Highlights
-
•
The current crisis related to the spread of COVID-19 has challenged epidemiologists and public health experts alike, leading to a rapid search for, and development of, new and innovative solutions to combat its spread.
-
•
A multidisciplinary approach needs to be followed for diagnosis, treatment and tracking, especially between medical and computer sciences, so, a common ground is available to facilitate the research work at a faster pace.
-
•
This review paper covers both medical and technological perspectives to facilitate the virologists, AI researchers and policymakers while in combating the COVID-19 outbreak.
-
•
This paper aimed to explore and understand how and which different technological tools and techniques have been used within the context of COVID-19.
-
•
Investigating artificial intelligence (AI) approaches for the diagnosis, anticipate infection and mortality rate by tracing contacts and targeted drug designing.
-
•
The impact of different kinds of medical data used in diagnosis, prognosis and pandemic analysis is also provided.
-
•
The investigation of this paper reveals several AI-based approaches that have been proposed as potential ways to help, with the COVID-19 pandemic, covering everything from initial diagnoses via image diagnostics up to the presentation of models that help to understand the spread of COVID-19 and identify potential new outbreak areas.
Keywords: Artificial intelligence, Computer-aided diagnosis, Deep learning, Machine learning, Infectious diseases, COVID-19, SARS-CoV-2
Abstract
While the world has experience with many different types of infectious diseases, the current crisis related to the spread of COVID-19 has challenged epidemiologists and public health experts alike, leading to a rapid search for, and development of, new and innovative solutions to combat its spread. The transmission of this virus has infected more than 18.92 million people as of August 6, 2020, with over half a million deaths across the globe; the World Health Organization (WHO) has declared this a global pandemic. A multidisciplinary approach needs to be followed for diagnosis, treatment and tracking, especially between medical and computer sciences, so, a common ground is available to facilitate the research work at a faster pace. With this in mind, this survey paper aimed to explore and understand how and which different technological tools and techniques have been used within the context of COVID-19. The primary contribution of this paper is in its collation of the current state-of-the-art technological approaches applied to the context of COVID-19, and doing this in a holistic way, covering multiple disciplines and different perspectives. The analysis is widened by investigating Artificial Intelligence (AI) approaches for the diagnosis, anticipate infection and mortality rate by tracing contacts and targeted drug designing. Moreover, the impact of different kinds of medical data used in diagnosis, prognosis and pandemic analysis is also provided. This review paper covers both medical and technological perspectives to facilitate the virologists, AI researchers and policymakers while in combating the COVID-19 outbreak.
1. Introduction
Coronaviruses are a large family of viruses that can cause severe illness to human beings [1]. Whilst we as humans do have experience with some coronaviruses, the current pandemic has been caused by a novel zoonotic disease [2]. Novel here means that humans have not yet been exposed to this virus, whereas zoonotic means it spread from animals to humans [3]. Due to the novelty of the virus, it appears to be the case that humans do not have any innate level of immunity, which would, in the case of exposure to other viruses, help lessen the spread and the effect. Viruses, especially novel viruses, have the potential to develop either into local epidemics, or more widespread pandemics [4]. For the purpose of this paper, a pandemic can be understood as an outbreak of any infectious disease that tremendously increases mortality and morbidity rate over the wider geographical region, whereas, an epidemic means an occurrence of disease spans over a passage of time in a limited area [5].
Pandemics and epidemics are not anything strange for the world, in just the more recent past, the Severe Acute Respiratory Syndrome (SARS) epidemic virus infected 8096 humans and caused the deaths of more than 770 persons, whereas the smallpox pandemic has infected millions of individuals and caused more than 500 million death world-wide throughout its existence [6]. The most severe viruses over the past century as well as their associated epidemics and pandemics are listed in Table 1 .
Table 1.
Deadliest Viruses Over Last Century.
| Year | Name | Spread by | Signs and Symptoms | Peak Pandemic Time period | Infected persons | Death toll/Fatality Rate (%) | Majorly Hit Areas | Ref |
|---|---|---|---|---|---|---|---|---|
| 1918 | Influenza (Spanish flu) | Contact with infected person | Fever, fatigue and chill | 1918 | 500M | 50 – 100M | Worldwide | [7] |
| 1920 | Rabies | Bite or scratch from rabid animal | Fever and tingling sensation around the wound. Fatal inflammation of the brain | 1920 | 29 M every year | 59,000/year | India and Africa | [8] |
| 1920 | HIV | Receiving unsafe injections, or blood transfusions | Influenza-like illness, swollen lymph nodes | 1981 – Present (1997 Peak) | 75M | 32M | Worldwide | [9] |
| – | Smallpox | Contact with infected person | Scars and often blindness | Unknown – 1979 | 300 M (in 20th century) | Worldwide | [6] | |
| 1950s | Hantavirus | Exposure to droppings of infected rodents | Fever, vomiting, abdominal pain and coughing | 1950, 1993 | 300,000/year | 30% – 50% | Korea, USA and China | [10] |
| 1950 | Dengue | Mosquitoes bite | Abdominal pain, fever, bleeding gum and vomiting | 1950 – Present | 100 M – 400 M every year | 2.5% | Philippines, Thailand and subtropical regions | [11] |
| 1967 | Marburg virus | Contact with blood, body fluid or infected tissue | Fever, myalgia, and maculopapular rash | 1967 – 1968, 1998 – 2000, 2004 - 2005 | 466 | 24% - 88% | Germany, Serbia, Congo, Angola and Uganda | [12] |
| 1973 | Rotavirus | Fecal-oral route | Diarrheal, dry mouth and fussiness | 2003, 2013 | – | 200,000 – 500,000/year | Worldwide | [13] |
| 1976 | Ebola virus | Contact with blood, broken skin, or mucous membranes | Fever, fatigue, muscle pain, Headache, sore throat, vomiting, rash | 2014 - 2016 | 31,095 | 12,950 | Sudan and Congo and West Africa | [14] |
| 2002 | SARS-CoV | Contact with infected person | Fever, chills, body aches and pneumonia | 2002 – 2004 | 8096 | Over 770 (9.63%) | Worldwide | [15] |
| 2012 | MERS-CoV | Contact with infected person | Respiratory problems, fever, cough | 2012 | 2494 | 858 | Middle East countries | [15] |
| 2019 | SARS-CoV-2 | Contact with infected person | Fever, dry cough, tiredness, and loss of taste | 2019 - Present | 18.92M | 710,916 (till August 6th, 2020) | First China, then worldwide | [16] |
SARS-CoV-2, the virus that is responsible for COVID-19 (this is the name of the disease), belongs to a large family of viruses that frequently causes severe respiratory symptoms [17]. In 2002 SARS-CoV, a predecessor to the current pandemic-causing virus began to spread; this virus was discovered in Guangdong, China. In 2012 another coronavirus (MERS-CoV) outbreak began in the Middle East, which caused a severe respiratory disease now known as Middle East Respiratory Syndrome [18]. This virus caused 858 deaths and infected 2494 humans. Now the world is grappling with another coronavirus which seems to have begun its spread in December 2019, though this is still disputed. COVID-19 is an infectious disease that spreads in humans mainly through direct or indirect contact with an infected person, respiratory droplets produced when an already infected human sneezes or talks, or aerosolized droplets (airborne transmission) [19]. The early symptoms include dry coughing, highly persistent fever, and respiratory difficulties which may lead to multiple organ failure or acute respiratory distress; in severe cases, death may also occur [20, 21].
As this pathogen is easily spread and grows exponentially and sustainably between humans, the manpower to combat this is limited. To put this another way, there is only a finite number of doctors, health care workers, and contact tracers and when the pandemic is at peak levels there may not be enough capacity to fully manage the pandemic. Thus, to this end, there is a distinct need for tools that can help improve these specialists’ ability to do their job. This can include the development of contact tracing applications, statistical dashboards and visualizations, machine learning models, or other sorts of AI-based tools.
To tackle the pandemic of COVID-19, scientists and medical experts are trying to come up with state-of-the-art solution to control the spread, identify infected patients, monitor virus growth and develop vaccines. Many researchers are working with computer-based diagnosis techniques using clinical blood samples, radiography images, and respiratory-related datasets. AI and machine learning techniques are helping medical personnel in monitoring, analysis and prediction of various critical applications such as survival mortality assessment, forecasting models, virions sequence formation and drug discovery models.
