Highlights
-
•
About two thirds of caregivers intend to vaccinate their children against COVID-19.
-
•
Most common reason for acceptance was to protect the child.
-
•
Most common reason for refusal was the vaccine’s novelty.
-
•
Child age, chronic illness, vaccination history affects willingness.
-
•
Caregiver gender, vaccination history, concern about infection affect willingness.
Keywords: COVID-19, Coronavirus, Vaccination, Pandemic, Child, Emergency Department
Abstract
Background
More than 100 COVID-19 vaccine candidates are in development since the SARS-CoV-2 genetic sequence was published in January 2020. The uptake of a COVID-19 vaccine among children will be instrumental in limiting the spread of the disease as herd immunity may require vaccine coverage of up to 80% of the population. Prior history of pandemic vaccine coverage was as low as 40% among children in the United States during the 2009 H1N1 influenza pandemic.
Purpose
To investigate predictors associated with global caregivers’ intent to vaccinate their children against COVID-19, when the vaccine becomes available.
Method
An international cross sectional survey of 1541 caregivers arriving with their children to 16 pediatric Emergency Departments (ED) across six countries from March 26 to May 31, 2020.
Results
65% (n = 1005) of caregivers reported that they intend to vaccinate their child against COVID-19, once a vaccine is available. A univariate and subsequent multivariate analysis found that increased intended uptake was associated with children that were older, children with no chronic illness, when fathers completed the survey, children up-to-date on their vaccination schedule, recent history of vaccination against influenza, and caregivers concerned their child had COVID-19 at the time of survey completion in the ED. The most common reason reported by caregivers intending to vaccinate was to protect their child (62%), and the most common reason reported by caregivers refusing vaccination was the vaccine’s novelty (52%).
Conclusions
The majority of caregivers intend to vaccinate their children against COVID-19, though uptake will likely be associated with specific factors such as child and caregiver demographics and vaccination history. Public health strategies need to address barriers to uptake by providing evidence about an upcoming COVID-19 vaccine’s safety and efficacy, highlighting the risks and consequences of infection in children, and educating caregivers on the role of vaccination.
1. Introduction
Over 100 different vaccine candidates have been developed since the genetic sequence for SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), was published in January 2020 [1]. Vaccination will be one of the most effective strategies in limiting the spread of the disease. This was different for the severe acute respiratory syndrome (SARS), Ebola, and Zika epidemics which ended before vaccine development was completed and a monovalent influenza A (H1N1) vaccine was not available before the first wave of the pandemic peaked but was later incorporated into commercially available seasonal influenza vaccines [2]. It is currently thought that developing a vaccine for SARS-CoV-2 will be relatively straightforward and attainable because the virus seems to be fairly stable [3].
Numerous factors affect caregiver acceptance of new vaccination programs, especially during pandemics. It is estimated that 40% of children (6 months to 17 years of age) in the United States received the pandemic H1N1 vaccination in 2009 [4] and factors like fear about the H1N1 illness, [5], [6] prior seasonal influenza vaccination experience, [6] and parental level of education [5] were associated with H1N1 vaccine uptake.
The objective of this study was to determine predictors associated with the willingness of caregivers to vaccinate their children against COVID-19, once a vaccine becomes available. Positive public opinion and trust in an expedited COVID-19 pandemic vaccine will be fundamental [7] since there is hesitancy about safety and need of new vaccines [8]. With predicted vaccine coverage of 55% to 82% of the population needed to provide herd immunity to SARS-CoV-2, [9] local health authorities such as those in the United States reported that it is unlikely herd immunity will be achieved given the current state of COVID-19 vaccine refusal [10]. Understanding factors associated with caregiver intentions to vaccinate children during a pandemic may support public health officials’ efforts towards broader acceptance of the vaccine and thus reach a higher level of immunity in the population.
2. Methods
2.1. Sample and procedures
This study is part of a larger COVID-19 Parental Attitude Study (COVIPAS) of caregivers presenting for emergency care for their children during the era of COVID-19. Using posters placed in waiting areas and patient rooms, as well as direct approach by healthcare team members, caregivers who arrived to 16 pediatric emergency departments (ED) in the USA (Seattle, Tacoma, Los Angeles, Dallas, Atlanta), Canada (Vancouver, Toronto, Saskatoon, Edmonton), Israel (Zerifin), Japan (Tokyo), Spain (Barcelona), and Switzerland (Zurich, Bern, Geneva, Bellinzona) were asked to take part in the survey. For infectious control purposes, caregivers used their own smartphones to complete the survey by logging into a secure online platform based on REDCap metadata-driven software (Vanderbilt University). Once a caregiver selected their study site, they provided consent for participation in the online survey, as approved by each site’s local Institutional Review Board (IRB). Five IRBs (in Switzerland and Spain) provided a waiver of consent whereby responding to the survey was considered consent to participate.
