Abstract
Introduction
The severity of coronavirus disease (COVID-19) in Japanese patients is unreported. We retrospectively examined significant factors associated with disease severity in symptomatic COVID-19 patients (COVID-Pts) admitted to our institution between February 20 and April 30, 2020.
Methods
All patients were diagnosed based on the genetic detection of severe acute respiratory syndrome coronavirus 2. Information on the initial symptoms, laboratory data, and computed tomography (CT) images at hospitalization were collected from the patients’ records. COVID-Pts were categorized as those with critical or severe illness (Pts-CSI) or those with moderate or mild illness (Pt-MMI). All statistical analyses were performed using R software.
Results
Data from 61 patients (16 Pt–CSI, 45 Pt-MMI), including 58 Japanese and three East Asians, were analyzed. Pt–CSI were significantly older and had hypertension or diabetes than Pt-MMI (P < 0.001, 0.014 and < 0.001, respectively). Serum albumin levels were significantly lower in Pt–CSI than in Pt-MMI (P < 0.001), whereas the neutrophil-to-lymphocyte ratio and C-reactive protein level were significantly higher in Pt–CSI than in Pt-MMI (P < 0.001 and P < 0.001, respectively). In the CT images of 60 patients, bilateral lung lesions were more frequently observed in Pt–CSI than in Pt-MMI (P = 0.013). Among the 16 Pt–CSI, 15 received antiviral therapy, 12 received tocilizumab, five underwent methylprednisolone treatment, six received mechanical ventilation, and one died.
Conclusions
The illness severity of Japanese COVID-Pts was associated with older age, hypertension and/or diabetes, low serum albumin, high neutrophil-to-lymphocyte ratio, and C-reactive protein.
Keywords: COVID-19, Illness severity, Body mass index, Systemic inflammation, Tocilizumab, Methylprednisolone
1. Introduction
Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly worldwide [1,2]. Globally, as of June 21, 2020, 8,650,917 confirmed cases of COVID-19, including 460,360 deaths, were reported to the World Health Organization (WHO) [3], with a mortality rate of approximately 5%. Pertaining to the clinical characteristics of COVID-19, many patients remain completely asymptomatic while retaining the full ability of transmitting the virus [4]. However, COVID-19 has a significantly higher morbidity and mortality rates in patients who ultimately develop symptoms. The majority of patients who rapidly develop acute respiratory distress syndrome (ARDS) require mechanical ventilation (MV), tend to remain ventilator-dependent for 10–14 days, and ultimately die [5,6]. Therefore, many patients have died of COVID-19 worldwide [3].
The total death rate from COVID-19 (7.53 per million people) in Japan [7] is extremely low compared to that in the USA, Brazil, and Russia (359.85, 230.31, and 54.63 per million population, respectively).
According to an article titled “Japan's Halfhearted Coronavirus Measures Are Working Anyway” by Foreign Policy [8], despite the indifferent attitude towards lockdown rules and poor testing, Japan appears to be skipping the worst phases of the pandemic. To the best of our knowledge, no report exists on the illness severity of COVID-19 in Japan. Therefore, we undertook this study, among mainly Japanese patients, to examine factors significantly associated with COVID-19 severity in symptomatic patients.
2. Materials and methods
2.1. Study approval
The protocol of this retrospective study was approved by the institutional review board of Osaka Habikino Medical Center on May 25, 2020 (approval no. 1023), and the study was conducted according to the tenets evinced in the 1964 Declaration of Helsinki and its later amendments. The requirement for informed consent was waived because of the retrospective study design and the use of anonymized patient data. Moreover, this study was performed in accordance with the institution's opt-out policy (http://www.ra.opho.jp/hospital/110/) as a substitute for directly acquiring informed consent.
2.2. Patient selection
We enrolled symptomatic patients with COVID-19 who were admitted to the study center between February 20 and April 30, 2020.
2.3. Detection of SARS-CoV-2
The genetic detection of SARS-CoV-2 was performed using loop-mediated isothermal amplification (LAMP; Loopamp® 2019-SARS-CoV-2 Detection Reagent Kit [9]) at our institution or using polymerase chain reaction (PCR; One-StepPrimeScript III RT-PCR kit [10]) at the Osaka Institute of Public Health.
