al. 2020).
Figure 4. Pregnant mice exhibit pulmonary injury 96 h post-exposure.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A) Lung wet/dry weight ratios at E18.5 are increased in pregnant mice exposed to Br2 and are reduced by tadalafil. B) Protein concentrations in the bronchoalveolar lavage fluid (BALF) of pregnant mice were increased at E18.5 and returned to the air control values following administration of tadalafil (TAD). C–F) PaO2 was unchanged, PaCO2 increased, [H+] increased and SaO2 was decreased in pregnant mice exposed to Br2 at E18.5. Administration of Tadalafil returned [H+] and SaO2 to their air control values but did not improve PaCO2; All data n=6–14 mice; ANOVA; means±S.E.M.
One possible explanation for the increased susceptibility of pregnant mice to halogens is that they inhale higher levels of bromine because of higher alveolar ventilation as compared to non-pregnant animals. To test this hypothesis we measured levels of a brominated fatty acids in the plasma, formed by the interaction of bromine and plasmalogens(Ford et al. 2016; Duerr et al. 2018). As shown in Figure 5, pregnant and non-pregnant mice exposed to bromine had similar levels of 18C brominated fatty acids in the plasma. Additionally, the survival curves observed in non-pregnant and pregnant mice exposed to Br2 show a delayed, as opposed to immediate increase in mortality in pregnant mice, the higher degree of lung injury was evident in pregnant mice at times distant (72 and 96h post exposure), and treatments specifically tailored to mechanisms we observed in pregnant mice only had therapeutic benefit only in pregnant mice, indicating sequential development of mechanisms of toxicity that are specific to pregnancy. These data suggest that changes in minute ventilation did not fully account for increased susceptibility of pregnant mice to bromine (Figure 5) and thus the differences in halogen toxodynamics in pregnancy may overshadow toxokinetic mechanisms.
Figure 5.
Levels of brominated fatty acids (18C = stearic acid) in the plasma of non-pregnant or pregnant mice at 1 hour post exposure to bromine (600 ppm for 30 min). Individual values for each mouse along with means ± SEM. There was no difference among the means of pregnant and non-pregnant mice for 18C brominated fatty acid. ANOVA, Tukey’s multiple comparisons test. n=6–8. NP: non-pregnant, P: pregnant, C: carbon.
Placental injury, fetal growth restriction, and a preeclamptic phenotype in pregnant mice:
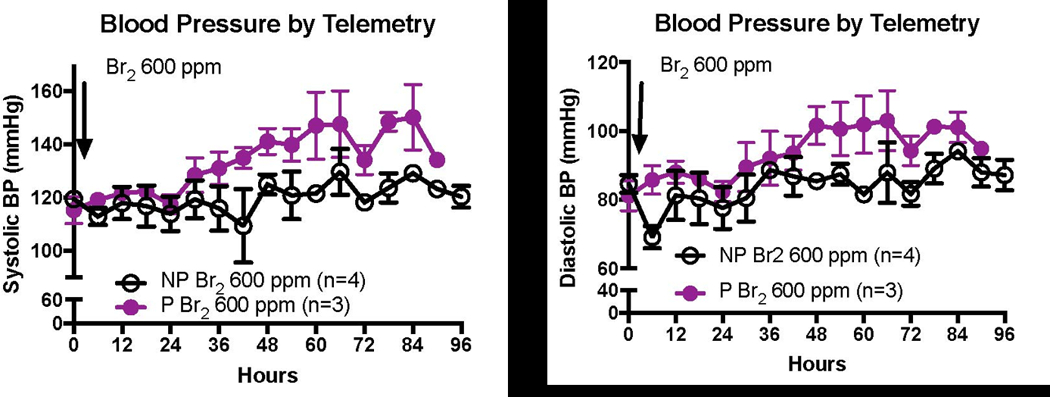
In addition to demonstrating an increase in systemic blood pressure acutely during bromine exposure, pregnant mice also had a 40% elevation in invasive diastolic aortic blood pressure compared to non-pregnant controls 4 days later on E 18.5(Lambert et al. 2017). The blood pressure values reported in Lambert et al. were obtained in anesthetized mice using a pressure transducer advanced into the aortic arch. Comparison of these preexisting blood pressure recordings in anesthetized mice to continuous blood pressure recordings in non-anesthetized mice using telemetry shown in (Figure 6) clearly demonstrate the limitations of performing these measurements under anesthesia. The measured BP of 75/50 in air exposed anesthetized mice (reported by Lambert et al. 2017) are well-below the expected normative values, while the BP of 120/80 in air exposed non-anesthetized mice are a good match to the expected values in mice. Additionally, in Br2 exposed anesthetized mice we only observed a significant increase of diastolic blood pressure, while in non-anesthetized Br2 exposed mice we observed significantly elevated both systolic and diastolic pressures as compared to air exposed non-anesthetized mice (Figure 6).
Figure 6. Exposure to Br2 increases the systemic blood pressure of pregnant mice.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes then returned to room air. At E18.5, a pressure-transducer catheter was inserted into the aortic arch via the carotid in anesthetized mice. Increases in both systolic and diastolic pressure were noted in pregnant mice.