The survey tool was available in English, French, German, Spanish, Japanese, Italian, and Hebrew. While sites began recruitment in a staggered fashion, surveys were obtained between March 26 and May 31, 2020. Only one caregiver completed the survey per visit, and due to restrictions to visitation in most sites, only one caregiver was in the room with the child.
3. Measures
The study-specific questionnaire was developed to include questions regarding demographic characteristics, information on the ED visit, and attitudes around COVID-19. The survey objective was to reflect caregiver opinions and actions during the pandemic. Literature related to the SARS epidemic in 2002–2003 helped inform questions in the survey. Pilot testing for face and content validity for all items of the survey, including those presented in this report, was completed a priori by 10 individuals representing the target group of caregivers and by 10 healthcare providers working in the ED environment who provided feedback that led to revisions and development of the final survey.
We asked caregivers to answer the question: “There is no vaccine/immunization currently available for Coronavirus (COVID-19). If a vaccine/immunization was available today, would you give it to your child?” followed by an open-ended question “Why?” or “Why not?”, with a free text box.
The description of each response was categorized into themes using an inductive approach by one author and reviewed for completeness by another author. The entirety of each response was analyzed and if more than one sentiment was expressed in the caregiver’s description, the individual response was coded to multiple themes. Free text responses that were blank were categorized as no comment. Themes were analyzed for frequencies of responses by participants.
3.1. Data analysis
Basic descriptive statistics and frequencies were used to describe all variables, comparing survey data from caregivers who would vaccinate their children against COVID-19 and those that would not. To determine which factors were significantly associated with the decision to vaccinate children, we used univariate analyses: Mann-Whitney test for comparing non-normal continuous variables, independent t-test for comparing normally distributed continuous variables, and Chi-square or Fisher’s exact test for categorical variables. We then used multivariable logistic regression analysis to estimate the adjusted odds ratio of agreeing to vaccinate children, using all the variables that showed significance (p < 0.1) in the univariate analysis. To compare caregiver concern of their child having COVID-19 (score 0–10) to willingness to vaccinate, we used the Mann-Whitney U test. All analyses were conducted with R version 3.5.1. A p-value<0.05 was considered statistically significant.
4. Results
A total of 1552 surveys were completed online. Eleven (0.7%) were excluded because they were completed by patients (n = 5) or completed halfway (n = 6). The median age of children was 7.5 (Standard Deviation (SD) = 5.0) years and the median age of caregivers was 39.9 (SD = 7.6) years. The vast majority of surveys were completed by parents (97.5%) as opposed to other caregivers. 184 (12%) had children with a chronic illness, and 28/184 (15%) had a potential contraindication to live vaccines (e.g. cancer, potentially receiving immunosuppressant medications). Of the included surveys, 317 (21%) were completed in the United States, 542 (35%) in Canada, 438 (28%) in Switzerland, 124 (8%) in Spain, 91 (6%) in Israel, and 29 (2%) in Japan. There were 1005/1541 (65.2%) caregivers who reported that they plan to vaccinate their child against COVID-19 and 509/1541 (33.0%) who do not plan to vaccinate their child against COVID-19. Twenty-seven (1.8%) caregivers did not answer this survey question.
Table 1 provides demographic information including a comparison between families who would or would not vaccinate their children against COVID-19, if a vaccine becomes available. In the univariate analysis, greater willingness to vaccinate was associated with older children (p = 0.009), children that were up-to-date on their vaccines (p < 0.001), children with no chronic illness (p = 0.096), when fathers completed the survey (p = 0.002), if the caregiver was older (p < 0.001), if the child (p < 0.001) or the caregiver (p < 0.001) reported they were immunized against influenza in the last year, and if the caregiver was more concerned about their child (p < 0.001) or themselves (p < 0.001) having COVID-19 when arriving to the ED.
Table 1.