2.4. Patient demographics, initial symptoms, and laboratory parameters
The patients’ medical records were reviewed to collect baseline data (age, sex, height, body weight, smoking history, and comorbidities) and details of initial symptoms at the time of hospitalization (fever (≥37.5 °C), cough, general fatigue, diarrhea, shortness of breath, taste dysfunction, muscle pain or body ache, sputum, headache, olfactory dysfunction, chills, rhinorrhea/nasal congestion, sore throat, and nausea/vomiting). Laboratory data were obtained from samples collected on the day of hospitalization, the day before, or the next day including the neutrophil-to-lymphocyte ratio (NLR), lymphocyte count (x 106/L), platelet count (x 106/L), serum albumin (s-Alb; g/dL), aspartate aminotransferase (AST; IU/L), alanine aminotransferase (ALT; IU/L), lactate dehydrogenase (LDH; IU/L), blood urea nitrogen (BUN; mg/dL), creatinine (CRN; mg/dL), C-reactive protein (CRP; mg/dL), random blood sugar (RBS; mg/dL), and ferritin (ng/mL).
In this study, the WHO body mass index (BMI) (kg/m2) categories [11], (underweight, <18.5; normal weight, 18.5–24.9; and overweight, ≥25.0) were used.
2.5. Severity of COVID-19
As specified in the National Institutes of Health (NIH) COVID-19 Treatment Guidelines [12], in this study, the illness severity of patients with COVID-19 at the time of hospitalization was stratified into the following four categories: asymptomatic or pre-symptomatic infection, mild/moderate, severe, and critical illness. Based on the Osaka Prefecture medical policy, only patients with COVID-19 who developed symptoms were admitted to this institution. Therefore, no patients had asymptomatic or presymptomatic infection in this study. As mentioned in the NIH COVID-19 Treatment Guidelines [12], because the criteria for each category may overlap or vary across guidelines and clinical trials, it would be difficult to clearly categorize the severity of COVID-19. Therefore, the illness severities of symptomatic patients with COVID-19 in this study were grouped into two (critical or severe illness [CSI] and moderate or mild illness [MMI]).
2.6. Computed tomography images
Computed tomography (CT) was performed either at another institution before admission or in our institution at the time of admission. CT images were reviewed by radiologists and physicians. In this study, we examined laterality, number of lobes affected, and area (%) of lesion to the total lung field. Furthermore, the area (%) of lesion to the total lung field was categorized as follows: 0%; ≥1% to <25%; ≥25% to <50%; and ≥50%.
2.7. Treatment
Antiviral therapy was administered to most patients with CSI and for some patients with MMI at the physician's discretion. Lopinavir and ritonavir were prescribed between March 1, 2020 and April 3, 2020; after which, favipiravir was administered.
Compassionate use of tocilizumab, which is a humanized anti-interleukin 6 (IL-6) receptor monoclonal antibody, was prescribed, as described previously [13], based on the following criteria: (1) findings of increased inflammation: CRP level >5 mg/dL or ferritin >1000 ng/mL; (2) requirement of oxygen supplementation or rapid progression, according to the evaluation of the chest CT images (>50% increase in infiltrates over 24–48 h). Patients with elevated procalcitonin and those who also had bacterial infections were excluded.
Furthermore, intravenous high-dose methylprednisolone pulse therapy (IHDMPPT) at 1 g per person for 3 consecutive days, with or without subsequent tapered lower dose steroid therapy, is frequently prescribed for ARDS treatment by physicians in Japan [14].
When there was room for intensive care at the high-care unit, MV was performed at this hospital; but with limited space, the patient was transferred to another institution for MV management.
2.8. Statistical analysis
All statistical analyses were performed using R software (R version 3.6.3, https://www.r-project.org/, accessed June 20, 2020). Correlation coefficients were calculated to examine the associations among various parameters. The differences in variables between CSI and MMI were evaluated using the chi-square or Mann–Whitney U test. Differences were considered statistically significant at P-values <0.05.
3. Results
3.1. Transmission route and detection method of SARS-CoV-2
Of the 61 patients with COVID-19 and with laboratory-confirmed SARS-CoV-2 infection admitted to this institution between February 20, 2020 and April 30, 2020, 20 had an unknown transmission route. The remaining patients’ transmission routes included family member or partner (N = 10), nightclub or restaurant in the entertainment district (N = 7), occupational exposure other than nightclub or restaurant in the entertainment district (N = 7), hospital or care service (N = 6), live music club (N = 4), recent travel (N = 4), and cruise ship (N = 3).
The most frequent method for detecting SARS-CoV-2 was PCR at the Osaka Institute of Public Health (N = 58). The confirmation of infection by the LAMP method was performed in-house for eight patients while in three patients from a cruise ship, the detection was by an unspecified method.
3.2. Initial symptoms of patients with COVID-19 at the time of hospitalization
In this study, all patients developed some kind of symptoms at the time of hospitalization. In the order of frequency, the percentages of occurrences of the symptoms were as follows: fever (≥37·5 °C) (83.6%); cough (73.8%); general fatigue (59.0%); diarrhea (37.7%); shortness of breath (37.7%); taste dysfunction (27.9%); muscle pain or body ache (24.6%); sputum (23.0%); headache (23.0%); smell dysfunction (21.3%); chills (18.0%); rhinorrhea or nasal congestion (16.4%); sore throat (13.1%); and nausea/vomiting (13.1%).