In order to help stop the pandemic, the early detection of positive cases is crucial as it facilitates the containment and isolation of the virus, thus reducing its spread. Currently, the standard procedure followed for identification of COVID-19 positive cases is performed by Reverse-Transcription Polymerase Chain Reaction (RT-PCR) [22]. To further improve and hasten the early detection process, there is room for the development of new and innovative tools that may improve the early detection and tracking of the virus [23, 24].
As there is a clear need for these innovative technical solutions, there has, logically, been a large amount of rapid-paced development of these tools. However, due to the speed and number of these developments, it is easy to become overwhelmed, thus limiting the ability to critically examine, explore, and understand what a given solution does, how it performs, and whether or not it may or may not be beneficial. Thus, with this in mind, the main objective of this comprehensive review is to provide an in-depth account of COVID-19 and various advancements in AI techniques that have been currently developed to help manage or combat COVID-19.
2. Methodology
The review was conducted between July and August 2020, using the keywords “COVID-19” AND (combined with one of the following: “AI”, “Machine Learning”, “Deep Learning”) as well as “COVID-19” AND “AI” AND (combined with one of the following: “Clinical Blood Samples”, “Forecasting Models”, “Drug Discovery Models”). This approach is, admittedly, broad, but allowed for a large number of relevant papers to be identified. Additionally, due to the nature of this study being a relatively new research subject, pre-print papers (such as those submitted to arxiv) were not precluded. As recommended by [25], we selected Google Scholar as our electronic bibliographic databases to remove any kind of biasness for any specific scientific publisher. We then excluded papers not written in English, duplicates, guest editorials, poster sessions, and blogs. Once the papers were identified, they were analyzed and grouped based on their aims and approaches.
2.1. Scope of the survey and contributions
Based on this conducted study, it is hoped that this paper will serve as a common ground for both medical experts and computer scientists to better understand the current state-of-the-art associated with COVID-19 and technological innovation. Although the research community has collected and shared adequate amount of information in the last few months, this review paper covered both medical and technological perspectives to facilitate the virologists, AI researchers and policymakers while in combating the COVID-19 outbreak. Due to a lack of in-depth understanding about COVID-19, numerous experts and hobbyists have taken advantage of different technological tools, such as open government data, big data, artificial intelligence (AI), etc. to help combat or understand the virus. A multidisciplinary approach needs to be followed for diagnosis, treatment and tracking, especially between medical and computer sciences, so, a common ground is available to facilitate the research work at a faster pace. This is interesting as COVID-19 is the first wide-spread global pandemic where these technological tools have been available, and accessible to such an extent that any stakeholder could, in theory, participate in the development of potential solutions.
With this in mind, the main contributions of this study are as follows:
-
•
Detailed history, characteristics, taxonomy, symptoms, behavior, and patterns of COVID-19 are outlined.
-
•
Presentation and discussion of AI (both traditional ML and advanced DL) approaches applied to COVID-19 in a variety of aspects and domains.
-
•
Description of major COVID-19 diagnosis techniques using clinical blood samples, radiography images, respiratory-related data for various critical applications such as survival mortality assessment, forecasting models, virions sequence formation and drug discovery models.
-
•
A detailed discussion on issues, challenges, and recommendations to tackle the pandemic through AI and timely facilitate effective decision making is presented.
In order to meet these objectives, the rest of the paper is organized as follows. Section 2 lays out the historical overview, origin and means of transmission, and global dispersion of the virus. Section 3 highlights the novel AI techniques that have been applied to COVID-19, and furthermore, it explores non-applied, but potentially useful, AI-based applications for vaccine discovery, mortality and survival rate prediction, and outbreak forecasting. Section 4 discusses the challenges in COVID-19 research. Finally, the review is concluded in Section 5 by presenting a summarized overview of the conducted studies.
3. Origin and overview of SARS-CoV-2
The type of coronaviruses that cause infections in humans belongs to a subfamily called Coronavirinae which is part of a larger Coronaviridae family. The coronavirus family is a very large family that is comprised of several viruses, such as Middle East Respiratory Syndrome virus (MERS-CoV), Severe Acute Respiratory Syndrome (SARS-CoV), and COVID-19 (SARS-CoV-2) [26].
The name Corona comes from the fact that the viral particle has spike-like projections or structures on its outer layer that can be seen under a scanning electron microscope. The RNA of coronaviruses is single-stranded, positive sense, has a diameter of around 80 to 120 nm and a nucleic acid length from 26 to 32 kbs [27]. They are classified under four variants or genera entitled as alpha (α), beta (β), gamma (γ) and delta (δ) [27]. It has been observed that mammals typically get infected by the α- and β-CoV while the other two (γ- and δ-CoV) mostly infect birds. From the α-CoV group, HCoV-NL63 and HCoV-229E have been found to affect humans, normally causing a mild respiratory illness, similar to the common cold. From the β-Cov group, SARS-CoV and MERS-CoV both tend to cause moderate to severe respiratory illnesses with a higher morbidity and mortality rate [28, 29].
The most recent addition to the coronaviruses is that of SARS-CoV-2, which became known in late 2019 after a number of a-typically presenting pneumonia cases were identified in Wuhan China; it was initially speculated that these cases might originate from the Huanan Seafood market [30]. The hallmark sign of many of the cases was the development of certain symptoms including dry cough with fever, breathing difficulty and a-typical presentation of pneumonia. On January 7th 2020, after a thorough analysis of the samples taken from nasopharyngeal swabs (collecting a clinical test sample of nasal secretions from the back of the nose and throat), the United States - Center for Disease Control (U.S. CDC) stated publicly that this disease appeared to be caused by the novel coronavirus [31]. After this, the International Committee on Taxonomy of Viruses (ICTV) named it the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [17], whereas the disease itself is named COVID-19.
In early 2020, due to the pervasive spread of COVID-19 around the globe, the WHO declared it a Public Health Emergency of International Concern (PHEIC) on 30th January 2020 after cases were reported in 18 different countries worldwide [32]. On the 11th of March 2020 the WHO upgraded the threat, declaring COVID-19 a global pandemic and a serious hazard to public health after a sharp incline in the number of cases reported outside of China [33].
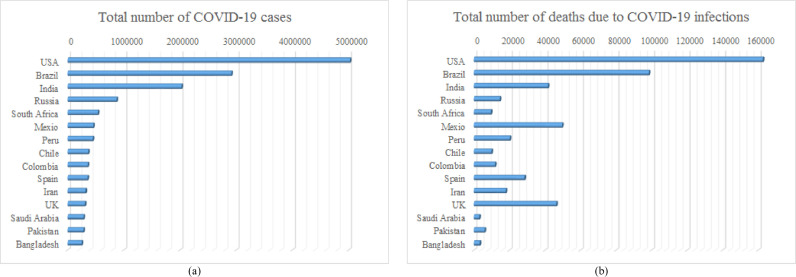
Among the 213 different countries, so far, the USA has seen the highest number of cases and fatalities, though other countries such as Brazil, India, Russia, South Africa, Mexico, Peru, Chile, Colombia, Spain, Iran, UK, Saudi Arabia, Pakistan, and Bangladesh have also registered a high number of cases; Fig. 1 shows the top 15 countries for coronavirus infections and deaths – based on the latest WHO situation reports [34]. The disease itself is so pervasive due to the ease with which it is able to transmit itself, via small respiratory droplets (such as those created when talking, sneezing, coughing, and breathing) and has a higher level of danger to a person's health due to the current absence of targeted pharmaceutical solutions such as vaccines or antiviral drugs.
Fig. 1.
COVID-19 transmission pattern in top 15 countries (August 6, 2020), (a) total number of COVID-19 infections, (b) total number of deaths due to COVID-19 infection.
4. AI-Based approaches for COVID-19
Pandemics are an ever-present threat to human society, health, and wellbeing and, therefore, attention must be dedicated to understanding how they occur, how they can be prevented, and how we can strategically mitigate the negative effects they cause. The current pandemic associated with COVID-19 is unique in that it represents the first and largest pandemic in modern times where new technological solutions, e.g. AI, are not only applicable to the pandemic, but able to be created by a large group of stakeholders from scientists to doctors to AI-hobbyists alike. As the pandemic has caused great disruption to normal day-to-day operations and created a sense of unknown amongst the public, many motivated scientists and citizens have tried to assist in the COVID-19 response by developing their own unique AI-based tools to solve a large number of problems, in a variety of applied domains, such as: COIVD-19 disease detection and classification, mortality rate prediction and severity assessment, outbreak forecasting and tracking, biological insight of SARS-Cov-2 strain, and drug discovery.