Factors associated with caregiver willingness to vaccinate their children against COVID-19. SD = standard deviation.
| Number of Surveys | Total population (n = 1541) | Not willing to vaccinate child against COVID-19 (n = 509) | Willing to vaccinate child against COVID-19 (n = 1005) | P value | |
|---|---|---|---|---|---|
| Child’s median age in years (SD) | 1532 | 7.50 (4.98) | 7.03 (4.94) | 7.74 (4.98) | 0.009 |
| Child’s gender female | 1533 | 733 (47.8%) | 248 (48.8%) | 477 (47.8%) | 0.748 |
| Child has chronic illness | 1532 | 184 (12.0%) | 71 (14.0%) | 109 (10.9%) | 0.096 |
| Child with chronic medication use | 1536 | 205 (13.3%) | 59 (11.6%) | 143 (14.3%) | 0.183 |
| Person completing the survey | 1540 | 0.002 | |||
| Father | 393 (25.5%) | 103 (20.2%) | 282 (28.1%) | ||
| Mother | 1109 (72.0%) | 396 (77.8%) | 695 (69.2%) | ||
| Other* | 38 (2.47%) | 10 (1.96%) | 27 (2.69%) | ||
| Caregiver’s median age in years (SD) | 1517 | 39.9 (7.58) | 39.0 (7.22) | 40.4 (7.72) | <0.001 |
| Caregivers with higher education** | 1517 | 1217 (80.2%) | 406 (80.4%) | 792 (80.3%) | 1.000 |
| Child’s vaccinations up to date | 1525 | 1352 (88.7%) | 407 (80.6%) | 930 (92.9%) | <0.001 |
| Child received influenza vaccine last 12 months | 1522 | 486 (31.9%) | 108 (21.3%) | 374 (37.5%) | <0.001 |
| Caregiver received influenza vaccine last 12 months | 1529 | 594 (38.8%) | 131 (25.8%) | 458 (45.7%) | <0.001 |
| Mean score 10-point Likert scale - caregiver concerned their child has COVID-19 (SD) | 1503 | 1.86 (2.78) | 1.36 (2.36) | 2.09 (2.93) | <0.001 |
| Mean score 10-point Likert scale- caregiver concerned they have COVID-19 (SD) | 1498 | 1.83 (2.64) | 1.37 (2.27) | 2.04 (2.77) | <0.001 |
grandparents or siblings
completed more education than high school studies
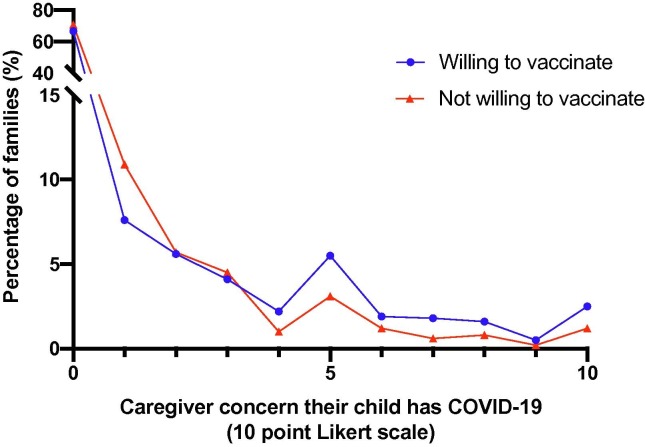
The multivariate logistic regression analysis (Table 2 ) revealed that factors predicting willingness to vaccinate against COVID-19 were the child’s age (Odds Ratio (OR) = 1.03, 95% confidence interval (CI) 1.00–1.05, p = 0.033), child’s vaccination was reported by caregivers to be up-to-date (OR = 2.57, 95% CI 1.81–3.68, p < 0.001), child (OR = 1.49, 95% CI 1.09–2.05, p = 0.013) and caregiver (OR = 2.08, 95% CI 1.55–2.8, p < 0.001) vaccination against influenza in the past year, and caregiver concern that the child had COVID-19 (OR = 1.08, 95% CI 1–1.17, p = 0.048). Fig. 1 depicts level of concern from COVID-19 in the child (0–10) and willingness to vaccinate against COVID-19 (p = 0.017). Factors predicting lack of willingness to vaccinate were mothers completing the survey (OR = 0.62, 95% CI 0.47–0.81, p < 0.001) and the child having a chronic illness (OR = 0.66, 95% CI 0.47–0.95, p = 0.022).
Table 2.
