Abstract
Background
The role of repeated prone positioning in intubated subjects with acute respiratory distress syndrome caused by COVID-19 remains unclear.
Methods
We conducted a retrospective observational cohort study of critically ill intubated patients with COVID-19 who were placed in the prone position between March 18, 2020 and March 31, 2020. Exclusion criteria were pregnancy, reintubation, and previous prone positioning at a referring hospital. Patients were followed up until hospital discharge. The primary outcome was oxygenation assessed by partial pressure of oxygen/fraction of inspired oxygen ratio (Pao2/Fio2) ratio. A positive response to proning was defined as an increase in Pao2/Fio2 ratio ≥20%. Treatment failure of prone positioning was defined as death or requirement for extracorporeal membrane oxygenation (ECMO).
Results
Forty-two subjects (29 males; age: 59 [52–69] yr) were eligible for analysis. Nine subjects were placed in the prone position only once, with 25 requiring prone positioning on three or more occasions. A total of 31/42 (74%) subjects survived to discharge, with five requiring ECMO; 11/42 (26%) subjects died. After the first prone positioning session, Pao2/Fio2 (mean (standard deviation)) ratio increased from 17.9 kPa (7.2) to 28.2 kPa (12.2) (P<0.01). After the initial prone positioning session, subjects who were discharged from hospital were more likely to have an improvement in Pao2/Fio2 ratio ≥20%, compared with those requiring ECMO or who died.
Conclusion
Patients with COVID-19 acute respiratory distress syndrome frequently responded to initial prone positioning with improved oxygenation. Subsequent prone positioning in subjects discharged from hospital was associated with greater improvements in oxygenation.
Keywords: acute respiratory distress syndrome (ARDS), COVID-19, mechanical ventilation, oxygenation, prone positioning
Editor's key points.
-
•
The role of repeated episodes of prone positioning in intubated subjects with ARDS secondary to COVID-19 remains unclear.
-
•
The authors report an observational cohort single-centre study of intubated COVID-19 subjects.
-
•
The primary outcome was Pao 2/Fio 2 ratio after initial proning.
-
•
After the initial proning session, improved oxygenation was more likely in subjects who survived to discharge after repeated prone positioning.
Coronavirus disease-19 (COVID-19) is a global pandemic that has affected more than 200 countries and territories worldwide, resulting in more than 1.1 million deaths.1
COVID-19 causes acute respiratory distress syndrome (ARDS) in approximately 20% of hospitalised subjects with COVID-19.2 , 3 ARDS has a high mortality rate (35–46%), particularly in subjects with a greater degree of lung injury.4 As of May 15, 2020, 57% of the 4855 UK hospitalised subjects with COVID-19 who required advanced respiratory support died.5 Management of respiratory failure in COVID-19 patients is largely supportive. One treatment recommended by the Surviving Sepsis Campaign (SSC) COVID-19 subcommittee is prone positioning.6 Before the COVID-19 pandemic, studies have shown that early prone positioning can improve the ratio of partial pressure of oxygen to the fraction of inspired oxygen (Pao 2/Fio 2 ratio) and reduce 28-day and 90-day mortality in severe ARDS.7, 8, 9 Although initial prone positioning improves oxygenation in both non-intubated10, 11, 12, 13, 14 and intubated15, 16, 17 patients with COVID-19, the physiological response to repeated prone positioning and its association with length of stay and mortality for COVID-19 has not been reported.
To prepare for the COVID-19 pandemic, we developed a treatment guideline and standardised approach to initiate prone positioning based on our previous research18 and input from an interdisciplinary team of respiratory therapists, nurses, and physicians. The aim of this study was to investigate the effect of prone positioning for patients with COVID-19 ARDS that required invasive mechanical ventilation.
Methods
Study design
This retrospective observational cohort study was approved by the Institutional Review Board in Rush University Medical Center (approval No. 20041301-IRB01; approved 4/17/2020).
Inclusion criteria
Adult subjects admitted to any of the adult ICUs at our facility with laboratory-confirmed COVID-19 infection requiring invasive mechanical ventilation with prone positioning between March 18, 2020 and March 31, 2020 were included in this study. COVID-19 was confirmed by a positive result on a reverse-transcriptase—polymerase-chain-reaction assay of a specimen collected on a nasopharyngeal swab.
Exclusion criteria
Individuals were excluded if they were: (1) pregnant; (2) intubated and placed in the prone position at least once at an outside hospital; (3) reintubated and placed in the prone position on their second intubation during hospitalisation.