3.3. Patient demographics, and laboratory data at the time of hospitalization
The 61 patients with COVID-19 were categorized based on illness severity into mild (N = 7), moderate (N = 38), severe (N = 11), and critical (N = 5) illness. Of the 61 patients, 58 and 3 were Japanese and East Asians, respectively.
The patient demographics and laboratory data at the time of hospitalization were compared between the 16 patients with CSI and 45 with MMI (Table 1 ). Patients with CSI were significantly older than those with MMI (P < 0.001). With regard to comorbidities, patients with CSI more frequently had hypertension and diabetes than those with MMI (P = 0.014 and < 0.001, respectively). No patient had chronic kidney disease, cerebrovascular disorders, or liver disorders.
Table 1.
Patient demographics and laboratory data at the time of hospitalization (N = 61).
| Total (%) | Severity of patients with COVID-19 |
P-value | ||
|---|---|---|---|---|
| CSI (%) | MMI (%) | |||
| Number of patients | 61 | 16 | 45 | |
| Patient demographics | ||||
| Age in years (mean [range]) | 47.51 (20–88) | 62.6 (33–81) | 42.1 (20–88) | <0.001 |
| Sex | ||||
| Female | 21 (34.4) | 3 (18.8) | 18 (40.0) | 0.220 |
| Male | 40 (65.6) | 13 (81.3) | 27 (60.0) | |
| Nationality | ||||
| East Asia | 3 (4.9) | 1 (6.3) | 2 (4.4) | 1.000 |
| Japanese | 58 (95.1) | 15 (93.8) | 43 (95.6) | |
| Smoking status | ||||
| Never | 30 (49.1) | 6 (37.5) | 24 (53.3) | 0.471 |
| Ever | 26 (42.6) | 9 (56.3) | 17 (37.8) | |
| Unknown | 5 (8.2) | 1 (6.3) | 4 (8.9) | |
| Mean time from onset to hospitalization (days, range) | 6 (1–19) | 8 (2–13) | 6 (1–19) | 0.407 |
| Mean body mass index (kg/m2, range) | 23·2 (16·9–38·9) | 25·0 (18·2–37·0) | 22·3 (16·9–38·9) | 0.298 |
| <18·5 | 3 | 1 | 2 | |
| 18·5–24·9 | 28 | 6 | 22 | |
| ≥25·0 | 21 | 8 | 13 | |
| Unknown | 9 | 1 | 8 | |
| Comorbidities: Yes | 28 (45.9) | 11 (68.8) | 17 (37.8) | 0.065 |
| Hypertension | 14 (23.0) | 9 (56.3) | 5 (11.1) | 0·014 |
| Diabetes | 9 (14.8) | 7 (43.8) | 2 (4.4) | <0·001 |
| Chronic lung disease | 9 (14.8) | 2 (12.5) | 7 (15.6) | 1.000 |
| Cardiovascular disease | 4 (6.6) | 1 (6.3) | 3 (6.7) | 1.000 |
| Acquired immune deficiency syndrome | 4 (6.6) | 1 (6.3) | 3 (6.7) | 1.000 |
| Hyperuricemia | 3 (4.9) | 2 (12.5) | 1 (2.2) | 0.167 |
| Hyperlipidemia | 2 (3.3) | 2 (12.5) | 0 | 0.066 |
| Mental disorders | 2 (3.3) | 0 | 2 (4.4) | 1.000 |
| Malignancy | 2 (3.3) | 1 (6.3) | 1 (2.2) | 0.459 |
| Pregnancy | 2 (3.3) | 0 | 2 (4.4) | 1.000 |
| Laboratory data (mean [range]) | ||||
| Neutrophil-to-lymphocyte ratio | 4.13 (0.8–35.8) | 7.59 (1.4–35.8) | 2.87 (0.8–9.2) | <0.001 |
| Lymphocyte count (x 106/L) | 1222.3 (175.0–3210.0) | 840.8 (175.0–3210.0) | 1361.1 (351.0–3210.0) | 0.001 |
| Platelet count (x 106/L) | 21.6 (10.4–51.6) | 20.0 (10.4–34.6) | 22.1 (12.4–51.6) | 0.476 |
| Serum albumin (g/dL) | 3.8 (2.2–4.9) | 3.2 (2.2–3.8) | 3.9 (3.1–4.9) | <0.001 |
| Aspartate aminotransferase (IU/L) | 38.6 (14.0–210.0) | 63.7 (16.0–210.0) | 29.7 (14.0–81.0) | 0.002 |
| Alanine aminotransferase (IU/L) | 38.1 (9.0–184.0) | 52.1 (17–184) | 33.1 (9.0–102.0) | 0.041 |
| Lactate dehydrogenase (IU/L) | 260.8 (134.0–903.0) | 368.9 (192.0–903.0) | 222.4 (134.0–458.0) | <0.001 |
| Blood urea nitrogen (mg/dL) | 12.2 (3.4–29.2) | 13.9 (8.0–29.2) | 11.6 (3.4–22.9) | 0.145 |
| Creatinine (mg/dL) | 0.7 (0.4–1.1) | 0.8 (0.4–1.1) | 0.7 (0.4–1.0) | 0.163 |
| C-reactive protein (mg/dL) | 3.6 (0.0–19.7) | 8.1 (2.3–19.7) | 2.1 (0.0–8.1) | <0.001 |
| Blood sugar (mg/dL) | 116.4 (74.0–368.0) | 151.6 (90.0–368.0) | 105.0 (74.0–253.0) | <0.001 |
CSI: critical or severe illness, MMI: moderate or mild illness.