Predictors of caregiver willingness to vaccinate their children against COVID-19 identified by multivariate logistic regression analysis.
| Odds Ratio | OR 95% CI | P value | |
|---|---|---|---|
| Child’s median age | 1.03 | (1.00 – 1.05) | 0.033 |
| Child has chronic illness | 0.66 | (0.47–0.95) | 0.022 |
| Child vaccinations up to date | 2.57 | (1.81–3.68) | <0.001 |
| Child vaccinated against influenza in the last 12 months | 1.49 | (1.09–2.05) | 0.013 |
| Mother completing the survey | 0.62 | (0.47–0.81) | <0.001 |
| Caregiver vaccinated against influenza in the last 12 months | 2.08 | (1.55–2.8) | <0.001 |
| Mean score 10 point Likert scale - caregiver concerned their child has COVID-19 | 1.08 | (1–1.17) | 0.048 |
| Mean score 10 point Likert scale- caregiver concerned they have COVID-19 | 1.05 | (0.97–1.14) | 0.220 |
Fig. 1.
Level of concern from COVID-19 in the child and willingness to vaccinate against COVID-19 if a vaccine was available. p-value is 0.017.
Table 3 provides the qualitative themes gained from responses of caregivers’ intent to vaccinate their children against COVID-19, with corresponding representative quotes for each of the themes identified in the two groups. Seven themes were identified among caregivers willing to vaccinate their children against COVID-19: protect the child, protect others, general vaccine acceptance, perceived pandemic severity, high risk child or family member, accepting but concerns of efficacy/safety, and desire to return to normal. Seven themes were identified among caregivers not willing to vaccinate: novelty, perceived child is not at risk to contract COVID-19, side effects/safety concerns, efficacy concerns, general vaccine refusal, perceived contraindication, and may vaccinate if more information available/recommended by healthcare provider. Reasons for their decision to vaccinate were provided by 789 (78.5%) of caregivers willing to provide vaccination for their child and 382 (75.0%) of those not willing. The most common considerations to immunize a child were protecting them and others (86.1% of caregivers providing reasoning), and the most common considerations not to immunize a child were the novelty of the vaccine and the caregivers’ notion that their children are at low risk to be sick with COVID-19 (82.7% of caregivers providing reasoning).
Table 3.
Reasons reported by caregivers for willingness to vaccinate or not vaccinate children against COVID-19.
| Reason to Vaccinate (n = 1005) | Example quote | Number of caregivers | Percent of available comments |
|---|---|---|---|
| Protect the child | “To give her immunity to COVID-19” | 492 | 62.4% |
| Protect others | ““Herd immunity matters” | 187 | 23.7% |
| General vaccine acceptance | “Vaccines work” | 109 | 13.8% |
| Perceived pandemic severity | “Seems more deadly of a virus” | 66 | 8.4% |
| High risk child or family members | “He has pre-existing lung issues” | 54 | 6.8% |
| Accepting, but concerns of efficacy/safety | “Actually I’d wait to see how most people reacted. So would not get right away but once I was sure it was safe” | 38 | 4.8% |
| Desire to return to normal life | “To get back to school” | 12 | 1.5% |
| No comment | 216 | ||
| Reason not to Vaccinate (n = 509) | Example quote | Number of caregivers | Percent of available comments |
| Novelty | “Not enough testing” | 197 | 51.6% |
| Perceived child not at risk to contract COVID-19 | “It doesn’t affect children as badly as adults” | 119 | 31.2% |
| Side effects/safety concerns | “Fear of side effects” | 84 | 22.0% |
| May vaccinate if more information available/recommended by healthcare provider | “I would want to wait until we know more before making such a decision” | 65 | 17.0% |
| Vaccine refusal in general | “Vaccines need to be abolished” | 40 | 10.5% |
| Efficacy Concerns | “You don’t know if it works” | 34 | 8.9% |
| Perceived Contraindication* | “I don’t think he can have vaccines while receiving chemo” | 7 | 1.8% |
| No comment | 127 | ||
(*) immunosuppression, child is too young, allergy
5. Discussion
Once a vaccine against COVID-19 is available and approved for use, in order to facilitate return to pre-pandemic activity, public health officials will need to enhance uptake of the new vaccine to ensure population immunity and mitigation of morbidity and mortality. Overcoming challenges in vaccine development and increasing uptake is crucial, especially during the pandemic and among children [11].