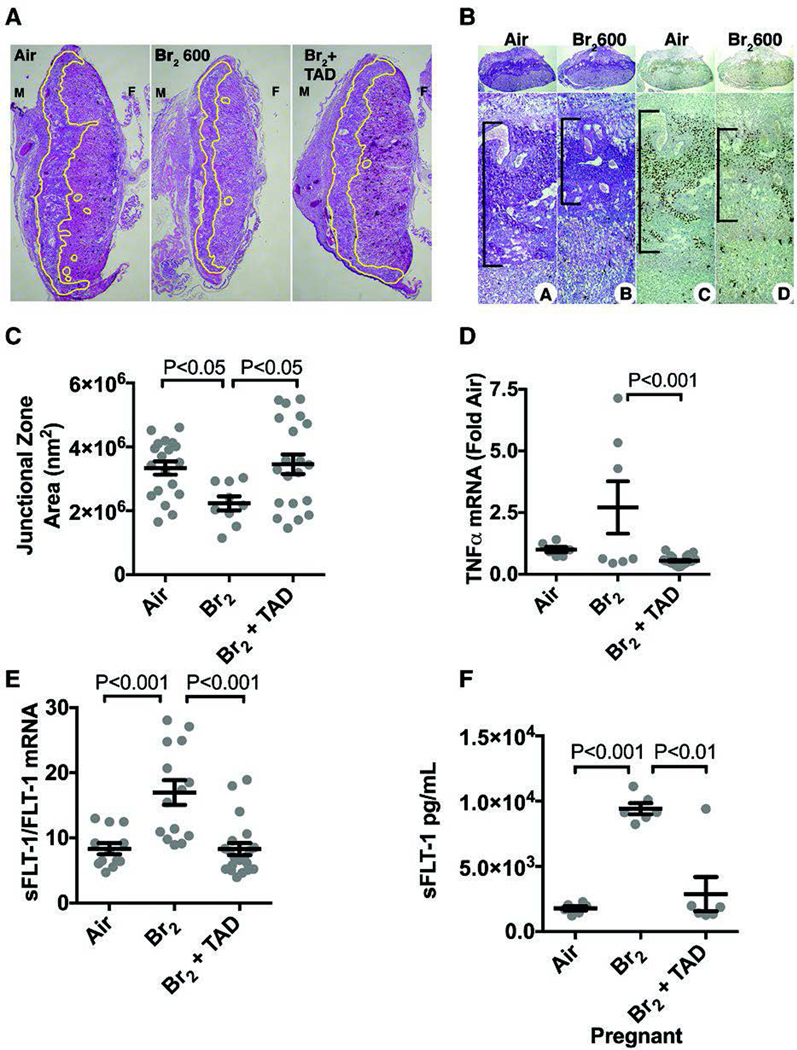
The systemic hypertension in Br2 exposed pregnant mice is concurrently observed along with hindered placental development. The characteristic markers of the junctional zone (the zone of trophoblast invasion into the decidua on the maternal side and the labyrinth on the fetal side) are glycogen containing cells detected by periodic acid Schiff (PAS) labeling and by CDX2 transcription factor. These markers were notably decreased in placentae of Br2 exposed pregnant mice as compared to placentae of air exposed mice (Figure 7)(Lambert et al. 2017). Quantitative evaluation of several similar sections revealed that there was a significant decrease in the area of junctional zone in placentae of Br2 exposed pregnant mice. Predictably, alterations in placental development were accompanied by severe fetal growth restriction with decreased fetal length and weights noted after bromine exposure as demonstrated in Figure 3(Lambert et al. 2017). Severe fetal growth restriction was also demonstrated in similar experiments with Br2 exposed pregnant mice (600 ppm, 30 minutes) and was also accompanied by a significantly decreased area of the junctional zone(Addis et al. 2020). This constellation of findings is reminiscent of preeclampsia, a pathological condition with a similar phenotype observed in human pregnancies also characterized by abnormal placentation, hypertension, and fetal growth restriction.
Figure 7. Exposure of pregnant mice to Br2 damages their placentas.
Pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air and received tadalafil (TAD) or vehicle. A–B) Representative H&E stained (A) placenta sections at E18.5 with the junctional zone demarcated with yellow highlighting (A) as well as (B) PAS staining (left) and CDX2 staining (right) of Br2-exposed pregnant mice revealed a reduced junctional zone (B: black bars) at E18.5; tadalafil administration restored junctional zones to normal size. C) Junctional zone areas at E18.5 for the indicated groups; n=9–20; ANOVA. D–E) TNFα mRNA (n=6–23) was reduced in Br2-exposed pregnant mice treated with tadalafil. sFLT-1/FLT-1 mRNA (=12–18) was increased in placentas of Br2-exposed pregnant mice at E18.5 (ANOVA), and was reduced to air control values by tadalafil. F) sFLT-1 in plasma at E18.5 increased in pregnant mice exposed to Br2 and was reduced with tadalafil; ANOVA. Only one placenta per pregnant mother was used. All data are means±S.E.M.
Endothelial dysfunction:
Endothelial dysfunction and alterations in angiogenic pathway mediators are thought to be integral to the development of human preeclampsia. The soluble form of the vascular endothelial growth factor receptor 1 (VEGFR1) also known as soluble fms-like tyrosine kinase-1 (sFlt-1) is overexpressed in preeclampsia and acts as a decoy receptor binding to VEGF and placental growth factor (PlGF), thus preventing downstream activation of the VEGFR1 pathway. Increased plasma levels of sFlt-1 as well as an increase in the ratio of sFlt-1:PlGF are observed prior to clinical manifestations of preeclampsia in humans(Levine et al. 2004). Similarly, in pregnant mice exposed to bromine the ratio of placental sFlt-1:VEGFR1 mRNA was increased and a five-fold increase in plasma sFlt-1 level was observed (Figure 7 E and F respectively)(Lambert et al. 2017). The increase in plasma sFlt-1 is progressive and manifests at 48 hours post-exposure continuing to increase linearly over time until sacrifice at 96h in surviving mice (R2= 0.8151, P<0.0001) (Figure 8) (Addis et al. 2020).
Figure 8.