Data are presented as n (%) and mean (range).
Mental disorders included panic disorder and obsessive compulsive disorder. Data were missing for each one patient with MMI in neutrophil-to-lymphocyte ratio and lymphocyte count, for three patients (one with CSI and two with MMI) in blood urea nitrogen, and for four (two with CSI and two with MMI) in blood sugar.
We examined the association of disease severity and laboratory data for NLR, s-Alb, AST, ALT, LDH, BUN, CRN, CRP, and RBS and found significantly lower s-Alb level (P < 0·001), and significantly higher levels of NLR, lymphocyte count, AST, ALT, LDH, CRP, and RBS level in patients with CSI than in those with MMI (P < 0.001, 0.001, 0.002, 0.041, <0.001, <0.001, and <0.001, respectively). Ferritin was examined in 15 of 16 patients with CSI and 17 of 45 with MMI. The mean (range) serum ferritin (ng/mL) levels in patients with CSI and MMI were 1253.5 (153.1–4383.4) and 313.5 (38.3–1203.0), respectively.
BMI (kg/m2) was examined in 52 patients, including 15 with CSI and 37 with MMI. The median (range) BMIs (kg/m2) in the overall, CSI, and MMI study populations were 23.2 (16.9–38.9), 25·0 (18.2–37.0), and 22.3 (16.9–38.9), respectively. The median BMI in the CSI group tended to be higher than that in the MMI group. Furthermore, a deviation from the normal BMI range was more frequently observed in the CSI group (9/15; 60.0%) than in the MMI group (15/37; 40.5%).
3.4. CT images at the time of hospitalization
Of the 61 patients with COVID-19, one was excluded from the analysis of CT images due to missing data; thus, the CT images of 60 patients obtained at the time of hospitalization were examined. Bilateral lung involvement was significantly more frequently observed in patients with CSI than in those with MMI (P = 0.013). Patients with CSI had a significantly higher number of affected lobes and a larger area (%) of lesion to the total lung field than those with MMI (P = 0.016 and < 0.001, respectively; Table 2 ).
Table 2.
Findings on computed tomographic scanning at the time of hospitalization.
| Total | CSI | MMI | P-value | |
|---|---|---|---|---|
| Number of patients | 60 | 16 | 44 | |
| Distribution | ||||
| None | 8 | 0 | 8 | 0.013 |
| Unilateral | 5 | 0 | 5 | |
| Bilateral | 47 | 16 | 31 | |
| Number of lobes affected | ||||
| 0 | 8 | 0 | 8 | 0·016 |
| 1 | 5 | 0 | 5 | |
| 2 | 4 | 0 | 4 | |
| 3 | 10 | 0 | 10 | |
| 4 | 5 | 2 | 3 | |
| 5 | 28 | 14 | 14 | |
| Area (%) of lesion to total lung field | ||||
| 0 | 8 | 0 | 8 | <0·001 |
| ≥1% to <25% | 26 | 1 | 25 | |
| ≥25% to <50% | 17 | 6 | 11 | |
| ≥50% | 9 | 9 | 0 | |
CSI: critical or severe illness, MMI: moderate or mild illness.
3.5. Treatment after hospitalization
As shown in Table 3 , 15 (93.8%) of 16 patients with CSI received antiviral therapy, 12 (75.0%) received tocilizumab, 6 (37.5%) received MV, and 5 (31.3%) underwent IHDMPPT. In contrast, 18 (40.0%) of the 45 patients with MMI received antiviral therapy, and none of the patients with MMI received tocilizumab, MV, or IHDMPPT. The 12 patients treated with tocilizumab are among the 13 patients described previously [13].
Table 3.