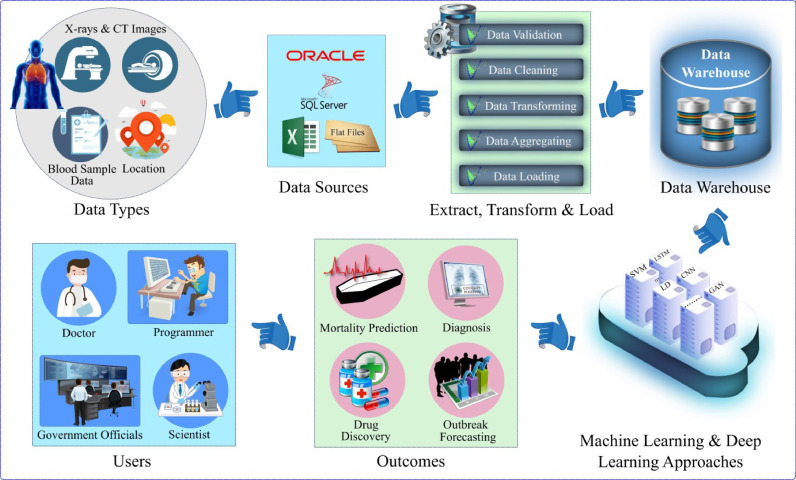
In order to explore the different approaches that are currently being developed and trialed, this section presents a selection of the different ways in which AI-based solutions have been applied to the COVID-19 pandemic. In order to provide a visual representation of how an AI-based COVID-19 system may work, a generic model has been created in Fig. 2 . The system can process various types of raw data including images, blood samples, crowed sourced data, even GPS information for tracking positive cases, etc., which can be stored in a repository. Data mining techniques are usually applied to clean the raw data that is and then stored in a database for further processing and retrieval. ML and DL methods can be applied to this data for better visualization, diagnosis, and forecasting, which can help the end-users making better decisions and taking actions to mitigate the disease.
Fig. 2.
Illustration of an AI-based solution for diagnosis, forecasting and mitigation of COVID-19.
4.1. COVID-19 diagnosis using radiography images
Early diagnosis of COVID-19 is important to ensure better outcomes for those who have been infected. At the beginning of the pandemic, due to the sense of the unknown that surrounded the new disease, one way that was being used to diagnose patients was via radiographic imagery (such as CT scans or X-Rays) of the lungs.
4.1.1. ML-BASED techniques and applications
Several experts have extensively applied various ML algorithms and approaches to COVID-19 radiographic imagery, such as Sethy et al. [35] who presented a COVID-19 diagnostic tool for the medical practitioner based on a Support Vector Machine (SVM) model. In this three-class problem, thirteen various pre-trained Convolutional Neural Network (CNN) models such as AlexNet, VGG16, VGG19, etc. extracted deep features from 381 chest X-ray (CXR) images, out of which 127 images belonged to COVID-19 infected patients, 127 images to other viral/bacterial pneumonia and 127 normal patients CXR. Finally, the SVM model was used to classify COVID-19 patients using extracted deep features. The comparative analysis in the study showed that ResNet50 plus SVM secured an average classification accuracy of 95.33% in 20 independent executions when the data was split into an 80:20 ratio of training and testing sets. Shi et al. [36] also proposed a model for assisting in the diagnosis of COVID-19 with a model called infection Size Aware Random Forest (iSARF). The proposed framework processed CT images of 1027 community-acquired pneumonia (CAP) patients and 1658 COVID-19 infected patients to obtain infections and lung fields’ segmentation using a modified version of V-net [37] (VB-Net). It identified the infected lesion size in each segmented lung and categorized it into four groups. Later, a Random Forest (RF) model was used for the final diagnosis in each group. From the experimental results, it was noted that the model provided an accuracy of 87.9%, a specificity of 83.3% and 90.7% sensitivity under 5-fold cross-validation to avoid any biasness in experimental tests. Other models that were identified from the literature review are expressed in Table 2 .
Table 2.
Machine Learning-Empowered COVID-19 Diagnostic Tools Using Radiography Images.
| Ref | Year | Model/Method Names | Task | Data Type | Classes | Acc | P | Sn | Sp | F1-score |
|---|---|---|---|---|---|---|---|---|---|---|
| [38] | May 2020 | Deep learning models (MobileNetV2, SqueezeNet) with Social Mimic Optimization method and SVM classifier | COVID-19 detection | X-ray images | 99.27 (CP) |
98.89 (CP) |
98.33 (CP) |
99.63 (CP) |
98.58 (CP) |
– |
| [39] | April 2020 | SVM with Multi-Level Thresholding | COVID-19 detection | X-ray images | 2 | 97.48 | – | 95.76 | 99.70 | – |
| [35] | April 2020 | SVM using ResNet50’s deep features | Detection of COVID-19 | X-ray images | 3 | 95.33 | – | 95.33 | – | 95.34 |
| [40] | April 2020 | ResNet152 model with SMOTE, and Random Forest and XGBoost classifiers | COVID-19 screening from healthy and other pneumonia cases | X-ray images | 3 | 97.70 | – | 97.7 | 98.8 | 97.7 |
| [41] | May 2020 | Clus-HMC framework and conventional ML algorithms (SVM, DT, MLP, kNN) and RF) | COIVD-19 identification in hierarchical and multi-class scenario | X-ray images | 7 | – | – | – | 89.0 | – |
| [42] | May 2020 | Residual Exempler Local Binary Pattern (ResExLBP) based on iterative Relief (IRF) feature selection and ML classifiers (DT, linear discriminant, SVM, kNN, and subspace discriminant (SD)) | COVID-19 Diagnosis. | X-ray images | 2 | 99.69 | – | 98.85 | 100.0 | – |
| [43] | July 2020 | Five ML-based approaches (Decision Tree, Ensemble, kNN, 3-naïve Bayes, and SVM) | Discriminate COVID-19 from other pneumonia patients | CT images | 2 | 91.94 | 90.63 | 93.54 | 90.32 | |
| [44] | April 2020 | Fast Fourier Transform (FFT)-Gabor scheme with SVM classifier | Identifying COVID-19 positive cases from radiography images dataset | CT Images | 2 | 95.37 | – | 95.99 | 94.76 | – |
| [45] | April 2020 | CNN-based transfer learning Fusion and Ranking techniques (VGG-16, GoogleNet and ResNet50) along with SVM classifier | COVID-19 classification | CT images | 2 | 98.27 | 97.63 | 98.93 | 97.60 | 98.28 |
| [46] | March 2020 | SVM with statistical feature extraction techniques (GLCM, LDP, GLRLM, GLSZM, DWT) | COVID-19 classification using segmented patches and feature extraction | CT images | 2 | 97.71 | 99.62 | 97.56 | 99.68 | 98.58 |
| [36] | March 2020 | Infection Size Adaptive Random Forest method (iSARF) with DT other ML techniques (LR, SVM, NN, SARF) | COVID-19 classification from community acquired pneumonia by acquiring lungs and infection fields segmentation | CT images | 2 | 87.90 | 90.70 | 83.30 | – |
Acc = Accuracy, P = Precision, R = Recall, Sn = Sensitivity, Sp = Specificity, AUC = Area under Curve, CP = Values specifically pertaining to COVID-19 class classification.
4.1.2. DL-BASED techniques and applications
There has also been a large number of DL-based approaches proposed to identify COVID-19 from radiographic imagery in the literature. For example, Brunese et al. [47] exploited the transfer learning technique and fine-tuned DL-based modified Visual Geometry Group (VGG-16) model. The proposed framework was divided into 2 models that considered 6523 chest X-rays (2753 belongs to patients with pulmonary diseases, 250 of COVID-19 infected patients, while 3520 related to healthy patients). The first model discriminates between healthy and pulmonary diseases patients, while the second model classifies the chest X-ray (CXR) of pulmonary disease patient as COVID-19 or other pneumonia. In the end, if a CXR is classified as COVID-19, the framework provides a visualization of the CXR highlighting the potentially SARS-CoV-2 infected area.
From the experimental data, it was observed that the discriminatory model (model 1) achieved accuracy, sensitivity, specificity and f-measure of 96%, 96%, 98% and 94% respectively, whereas the disease classification model (model 2) yielded an accuracy of 98%, sensitivity of 87%, specificity of 94%, and an f-measure of 89%.