In our international sample, two-thirds of caregivers reported willingness to vaccinate their children against COVID-19, once a vaccine is available. Independent factors associated with increased uptake included the age of the child, children who are up-to-date on their immunization schedule, if the child or caregiver were vaccinated against influenza in the past year, and if when completing the survey in the ED they were concerned their child may have COVID-19. Similar factors were reported regarding uptake of the pandemic H1N1 vaccine, such as an individual’s vaccination in the past, [12], [5], [6], [13] as well as an older age of the individual responder [12] and their child [14].
The rate of intent to vaccinate in our sample was somewhat lower than a recent nationally representative sample from the US in which 80% of parents stated they would vaccinate their child against COVID-19 [17]. Among adults, 81% of Australian residents suggested they ‘definitely’ or ‘probably’ plan to get vaccinated against COVID-19, [15] 65% in a recent study in Saudi Arabia, [16] three quarters of an online sample from France, [18] 69% in the United Kingdom, [19] and 65% in Ireland [19]. Interestingly, the rate of children that obtained vaccination after the H1N1 pandemic was 40% in the US, [4] perhaps representing a divide between reported intentions and true vaccination rates, or differences in risk perceptions between these two diseases.
Fear of the pandemic’s severity as a primary motivation for COVID-19 vaccination identified in our global sample is similar to prior notions of parental plan to vaccinate their child against H1N1 [5], [6], [20]. Perceived risk from the illness, [12] knowledge of the disease, [5] and understanding that vaccines are effective prevention strategies [12] were associated with increased pandemic H1N1 vaccination uptake [5], [6]. In our sample, ‘protection of the child’ was the most common theme caregivers reported as a reason to vaccinate. Similarly, caregivers who perceived that the child was not at risk to contract COVID-19 reported lack of willingness to vaccinate in the future (31% of those providing reasoning for not vaccinating). This is similar to the H1N1 pandemic, where a strong association was noted between vaccine intentions and fear of the adult and child catching the disease, [6] as well as a report that concerns of a COVID-19 outbreak in Australia were associated with enhanced willingness to get vaccinated [15]. However, as we found a correlation between caregivers’ concern the child already contracted COVID-19 and their willingness to vaccinate the child, there may be a gap in understanding the role of a vaccine as a preventive measure, and greater education of caregivers is warranted on the utility of vaccines. “Protection of others” was the second most common theme in our willing cohort of caregivers, possibly reflecting either a wish to return to social and physical contact with family and friends, or an altruistic motive by caregivers [21].
Of interest, caregivers of children with chronic illness, a group that may benefit from protection against the virus and who are likely more familiar with the medical system, reported lower rate of intent to vaccinate their children against COVID-19. This is in contrast to findings in one ED in Canada form the early 2000 s in which children with chronic illness were reported to be more likely than those without a chronic illness to be immunized against seasonal influenza [22]. While this may reflect general concerns of using live vaccines in children with immunocompromised states (15% of those with chronic illness in our cohort), public health programs should focus their educational efforts on the importance of COVID-19 vaccine protection among children with chronic illness.
We also report that fathers were more willing than mothers to vaccinate in this survey, congruent with the gender difference seen among adults considering H1N1 vaccination [12]. Similarly, in a discrete choice experiment from the Netherlands on pandemic vaccination decision making, females who stated that they were never in favor of vaccination made different trade-offs than males who stated that they were (possibly) willing to get vaccinated [23]. Risk taking behaviours of fathers may be different than those of mothers, similar to findings related to child play and pediatric trauma prevention, [24] highlighting that vaccine information targeted to families should address these different perspectives in order to encourage the highest vaccination acceptability possible.
Level of education was not a factor associated with intent to vaccinate against COVID-19 in our global cohort, in contrast to prior reports [5], [14] that parents with a post-secondary education were more willing to vaccinate their children during the H1N1 pandemic. We did not collect income data and could not assess concerns about out-of-pocket expenses, both of which have been reported previously as important factors for H1N1 vaccine uptake [25] and intent to receive a future COVID-19 vaccine [16].
Similar to reports after the H1N1 pandemic experience, we found that vaccine safety is a fundamental determinant for decision making [13]. Concerns over adverse effects are also reported as the most common reason (68–86%) parents refuse vaccination for their children in non-pandemic situations [26], [27]. The rate of reporting potential side effects as a reason not to vaccinate was meaningfully lower in our cohort (20%) compared to concerns parents shared during H1N1 (80%), [13] perhaps because the COVID-19 vaccine was not available during this survey, and no reports of side effects were known. The notion of “novelty”, reported by half of caregivers providing reasoning for not planning to vaccinate in our sample, may be a surrogate for safety concerns, and hope for more experience with a future vaccine before caregivers expose their children to the new product.