Prone positioning protocol
A checklist with an accompanying education video was created to assure consistent prone positioning (Supplementary material).19 Considering the volume of subjects that required prone positioning, a multidisciplinary team led by a respiratory therapist was ultimately established to complete all prone and supine sessions. The team was trained using a volunteer to simulate a patient who was intubated.18 , 19 A treatment guideline was established (Supplementary material), based on the PROSEVA study7and consensus among physician, nursing, and respiratory care leadership at our institution. Intubated subjects diagnosed with ARDS were placed in the prone position by the team when a patient had a Pao 2/Fio 2 ratio of <20 kPa with PEEP set ≥10 cm H2O and Fio 2≥0.6. Prone positioning was maintained for at least 16 h, except if cardiopulmonary resuscitation was needed. Prone positioning was terminated when Pao 2/Fio 2 ratio remained >20 kPa in the supine position or if extracorporeal membrane oxygenation (ECMO) or palliative care was needed. Lung protective ventilation (tidal volume targeted at 6 ml kg−1 of predicted body weight, plateau pressure ≤30 cm H2O, and ARDS network high-PEEP low-Fio 2 tables)20 were utilised for all subjects. ECMO was considered if oxygenation could not be maintained under lung protective ventilation with prone positioning, paralysis, and inhaled pulmonary vasodilators.
Data collection
Subject characteristics including age, sex, race, laboratory results, microbiology findings, and diagnosis were collected. COVID-19 related risk factors including age, pre-existing pulmonary disease, chronic kidney disease, diabetes, hypertension, cardiovascular disease, and immunosuppression were also recorded. Pre- and post-prone positioning changes in vital signs, arterial blood gases, ventilator settings, respiratory mechanics (plateau pressure and respiratory system static compliance) and ventilatory ratio (calculated as: [minute volume (mL/min) × Paco 2 (mm Hg)]/[predicted body weight × 100 (mL/min) × 37.5 (mmHg)]) for the first three prone positioning sessions (if applicable) for each individual were recorded. Laboratory tests included creatine kinase myocardial band, lactate dehydrogenase, C-reactive protein, D-dimer, troponin, ferritin, and absolute lymphocyte within 24–48 h of pre- and post-prone positioning for the first prone positioning session. Use of sedatives and paralytics was also recorded pre and post the first prone positioning session. Patient outcomes, including mechanical ventilation duration, successful extubation, escalation of care to ECMO, survival, and length of stay in ICU and hospital were collected. Each patient was followed until hospital discharge.
Primary outcome
The primary outcome was oxygenation, assessed by Pao 2/Fio 2 ratio, before and after the initial prone positioning manoeuvre. A positive response was defined a priori as an increase in Pao 2/Fio 2 ratio ≥20%.
Secondary outcomes
We assessed the following secondary outcomes:
-
1.
Serial Pao 2/Fio 2 ratios were assessed after repeated prone positioning, compared between subjects discharged to home or long-term care facility versus those who died or required ECMO.
-
2.
Haemodynamic (heart rate, arterial blood pressure) and ventilatory parameters (tidal volume, ventilatory frequency, PEEP, plateau pressure and ventilatory ratio) after repeated prone positioning.
Statistical analysis
Continuous variables were expressed as mean (standard deviation) or median (inter-quartile range), depending on the normality of distribution; the Kolmogorov–Smirnov test was used to test normality of distribution for continuous variables. Continuous variables were compared pre- and post-prone positioning by the paired t-test or Wilcoxon signed rank test for the same prone positioning session. The changes in oxygenation after the first three prone positioning sessions was compared by repeated measures analysis of variance or the Friedman test. Comparisons between two groups (treatment success vs failure) were analysed by an independent t-test or the Mann–Whitney test. Differences in categorical variables were assessed using the χ2 or Fisher's exact test. Binary stepwise logistic regression was performed to assess the impact of a number of factors on the likelihood of treatment failure. Data analysis was conducted with SPSS statistical software (SPSS 26.0: SPSS; Chicago, IL, USA) and a P-value of <0.05 was considered to be statistically significant.
Results
Subject characteristics
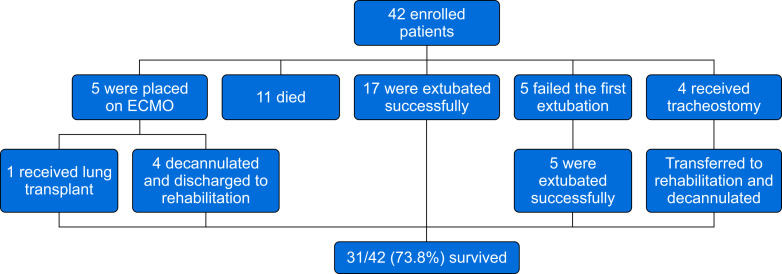
Between March 18, 2020 and March 31, 2020, 50 subjects with laboratory-confirmed COVID-19 were intubated and admitted to intensive care. Eight subjects were excluded for not meeting the criteria for prone positioning (n=6), being placed in the prone position after reintubation (n=1), or being intubated and placed in the prone position at an outside hospital for >24 h (n=1). Of the 42 subjects eligible for analysis (Table 1 ), 26 were intubated at our institution because of refractory hypoxaemia; 16 subjects were intubated elsewhere before transfer to our institution. Individuals underwent three (two to six) prone positioning manoeuvres for 16.1 (16–17) h, with 25 subjects requiring prone positioning on at least three occasions. No major complications, including pneumothorax, were observed. A total of 31/42 (74%) subjects survived to discharge (Fig 1 ) requiring intensive care for 21.5 (14.8–31.5) days. Five subjects were placed on ECMO. Eleven subjects died, nine of whom died within 28 days of ICU admission.