Similarly, to identify SARS-CoV-2 infected cases from CXR images, various CNN frameworks such as VGG19, MobileNet v2, Inception, Xception, and Inception ResNet v2 have been evaluated using transfer learning by Apostolopoulos and Mpesiana [48]. From their conclusion, it was observed that MobileNet v2 outperformed the other tested state-of-the-art architectures by securing 99.10% sensitivity, 97.09% specificity, 97.40% accuracy on the two-class problem and 92.85% accuracy on the three-class problem. In that paper, the models were trained and evaluated on a dataset of 1427 CXR images that constitutes 700 images of bacterial pneumonia infected patients, 224 images of COVID-19 infected cases, and 504 belonging to normal patients. MobileNet v2 also performed well yielding a sensitivity, specificity, two-class problem accuracy, and three-class problem accuracy of 98.66%, 96.46%, 96.78% and 94.72%. For this model, the dataset was composed of 714 viral pneumonia CXRs images, 224 COVID-19 cases images, and 504 normal patients CXRs.
Jaiswal et al. [49] applied the deep transfer learning (DTL) technique with the pre-trained DenseNet201 model on chest CT images. In this study, a total of 1262 chest CT images related to SARS-CoV-2 positive cases and 1230 CT images of other patients were utilized and diversified using data augmentation technique to train and test the pre-trained DenseNet201 with additional CNN structure. The proposed tool achieved better performance on the testing set in terms of accuracy, precision, recall, f-measure and specificity of 96.25%, 96.29%, 96.29%, 96.29% and 96.21% respectively.
Xu et al. [50] developed a 3D CNN-based DL model to differentiate COVID-19 infected cases from influenza-A caused viral pneumonia (IAVP) and healthy person's by using a dataset consisting of 618 CT samples (224 IAVP CT images, 219 samples from 110 COVID-19 infected patients, and 175 healthy cases instances) from three Chinese hospitals. The images were segmented and features were extracted using ResNet. Later, the location-attention classification model classified the segmented CT images with an overall accuracy of 86.7%.
Other identified DL-based methods and approaches are highlighted in Table 3 . COVID-19 DIAGNOSIS USING CLINICAL BLOOD SAMPLES DATA
Table 3.
Deep Learning-Empowered COVID-19 Diagnostic Tools Using Radiography Images.
| Ref | Year | Model/Method Names | Task | Data Type | Classes | Acc | P | Sn | Sp | F1-score |
|---|---|---|---|---|---|---|---|---|---|---|
| [49] | July 2020 | DenseNet201 based transfer learning and CNN | Detection and diagnosis of COVID-19 | CT images | 2 | 96.25 | 96.29 | 96.29 | 96.21 | 96.29 |
| [51] | July 2020 | Customized CNN-based ResNet50 | COVID-19 detection from multiclass | CT images | 3 | 91.0 | – | 92.1 | 90.29 | – |
| [52] | June 2020 | Deep CNN-based network | COVID-19 detection from multiclass | CT images | 4 | – | – | 90.19 | 95.76 | – |
| [53] | June 2020 | COVID-19Net and DenseNet121-FPN | COVID-19 prognostic and diagnostic analysis | CT images | 2 | 78.32 | – | 80.39 | 76.61 | 77.0 |
| External Validation. set 2 | 2 | 80.12 | – | 79.35 | 81.16 | 82.02 | ||||
| [50] | June 2020 | 3D DL model | Early stage COVID-19 classification from other viral pneumonia and healthy cases | CT images | 3 | 86.70 | 86.80 | 86.6 | – | 86.7 |
| [54] | June 2020 | Contrastive self-supervised learning (CSSL) fine-tuned ImageNet-pretrained model (DenseNet169, ResNet50) | COVID-19 classification | CT images | 2 | 89.10 | – | – | – | 89.60 |
| [55] | May 2020 | 3D CNN-based network | COVID-19 diagnosis using chest CT infiltrative biomarkers | CT images | 2 | 70.0 | – | – | – | – |
| [56] | May 2020 | GAN, ResNet-32 and variant of 3D U-Net architectures | Effect of synthetic data enhancement on COVID-19 classification | CT Images | – | – | – | – | – | – |
| [57] | May 2020 | CNN and MLP (SVM and Random Forest) based model | COVID-19 diagnosis | CT images + clinical data | 2 | 83.5 | 81.9 | 84.3 | 82.8 | – |
| [58] | May 2020 | Transfer learning ResNet-32 based Deep CNN model | COVID-19 classification | CT images | 2 | 93.02 | 95.19 | 91.48 | 94.78 | – |
| [59] | May 2020 | Various DL-based segmentation models (U-net, DRUNET, FCN, SegNet & DeepLabv3) and 3D ResNet-18 classification model | Diagnosis, severity and prognosis prediction of COVID-19 among other pneumonia. Later tested on external validation set | CT images with metadata | 3 | 92.49 | – | 94.93 | 91.13 | – |
| [60] | April 2020 | Multitask deep learning model (Encoder, 2 decoders, MLP, and CNN) | COVID-19 classification and segmentation | CT images | 4 | 86.0 | – | 94.0 | 79.0 | – |
| [61] | April 2020 | EfficientNet B4 with Fully-connected NN | Develop and evaluate AI network for COVID-19 classification from other pneumonia | CT images | 2 | 96.0 | 95.0 | 96.0 | – | |
| External dataset | 87.0 | 89.0 | 86.0 | |||||||
| [62] | April 2020 | 3D DL models | COVID-19 detection, and infected region identification. | CT images | 3 | 86.70 | 86.87 | 98.20 | 92.20 | 86.70 |
| [63] | April; 2020 | CNN-based Deep learning networks (Inception-V4 and AlexNet) | Diagnosis and prognosis of COVID-19 case | CT images | 2 | 94.74 | – | 87.37 | 87.45 | – |
| [64] | April 2020 | Multi-objective differential evolution-based CNN | COVID-19 patients’ classification and disease evaluation | CT images | 2 | 93.50 | – | 91.00 | 91.00 | 89.90 |
| [65] | April 2020 | Modified Deep Transfer Learning based Inception model | Diagnosis of COVID-19 by extracting graphical features from CT images. Later tested on external validation set | CT images | 2 | 89.50 | 88.00 | 87.00 | 77.00 | |
| External dataset | 79.3 | 83.0 | 67.0 | 63.0 | ||||||
| [66] | March 2020 | Composite hybrid feature extraction (CHFS) based Stack Hybrid Classification (SHC) composed of CNN and ML models (SVM, RF) | Predicting recurrences in no recurrence COVID-19 and SARs cases | CT images | 2 | 96.07 | 96.10 | 96.10 | 96.10 | |
| [67] | March; 2020 | ResNet-50 | Classification of COVID-19 from other viral and infectious diseases | CT images | 5 | 98.80 (CP) | 94.50 (CP) | 98.20 (CP) | 98.90 (CP) | – |
| [68] | March 2020 | Variants of DL models (Segmentation; 3D U-Net++, U-Net, FCN-8 s, V-Net. Classification; Inception networks, ResNet-50 and DPN-92) | COVID-19 detection | CT images | 2 | – | – | 97.40 | 92.20 | – |
| [69] | March 2020 | Deep Learning based COVNet | COVID-19 detection from community acquired non-pneumonia and pneumonia lungs CT images | CT images | 3 | – | – | 90.0 (CP) | 96.0 (CP) | – |
| [70] | March 2020 | Weakly supervised pre-trained 2D UNet-based 3D deep CNN called DeCoVNet | Rapid diagnostic tool for COVID-19 detection using 3D CT images | CT images | 2 | 90.10 | 84.0 | 90.70 | 91.10 | – |
| [71] | Feb;2020 | DL-based Details Relation Extraction NN (DRE-Net) | Online and fast diagnosis of COVID-19 from bacterial pneumonia cases using 3D CT images | CT images | 2 | 94.0 | 96.0 | 93.0 | – | 94.0 |
| [72] | July 2020 | CNN-based Deep Convolutional CAPSNET | COVID-19 diagnosis | X-ray images | 2 | 97.23 | 97.08 | 97.42 | 97.04 | 97.24 |
| 3 | 84.22 | 84.61 | 84.22 | 91.79 | 84.21 | |||||
| [73] | July 2002 | DL-based 2D curvelet transform-CSSA-EfficientNet-B0 | COVID-19 detection | X-ray images | 3 | 99.69 | 99.62 | 99.44 | 99.81 | 99.53 |
| [74] | July 2020 | Deep transfer learning-based customized CNN model | COVID-19 detection | X-ray images | 2 | 97.40 | – | 97.09 | 97.29 | 96.96 |