Among a US nationally representative sample, almost 20% of parents reported that they will not vaccinate against COVID-19, with reasons being the vaccine’s novelty (82%), possible side effects (80%) and general vaccine avoidance (72%) [16]. Our findings suggest a higher rate of COVID-19 vaccine rejection among caregivers, likewise attributed to concerns of novelty (51.6%), safety (22.0%), and general vaccine avoidance (10.5%), but also a lack of perceived threat of COVID-19 infection among children (31.2%). While it is recognized that education is just one of many arms of intervention in combatting the complex factors associated with vaccine hesitancy, [28] public health efforts should aim to provide greater education on the risks and consequences of disease in children, [29] particularly the potential to infect family members and those at risk, as this was the second most reported reason for COVID-19 vaccine refusal.
Vaccine effectiveness was also important for the majority of parents (58%) during the H1N1 pandemic, [13] and 17% of those providing reasoning for refusal to vaccinate against COVID-19 responded that they may vaccinate if more information became available. Social legitimacy, [14], [30] following large groups getting vaccinated, [12] and hearing recommendations from healthcare providers [12] may empower parents to vaccinate their children against COVID-19.
Our findings provide specific ways in which public health programs internationally may optimize future COVID-19 vaccine uptake including targeted education in a manner that recognizes caregiver concerns, child age and chronic illness status, previous vaccine history, and perception of infection risk as determinants of preference. Although vaccine hesitancy is pervasive worldwide across all socioeconomic groups, it varies between countries and is context-specific even among individuals belonging to the same country [28], [31]. Further regional analysis may help public health programs with targeted campaigns, though this was not possible in our analysis given that larger samples are needed from those regions. We identified potential barriers to a pandemic vaccine’s uptake, which are largely similar to reasons reported by parents who generally oppose vaccination, [25] particularly highlighting the need for strategies to inform caregivers on the role of vaccines in prevention and to not understate the risks of COVID-19 infection in children.
6. Limitations
Our study has several limitations. First, the population of parents and other caregivers responding to the survey is not representative of all caregivers in the six countries where the survey took place, as we administered the survey in a hospital ED setting during the peak of COVID-19. ED access patterns by caregivers may have been influenced by the pandemic, resulting in delayed or omitted visits due to stay-in-place orders by local governments, or children who may not have ordinarily presented to the ED but did because their primary health care provider was unavailable. Moreover, not all parents completed the survey and a few (2.5%) respondents were caregivers other than parents (e.g. grandparents who may not be decision makers for future vaccination. Also, requiring a smartphone to complete the survey may prohibit participation for some. However, one of the similarities of our cohort to the general population of caregivers is the 10–13% rate of caregivers rejecting vaccines [32]. Our multicenter trial in 16 EDs may help generalize the findings to other centers/countries once a vaccine is available. Future research must take into account regional and geographic differences, especially when trying to determine parental rationales for not planning to vaccinate their children.
Secondly, caregivers shared their considerations in regards to vaccinating their child at times of intense uncertainty during a period of major change in daily activities (no school, work-at-home), and their willingness to vaccinate against COVID-19 may be different when community life returns to a new normal activity and the numbers of infected patients drop. Throughout the period of survey data collection, communications from local authorities had evolved and factors including the availability of COVID-19 testing for children had changed over time. Finally, we proposed a hypothetical vaccine, and once available and tested, caregivers may learn new information that may change their mind with regards to vaccinating their children.
7. Conclusions
About two thirds of caregivers in a global sample intend to vaccinate their children against COVID-19, once available. Child’s age, child vaccination status including previous influenza vaccination history, caregiver gender, and concerns of COVID-19 infection were factors associated with intent to vaccinate a child. Public health effort should aim to educate caregivers about the role of vaccination, and to focus on families such as those with children with a chronic illness. Communicating safety and healthcare recommendations will likely be instrumental in promoting vaccine uptake.
All authors attest they meet the ICMJE criteria for authorship.