Table 1.
Overall patient characteristic information and comparisons between groups of treatment success and treatment failure. BMI, body mass index; CK-MB, creatine kinase myocardial band; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; Cst, static compliance; ECMO, extracorporeal membrane oxygenation; Fio2, fraction of inspired oxygen; PBW, predicted body weight; LDH, lactate dehydrogenase; Pao2, partial pressure of oxygen; PEEP, positive end expiratory pressure; Pplat, plateau pressure; SOFA, sequential organ failure assessment; Vt, tidal volume.
| Overall |
Treatment success |
Treatment failure |
P | |
|---|---|---|---|---|
| N=42 | N=26 | N=16 | ||
| Age, yr | 58.5 (51.8–69.3) | 57.0 (49.8–65.8) | 61.5 (52.3–72.0) | 0.27 |
| Gender, male, n (%) | 29 (69) | 16 (61.5) | 13 (81.3) | 0.09 |
| Ethnicity, n (%) | 0.80 | |||
| African American | 16 (38) | 11 (42.3) | 5 (31.3) | |
| Hispanic/Latino | 16 (38) | 10 (38.5) | 6 (37.5) | |
| Caucasian | 4 (9.5) | 2 (7.7) | 2 (12.5) | |
| Asian | 3 (7) | 1 (3.8) | 2 (12.5) | |
| Other | 3 (7) | 2 (7.7) | 1 (6.3) | |
| Height, cm | 171.6 (10.7) | 170.1 (164.5–176.5) | 170.1 (165.7–180.2) | 0.19 |
| Weight, kg (SD) | 100.6 (19.4) | 103.9 (20.0) | 95.2 (17.7) | 0.16 |
| PBW, kg (SD) | 66.0 (10.9) | 64.5 (11.8) | 68.5 (9.1) | 0.25 |
| BMI, kg m−2 (SD) | 34.2 (7.5) | 35.8 (7.9) | 31.6 (6.2) | 0.08 |
| BMI ≥35 kg m−2 (%) | 14 (33.3) | 11 (42.3) | 3 (18.8) | 0.18 |
| COVID-19 epidemiological risk factors | ||||
| Age ≥55 yr, n (%) | 26 (61.9) | 14 (53.8) | 12 (75.0) | 0.21 |
| Hypertension, n (%) | 25 (59.5) | 15 (57.7) | 10 (62.5) | 1.0 |
| Diabetes mellitus with A1C >7.6%, n (%) | 15 (35.7) | 10 (38.5) | 5 (31.3) | 0.75 |
| Cardiovascular disease, n (%) | 13 (31) | 7 (26.9) | 6 (37.5) | 0.51 |
| Pre-existing pulmonary disease, n (%) | 9 (21.4) | 5 (19.2) | 4 (25.0) | 0.71 |
| COPD, n (%) | 3 (7.1) | 2 (7.7) | 1 (6.3) | 1.0 |
| Asthma, n (%) | 5 (11.9) | 4 (15.4) | 1 (6.3) | 0.63 |
| Immunosuppression, n (%) | 4 (9.5) | 4 (15.4) | 0 | 0.28 |
| Chronic kidney disease, n (%) | 4 (9.5) | 3 (11.5) | 1 (6.3) | 1.0 |
| Intubated and transferred from outside hospital, n (%) | 16 (38.1) | 8 (30.8) | 8 (50.0) | 0.33 |
| From intubation to 1st prone, h | 25.7 (8.9–55.1) | 23.5 (8.0–50.9) | 42.0 (19.9–94.0) | 0.20 |
| Duration for the 1st prone, h | 16.1 (16–17) | 16.2 (16.0–17.0) | 16.0 (15.7–16.6) | 0.41 |
| Ventilator settings and respiratory mechanics before the 1st prone positioning | ||||
| Vt, ml kg-1 of PBW (n=39) | 6.0 (5.9–6.4) | 6.0 (5.9–6.5) | 5.9 (5.1–6.3) | 0.21 |
| PEEP, cm H2O | 15 (13.5–16) | 14 (12–16) | 16 (14–18) | 0.38 |
| Pplat, cm H2O (SD) (n=39) | 27.7 (4.0) | 27.1 (3.8) | 28.8 (4.3) | 0.20 |
| Cst, ml cm H2O−1 (SD) (n=39) | 33.7 (11.1) | 34.3 (11.6) | 32.7 (10.6) | 0.67 |
| Laboratory tests | ||||
| D-dimer, ng ml−1 (n=18) | 5.0 (0.75–10.46) | 1.60 (0.65–7.71) | 8.81 (2.18–14.19) | 0.22 |
| CK-MB, U L−1 (n=30) | 232 (134.5–560) | 209.0 (129.0–585.5) | 255.0 (130.5–672.0) | 0.85 |
| CRP, mg L−1 (SD) (n=37) | 220.0 (107.4) | 203.3 (107.8) | 247.2 (104.8) | 0.23 |