Br2-exposed pregnant mice exhibit progressively increased circulating sFLT-1 (soluble fms-like tyrosine kinase 1) with a concomitant increase of lung wet/dry weight. Non-pregnant and pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. sFLT-1 levels in the plasma of (A) pregnant Br2-exposed mice increased 48-hour post-exposure vs air controls and continued to increase until euthanasia at 96 hours. This increase was linear (R2=0.8151, P<0.0001). Similarly exposed (B) non-pregnant mice exhibited no increase at any time point; n=6 to 8; ANOVA. Lung wet:dry weight ratios of (C) pregnant Br2-exposed mice increased 72-hour post-exposure compared with air controls and continued to increase until euthanasia at 96 hours. Similarly exposed (D) non-pregnant mice exhibited no increase at any time point; n=6 to 8; ANOVA. All data are individual values and means±SEM. sFLT-1 indicates soluble fms-like tyrosine kinase 1.
The activation of VEGFR by VEGF stimulates the Akt pathway which, among other effects, improves survival and barrier function of endothelial cells as well as increases production of the endothelial nitric oxide synthase (eNOS). The activity of eNOS is required for endothelial production of NO which, in turn, acts within the vascular smooth muscle cells to regulate vasodilation through a cyclic guanosine monophosphate (cGMP)-dependent mechanism. Examination of cGMP levels in the placenta, lung, and aorta of bromine-exposed pregnant mice revealed decreased levels potentially providing a mechanistic linkage between placental sFlt-1 over-expression and systemic hypertension after bromine injury via endothelial dysregulation (Figure 7)(Lambert et al. 2017). In pregnant mice exposed to Br2 and subsequently treated with exogenous VEGF-121 (a mouse VEGF splice variant) survival to 120 hours was increased to 76% from 48% survival in the vehicle control cohort(Addis et al. 2020). No difference in survival was noted in non-pregnant mice treated with VEGF-121 suggesting that there is a pregnancy-specific alteration in VEGF signaling after Br2 exposure. Similarly, maternal body weight gain was partially rescued by VEGF-121 treatment in pregnant animals. Significantly, at 120 hours after Br2 exposure pregnant mice treated with VEGF-121 had reduced lung wet:dry weight ratios compared to vehicle treated pregnant mice and no significant difference was noted between wet:dry weight ratios of air-exposed mice and pregnant Br2 exposed mice treated with VEGF-121(Addis et al. 2020). Further, BAL fluid protein content, cell count, and neutrophil counts were simultaneous decreased after VEGF-121 treatment while weights of delivered fetuses increased compared to vehicle control animals accompanied by a significant increase in the area of the junctional zone(Addis et al. 2020). Taken together, these findings suggest that decreased VEGF signaling is involved to some degree in the pregnancy-specific phenotype observed post Br2 exposure.
Cardiovascular Derangements:
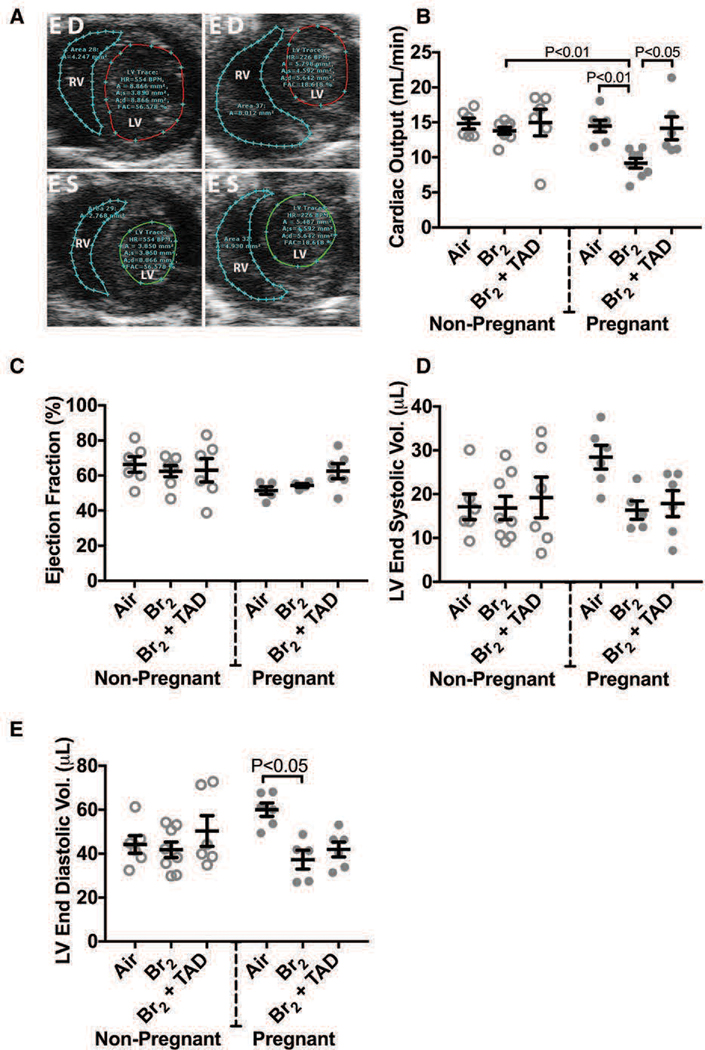
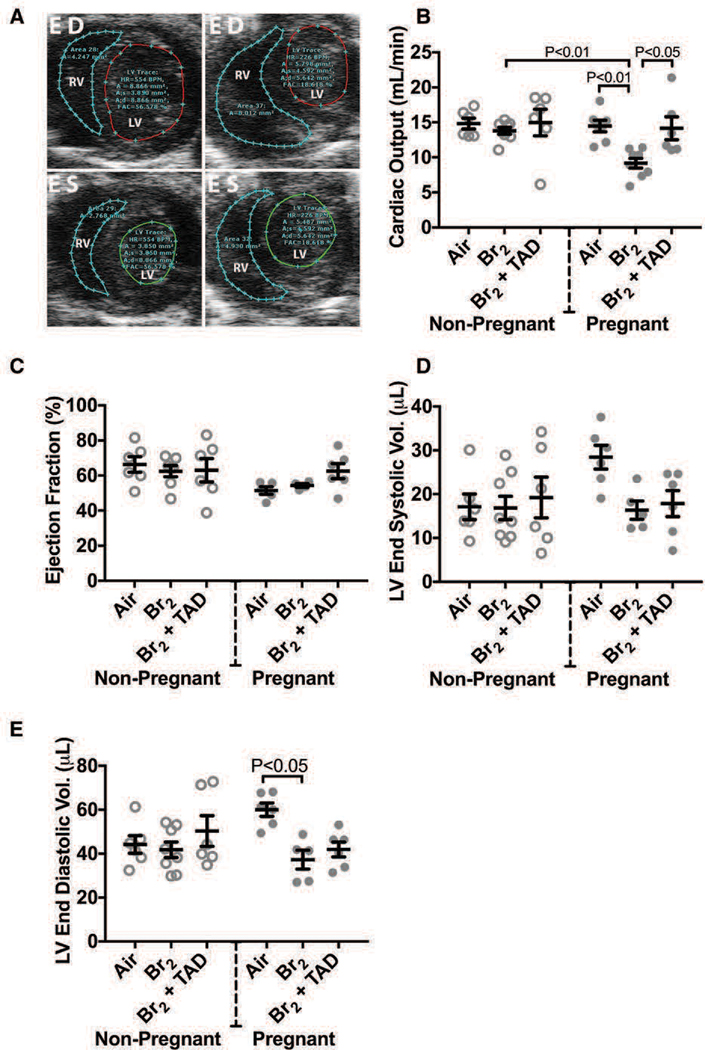
In addition to the acute hemodynamic derangements observed during the exposure period, cardiac function progressively deteriorates in bromine exposed pregnant mice, resulting in significant deficiency in left ventricular end diastolic volume and in cardiac output. In normal pregnancy, cardiac output (CO) increases to meet metabolic demands, with stroke volume (SV) rising concurrently with expansion of the blood volume. The SV increase stems from a combinatory effect of increased end-diastolic volume (increased preload) and increased myocardial mass. Human pregnancy also leads to an increase in heart rate (HR), particularly later in the gestation. With the exception of increased heart rate, these anticipated findings were observed in the pregnant, air control group which demonstrated increased echocardiographic derived estimations of CO, SV, and left