8. Data statement
The data will not be shared nor disseminated to study participants/patient organizations
CRediT authorship contribution statement
Ran D. Goldman: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Supervision, Project administration. Tyler D. Yan: Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Project administration. Michelle Seiler: Investigation, Resources, Writing - review & editing. Cristina Parra Cotanda: Investigation, Resources, Writing - review & editing. Julie C. Brown: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing. Eileen J. Klein: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing. Julia Hoeffe: Investigation, Resources, Writing - review & editing. Renana Gelernter: Investigation, Resources, Writing - review & editing. Jeanine E. Hall: Investigation, Resources, Writing - review & editing. Adrienne L. Davis: Investigation, Resources, Writing - review & editing. Mark A. Griffiths: Investigation, Resources, Writing - review & editing. Ahmed Mater: Investigation, Resources, Writing - review & editing. Sergio Manzano: Investigation, Resources, Writing - review & editing. Gianluca Gualco: Investigation, Resources, Writing - review & editing. Naoki Shimizu: Investigation, Resources, Writing - review & editing. Thomas L. Hurt: Conceptualization, Methodology. Sara Ahmed: Conceptualization, Methodology. Matt Hansen: Investigation, Resources, Writing - review & editing. David Sheridan: Investigation, Resources, Writing - review & editing. Samina Ali: Investigation, Resources, Writing - review & editing. Graham C. Thompson: Investigation, Resources, Writing - review & editing. Nathalie Gaucher: Investigation, Resources, Writing - review & editing. Georg Staubli: Formal analysis, Data curation, Investigation, Resources, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to Dr. Julie A. Bettinger for reviewing this manuscript
References
- 1.Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 2.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 3.Mahase E. Covid-19: What do we know so far about a vaccine? BMJ. 2020;369 doi: 10.1136/bmj.m1679. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Final estimates for 2009-10 seasonal Influenza and Influenza A (H1N1) 2009 monovalent vaccination coverage - United States, August 2009 through May, 2010; May 13, 2011. https://www.cdc.gov/flu/fluvaxview/coverage_0910estimates.htm
- 5.Setbon M., Raude J. Factors in vaccination intention against the pandemic influenza A/H1N1. The European Journal of Public Health. 2010;20(5):490–494. doi: 10.1093/eurpub/ckq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin G.J., Potts H.W.W., Michie S. Likely uptake of swine and seasonal flu vaccines among healthcare workers. A cross-sectional analysis of UK telephone survey data. Vaccine. 2011;29(13):2421–2428. doi: 10.1016/j.vaccine.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Fadda M., Albanese E., Suggs L.S. When a COVID-19 vaccine is ready, will we all be ready for it? Int J Public Health. 2020;65(6):711–712. doi: 10.1007/s00038-020-01404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé E., Laberge C., Guay M., Bramadat P., Roy R., Bettinger J.A. Vaccine hesitancy: An overview. Human Vaccines & Immunotherapeutics. 2013;9(8):1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffer DeRoo S., Pudalov N.J., Fu L.Y. Planning for a COVID-19 Vaccination Program. JAMA. 2020;323(24):2458. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 10.Cohen E. Fauci says COVID-19 vaccine may not get US to herd immunity if too many people refuse to get it. CNN [Internet]. 2020 June 28 [cited 2020 Sept 15]. Available from: [Google Scholar]
- 11.Nicholson A., Shah C.M., Ogawa V.A. National Academies Press (US); Washington (DC): 2019. Exploring Lessons Learned from a Century of Outbreaks: Readiness for 2030: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 12.Bish A., Yardley L., Nicoll A., Michie S. Factors associated with uptake of vaccination against pandemic influenza: A systematic review. Vaccine. 2011;29(38):6472–6484. doi: 10.1016/j.vaccine.2011.06.107. [DOI] [PubMed] [Google Scholar]
- 13.Torun S.D., Torun F., Catak B. Healthcare workers as parents: attitudes toward vaccinating their children against pandemic influenza A/H1N1. BMC Public Health. 2010;10(1) doi: 10.1186/1471-2458-10-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akıs S., Velıpasaoglu S., Camurdan A.D., Beyazova U., Sahın F. Factors associated with parental acceptance and refusal of pandemic influenza A/H1N1 vaccine in Turkey. Eur J Pediatr. 2011;170(9):1165–1172. doi: 10.1007/s00431-011-1425-6. [DOI] [PubMed] [Google Scholar]
- 15.Faasse K., Newby J. Public perceptions of COVID-19 in Australia: perceived risk, knowledge, health-protective behaviours, and vaccine intentions. medRxiv. 2020 doi: 10.3389/fpsyg.2020.551004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padhi B.K., Al-Mohaithef M. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. medRxiv. 2020 doi: 10.2147/JMDH.S276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thunstrom L., Ashworth M., Finnoff D., Newbold S. Hesitancy towards a COVID-19 vaccine and prospects for herd immunity. SSRN Electron J. 2020 doi: 10.1007/s10393-021-01524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward J., Alleaume C., Peretti-Watel P. The French public’s attitudes to a future COVID-19 vaccine: the politicization of a public health issue. SocArXiv. 2020 doi: 10.1016/j.socscimed.2020.113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, Vallieres, Bentall et al. Preparing for a COVID-19 vaccine: Identifying and psychologically profiling those who are vaccine hesitant or resistance in two general population samples. PsyArXiv 2020.