| LDH, U L−1 (n=29) | 574 (449–705) | 528.5 (389.0– 632.8) | 670.0 (559.5–844.0) | 0.13 |
| Troponin, ng ml−1 (n=35) | 0.05 (0.02–0.15) | 0.04 (0.02–0.14) | 0.07 (0.02–0.25) | 0.35 |
| Ferritin, μg L−1 (SD) (n=36) | 1842 (1153.7) | 1753.4 (1226.6) | 1998.6 (1040.5) | 0.55 |
| Absolute lymphocyte, ×109 (n=38) | 0.94 (0.60–1.51) | 0.97 (0.73–1.63) | 0.89 (0.46–1.35) | 0.87 |
| pH (SD) (n=36) | 7.30 (0.08) | 7.30 (0.09) | 7.31 (0.06) | 0.85 |
| HCO3, mmol l−1 (SD) (n=36) | 25.1 (4.7) | 24.4 (4.0) | 26.6 (6.0) | 0.33 |
| Paco2, kPa (n=36) | 7.2 (5.7–7.9) | 6.4 (5.5–8.2) | 7.2 (5.7–7.6) | 0.56 |
| Pao2/Fio2, kPa (SD) (n=36) | 17.9 (7.2) | 18.7 (7.6) | 16.4 (6.6) | 0.44 |
| SOFA score (SD) | 6.8 (2.5) | 6.4 (2.2) | 7.4 (3.0) | 0.23 |
| Pao2/Fio2 improvement at the first three prone positions, kPa | ||||
| 1st prone (n=36) | 7.3 (2.1–16.7) | 7.3 (3.5–17.9) | 3.1 (1.3–16.5) | 0.56 |
| 2nd prone (n=27) | 4.0 (−0.2–18.1) | 10.7 (3.7–19.0) | 1.4 (−1.6–3.4) | <0.01 |
| 3rd prone (n=20) | 6.3 (−0.4–16.0) | 10.2 (5.2–18.3) | 0.5 (−1.4–2.9) | 0.03 |
| Pao2/Fio2 improvement at the first three prone positions, % | ||||
| 1st prone (n=36) | 48.2 (15.8–110.3) | 63.8 (19.3–108.9) | 41.3 (9.5–113.8) | 0.73 |
| 2nd prone (n=27) | 18.3 (−0.6–102.7) | 54.4 (14.0–127.7) | 7.6 (−15.6–7.9) | <0.01 |
| 3rd prone (n=20) | 36.7 (−1.5–95.4) | 50.8 (22.2–102.9) | 3.2 (−12.3–27.3) | 0.04 |
| Ventilatory ratio changes at the first three prone positions | ||||
| 1st prone (n=33) | 0.17 (0.06–0.36) | 0.12 (−0.11–0.33) | 0.35 (−0.01–0.93) | 0.13 |
| 2nd prone (n=22) | 0.03 (−0.11–0.26) | 0.08 (−0.19–0.28) | 0 (−0.10–0.22) | 0.63 |
| 3rd prone (n=17) | 0.08 (−0.20–0.26) | −0.04 (−0.20–0.20) | 0.23 (−0.17–0.30) | 0.48 |
| Number of prone positioning sessions during intubation | 3.0 (2.0–6.0) | 3.0 (1.75–6.25) | 3.50 (2.0–4.75) | 0.80 |
| Antivirus medication use, n (%) | ||||
| Remdesivir | 1 (2.4) | 1 (3.8) | 0 | 1.0 |
| Tocilizumab | 18 (42.9) | 12 (46.2) | 6 (37.6) | 0.75 |
| Hydroxychloroquine | 40 (95.2) | 25 (96.2) | 15 (93.8) | 1.0 |
| Azithromycin | 37 (88.1) | 23 (88.5) | 14 (87.5) | 1.0 |
| Corticosteroids use, n (%) | ||||
| Dexamethasone | 6 (14.3) | 3 (11.5) | 3 (18.8) | 0.66 |
| Prednisone | 2 (4.8) | 1 (3.8) | 1 (6.3) | 1.0 |
| Hydrocortisone | 19 (45.2) | 12 (46.2) | 7 (43.8) | 1.0 |
| Methylprednisolone | 8 (19.0) | 5 (19.2) | 3 (19.8) | 1.0 |
| Bronchoscopy, n (%) | 13 (31.0) | 10 (38.5) | 3 (18.8) | 0.30 |
| For diagnosis | 3 | 3 (11.5) | 0 | 0.42 |
| For secretion management | 8 | 6 (23.1) | 2 (12.5) | |
| Other | 2 | 1 (3.9) | 1 (6.3) | |
Fig 1.
Clinical outcomes. ECMO, extracorporeal membrane oxygenation.
Primary outcome: oxygenation after initial prone positioning
The Pao 2/Fio 2 ratio improved from 17.9 (7.2) to 28.2 (12.2) kPa within 81 (61–119) minutes of prone positioning in 36 subjects who had complete arterial blood gas data (P<0.01). The Pao 2/Fio 2 ratio improved ≥20% in 26/36 (72%) subjects. After being returned to the supine position, improvements in Pao 2/Fio 2 ratio persisted (Table 2 ).
Table 2.