In-patient treatment.
| Treatment | Total (%) | CSI (%) | MMI (%) |
|---|---|---|---|
| Number of patients | 61 | 16 | 45 |
| Antibiotics | 6 (9.8) | 6 (37.5) | 0 |
| Antiviral therapy | 33 (54.1) | 15 (93.8) | 18 (40.0) |
| Lopinavir and ritonavir | 8 (13.1) | 4 (25.0) | 4 (8.9) |
| Favipiravir | 20 (32.8) | 13 (81.3) | 7 (15.6) |
| Ciclesonide inhalant | 29 (47.5) | 14 (87.5) | 15 (33.3) |
| Tocilizumab | 12 (19.7) | 12 (75.0) | 0 |
| IHDMPPT | 5 (8.2) | 5 (31.3) | 0 |
| Mechanical ventilation (MV) | 6 (9.8) | 6 (37.5) | 0 |
| Median time to MV from admission (hours, range) | 18.5 (4–71) | 18.5 (4–71) | – |
CSI: critical or severe illness, MMI: moderate or mild illness.
IHDMPPT: Intravenous high-dose methylprednisolone pulse therapy of 1 g per person for three consecutive days.
3.6. Characteristics of COVID-19 patients who received MV and/or IHDMPPT
Table 4 shows the characteristics of the eight COVID-19 patients whose clinical condition worsened after hospitalization, necessitating MV and/or IHDMPPT. These eight patients, constituting half (50%) of the CSI group included: all the 5 patients with critical illness and 3 of the 11 patients with severe illness. All of the 5 critically ill patients were men, had BMI outside the normal range (≥18.5 to <25 kg/m2) and had diabetes.
Table 4.
Characteristics of the eight patients with COVID-19 who received mechanical ventilation and/or high-dose methylprednisolone treatment.
| Severity | Critical illness |
Severe illness |
||||||
|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
| Age (years) | 79 | 71 | 70 | 48 | 81 | 33 | 79 | 62 |
| Sex | Male | Male | Male | Male | Male | Male | Male | Female |
| Nationality | Japanese | Japanese | Japanese | Japanese | Japanese | Japanese | Japanese | Japanese |
| Body mass index (kg/m2) | 25.0 | 25.1 | 25.9 | 37.0 | 18.2 | 25.7 | 21.5 | 19.0 |
| Smoking status | Ever | Ever | Never | Ever | Ever | Ever | Never | Ever |
| Comorbidities | ||||||||
| Hypertension | + | + | – | + | – | – | + | + |
| Diabetes | + | + | + | + | + | – | – | – |
| Chronic lung disease | – | – | + | – | – | – | – | – |
| Findings on computed tomographic scanning | ||||||||
| Laterality | Bilateral | Bilateral | Bilateral | Bilateral | Bilateral | Bilateral | Bilateral | Bilateral |
| Number of lobes affected | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Area (%) of lesion to total lung field | ≥25%, <50% | ≥50% | ≥50% | ≥25%, <50% | ≥50% | ≥25%, <50% | ≥25%, <50% | ≥50% |
| Laboratory data at the time of hospitalization | ||||||||
| Neutrophil-to-lymphocyte ratio | 4.5 | 1.4 | 5.8 | 4.8 | 10.6 | 2.8 | 1.57 | 14.9 |
| Serum albumin (g/dL) | 3.0 | 3.6 | 2.8 | 3.7 | 2.2 | 3.8 | 4.5 | 3.1 |
| C-reactive protein (mg/dL) | 8.92 | 7.47 | 10.30 | 3.78 | 15.26 | 3.77 | 8.57 | 5.06 |
| Ferritin (ng/mL) | 1238.9 | 4383.4 | 1166.6 | 3355.4 | 638.6 | 420.5 | 1110·7 | 1208·9 |
| Antiviral therapy | ||||||||
| Lopinavir and ritonavir | + | + | + | – | – | + | – | – |
| Favipiravir | – | + | + | + | + | – | + | + |
| Tocilizumab | + | + | – | + | + | – | + | + |
| Time to initiation of tocilizumab from admission (hours) | 19 | 16 | NA | 24 | 4 | NA | 28 | 5 |
| IHDMPPT, | – | – | – | + | + | + | + | + |
| Time to IHDMPPT initiation from admission (days) | NA | NA | NA | 6 | 2 h | 5 | 4 | 7 |
| Mechanical ventilation (MV) | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Time to initiation of MV from admission (hours) | 4 | 20 | 17 | 42 | NA | NA | 71 | 12 |
| Tracheotomy | + | + | + | – | NA | NA | – | – |
| Prognosis | Alive | Alive | Alive | Dead | Alive | Alive | Alive | Alive |
NA: not applicable.
IHDMPPT: intravenous high-dose methylprednisolone pulse therapy of 1 g per person for three consecutive day.