| [75] | July 2020 | CNN-based Confidence-aware anomaly detection (CAAD) model with EfficientNet | Anomaly detection and classification of viral pneumonia from non-viral pneumonia | X-ray images | 2 | 72.77 | – | 71.70 | 73.83 | – |
| [76] | June 2020 | DL-based CNN Truncated Inception Net | COVID-19 cases detection | X-ray images | 2 | 98.77 | 99.0 | 95.0 | 99.0 | 97.0 |
| [77] | June 2020 | Deep CNN based Inception V3 model | COVID-19 classification using transfer learning and data augmentation | X-ray images | 3 | 98.0 | – | – | – | – |
| [47] | June 2020 | VGG-16 | Pneumonia detection, COVID-19 identification and localization | X-ray images | 3 | 97.0 | – | 92.0 | 96.0 | 92.0 |
| [78] | June 2020 | Various pre-trained DL techniques (AlexNet, DenseNet201, RestNet18, and Deep CNN Sgdm-SqueezNet) | COVID-19 detection using image augmentation | X-ray images | 2 | 98.3 | 100.0 | 96.7 | 100 | 98.3 |
| 3 | 98.3 | 100.0 | 96.7 | 99 | 98.3 | |||||
| [79] | June 2020 | Pretrained Xception-based Deep CNN CoroNet | COVID-19 detection | X-ray images | 2 | 99.0 | 98.3 | 99.3 | 98.6 | 98.5 |
| 3 | 95.0 | 95.0 | 96.9 | 97.5 | 95.6 | |||||
| 4 | 89.6 | 90.0 | 89.9 | 96.4 | 89.8 | |||||
| [80] | June 2020 | Transferable multi-receptive feature optimizer with Deep CNN-based CovXNet | COVID-19 detection with other pneumonias | X-ray images | 2 | 98.1 | 98.0 | 98.5 | 97.9 | 98.3 |
| 3 | 95.1 | 94.9 | 96.1 | 94.3 | 95.5 | |||||
| 4 | 91.70 | 92.90 | 92.10 | 93.60 | 92.60 | |||||
| [81] | June 2020 | Customized CNN and pre-trained ImageNet models (VGG16, VGG-19, Inception-V3, etc.) | COVID-19 screening by learning modality-specific features | X-ray images | 3 | 99.01 | 99.01 | 99.01 | – | 99.01 |
| [82] | May 2020 | CNN-based MobileNet v2 | Extracting COVID‑19 Biomarkers and classification of pulmonary diseases | X-ray images | 2 | 99.18 | – | 97.36 | 99.42 | – |
| 7 | 87.66 | |||||||||
| [83] | May 2020 | Concatenated Xception and ResNet50V2 Networks | COVID-19 cases identification from normal and other pneumonia infected patients’ X-rays | X-ray images | 3 | 91.4 | 72.8 | 87.3 | 94.2 | – |
| [84] | May 2020 | Weakly labeled data augmentation with different deep learning models | COVID-19 detection | X-ray images | 2 | 99.26 | – | – | – | – |
| [85] | May 2020 | Modified InceptionV3 model | COVID-19 screening | X-ray images | 4 | 76 | – | 93.0 | 91.8 | – |
| [86] | May 2020 | CNN-based COVID-Net | COVID-19 classification from normal and other pneumonia cases | X-ray images | 3 | 93.30 | 98.90 | 91.0 | – | – |
| [87] | April 2020 | CNN-based COVID-CAPS | COVID-19 identification | X-ray images | 5 | 98.30 | – | 80.0 | 98.60 | – |
| [88] | April 2020 | Resnet50 and VGG16 plus Customized CNN | Diagnosing COVID-19 | X-rays images | 2 | 91.24 | – | 78.79 | 93.12 | – |
| [89] | April 2020 | Tailored CNN models (ResNet34, ResNet50, DenseNet169, VGG-19, Inception ResNetV2, and RNN-LSTM architectures) | COVID-19 screening, detection and infection evaluation | X-ray images | 3 | 84 | – | – | – | – |
| [90] | April 2020 | ResNet-50 | COVID-19 diagnosis from viral pneumonia | X-ray images | 4 | 99.0 | 99.0 | 99.80 | – | 99.80 |
| [91] | April 2020 | GAN with fine-tuned deep transfer models (AlexNet, GoogLeNet, Squeeznet and ResNet18) | Effects of data augmentation on COVID-19 detection from x-rays images dataset | X-ray images | 2 | 99.00 | 98.97 | 98.97 | – | 98.97 |
| [92] | April 2020 | Light weight Deep Neural Network (DenseNet-121, SqueezeNet, MobileNetV2) | On-device COVID-19 screening using X-rays and noisy snapshots of chest x-rays | X-rays Noisy snapshots | 2 | 89.7 | – | – | – | – |
| X-ray images | 2 | 93.50 | – | – | – | – | ||||
| [93] | April 2020 | GAN with deep transfer models (Alexnet, Googlenet, and Restnet18) | COVID-19 detection | X-ray images | 2 | 100 | 100 | 100 | – | 100 |
| 3 | 85.2 | 85.2 | 85.2 | – | 85.2 | |||||
| 4 | 80.6 | 84.2 | 80.6 | – | 82.3 | |||||
| [94] | April 2020 | Shallow light-weight CNN-tailored model | COVID-19 screening | X-ray images | 2 | 96.92 | 100 | 94.20 | 100 | 97.01 |
| [95] | April 2020 | CNN-based DarkCovidNet model | COVID-19 classification | X-ray images | 3 | 98.08 | 98.03 | 95.13 | 95.30 | 96.51 |
| [96] | April 2020 | Fine-tuned Deep Bayes-SqueezeNet-based COVIDiagnosis-Net | COVID-19 Diagnosis using augmented dataset | X-ray images | 3 | 98.26 | – | – | 99.13 | 98.25 |
| [97] | March 2020 | CNN-based Decompose, Transfer, and Compose (DeTraC) model | COVID-19 disease recognition from other pneumonia cases | X-ray images | 3 | 95.12 | – | 97.91 | 91.87 | – |
| [48] | March 2020 | Transfer learning based CNN models (VGG19, MobileNet v2, Inception, Xception, Inception ResNet v2) | COVID-19 detection | X-ray images | 2 | 96.78 | – | 98.66 | 96.46 | – |
| [98] | March 2020 | Deep CNN-based COVID-ResNet | COVID-19 Classification from other pneumonia cases | X-ray images | 4 | 96.23 | 96.86 | 96.92 | – | 96.89 |
| [99] | March 2020 | Drop-weights based Bayesian CNN network (BCNN) | Improving classification accuracy for COVID-19 diagnosis from other bacterial, viral pneumonia and healthy cases | X-ray images | 4 | 89.82 | – | – | – | – |
| [100] | March 2020 | CNN-based COVIDX-Net includes seven different architectures (VGG19, DenseNet121, InceptionV3, ResNetV2, Inception-ResNetV2, Xeption, MobileNet2) | To diagnose COVID-19 in X-ray images | X-ray images | 2 | 90.00 | 83 | 100 | – | 91.00 |
| [101] | March 2020 | CNN-based three different models (ResNet50, InceptionV3 and Inception-ResNetV2) | COVID-19 detection | X-ray images | 2 | 98.0 | 100 | 96.0 | 100 | 98.0 |
| [102] | July 2020 | GAN with CNN and ConvLSTM-based deep learning models | COVID-19 infection detection | X-ray and CT images | 2 | 99.0 | 97.7 | 100 | 97.8 | 99.0 |
| [103] | June 2020 | Fine-tuned DL networks (ResNet, Inception-v3, Inception ResNet-v2, DenseNet169, and NASNetLarge) | COVID-19 classification | X-ray and CT images | 2 | 98.0 | 88.0 | 90.0 | 95.0 | 89.0 |
| 3 | 96.0 | 93.0 | 90.0 | 94.0 | 91.0 | |||||
| [104] | May 2020 | Commercialized deep learning-based CAD system | COVID-19 infected patient identification | X-ray images | 2 | – | – | 68.8 | 66.7 | – |
| CT images | 2 | – | – | 81.5 | 72.3 | |||||
| [105] | May 2020 | Seven deep learning models (VGG16, VGG19, DenseNet201, Inception_ResNet_V2, Inception_V3, Resnet50, and MobileNet_V2) | COVID-19 detection and classification | X-ray & CT images | 3 | 92.60 | 93.85 | 82.80 | 97.37 | 87.98 |
| [106] | April 2020 | Multi-task DL methods; NABLA-N (for region segmentation) and Inception Residual Recurrent Convolutional Neural Network | Identification of COVID-19 patients and infected region localization | X-ray images | 2 | 84.67 | ||||
| CT images | 2 | 98.78 | – | – | – | – | ||||
| [107] | April 2020 | Seven pre-trained architectures of CNN (AlexNet, SqueezeNet, GoogleNet, VGG-16, MobileNetV2, ResNet18, ResNet50, DesnseNet201) | Diagnosing COVID-19 disease from normal and other viral and bacterial pneumonia cases | X-ray and CT images | 2 | 98.75 | 96.43 | 100 | 97.50 | 98.18 |
| 3 | 97.20 | 97.67 | 97.50 | 98.51 | 97.50 | |||||
| 4 | 80.95 | 82.52 | 82.20 | 93.48 | 82.23 | |||||
| [108] | March 2020 | CNN and Pre-trained AlexNet | COVID-19 Diagnosis | X-ray images | 2 | 98.0 | – | 100.0 | 96.0 | – |
| CT images | 2 | 94.1 | – | 90.0 | 100.0 | – |
Acc = Accuracy, P = Precision, R = Recall, Sn = Sensitivity, Sp = Specificity, AUC = Area under Curve, CP = Values specifically pertaining to COVID-19 class classification.