Vital signs, respiratory mechanics, and arterial blood gases at phases of pre- vs post-prone positioning and pre- vs post-supine positioning for the first prone positioning session. The significance level is 0.05. Significance values have been adjusted by the Bonferroni correction for multiple tests. ∗Compared with pre-prone (w/in 1 h) P<0.05. †Compared with post-prone (w/in 2 h) P<0.05. DBP, diastolic blood pressure; Fio2, fraction of inspired oxygen; HR, heart rate; Pao2, partial pressure of oxygen; Paco2, partial pressure of carbon dioxide; PEEP, positive end expiratory pressure; SBP, systolic blood pressure; Spo2, saturation of pulse oximetry; VF, ventilatory frequency.
| Pre-prone (w/in 1 h) |
Post-prone (w/in 2 h) |
Post-prone (4 h after) |
Pre-supine (0.5–2 h before) |
Post-supine (0.5–2 h after) |
N | P | |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| HR, bpm | 82.0 (76.0–94.0) | 85.0 (77.0–103.5) | 87.0 (76.0–100) | 83.0 (72.0–92.0) | 80.0 (67.0–88.5)† | 41 | <0.01 |
| SpO2, % | 96.0 (93.0–99.0) | 97.5 (95–99) | 97. 0 (95.0–99.0) | 98.0 (96.0–99.0) | 96.5 (94.0–99.0) | 41 | 0.21 |
| SBP, mm Hg | 113.0 (102.0, 131.0) | 119.0 (106.0, 129.0) | 117.0 (107.0, 130.0) | 121.0 (103.0, 130.0) | 116.0 (98.0, 132.0) | 39 | 0.94 |
| DBP, mm Hg | 60.0 (55.0, 65.0) | 59.0 (54.0, 68.0) | 59.0 (54.0, 67.0) | 63.0 (55.0, 69.0) | 59.0 (53.0, 65.0) | 39 | 0.68 |
| Tidal volume set, ml kg−1 | 6.0 (5.85–6.39) | 6.0 (5.84–6.18) | 6.0 (5.91–6.33) | 6.02 (5.91–6.27) | 6.0 (5.91–6.27) | 36 | 0.34 |
| VF set, bpm | 20.0 (16.0–25.0) | 22.0 (16.0– 28.0) | 24.0 (18.0–28.0)∗ | 25.0 (22.0–30.0) | 25.0 (22.0–29.0) | 41 | <0.01 |
| VF measure, bpm | 22.0 (17.0–27.0) | 24.0 (16.5–28.5) | 24.0 (20.0–28.0)∗ | 26.0 (22.0–30.0) ∗,† | 26.0 (22.0–29.0)∗ | 41 | <0.01 |
| PEEP set, cm H2O | 16.0 (13.0–16.0) | 16.0 (14.0–16.0) | 14.0 (14.0–16.0) | 14.0 (13.0–16.0) | 14.0 (14.0–16.0) | 41 | 0.13 |
| Plateau pressure, cm H2O | 27.5 (26.0–30.0) | 28.0 (24.0–30.0) | 27.0 (24.0–30.0) | 26.50 (25.0–29.0) | 27.0 (23.0–30.0) | 28 | 0.62 |
| Respiratory system static compliance, ml cm H2O−1 | 29.2 (23.3–35.5) | 29.2 (24.0–36.2) | 29.6 (25.5–35.0) | 27.7 (26.5–33.0) | 29.3 (25.7–37.3) | 27 | 0.38 |
| Fio2 | 0.80 (0.60–1.0) | 0.60 (0.50–0.70) | 0.55 (0.40–0.70) ∗ | 0.50 (0.40–0.60)∗,† | 0.50 (0.45–0.60)∗ | 40 | <0.01 |
| Pao2/Fio2, kPa | 17.5 (11.6–19.2) | 27.7 (19.5–35.7)∗ | 26.1 (17.9–33.1)∗ | 32 | <0.01 | ||
| Pao2, kPa | 11.8 (9.3–14.2) | 14.5 (10.2–20.4)∗ | 13.5 (10.3–17.3) | 32 | <0.01 | ||
| pH | 7.31 (7.23–7.36) | 7.31 (7.24–7.36) | 7.33 (7.30–7.37) | 32 | 0.13 | ||
| Paco2, kPa | 7.2 (5.7–7.9) | 6.8 (6.0–7.7) | 6.3 (5.5–6.8) | 32 | 0.29 | ||
| HCO3−, mmol L−1 | 26.0 (21.8–27.2) | 24.5 (21.80–7.6) | 23.2 (20.7–27.0)∗ | 32 | 0.01 | ||
| Ventilatory ratio | 1.79 (1.42–2.37) | 1.97 (1.61–2.76) | 1.82 (1.64–2.24) | 32 | 0.03 |
Secondary outcomes
Serial Pao2/Fio2 ratios after repeated prone positioning sessions
Twenty-five subjects were placed in prone positioning three or more times. Similar changes in arterial blood gases were observed for the first three prone positioning sessions, although the reduction in Fio 2 was more pronounced after the first prone positioning session than subsequent sessions (Table 3 ).