Three of the five critically ill patients received tracheotomies due to upper respiratory obstruction after MV management (Cases 1 and 2 were treated with tocilizumab), and due to prolonged MV management (Case 3). One patient died suddenly of upper respiratory obstruction (Case 4, treated with tocilizumab and two cycles of IHDMPPT). Case 5, who was admitted after transportation via ambulance, received immediate IHDMPPT and subsequent tocilizumab and improved without MV. Of the three patients with severe illness, Case 6 received IHDMPPT after being transferred to another hospital due to worsening respiratory failure, but subsequently improved without MV. Cases 7 and 8 received tocilizumab at the study center; following transfer to another hospital for MV, these two patients received IHDMPPT and subsequently improved after extubation.
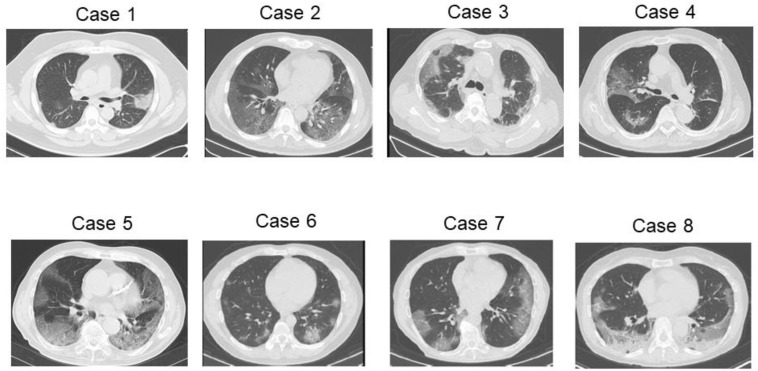
Fig. 1 shows the CT images of the eight patients with the spreading of the shadows of ground glass opacities throughout the lung lobes.
Fig. 1.
Computed tomography images of five critically ill patients with coronavirus disease. Case 1, prominent subsegmental areas of consolidation with a clear margin in the left lung; Case 2, multiple patchy areas of pure ground glass opacities with a crazy-paving appearance; Case 3, multiple areas of consolidation with interlobular septal thickening are seen on both the lungs with a prominent peripheral distribution; Case 4, ground glass opacities with prominent vascular enhancement in the right lung; Case 5: opacities indicating consolidation in both lungs and the presence of an air bronchogram with a crazy-paving appearance; Case 6: bilateral prominent subsegmental areas of consolidation with left-sided predominance; Case 7: a crazy-paving appearance with a bilateral prominent peripheral distribution in the lungs; and Case 8: consolidation with higher density areas with a bilateral prominent peripheral distribution in the lungs.
4. Discussion
In this study, we evaluated the findings of 61 symptomatic patients with confirmed SARS-CoV-2 infection. Since the majority (N = 58) of the 61 patients were Japanese, the severity of COVID-19 in this study seemed to be mainly from Japanese patients. To the best of our knowledge, this is the first study to describe in detail, the factors associated with both disease severity and the treatment approach, including tocilizumab and IHDMPPT among Japanese COVID-19-pts.
The severity of COVID-19 correlated significantly with older age; hypertension and/or diabetes as comorbidities; high NLR, LDH, CRP, and RBS levels; low s-Alb level; and the number of affected lobes and the area (%) of lesion to the total lung field on CT images. All the patients with critical illness were Japanese men with BMI outside the normal range (≥18.5 to <25 kg/m2) and with diabetes.
Similar to previous studies [15,16], regarding the CT findings in COVID-19, the chest CT of most patients in this study were characterized by bilateral ground-glass lesions located in the sub-pleural area of the lung. Area of lesion to the total lung field in patients with CSI was significantly larger than that in those with MMI. However, it is necessary to focus more attention to patients with relatively small areas of lesion because of the possibilities of rapidly worsening condition.
A recent study [17] reported the clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on a cruise ship in Japan. In that study [17], the 104 patients with COVID-19 were categorized as having asymptomatic (n = 43; 41%), mild (n = 41; 39%), and severe illness (n = 20; 19%), and the study had a lower proportion of males (44%) among the asymptomatic patients than that (65.6%) in this study. The frequency of diabetes in asymptomatic patients was 12% in this previous study [17], whereas the frequency in this study was 4.4% and 43.8% in patients with MMI and CSI, respectively. Thus, the frequencies of male sex and diabetes increased with a higher disease severity.
Previous studies [18,19] reported that NLR can be used to identify high-risk patients with moderate–severe ARDS, with an optimal threshold value of 9.8 [18] or 3.13 [19]. Wang et al. [2] reported that several patients with COVID-19 had an increased neutrophil count and a decreased lymphocyte count during the severe disease phase, which indicated the potentially critical condition and serious disturbance of the internal environment in severely ill patients. Thus, NLR as a biomarker may be helpful for assessing the illness severity in patients with COVID-19. Huang et al. [20] reported an inverse relationship between s-Alb level and mortality risk in COVID-19 patients. Their study indicated that a low s-Alb level might be attributable to systemic inflammation in COVID-19 and correlated with high NLR and CRP levels.