Many developing countries and rural areas may not have X-rays or CT scan machines readily available; therefore, researchers have also practiced ML and DL approaches on clinical blood reports to segregate COVID-19 patients. A COVID-19 positive cases predictor based on five distinct ML approaches (namely gradient boosting trees, logistic regression, random forest, neural networks, and SVM) were evaluated by Batista et al. [109]. In this study, blood sample data of 235 adult patients was collected from a hospital in Brazil, out of which 102 patients were confirmed to be COVID-19 positive. The collected dataset was divided into a 70:30 ratio for training and testing and the models were tested under 10-fold cross-validation. Each sample had 15 features including red blood cells, red cell distribution width (RDW), c-reactive protein (CPR), basophils, lymphocytes, leukocytes, platelets, mean corpuscular volume (MCV), eosinophils, hemoglobin, mean corpuscular hemoglobin, monocytes, mean corpuscular hemoglobin concentration, gender and age. From the findings, it was observed that the SVM model produced satisfactory results similar to other state-of-the-art techniques. For the same dataset it achieved an accuracy of 84.7%, sensitivity of 67.7%, specificity of 85.0%, F1-score of 72.4%. Moreover, the SVM approach also exhibited a positive predicted value (PPV), negative predicted value (NPV) and brier score of 77.8%, 77.3% and 16.0% respectively.
Table 4 illustrates other identified COVID-19 detection applications based on ML approaches that use blood sample data.
Table 4.
Machine Learning-Empowered COVID-19 Diagnostic Applications Using Clinical Blood Samples Data.
| Ref | Year | Model/Method Names | Task | Data Type | Classes | Acc | P | Sn | Sp | F1-score |
|---|---|---|---|---|---|---|---|---|---|---|
| [110] | July 2020 | Random Forest and other ML models (Decision Tree, Extremely Randomized Trees, kNN, Logistic Regression, Naïve Bayes, and SVM) | Diagnosis of COVID-19 from routine blood exams’ hematochemical values | Text | 2 | 82.0 | 83.0 | 92.0 | 65.0 | – |
| [111] | July 2020 | Gradient Boosted Decision Tree and other ML models (Decision Tress, Logistic Regression, and Random Forest) | COVD-19 diagnosis using 27 routine laboratory tests | Text | 2 | – | – | 76.1 | 80.8 | – |
| [112] | June 2020 | Supervised ML-based models (Adaboost, Decision Tree, Logistic Regression, Multinomial Naïve Bayes, Random Forest, Stochastic Gradient Boosting, and SVM) with Term Frequency/Inverse Document Frequency feature engineering | COVID-19 classification from other viral pneumonia using clinical reports | Text | 4 | 96.2 | 94.0 | 96.0 | – | 95.0 |
| [109] | April 2020 | SVM and other ML models (Random Forest, LR etc.) | Diagnosis of COVID-19 | Text | 2 | 84.7 | 77.8 | 67.70 | 85.0 | 72.4 |
| [113] | March 2020 | Random Forest | COVID-19 Identification using clinical blood test data, each with 49 parameters. Later, tested on external validation set | Text | 4 | 95.95 | 95.12 | 96.97 | – |
Acc = Accuracy, P = Precision, R = Recall, Sn = Sensitivity, Sp = Specificity, AUC = Area under Curve.
4.2. COVID-19 diagnosis using respiratory and coughing wave data
Another type of COVID-19 diagnostic tool that has been developed is related to respiratory wave data, such as developed in Wang et al. [114]. In this paper, the authors proposed a respiratory pattern classification model that was based on a novel GRU neural network [115], which extends GRU networks with bidirectional and attentional mechanisms (BI-AT-GRU) by using time-series real-world and stimulated respiratory data. The model detects and distinguishes the Tachypnea respiratory pattern, a more rapid respiration symptom that occurs in COVID-19 infected patients, from 6 other viral infection patterns. A novel Respiratory Stimulated Model (RSM) was used to generate stimulated breathing patterns to fill the scarce real-world data. The trained model was evaluated on real-world data captured by a depth camera. The proposed BI-AT-GRU model resulted in precision, recall, f1-score, and accuracy of 94.4%, 95.1%, 94.8% and 94.5% respectively.
Table 5 lists the other identified COVID-19 AI-based applications that utilize respiratory or coughing data.
Table 5.
Machine Learning and Deep Learning-Empowered COVID-19 Diagnostic Applications Using Respiratory Data.
| Ref | Year | Model/Method Names | Task | Modality/Data Type | Classes | Acc | P | Sn | Sp | F1-score |
|---|---|---|---|---|---|---|---|---|---|---|
| [116] | June 2020 | Deep Transfer Learning-based Multi-Class classifier (DTL-MC) | COVID-19 diagnosis from cough samples by examining the dissimilarities of pathomorphological alterations in respiratory system | Coughing/Sound waves | 2 | 92.85 | 91.43 | 94.57 | 91.14 | 92.97 |
| 4 | 92.64 | 89.91 (CP) | 89.14 (CP) | 96.67 (CP) | 89.52 (CP) | |||||
| [117] | June 2020 | CNN-based VGGish model as feature extractor along with SVM and Logistic Regression classifiers | COVID-19 diagnosis from coughing samples | Coughing and breathing/Sound waves | 2 | – | 80.0 | 72.0 | – | – |
| [118] | June 2020 | Bi-Directional Gate Recurrent Unit with attention mechanism | COVID-19 detection by analyzing thermal and corresponding RBG videos | Breathing/Thermal videos | 2 | 83.69 | – | 90.23 | 76.31 | 84.61 |
| [114] | Feb, 2020 | Bidirectional and Attentional Gated Recurrent Unit Neural Network (BI-AT-GRU) | COVID-19 detection | Breathing patterns | 2 | 94.5 | 94.4 | 95.1 | – | 94.8 |
Acc = Accuracy, P = Precision, R = Recall, Sn = Sensitivity, Sp = Specificity, AUC = Area under Curve, CP = Values specifically pertaining to COVID-19 class classification.
4.3. DISEASE severity and survival-mortality assessment models
Outside of just diagnosis of COVID-19, AI-based tools have also been applied to identifying the severity of the disease in a patient, as well as to develop and optimize treatment strategies. For example, in order to attempt to help to reduce the mortality of COVID-19, and to optimize potential treatment strategies, a novel DL-based framework, combined with a multivariate logistic regression model, was proposed by Bai et al. [119]. Multi-layer perceptron (MLP) was used to convert each statistical sample containing 75 clinical data characteristics to a 40-dimensional feature vector. The proposed model was then evaluated using a dataset of 133 patients and achieved an overall accuracy of 89.1% and 95.4% AUC under 5-fold cross-validation repeated 5 times. The researchers found six key risk factors (age, lymphocyte, comorbid with hypertension, albumin, hypersensitive C-reactive protein level, and progressive consolidation in CT images) plus fibrosis in CT as a predictive factor for malignant progression.