Table 3.
The pre- and post-prone positioning changes of vital signs, ventilator settings and arterial blood gases during the first three prone positioning sessions. DBP, diastolic blood pressure; Fio2, fraction of inspired oxygen; HR, heart rate;Pao2, partial pressure of oxygen; Paco2, partial pressure of carbon dioxide; PEEP, positive end expiratory pressure; SBP, systolic blood pressure; Spo2, saturation of pulse oximetry; VF, ventilatory frequency.
| N | Pre and post changes in 1st prone position | Pre and post changes in 2nd prone position | Pre and post changes in 3rd prone position | P | |
|---|---|---|---|---|---|
| Vital signs | |||||
| HR, beats min−1 | 25 | 1.0 (−3.0–12.0) | 0 (−3.0–4.0) | 2.0 (−4.0–8.0) | 0.04 |
| VF, bpm | 25 | 0 (0–3.5) | 0 (0–1.0) | 0 (0–0) | 0.24 |
| SBP, mm Hg | 25 | 3.0 (−8.5, 20.5) | 4.0 (−9.0, 18.0) | 9.0 (−4.5, 16.5) | 0.96 |
| DBP, mm Hg | 25 | 1.0 (−4.5, 10.5) | 0 (−4.5, 7.5) | 3.0 (0, 9.5) | 0.42 |
| SpO2, % | 25 | 1.0 (−0.5–4.0) | 2.0 (0–5.0) | 1.0 (0–3.0) | 0.15 |
| Ventilator settings and respiratory mechanics | |||||
| Tidal volume, ml kg−1 | 20 | 0 (0–0) | 0 (−0.4–0) | 0 (0–0) | 0.85 |
| PEEP, cm H2O | 25 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.55 |
| Fio2 | 24 | −0.15 (−0.4–0) | 0 (−0.10–0) | 0 (−0.08–0.08) | <0.01 |
| Plateau pressure, cm H2O | 18 | 0 (−1.0–1.0) | 0 (−0.25–3.0) | 0 (−0.25–1.0) | 0.82 |
| Respiratory system static compliance, ml cm H2O−1 | 17 | 0 (−4.7–3.7) | −1.6 (−5.7–1.4) | 0 (−2.2–2.8) | 0.90 |
| Ventilatory ratio | 13 | 0.27 (0.03–0.41) | 0 (−0.12–0.27) | −0.04 (–0.23–0.18) | 0.06 |
| Arterial blood gases | |||||
| Pao2/Fio2 change | 17 | 7.8 (2.5–17.9) | 7.5 (1.9–18.5) | 4.0 (−0.7–15.2) | 0.66 |
| Pao2/Fio2 change, % | 17 | 71.9 (16.5–142.9) | 36.4 (9.5–126.4) | 15.9 (−2.9–88.7) | 0.59 |
| Pao2, kPa | 17 | 2.0 (−0.9–8.8) | 4.4 (−0.7–10.7) | 2.4 (−0.5–6.8) | 0.94 |
| pH | 17 | −0.02 (−0.04–0.05) | −0.01 (−0.05–0.02) | 0.01 (−0.03–0.03) | 0.87 |
| Paco2, kPa | 17 | 0.3 (−0.8–1.0) | 0.3 (−0.2–0.7) | −0.1 (−0.5–0.4) | 0.65 |
| HCO3−, mmol L−1 | 17 | −0.6 (−1.4–0.8) | 0 (−0.9–0.9) | 0.1 (−0.4–0.9) | 0.29 |
Respiratory mechanics
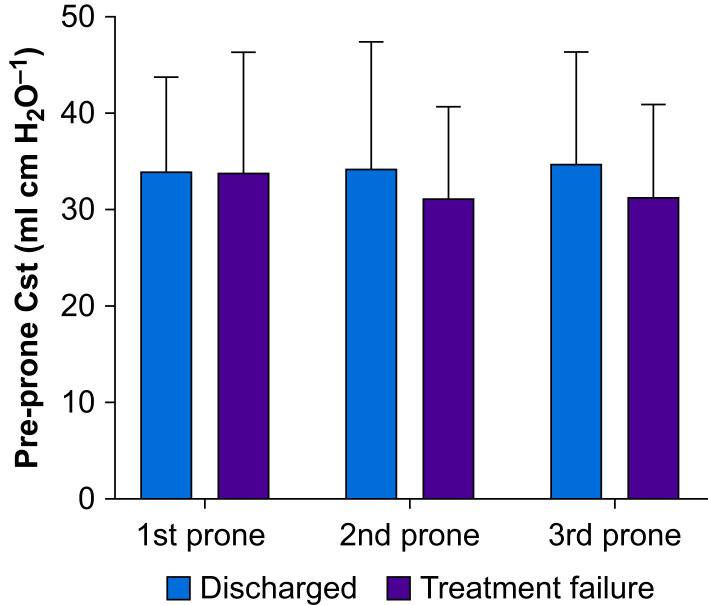
Tidal volume, PEEP, plateau pressure (Table 2), and respiratory system static compliance (Fig 2 ) were similar throughout repeated prone positioning manoeuvres. Both set and measured ventilatory frequencies were increased after prone positioning (P<0.01). The ventilatory ratio also improved after prone positioning (Table 2, Table 3).