In this study, patients with CSI had high NLR and CRP levels and low s-Alb levels, similar to those in previous studies [[18], [19], [20]]. High NLR is associated with systemic inflammation [21,22]. The BMI (kg/m2) of symptomatic patients with COVID-19 in this study tended to be higher than the average [22.7 (22.3–23.0)] for Japanese adults aged 18 years or older [23]. Hajifathalian et al. [24] reported an association of obesity with a significantly higher rate of ICU admission or death. In their report [24], BMI (kg/m2) categories were defined as normal (including overweight), BMI >18.5 and < 30 (reference category), underweight (BMI <18.5), and obese (BMI >30). However, their study results [24] could not possibly be extrapolated to the Japanese population.
BMI, for most of the 61 patients in this study were categorized as stated above. However, being overweight would be a very important factor for critical illness in patients with COVID-19. A meta-analysis indicated an association of obesity [25] with immune responses that are characterized by chronic systemic inflammation, which plays a crucial role in the development of various metabolic abnormalities related to obesity. However, in elderly patients, being underweight (BMI <18.5 kg/m2) may be important, as shown in critically ill patients in this study. Low BMI in elderly patients would suggest sarcopenia [26]. In their review, Lo et al. [27] showed that inflammation in sarcopenia is associated with an age-dependent increment in the levels of proinflammatory cytokines in older patients.
Furthermore, aging is associated with the development of a low-level, systemic, chronic inflammation known as “inflammaging,” [28] which is a highly significant risk factor for both morbidity and mortality in older adults. Ferrucci et al. [29] hypothesized that inflammaging affects cerebrovascular disease, multimorbidity, and frailty by inhibiting growth factors and increasing catabolism. COVID-19 is characterized by a high mortality rate in elderly men with aging-related comorbidities, including diabetes and hypertension. In patients with SARS-CoV-2 infection and the above-described chronic inflammation, an excessive immunoreaction, such as cytokine storm, would be induced. Subsequently, the clinical condition of patients with COVID-19 would rapidly worsen.
However, there remains an unanswered question on the reasons why COVID-19 mortality rate in Japan is lower than that in other regions besides Asia. As shown in this study, in Japan, glucocorticoid therapy (GCT), frequently used in clinical practice, can reduce mortality. However, GCT did not show a positive impact on the clinical outcomes of patients with the Middle East Respiratory Syndrome [30]. Recently, Wu et al. [6] reported favorable data on the use of methylprednisolone that revealed a decreased mortality rate (62%) in critically ill patients with ARDS. Furthermore, the RECOVERY trial [31] showed that dexamethasone 6 mg once daily for 10 days reduced deaths among patients on MV and those receiving oxygen only. Thus, GCT can have beneficial effects in COVID-19 patients with ARDS.
Recently, it was reported that the COVACTA trial on tocilizumab [32] did not meet its primary endpoint of improved clinical status in patients with COVID-19 with associated pneumonia, or the key secondary endpoint of reduced patient mortality. However, a recent study [33] showed that high dose methylprednisolone followed by tocilizumab improved the clinical status of COVID-19 patients with cytokine storm syndrome. Further examination of the efficacy of tocilizumab combined with GCT in COVID-19 patients would be needed.
This study had several limitations. First, this was a retrospective study at a single center. Second, the number of participants in this study was small. Third, we did not evaluate the detailed side effects of tocilizumab and methylprednisolone. Finally, we could not evaluate all the risk factors associated with COVID-19 severity in multivariate analysis owing to many missing data.
In conclusion, the significant factors associated with COVID-19 severity in Japanese patients were similarly as in previous studies. Further examination of treatment including GCT and tocilizumab for COVID-19 will be needed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
TN, YT, TA, HK, TH, and TT contributed to study conception and design. TY, YT, TA, HK, HM, MK, TH, KK, YK, SM, SH, and HS contributed to data collection and analysis. HM, YT, TA, KK, HK, HS, YK, TH, TN and TT contributed to the drafting and editing of the manuscript. TN, YT, TA, HK, HS, TH, HM, and TT revised the manuscript. YT, SY, TH, TT, and TN provided study supervision. TH, HS and TT conducted the statistical analyses. All authors read and approved the final manuscript. All authors meet the ICMJE authorship criteria.