ventricular end diastolic volume (LVEDV) compared to non-pregnant air-exposed control animals (Figure 9). Conversely, at E 18.5 CO and SV were significantly reduced in pregnant mice exposed to bromine with values approaching those of non-pregnant mice in both air and bromine arms of the study(Lambert et al. 2017). Cardiac chamber dimensions were similar between groups with the exception of a decreased in LVEDV observed in pregnant mice exposed to bromine compared to the normal increased LVEDV noted in pregnant mice exposed to air. In all groups left ventricular ejection fraction was preserved. Cardiac output measurements performed on the pulmonary circulation (right ventricular output) also were increased in pregnancy reflecting the interdependence of the systemic and pulmonary circulatory systems in series. Pregnant mice exposed to bromine however demonstrated an increase in right ventricular systolic pressure (RVSP) as determined by both invasive ventricular pressure monitoring along with echocardiographic Doppler-based estimation (Figure 9). This increase in RVSP was not noted in air-control pregnant mice.
Figure 9. Br2-exposed pregnant mice exhibit diminished cardiac function at E18.5.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A) Representative echosonography LV and RV traces at E18.5 of pregnant mice exposed to air or Br2 with demarcation of ventricular sizes. HR = Heart rate, A = Area, A;s = Area systole, A;d = Area diastole, FAC = Fractional area change. B) Cardiac output (LV) determined by echosonography was decreased in Br2-exposed pregnant mice, which increased following administration of tadalafil; ANOVA: n=6–8. C) Ejection fraction (LV) was unchanged in all groups; ANOVA. D) LV end-systolic volume was similar in all groups. E) However, LV end-diastolic volume was diminished in Br2-exposed pregnant mice; n=6–8; ANOVA. All data are means±S.E.M.
Notably, it is well-documented that exposure to halogens results in acute cardiotoxicity in non-pregnant animals. Bromine exposures of greater duration (600 ppm for 45 minutes) have been demonstrated to cause severe cardiac injury in non-pregnant rats and chlorine exposures in rats (600 ppm for 30 minutes) resulted in acute biventricular failure (Zaky, Ahmad, et al. 2015; Zaky, Bradley, et al. 2015; Ahmad et al. 2019).
Mechanisms of injury in non-pregnant animals includes inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), loss of cardiac ATP activity, and myocyte death as was documented in rats exposed to chlorine gas(Ahmad et al. 2015). Significantly, cardiac injury was observed even when the inspired oxygen concentration was increased to correct for chlorine-induced hypoxia(Zaky, Bradley, et al. 2015). Higher levels of chlorine exposure (600 ppm for 30 minutes) resulted in lethal biventricular failure as noted above. Conversely, a lower exposure dose (500 ppm) resulted in diastolic dysfunction and a hyperdynamic LV despite an ex vivo retrograde perfused heart preparation indicative of reduced contractile force(Zaky, Bradley, et al. 2015). The increased LVEF observed was surmised by the authors to be related to afterload reduction (decreased measured systemic blood pressures) in combination with a hyperadrenergic state leading to increased inotropy(Zaky, Bradley, et al. 2015). It is notable that echocardiographic examinations in these animals were conducted under ketamine anesthesia, an anesthetic with well-described sympathomimetic effects.
Bromine also has cardiotoxic effects in rats evidenced by both decreased SERCA activity and increased calcium sensitive LV calpain activity. Cytoskeletal disruption was observed along with mitochondrial damage, neutrophil infiltration, and evidence of contraction band necrosis(Ahmad et al. 2019). Exposed non-pregnant rats had echocardiographic evidence of both systolic and diastolic LV dysfunction at 1-week post exposure to 600 ppm for 45 minutes(Ahmad et al. 2019).