Fig 2.
Respiratory system compliance and prone positioning. Using the change in Pao2/Fio2 ratio pre- and post-prone positioning ≥20% as the response criteria, 26 subjects met the criteria in the first prone positioning session (n=36) whereas 13 and 11 subjects responded in the second (n=27) and third (n=20) prone positioning sessions, respectively. Responders' respiratory system compliance before each prone positioning in the three sessions was similar to non-responders. Cst, compliance of respiratory system.
Haemodynamic and laboratory parameters
Vasopressor requirements did not alter after prone positioning, although the propofol dose decreased after the first prone positioning session (Supplementary Table S1). Laboratory test results were similar throughout (Supplementary Table S2).
Oxygenation after prone positioning and outcome
After the initial prone positioning session, subjects who were discharged from hospital were more likely to have an improvement in Pao 2/Fio 2 ratio ≥20% compared with those requiring ECMO or who died after both the second (11/16 vs 2/11, P=0.01) and third (9/12 vs 2/8, P=0.07) prone positioning sessions, respectively. In the second prone positioning session, the Pao 2/Fio 2 improvement was higher in the treatment success group than in the treatment failure group (10.7 [3.7–19.0] vs 1.4 [−1.6–3.4] kPa, P<0.01). This was also observed during the third prone positioning session (10.2 [5.2–18.3] vs 0.5 [−1.4–2.9] kPa, P=0.03); Table 1). In the logistic regression analysis, Pao 2/Fio 2 ratio incremental change in the second prone positioning session was associated with treatment success (odds ratio, 1.03; 95% confidence interval, 1.0–1.05; P=0.03).
Discussion
We found that in COVID-19 subjects placed in prone positioning, oxygenation improved and better oxygenation responses were associated with overall better outcomes. This is the first report, to our knowledge, on changes in Pao 2/Fio 2 ratio after repeated prone positioning sessions and the associated outcomes of those changes. Although high mortality from severe COVID-19 has been reported,5 , 21, 22, 23 our 28-day ICU mortality was 21.4%, which is similar to the PROSEVA study. In PROSEVA, 62.4% of subjects had ARDS as a result of pneumonia,7 whereas all of our subjects had virus-induced ARDS. The pre-prone positioning Pao 2/Fio 2 ratio in our study was higher than that in the PROSEVA study, possibly because of the higher level of PEEP we utilised. If the five subjects we placed on ECMO were grouped with those who died, to simulate the incidence of ECMO utilisation in Italy during the pandemic (1%),24 our mortality rate (38.1%, 16/42) is still lower than reported mortality for intubated COVID-19 subjects with severe ARDS.5 , 21, 22, 23
Similar to the findings from other studies,11, 12, 13 , 15, 16, 17 Pao 2/Fio 2 ratio improved after the first prone positioning session in our study, but we did not find differences in improvement of Pao 2/Fio 2 ratio between treatment success and failure groups, as also reported by Meenen and colleagues.25 Nevertheless, our study also showed that survivors responded to prone positioning on the second and third prone positioning cycles, in contrast to little or no response in those who ended up being placed on ECMO or those who died. Our findings suggest that the oxygenation response to prone positioning, after each cycle, may be helpful in guiding decisions regarding facility transfer or earlier escalation to ECMO.
COVID-19 ARDS has been proposed to be an atypical form of ARDS in terms of recruitability.26 Gattinoni and colleagues27 indicated that intubated COVID-19 subjects whose respiratory system compliance was high (50.2 [14.3] ml cm H2O−1) had lower recruitability, which was consistent with two other European reports.17 , 27 In our study, however, the respiratory system compliance was lower (median: 29.2 ml cm H2O−1) than that reported by Gattinoni and colleagues,17 , 27 , 28 but similar to four other studies.15 , 16 , 22 , 29 Additionally, in subjects who responded to prone positioning (defined as Pao 2/Fio 2 ratio improvement of ≥20%), we found no difference in pre-prone positioning respiratory system compliance when compared with non-respondents during the first three prone positioning sessions. The same result was found between the treatment success and treatment failure groups. It should be noted that body mass index (BMI) was ∼1.3 times larger in our study compared with BMI in the European ARDS population reported by Guérin and colleagues8 and others.28 This might explain the low compliance found in our subjects. A trend of higher BMI was seen in the treatment success group, which may reflect the obesity survival paradox described in pneumonia.30 Future studies are needed to validate this finding in subjects with COVID-19.
We used ventilatory ratio to evaluate dead space.29 , 31 The pre-prone positioning ventilatory ratio in our subjects was higher than in the subjects in a preceding study which used similar volume settings.15 This might be explained by the higher acuity of subjects in our study, as evidenced by the need for higher PEEP, higher plateau pressures, lower compliance, and lower Pao 2/Fio 2 ratio before initial prone positioning. After prone positioning, the ventilatory ratios were increased within 1 h. This might be explained by alveolar over-distension caused by the same (pre-prone positioning) PEEP being applied during post-prone positioning. PEEP was reduced around 4 h after prone positioning in our study, as a result of the improvement of oxygenation. It was also maintained at that level after supine positioning, and interestingly, ventilatory ratios returned to pre-prone positioning levels. This finding suggests that close monitoring of changes in dead space and timely reduction in PEEP during prone positioning is needed, which might help to avoid alveolar over-distension.