Albahri et al. [120] also proposed an ML-based rescue framework that was combined with a novel multi-criteria decision-making (MCDM) model to identify and prioritize severely infected COVID-19 patients who are most suitable for relevant convalescent plasma (CP) transfusion.
Chen et al. [121] developed a fatality rate predictive model for severe SARS-CoV-2 patients based on five distinct ML approaches, namely: elastic net, bagged flexible discriminant analysis (bagged-FDA), logistic regression, random forest and partial least squares regression. The model used data of 183 severely infected COVID-19 patients, out of which 115 survived and found four key features and clinical pointers (age, d-dimer level, lymphocyte count, and high-sensitivity C-reactive protein level) for survival/mortality assessment. Moreover, the developed models were evaluated on an external validation set that consisted of 64 severe COVID-19 confirmed cases, out of which 33 were survivals. The experimental results concluded that the logistic regression model was to be preferred over other ML models due to its high interpretability and simplicity; it obtained sensitivity, specificity and AUC of 89.2%, 68.7% and 89.5% respectively.
Table 6 shows other identified AI-based approaches related to severity and fatality assessment models.
Table 6.
Machine Learning and Deep Learning Approaches to Predict COVID-19 Infected Patient's Survival and Mortality Rate and Disease Severity Assessment.
| Ref | Year | Model/Method Names | Task/Problem | Data Type |
|---|---|---|---|---|
| [122] | July 2020 | Deep transfer learning based VGG16 model | Severity assessment of COVID-19 infection in lungs | Radiography images |
| [123] | July 2020 | Several ML-based approaches (Adaboost Pregressor, Decision Tree, Elastic Net, Huber Regression, Random Forest, SVM and others) | Mortality rate prediction by analysis the impact of atmospheric temperature and humidity on COVID-19 transmission | Textual and Time series |
| [124] | July 2020 | AdaBoost with fine-tuned Random Forest model | Possible outcome (death, recovery) and COVID-19 disease severity prediction | Text |
| [120] | June 2020 | Multi-criteria-decision-method (MCDM) and ML methods | Identifying and prioritizing critical COVID-19 patients for convalescent plasma transfusion | Blood sample Images |
| [125] | June 2020 | Multiple linear regression (Generalised linear model, Conditional inference trees, Penalized binomial regression, and SVM with linear kernel) | COVID-19 diagnosis, volume of disease and severity prediction | CT images and Clinical data |
| [121] | April 2020 | Partial Least Squares Regression, Logistic Regression, Random Forest, Elastic Net, and Bagged Flexible Discriminant Analysis | Mortality prediction among severely ill COIVD-19 patients | Time series |
| [126] | April 2020 | SVM | Recovery prediction of COVID-19 patients | Text |
| [127] | April | SVM | Detection of critical cases from mild symptom COVID patients | Text |
| [119] | March 2020 | Deep Learning based models with Multivariate Logistic Regression network (MLP + LSTM) | Predict COVID-19 mild severity patients | Text & CT images |
| [128] | March 2020 | Random Forest and Linear Regression | Hospital stay prediction of COVID-19 infected patient | – |
| [129] | March 2020 | Random Forest | Identification of risk factors linked with mortality of coronavirus infected persons | Text |
| [130] | March 2020 | Random Forest | Severity assessment of COVID-19 infection | CT images |
| [131] | March 2020 | XGBoost | Mortality survival ratio prediction of severely infected COVID-19 | Time series |
4.4. COVID-19 outbreak forecasting models
One of the first areas where AI was applied to the COVID-19 pandemic was related to the development of outbreak forecasting models, which had the potential to help decision makers understand the potential progression of COVID-19 in their area. One such approach was developed by Kavadi et al. [132] who suggested a partial derivative regression and non-linear machine learning (PDR-NML) model to predict the COVID-19 transmission dynamics in India. The presented model achieved better performance in terms of accuracy and prediction time over linear regression and state-of-the-art AI-based models. It secured an accuracy of 99.7% with a prediction time of 5750 ms for 5000 samples. Another approach was offered by Carrillo-Larco and Castillo-Cara [133] who presented a model based on k-means clustering that categorized countries sharing similar numbers of confirmed SARS-CoV-2 cases. In this study, researchers not only used COVID-19 prevalence data (deaths, confirmed cases etc.), but also considered open access predictors like the prevalence of diseases such as HIV/AIDS, diabetes, and tuberculosis in 156 countries along with their standard health system metrics, air quality, and social-economic parameters such as the gross domestic production.
A third approach was developed by Hu et al. [134], who attempted to develop an AI-based modified auto-encoder (MAE) that modeled multiple public health interventions by using real data to forecast a potential COVID-19 pandemic outbreak in a large geographic region and subsequently calculating the potential impact of interventions to curb the pandemic. The proposed architecture consisted of two single auto-encoders, each having three feedforward neural network layers. The used data pertained to confirmed positive COVID-19 cases and COVID-19 attributed deaths across 152 countries over the period of January 20th to March 16th, 2020. The estimate includes pandemic peak time, end time, and future cases. It concludes that MAE performed better in forecasting daily new cases in China (with an error of 0.00134) when compared with susceptible-exposed-infected-recovered (SEIR) model as used by Sameni [135] .
Beyond conventional ML-based approaches, advanced DL techniques have also been used to estimate future cases. Paul et al. [136] suggested a convolutional LSTM-based multivariate spatiotemporal model to forecast the pandemic outbreak at the world-level. The proposed framework converted the spatial features into groups of temporal/non-temporal component-based 2D images and used data from USA and Italy to train the network. The model performed well for predicting the number of cases over a 5-days period, with a mean absolute percent error (MAPE) of 0.3% and 5.57% for Italy and USA respectively.
Table 7 lists more COVID-19 outbreak forecasting models based on ML and DL techniques
Table 7.
Machine Learning and Deep Learning Approaches for Risk Assessment and COVID-19 Outbreak Prediction.
| Ref | Year | Model/Method Names | Task/Problem | Data Type |
|---|---|---|---|---|
| [137] | July 2020 | Least square-SVM and Autoregressive Integrated Moving Average (ARIMA) | Predicting number of COVID-19 confirmed cases in next one-month | Time series |
| [138] | July 2020 | 5 DL-based methods (Gated Recurrent Units, Long short-term memory (LSTM), Bidirectional LSTM, Recurrent Neural Network, and Variational Auto-Encoders) | Short-term forecasting of recovered and new contaminated cased | Time series |
| [139] | July 2020 | ML-based FbProphet model | COVID-19 epidemic trend prediction | Time series |
| [140] | July 2020 | Artificial Neural Network based adaptive incremental deep learning model | Risk based Population Compartmentalization to reduce the number of deaths, while monitoring, forecast and growth stimulation of COVID-19 disease. | Time series |
| [133] | June 2020 | K-mean with PCA | Clustering countries in groups according to COVID-19 positive cases. | Text |
| [132] | June 2020 | Partial derivative regression and nonlinear ML method (PDR-NML) | COVID-19 pandemic outbreak prediction across globe | Text |
| [141] | June 2020 | ML-based models (SVM, Linear Regression, and Polynomial Regression) | COVID-19 epidemic prediction, transmission rate analysis, and growth rates and migration type analysis. | Text |
| [134] | May 2020 | Modified Auto-Encoders | To estimate pandemic transmission and evaluate interventions and measurements to halt COVID-19 spread | Time series |
| [142] | May 2020 | Unsupervised Self-Organizing Maps | Spatially grouping countries that share similar COVID-19 cases | Time series |
| [143] | May 2020 | ML-based method with Cloud computing | Potential threat and growth prediction of COVID-19 | Time series |
| [144] | April 2020 | Linear Regression with LSTM | Predicting outbreak trends and COVID-19 incidence in Iran. | Time series |
| [145] | April 2020 | Regression tree and Wavelet transform methods | Risk assessment and forecasting COVID-19 outbreak in multiple countries | Time series |
| [146] | April 2020 | SEIR, SIR models and Neural Network | Forecast COVID-19 spread in Italy, South Korea, USA, and Wuhan (China) | Time series |
| [147] | April 2020 | Hybridized DL-based Composite Monte-Carlo (CMC) with Fuzzy rule induction | Forecasting future possibilities w.r.t COVID-19 epidemic | Time series |
| [148] | April 2020 | SEIR and Regression Model | COVID-19 outbreak prediction in India | Time series |
| [149] | April 2020 | Topological Autoencoder (Simplified Soft-supervisied-TA) | Visualization of COVID-19 transmission across globe | Time series |
| [150] | April 2020 | Variational-LSTM autoencoder | Predict COVID-19 pandemic spread across globe | Time series |
| [151] | April 2020 | Distinct ML models (RF, MLP, LSTM-R, LSTM-E, M-LSTM) | Forecast COVID-19 cases in Iran | Text |
| [152] | April 2020 | RNN-GRU(LSTM) and FCNN | Forecasting COVID-19 epidemic curve | Time series |
| [153] | April 2020 | Augmented ARGONet, LASSO, GLEAM Data augmentation (Bootstrap & random Gaussian noise) | COVID-19 outbreak prediction in real time | Text |
| [136] | April 2020 | Conv-LSTM | Estimate COVID-19 | Geospatial data (text and images) |
| [154] | April 2020 | Curve fitting with LSTM | Forecast COVID-19 cases in India and effectiveness of lockdown and social distancing | Time series |
| [155] | April 2020 | Simple and Adjusted SEIR network, and Logistic growth model | Predicting COVID-19 worldwide outbreak | Time series |
| [156] | March 2020 | SEIR | COVID-19 pandemic outbreak transmission and effectiveness of control measures | Time series |
| [157] | March 2020 | MLP, LSTM, Gate Recurrent Unit (GRU) and Deep-CNN-based multiple input model | COVID-19 outbreak prediction in China | Time series |
| [158] | March 2020 | LSTM with customized SEIR | Forecast COVID-19 epidemic curve in China | Time series |
4.5. VIRIONS sequence formation and drug discovery models
A final area where AI is being applied in response to the COVID-19 pandemic is related to the development of potential medications and vaccinations. One such example, related to vaccine development, was proposed by Ong et al. who offered a supervised machine learning reverse vaccinology model (Vaxign-ML) [159] for the reverse vaccinology system Vaxign [160]. In [161], Ong et al. trained five traditional ML algorithms (SVM, RF, k-nearest neighbor, logistic regression, and extreme gradient boosting (XGB) [162] on an extracted protein dataset, which was annotated with physicochemical and biological features, under 5-fold cross-validation. It was found that the XGB model performed well by obtaining F1-score of 94%.