Declaration of competing interest
T.H. received honoraria and research funding as the primary investigator in this institution from Ono Pharmaceutical Co. Ltd. (Osaka, Japan), Lilly Japan Co. Ltd. (Hyogo, Japan), AstraZeneca Co. Ltd. (Osaka, Japan), Taiho Pharmaceutical Co. Ltd. (Tokyo, Japan), Chugai Pharmaceutical Co. Ltd. (Tokyo, Japan), and MSD Oncology Co. Ltd. (Tokyo, Japan). TH did not receive honoraria or research funding associated with this study. The other authors have no conflicts of interest to declare.
Acknowledgments
The authors thank the patients and their families and are grateful to all members of the COVID-19 care team at Osaka Habikino Medical Center.
The authors thank the medical team of Emergency and Critical Care Medicine in Kansai Medical University, Emergency and Critical Medical Care Center in Osaka City General Hospital, Osaka Prefectural Nakakawachi Emergency and Critical Care Center, Emergency and Critical Care Center in Osaka General Medical Center, and respiratory medicine in Yao Tokushukai Hospital for their devoted treatment.
The authors also appreciate Mrs. Yuko Tani for her assistance in summarizing the research data.
The authors thank Editage (www.editage.jp) for English language editing.
Role of the funding source: This authors received no any funding for this study.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 4.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Change Data Lab . 2020. Co Global Change Data Lab. Coronavirus pandemic: daily updated research and data. 2020ronavirus pandemic: daily updated research and data.https://ourworldindata.org/grapher/total-covid-deaths-per-million?tab=table [Google Scholar]
- 8.Foreign Policy . 2020. Japan's halfhearted coronavirus Measures are working Anyway.https://foreignpolicy.com/2020/05/14/japan-coronavirus-pandemic-lockdown-testing/ [Google Scholar]
- 9.Eiken . 2020. Eiken genome site.http://loopamp.eiken.co.jp/ [Google Scholar]
- 10.Takara-Bio . 2020. One step PrimeScript™ III RT-qPCR mix.http://catalog.takara-bio.co.jp/product/basic_info.php?unitid=U100009388 [Google Scholar]
- 11.World Health Organization. Body mass index - BMI. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [accessed August 31, 2020].
- 12.National Institutes of Health . 2020. Management of persons with COVID-19.https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ [Google Scholar]
- 13.Hashimoto S., Kitajima H., Arai T., Tamura Y., Nagai T., Morishita H., et al. A retrospective study evaluating efficacy and safety of compassionate use of tocilizumab in 13 patients with severe-to-critically ill COVID-19: analysis of well-responding cases and rapidly-worsening cases after tocilizumab administration 2020. medRxiv. 2020 doi: 10.1101/2020.06.24.20134288. [DOI] [Google Scholar]
- 14.Kinoshita Y., Ishii H., Kushima H., Watanabe K., Fujita M. High-dose steroid therapy for acute respiratory distress syndrome lacking common risk factors: predictors of outcome. Acute Med Surg. 2018;5:146–153. doi: 10.1002/ams2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (covid-19) pneumonia in 62 patients in wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Liu B., Yu Y., Wang X., Du Y., Gu J., et al. The characteristics and clinical value of chest CT images of novel coronavirus pneumonia. Clin Radiol. 2020;75:335–340. doi: 10.1016/j.crad.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K., et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20:1043–1050. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma A., Cheng J., Yang J., Dong M., Liao X., Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Crit Care. 2020;24:288. doi: 10.1186/s13054-020-03007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y., et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Diao J., Qi C., Jin J., Li L., Gao X., et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18:75. doi: 10.1186/s12872-018-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Zhou S., Liu Y., Zhai L., Sun X. Prognostic value of systemic inflammatory markers in ovarian Cancer: a PRISMA-compliant meta-analysis and systematic review. BMC Canc. 2018;18:443. doi: 10.1186/s12885-018-4318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Mean body mass index trends among adults, age-standardized (kg/m2) estimates by country. https://apps.who.int/gho/data/node.main.A904?lang=en [accessed August 31, 2020].
- 24.Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T., et al. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York city. Obesity. 2020;28:1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamjane N., Benyahya F., Nourouti N.G., Mechita M.B., Barakat A. Cardiovascular diseases and metabolic abnormalities associated with obesity: what is the role of inflammatory responses? A systematic review. Microvasc Res. 2020;131:104023. doi: 10.1016/j.mvr.2020.104023. [DOI] [PubMed] [Google Scholar]
- 26.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., et al. Sarcopenia in asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Lo J.H., U K.P., Yiu T., Ong M.T., Lee W.Y. Sarcopenia: current treatments and new regenerative therapeutic approaches. J Orthop Transl. 2020;23:38–52. doi: 10.1016/j.jot.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 31.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche Roche provides an update on the phase iii covacta trial of actemra/roactemra in hospitalised patients with severe covid-19 associated pneumonia. 2020. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm
- 33.Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J., et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]