It is likely that the mechanism of gradually developing myocardial function deficit in pregnant mice exposed to Br2 is different from the mechanisms of the aforementioned acute injury described in non-pregnant rats. The interplay between VEGF signaling in endothelial cells and cardiomyocyte remodeling may further explain some of the observed cardiovascular effects of halogen exposure. Bromine-induced placental injury driving sFlt-1 overexpression may decrease VEGFR2 signaling, thus reducing positive downstream effects that are linked to physiologic cardiomyocyte remodeling and instead preferentially inducing a pathological phenotype(Kivela et al. 2019; Ueda et al. 2019).
Inflammatory Response:
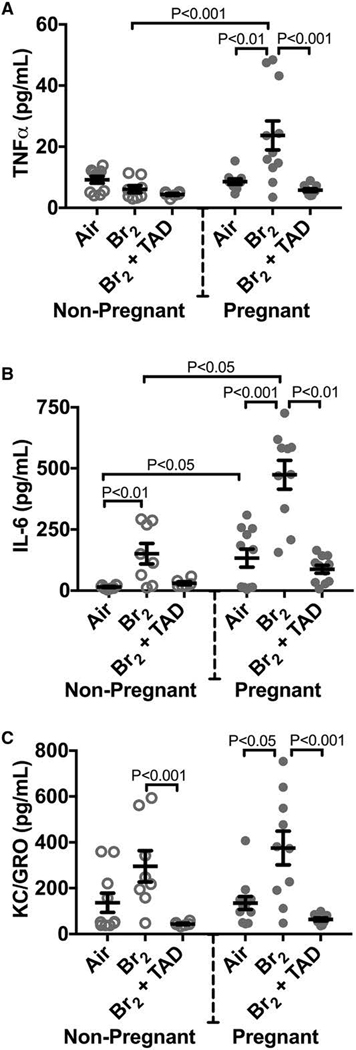
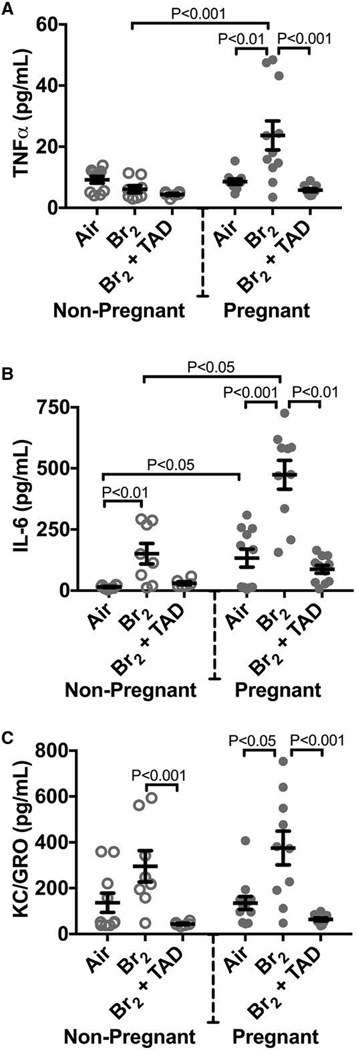
Bromine and chlorine exposure both trigger acute inflammatory responses in mice and rats (Song et al. 2011; Aggarwal et al. 2016; Aggarwal et al. 2018). In pregnant mice exposed to bromine this pro-inflammatory milieu is observed to persist after the acute period post-insult, offering evidence that pregnancy may both potentiate and prolong pulmonary injury and dysfunction. At E 18.5 pregnant mice exposed to bromine demonstrated increased plasma tumor necrosis factor alpha (TNFα) and keratinocyte chemoattractant/growth related oncogene (KC/GRO) compared to non-pregnant controls. Both pregnant and non-pregnant animals had elevations in interleukin-6 (IL-6) at the same time point. In the placenta, pregnant mice exposed to bromine had elevated TNFα and IFNγ mRNA expression compared to air controls indicative of local placental injury and inflammation (Figure 10).
Figure 10. Pregnant mice exposed to Br2 demonstrated systemic inflammation at E18.5.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A–C) Plasma TNFα, IL-6, and KC/GRO increased in pregnant mice exposed to Br2 but only IL-6 increased in non-pregnant mice post-Br2. All three cytokines in pregnant mice exposed to Br2 returned to air control values following tadalafil administration; n=6–12 per group; ANOVA. All data are means±S.E.M.
Potential therapeutic targets:
Intervention to address alterations in the cGMP-mediated vasodilatory response via administration of the phosphodiesterase-5 inhibitor tadalafil demonstrates promise and the supporting data sheds additional light on one of the underlying mechanisms by which halogen exposure exerts a pathological influence.
Tadalafil is a phosphodiesterase-5 specific inhibitor currently approved for clinical use to treat erectile dysfunction, benign prostatic hypertrophy, and used “off-label” for the treatment of pulmonary hypertension. With phosphodiesterase-5 inhibition the breakdown of cGMP is impeded, resulting in a functional increase in the availability of cGMP to serve as a vasoactive mediator. Pregnant mice exposed to bromine demonstrate decreased levels of cGMP. In pregnant mice administered tadalafil 1-hour post-exposure and then once every subsequent 24-hours they demonstrated increased cGMP levels accompanied by a significantly improved overall mortality rate resulting in 80% survival and bringing the survival curve back into alignment with that observed in non-pregnant mice post-exposure (Figure 3). Pregnant mice administered tadalafil demonstrated a less pronounced decrease in maternal weight at 4 days post-exposure compared with control animals. Finally, tadalafil administration improved fetal length and fetal weight. These findings suggest that impaired cGMP-mediated vasodilation may be at least in part a mechanistic driver for not only the maternal phenotype demonstrated, but also the observed impairment of fetal growth and development. Downstream vasoactive modulation of the placental vasculature may be a significant component of the toxic effects of halogen gas on the fetus.