A limitation of this single-centre study was that we did not transport subjects for a CT scan to investigate pulmonary morphology that might explain why some subjects did not respond to being placed in the prone position. Second, we used ventilatory ratio as a surrogate assessment of dead space.29 Future studies are needed to understand if ventilatory ratio is an acceptable way to approximate dead space in subjects with COVID-19.28 Lastly, some data were missing as a result of the immediate need for prone positioning and increased staff workload.
In summary, prone positioning improved oxygenation for patients with COVID-19 ARDS who required invasive mechanical ventilation. Serial assessment of the Pao 2/Fio 2 ratio may help guide decisions for earlier escalation of treatment, including ECMO.
Authors' contributions
Conceived the study: JL, TW
Designed the study: JL
Validated the data: JL, AAA, RK, AEA
Analysed the data: JL
Interpreted the data: JL, TW, FC, JBS, RK, SS, SHM
Drafted the manuscript: JL, TW
Revised and approved the manuscript: TW, FC, RK, SS, SHM, AAA, RK, AEA
Substantially revised and approved the manuscript: JL, JBS
Declarations of interest
JBS discloses a relationship with Ventec Life Systems and Teleflex. JL discloses research support from Fisher & Paykel Healthcare and Rice Foundation outside the submitted work. All other authors declare that they have no conflicts of interest.
Acknowledgements
We thank all the respiratory therapists in the Respiratory Care Department in Rush University Medical Centre for their dedicated and diligent work during the COVID-19 pandemic. We also thank Sharon Foley, Guoqiang Jing, and Rongshou Zheng for consultancy in data analysis.
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.09.042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. WHO Coronavirus disease (COVID-19) dashboard. Available from: https://covid19.who.int/. (accessed 22 October, 2020).
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 subjects hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for subjects with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Intensive Care National Audit & Research Centre. COVID-19 report. Available from: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. (accessed 22 May 2020).
- 6.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 8.Guérin C., Beuret P., Constantin J.M., et al. A prospective international observational prevalence study on prone positioning of ARDS subjects: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44:22–37. doi: 10.1007/s00134-017-4996-5. [DOI] [PubMed] [Google Scholar]
- 9.Munshi L., Del Sorbo L., Adhikari N.K.J., et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 10.Caputo N.D., Strayer R.J., Levitan R. Early self-prone positioning in awake, non-intubated subjects in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elharrar X., Trigui Y., Dols A.M., et al. Use of prone positioning in nonintubated subjects with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323:2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q., Wang T., Qin X., Jie Y., Zha L., Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24:250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartini C., Tresoldi M., Scarpellini P., et al. Respiratory parameters in subjects with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppo A., Bellani G., Winterton D., et al. Feasibility and physiological effects of prone positioning in non-intubated subjects with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8:765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziehr D.R., Alladina J., Petri C.R., et al. Respiratory pathophysiology of mechanically ventilated subjects with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan C., Chen L., Lu C., et al. Lung recruitability in SARS-CoV-2 associated acute respiratory distress syndrome: a single-center, observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsetti A., Damia Paciarini A., Marini B., et al. Prolonged prone position ventilation for SARS-CoV-2 subjects is feasible and effective. Crit Care. 2020;24:225. doi: 10.1186/s13054-020-02956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obaidan A., Scott J.B., Mirza S.H., Aljoaid A., Tailor R., Vines D.L. Evaluation of a training method to improve knowledge and confidence of prone positioning. Respir Care Ed Annu. 2018;27:3–15. [Google Scholar]
- 19.Available from: https://www.youtube.com/watch?v=lcBPaHQUvXY&feature=youtu.be (accessed 31 May 2020).
- 20.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in subjects with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill subjects with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill subjects in the Seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill subjects with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 subjects infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Meenen D.M., Roozeman J.P., Serpa Neto A., et al. Associations between changes in oxygenation, dead space and driving pressure induced by the first prone position session and mortality in subjects with acute respiratory distress syndrome. J Thorac Dis. 2019;11:5004–5013. doi: 10.21037/jtd.2019.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roesthuis L., van den Berg M., van der Hoeven H. Advanced respiratory monitoring in COVID-19 subjects: use less PEEP! Crit Care. 2020;24:230. doi: 10.1186/s13054-020-02953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X., Liu X., Xu Y., et al. Ventilatory ratio in hypercapnic mechanically ventilated subjects with COVID-19 associated ARDS. Am J Respir Crit Care Med. 2020;201:1297–1299. doi: 10.1164/rccm.202002-0373LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie W., Zhang Y., Jee S.H., Jung K.J., Li B., Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61. doi: 10.1186/1741-7015-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha P., Calfee C.S., Beitler J.R., et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.