- 20.Schwarzinger M, Flicoteaux R, Cortarenoda S, Obadia Y, Moatti JP. Low acceptability of A/H1N1 pandemic vaccination in french adult population: Did public health policy fuel public dissonance? PLoS One 2010;5:e10199. [DOI] [PMC free article] [PubMed]
- 21.Shim Eunha, Chapman Gretchen B., Townsend Jeffrey P., Galvani Alison P. The influence of altruism on influenza vaccination decisions. J. R. Soc. Interface: 2012;9(74):2234–2243. doi: 10.1098/rsif.2012.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant V.J., Le Saux N., Plint A.C. Factors influencing childhood influenza immunization. CMAJ. 2003;168:39–41. [PMC free article] [PubMed] [Google Scholar]
- 23.Determann D, Korfage IJ, Lambooij MS, et al. Acceptance of vaccinations in pandemic outbreaks: A discrete choice experiment. PLoS One 2014;9:e102505. [DOI] [PMC free article] [PubMed]
- 24.Brussoni M., Olsen L. Striking a balance between risk and protection: fathers' attitudes and practices toward child injury prevention. J Dev Behav Pediatr. 2011;32:491–498. doi: 10.1097/DBP.0b013e31821bd1f5. [DOI] [PubMed] [Google Scholar]
- 25.Lau J. T F, Yeung N. C Y, Choi K C, Cheng M. Y M, Tsui H Y, Griffiths S. Acceptability of A/H1N1 vaccination during pandemic phase of influenza A/H1N1 in Hong Kong: population based cross sectional survey. BMJ. 2009;339(oct27 1):b4164. doi: 10.1136/bmj.b4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmon Daniel A., Moulton Lawrence H., Omer Saad B., deHart M. Patricia, Stokley Shannon, Halsey Neal A. Factors Associated With Refusal of Childhood Vaccines Among Parents of School-aged Children: A Case-Control Study. Arch Pediatr Adolesc Med. 2005;159(5):470. doi: 10.1001/archpedi.159.5.470. [DOI] [PubMed] [Google Scholar]
- 27.Wenger O.K., McManus M.D., Bower J.R., Langkamp D.L. Underimmunization in Ohio's Amish: Parental Fears Are a Greater Obstacle Than Access to Care. Pediatrics. 2011;128(1):79–85. doi: 10.1542/peds.2009-2599. [DOI] [PubMed] [Google Scholar]
- 28.Dubé Eve, Gagnon Dominique, Nickels Emily, Jeram Stanley, Schuster Melanie. Mapping vaccine hesitancy—Country-specific characteristics of a global phenomenon. Vaccine. 2014;32(49):6649–6654. doi: 10.1016/j.vaccine.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung Eva W., Zachariah Philip, Gorelik Mark, Boneparth Alexis, Kernie Steven G., Orange Jordan S., Milner Joshua D. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 2020;324(3):294. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung Minsoo, Lin Leesa, Viswanath K. Associations between health communication behaviors, neighborhood social capital, vaccine knowledge, and parents’ H1N1 vaccination of their children. Vaccine. 2013;31(42):4860–4866. doi: 10.1016/j.vaccine.2013.07.068. [DOI] [PubMed] [Google Scholar]
- 31.Larson Heidi J., Jarrett Caitlin, Eckersberger Elisabeth, Smith David M.D., Paterson Pauline. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy Allison M., Brown Cedric J., Gust Deborah A. Vaccine Beliefs of Parents Who Oppose Compulsory Vaccination. Public Health Rep. 2005;120(3):252–258. doi: 10.1177/003335490512000306. [DOI] [PMC free article] [PubMed] [Google Scholar]