The VEGF pathway provides another avenue to develop targeted therapeutics for the pregnant patient after halogen exposure. Modulation of VEGF signaling is now common in the development of antiangiogenic, antineoplastic pharmacologics and consequently significant work exists describing the various VEGF subtypes including PlGF. VEGF signaling in pregnancy is intricate and made more complex by the fluctuations in signaling necessary to facilitate the various stages of placental development. The findings that sFlt-1 and the ratio of sFlt-1 to PlGF are both increased after halogen exposure suggest that pharmacologically increasing VEGF signaling may prove beneficial in attenuating endothelial dysfunction. As discussed above, treatment of pregnant mice exposed to Br2 with VEGF-121 improved mortality, increased maternal weight gain, improved indices of pulmonary endothelial dysfunction, and improved placental and fetal development while treatment of non-pregnant animals proved ineffectual(Addis et al. 2020). Additional research is necessary to explore whether this is a common feature of other halogen gasses and to further understand the underlying mechanisms prior to potentially translating these results to the bedside.
Discussion:
The safety profile of modern inhalational anesthetic agents is markedly improved and the specter of a large-scale military deployment of chemical weapons during trench warfare has faded as military tactics and security threats have evolved. Halogens, however, remain an important component of modern global industry and accidental or terrorism-related exposures will likely continue to occur. Exposure to Cl2 and Br2 due to cigarette smoking is overlooked and warrants potential further exploration. In our efforts to further understand the pathophysiologic effects of halogens it is important to consider the implications of exposure to the parturient and fetus. The current evidence suggests a different pattern of lung injury occurs post-exposure in pregnant mice than is observed in non-pregnant mice. Rather than acute lung injury with gradual resolution in sub-lethal exposures, the injury incurred in pregnant mice seems to progress and gradually worsen past the 24-hour point up until euthanasia or death. The systemic consequences of halogen gas exposure are remarkably similar in phenotype to preeclampsia with a concurrent biomarker profile suggestive of a similar angiogenic imbalance as is characteristic of preeclampsia. The cumulative effects of progressive systemic hypertension, pulmonary edema, and uncompensated respiratory acidosis and cardiac dysfunction resulted in increased mortality in exposed pregnant mice. Worsening hypertension, pulmonary edema, and cardiac function in preeclamptic human patients portend worse clinical outcomes and thus it is reasonable to surmise that halogen exposure in pregnant humans may lead to similar negative clinical outcomes.
Severe preeclampsia is generally considered to develop over a period of weeks with elevations in sFlt-1 noted 5-weeks before the onset of clinical symptoms(Levine et al. 2004). Historical attempts to create rodent models of preeclampsia have struggled with the lack of pregnancy specific phenotype development (for example, non-pregnant animals developing hypertension), prolonged onset prior to clinical effect, or the necessity of performing a survival surgery in the animal. One frequently employed rodent model of preeclampsia relies on reduced uteroplacental perfusion (RUPP) achieved by uterine artery ligation(Marshall et al. 2018). RUPP induced uteroplacental insufficiency alters trophoblast differentiation, placental gene expression, fetal capillary network development, and the cellular makeup of the placental junctional zone(Natale et al. 2018). Bromine exposure appears to reproducibly trigger a pregnancy-specific phenotype and has marked effects on junctional zone histology and fetal growth and weight. This suggests a similar pathway related to uteroplacental insufficiency is shared between RUPP and bromine exposure. Furthermore, the rapidity and severity of sFlt-1 elevation concurrent with the acute onset of symptoms reminiscent of severe preeclampsia is a remarkable response to elicit from a single 30-minute bromine exposure.
In addition to an elevation of plasma sFlt-1, exogenous VEGF administration can partially rescue key components of the halogen-exposure phenotype but only in pregnant mice. This suggests that the VEGF signaling pathway is involved and may be at least partially responsible for the increased permeability of the blood gas barrier and consequent pulmonary edema. Despite this, the mechanisms by which halogen exposure trigger increased placenta secretion of sFLT-1 remain unclear. Potential mechanisms include interruption of placental blood flow with relative tissue hypoxia within the uteroplacental unit or toxic effects of brominated lipids within the placenta.
It is important to further understand the underlying pathways by which halogen exposure triggers this constellation of findings in such an acute fashion. Finally, investigative efforts to elucidate the mechanistic underpinnings of halogen toxicity may concurrently help us better understand the pathophysiology of preeclampsia, particularly as it relates to pulmonary edema and cardiovascular injury.
Conclusion:
Pregnancy may potentiate, exacerbate, and prolong the acute lung injury incurred during exposure to the halogen gases. Endothelial dysfunction with increased pulmonary endothelial permeability seems to contribute to the resultant pulmonary edema and manifests approximately 24-hours post-exposure in rodent models. Additionally, halogen exposure may precipitate a preeclamptic-like syndrome characterized by systemic and pulmonary hypertension, placental injury, endothelial dysfunction, fetal growth restriction, and a decreased cardiac output culminating to impart a higher risk of mortality to the parturient and fetus. Angiogenic imbalance reflected by an increase in sFlt-1 implicates VEGF signaling pathways as playing an important role in the preeclamptic-like syndrome observed in halogen exposed pregnant mice. Additional research is indicated to further characterize the mechanisms driving the phenotype and to explore possible therapeutic targets and develop interventions to mitigate risk to the parturient and fetus in the event of halogen gas exposure.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health under award number T32HL129948. Supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute of Environmental Health Sciences (NIEHS), Grant Numbers (UO1 ES026458, UO1 ES027697) the National Heart, Lung and Blood Institutes.
References
- Addis DR, Lambert JA, Ren C, Doran S, Aggarwal S, Jilling T, Matalon S. 2020. Vascular Endothelial Growth Factor-121 Administration Mitigates Halogen Inhalation-Induced Pulmonary Injury and Fetal Growth Restriction in Pregnant Mice. Journal of the American Heart Association. 9(3):e013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Ahmad I, Lam A, Carlisle MA, Li C, Wells JM, Raju SV, Athar M, Rowe SM, Dransfield MT et al. 2018. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight. 3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. 2016. Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury. Antioxid Redox Signal. 24(2):99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schoneich C, Reisdorph N, Powell RL, Chandler JD et al. 2015. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol. 52(4):492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N et al. 2019. Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats. Am J Physiol Heart Circ Physiol. 316(1):H212–h223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azman MA, Yasir MS, Rahman IA, Hamzah S, Rahman SA, Elias MS, Abdullah NA, Hashim A, Shukor SA. 2016. Concentration of trace elements on branded cigarette in Malaysia. AIP Conference Proceedings. 1704(1):040007. [Google Scholar]
- BBC News. 2011. Bromine chemical leak in Russian city of Chelyabinsk. [accessed 2019 October 22]. https://www.bbc.com/news/world-europe-14755874.
- Bedson R, Riccoboni A. 2013. Physiology of pregnancy: clinical anaesthetic implications. BJA Education. 14(2):69–72. [Google Scholar]
- Bessac BF, Jordt SE. 2008. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda). 23:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Reproductive Health in Emergency Preparedness and Response. [Google Scholar]
- Centers for Disease Control and Prevention National Institute for Occupational Safety and Health. 2014. Bromine: Immediately Dangerous to Life or Health Concentrations (IDLH). [accessed 01/30/2020]. https://www.cdc.gov/niosh/idlh/7726956.html.
- Chestnut DH. 2019. Chestnut’s obstetric anesthesia : principles and practice. 6th edition. ed. St. Louis, MO: Elsevier. [Google Scholar]
- Chisholm K, Cook A, Bower C, Weinstein P. 2008. Risk of birth defects in Australian communities with high levels of brominated disinfection by-products. Environ Health Perspect. 116(9):1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen NG, Hansen TG, Disma N. 2019. Anesthesia Neurotoxicity in the Developing Brain: Basic Studies Relevant for Neonatal or Perinatal Medicine. Clin Perinatol. 46(4):647–656. [DOI] [PubMed] [Google Scholar]
- Coulliette AD, Peterson LA, Mosberg JA, Rose JB. 2010. Evaluation of a new disinfection approach: efficacy of chlorine and bromine halogenated contact disinfection for reduction of viruses and microcystin toxin. Am J Trop Med Hyg. 82(2):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianati E, Wade MG, Hales BF, Robaire B, Plante I. 2017. From the Cover: Exposure to an Environmentally Relevant Mixture of Brominated Flame Retardants Decreased p-beta-Cateninser675 Expression and Its Interaction With E-Cadherin in the Mammary Glands of Lactating Rats. Toxicol Sci. 159(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA. 2018. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J Lipid Res. 59(4):696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth PG. 2016. Of Penguins, Pinnipeds, and Poisons: Anesthesia on Elephant Island. Anesthesiology. 125(1):25–33. [DOI] [PubMed] [Google Scholar]
- Floyd R, Johnson JD, Sharp DG. 1976. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 31(2):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. 2016. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res. 57(8):1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretwell S. 2015. Graniteville: 10 years later, deadly train wreck haunts SC town. The Charlotte Observer. https://www.charlotteobserver.com/news/local/article9252647.html. [Google Scholar]
- Häsänen E, Manninen PKG, Himberg K, Väätäinen V. 1990. Chlorine and bromine contents in tobacco and tobacco smoke. Journal of Radioanalytical and Nuclear Chemistry. 144(5):367–374. [Google Scholar]
- Horton BJ, Luben TJ, Herring AH, Savitz DA, Singer PC, Weinberg HS, Hartmann KE. 2011. The effect of water disinfection by-products on pregnancy outcomes in two southeastern US communities. J Occup Environ Med. 53(10):1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR et al. 2009. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 374(9688):451–458. [DOI] [PubMed] [Google Scholar]
- Jilling T, Ren C, Yee A, Aggarwal S, Halloran B, Ambalavanan N, Matalon S. 2018. Exposure of neonatal mice to bromine impairs their alveolar development and lung function. Am J Physiol Lung Cell Mol Physiol. 314(1):L137–l143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela R, Hemanthakumar KA, Vaparanta K, Robciuc M, Izumiya Y, Kidoya H, Takakura N, Peng X, Sawyer DB, Elenius K et al. 2019. Endothelial Cells Regulate Physiological Cardiomyocyte Growth via VEGFR2-Mediated Paracrine Signaling. Circulation. 139(22):2570–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Egi M, Mizobuchi S. 2017. Haptoglobin Administration in Cardiovascular Surgery Patients: Its Association With the Risk of Postoperative Acute Kidney Injury. Anesth Analg. 124(6):1771–1776. [DOI] [PubMed] [Google Scholar]
- Lam A, Vetal N, Matalon S, Aggarwal S. 2016. Role of heme in bromine-induced lung injury. Ann N Y Acad Sci. 1374(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JA, Carlisle MA, Lam A, Aggarwal S, Doran S, Ren C, Bradley WE, Dell’Italia L, Ambalavanan N, Ford DA et al. 2017. Mechanisms and Treatment of Halogen Inhalation-Induced Pulmonary and Systemic Injuries in Pregnant Mice. Hypertension. 70(2):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG. 1894. Chloroform Vapour as an Irritant to the Larynx. The Lancet. 143(3691):1354–1356. [Google Scholar]
- Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. 2008. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 295(5):L733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH et al. 2004. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 350(7):672–683. [DOI] [PubMed] [Google Scholar]
- Makarovsky I, Markel G, Hoffman A, Schein O, Brosh-Nissimov TM, Finkelstien A, Tashma Z, Dushnitsky T, Eisenkraft A. 2007. Bromine--the red cloud approaching. Isr Med Assoc J. 9(9):677–679. [PubMed] [Google Scholar]
- Marshall SA, Hannan NJ, Jelinic M, Nguyen TPH, Girling JE, Parry LJ. 2018. Animal models of preeclampsia: translational failings and why. Am J Physiol Regul Integr Comp Physiol. 314(4):R499–R508. [DOI] [PubMed] [Google Scholar]
- McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, Grobler A, Stargatt R, Hunt RW, Sheppard SJ et al. 2019. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 393(10172):664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann ME, Soriano SG. 2019. Does general anesthesia affect neurodevelopment in infants and children? BMJ. 367:l6459. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. 2004. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data.(350):1–36. [PubMed] [Google Scholar]
- Müller ALH, Bizzi CA, Pereira JSF, Mesko MF, Moraes DP, Flores EMM, Muller EI. 2011. Bromine and chlorine determination in cigarette tobacco using microwave-induced combustion and inductively coupled plasma optical emission spectrometry. Journal of the Brazilian Chemical Society. 22:1649–1655. [Google Scholar]
- Müller ALH, Müller CC, Antes FG, Barin JS, Dressler VL, Flores EMM, Müller EI. 2012. Determination of Bromide, Chloride, and Fluoride in Cigarette Tobacco by Ion Chromatography after Microwave-Induced Combustion. Analytical Letters. 45(9):1004–1015. [Google Scholar]
- Natale BV, Mehta P, Vu P, Schweitzer C, Gustin K, Kotadia R, Natale DRC. 2018. Reduced Uteroplacental Perfusion Pressure (RUPP) causes altered trophoblast differentiation and pericyte reduction in the mouse placenta labyrinth. Scientific Reports. 8(1):17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee on Acute Exposure Guideline Levels. 2010. Bromine acute exposure guideline levels. Acute exposure guideline levels for selected airborne chemicals Vol 9. Washington (DC): National Academies Press. [Accessed 2020 Jan 30]. https://www.ncbi.nlm.nih.gov/books/NBK208163/. [Google Scholar]
- Rozov LA, Huang C, Vernice GG, inventors; Anaquest, Inc., Liberty Corner, NJ, assignee. 1993. April 27. Synthesis of desflurane. patent 5,205,914.
- Rozov LA, Quiroz F, Vernice GG, inventors; Ohmeda Pharmaceutical Products Division Inc., Liberty Corner, N.J., assignee. 1995. May 16. Preparation of isoflurane. patent 5,416,244.
- Ryter SW, Tyrrell RM. 2000. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 28(2):289–309. [DOI] [PubMed] [Google Scholar]
- Sherer O, Kuhn H, inventors; Farbwerke Hoechst Aktiengesellschaft formerly Meister Lucius & Bruning, Frankfurt, Germany, assignee. 1960. November 8. Process for preparing 1,1,1-trifluoro-2-chloro-2-bromethane from 1,1,2-triflu-oro-1-bromo-2-chlorethane. patent 2,959,624.
- Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. 2011. Postexposure administration of a {beta}2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol. 45(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squadrito GL, Postlethwait EM, Matalon S. 2010. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol. 299(3):L289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling CW, Raventos J, inventors; Imperial Chemical Industries Ltd, London, England, assignee. 1958. August 26. 1,1,1-trifluoro-2-bromio-2-chloroethane and a process of making it. patent 2849502.
- Suckling CW, Raventos J, inventors; Imperial Chemical Industries Ltd., London, England, assignee. 1960. January 12. Process for the preparation of 1,1,1-trifluoro-2-bromo2-chloroethane. patent 2,921,098.
- Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR et al. 2016. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. Jama. 315(21):2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell RC, inventor; Medeva Pharmaceuticals PA, Inc., Bethlehem, PA, assignee. 1999. October 19. Method for the preparation of sevoflurane. patent 5,969, 193.
- The International Bromine Council. About Bromine: Production. [accessed 2019 October 22]. https://www.bsef.com/about-bromine/production/.
- Ueda K, Toko H, Komuro I. 2019. Endothelial Cell-Derived Angiocrines Elicit Physiological Cardiomyocyte Hypertrophy. Circulation. 139(22):2585–2587. [DOI] [PubMed] [Google Scholar]
- Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. 2010. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 77(10):913–920. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Culley DJ. 2019. GAS, PANDA, and MASK: No Evidence of Clinical Anesthetic Neurotoxicity! Anesthesiology. 131(4):762–764. [DOI] [PubMed] [Google Scholar]
- Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, Figdor CG. 2001. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 98(6):1802–1811. [DOI] [PubMed] [Google Scholar]
- Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J et al. 2018. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 129(1):89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Chlorine Council. World Chlorine Council: Sustainable Future. [Google Scholar]
- World Health Organization. 2018. Bromine as a Drinking Water Disinfectant. [Google Scholar]
- WVTM 13. 2019. Chemical spill at Birmingham Water Works plant sends 50 to area hospitals. [updated 02/28/2019; accessed 2019 October 22]. https://www.wvtm13.com/article/chemical-spill-at-birmingham-water-works-plant-sends-people-to-the-hospital/26551385.
- Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. 2010. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 7(4):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaky A, Ahmad A, Dell’Italia LJ, Jahromi L, Reisenberg LA, Matalon S, Ahmad S. 2015. Inhaled matters of the heart. Cardiovasc Regen Med. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell’Italia LJ, Matalon S, Ahmad S. 2015. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]