Another approach is offered by Magar et al. [163] who established a machine learning approach for determining COVID-19 inhibitory synthetic antibodies. In this case, graph featurization along with various ML schemes screened the dataset of virus-antibody sequences and revealed 8 stable antibodies as potential COVID-19 inhibitors.
Other identified approaches for the development of potential COVID-19 medications and vaccinations are highlighted in Table 8 .
Table 8.
Machine Learning and Deep Learning Approaches for Drug Discovery and Protein Sequence Detection to Combat COVID-19 Pandemic.
| Ref | Year | Model/Method Names | Task/Problem |
|---|---|---|---|
| [161] | July 2020 | ML-based Vaxign-ML reverse vaccinology tool | To predict SARS-CoV-2 vaccine candidate |
| [164] | May 2020 | Semi-Supervised Variational AutoEncoder and LSTM model | To find Simplified Molecular-Input Line-Entry System (SMILE) fingerprint of molecules for drug discovery |
| [165] | May 2020 | GAN | Designing of non-Cov SARs-CoV-2 drug compound |
| [166] | March 2020 | Pre-trained Deep learning based Molecule Transformer-Drug Target Interaction (MT-DTI) | Identify antiviral drug availability to coop SARS-COV-2 |
| [163] | March 2020 | XGBoost, RF, Multilayer perceptron, SVM and LR | Discover potentially inhibitory antibodies for COVID-19 |
| [167] | March 2020 | Adaptive Network-based Fuzzy Inference System (ANFIS), Multi Layered Perception (MLP) | CRISPR-based nucleic acid detection |
| [168] | Feb, 2020 | GAN | Generate COVID-19 drug compound formation |
5. Discussion
The investigation of this paper reveals several AI-based approaches that have been proposed as potential ways to help, with the COVID-19 pandemic, covering everything from initial diagnoses via image diagnostics up to the presentation of models that help to understand the spread of COVID-19 and identify potential new outbreak areas. By providing this comprehensive overview and identification of the current state-of-the-art, this structured review may help assist medical and research stakeholders currently involved in the COVID-19 response.
When it comes to potential barriers to the use of AI for COVID-19 responses, one of the foremost inaccuracies is related to the failure of forming diagnostic programs that fail differentiate by symptomatic basis. Therefore, in order to attain more accurate and precise prediction models, the patient's history of other existing ailments such as diabetes, heart, liver or other chronic conditions along with his age and gender should also be taken into account. The significance of these factors greatly influences the characteristics of COVID-19 for a specific patient compared with other pneumonia diseases known so far. As many countries around the world are opening up their data related to COVID-19, it becomes possible for anyone to create new AI-based services, and, additionally, if these statistics are provided in an effective way, tools for the prediction of COVID-19 diagnoses and spread could, in theory, be developed.
Another issue regarding the COVID-19 pandemic is urgency. As the government needs high-quality statistical data in almost real-time to understand the current epidemiological situation in their area and to regulate quarantine in that same area, effort should be focused on improving the availability and provision of data first. As at the beginning of the pandemic, data was often not of high quality (though this depends on a large amount on which country's data is being examined) the initially used scientific datasets were often constructed in a quick manner and may not be as precise or adequate as one would ideally like.
While considering the statistical analysis of the COVID-19 disease, the crucial challenge is to secure real-time high-quality data. As this disease is still in a rapid growth phase, the fast nature of data makes it extremely difficult to produce reliable models. Collection of regularly updated region-specific values of COVID-19 is crucial in order to overcome this issue.
A final challenge, and one of the most important, is the lack of understanding of epidemiological theory, beliefs, and knowhow of many AI researchers. This leads to a situation where many of the AI-based systems are created without input from medical professionals, thus limiting their usefulness and reliability. Thus, to this end, it is important that any implemented AI-based system aims to involve medical experts in the design and implementation.
Whilst it does appear to be the case that many AI-based approaches have been proposed, there is a limited real-world implementation of these tools. What this paper has shown, however, is that there is clearly a wide variety of potential methods that could be applied. Moving forward, it is imperative for AI-designers and researchers to work together with medical professionals to create and develop these systems that are applicable to real-world datasets and relevant to the work of the given stakeholder group (e.g. policymakers or health care workers). AI is not a cure-all that can fix the COVID-19 crisis, but it does have the potential to augment a given person's ability to handle and manage the crisis, thus, potentially, limiting the spread of the virus and leading to potentially better outcomes for infected patients.
6. Conclusion
As COVID-19 has permeated throughout the world, there is no one who has not felt its impact in some way. Thus, scientific and medical researchers are working rapidly to find a viable cure against the disease. One tool that may well help aid this effort is artificial intelligence (AI) by assisting front-line workers in numerous ways.
This paper has initially presented the details of the COVID-19 viruses including taxonomy, signs and symptoms, cause of spread, affected body organ, and hazardous impact on human society over the last century. Secondly, we mentioned the structural genome and origin of COVID-19 infectious virus, compared it with other Coroviridae family viruses, its transmission behavior and impact on global health. We presented an overview of potential use cases and AI-based implementations currently being developed and trialed. For instance, technological experts have developed AI-based programs for the rapid analysis, scanning and diagnosis of the coronavirus through pneumonia radiographic images or clinical blood sample data. These approaches offer an additional way to detect COVID-19, in addition to the more commonly used PCR test.
As testing capabilities continue to improve, AI-based tools become more useful to assist in clinical settings rather than to replace medical professionals. However, where AI is likely to see an increased use and benefit moving forward in the future, especially as more and higher quality data becomes available, is its use in predicting potential new areas of outbreak and forecasting the spread of COVID-19. These models are based on parameters such as the number of positive patients in different regions of a city and tracking their movements to anticipate the potential spread through contact. For example, it could be possible to apply an AI-based model to data gathered via a contact tracing application or sewer COVID-19 prevalence data, though other data could also be used, to better predict the scale and size of a new outbreak. The results thus obtained could help to manage and implement precautionary actions by defining guidelines in pandemic affected areas. It is important that AI-based tools are relevant, accurate, and impactful. In order to do this, cooperation is needed between AI-based researchers, medical professionals, and governmental agencies. Thus, further research should identify real-world empirical examples of AI being used on real-world data. This paper has made initial steps in gathering and highlighting the current state-of-the-art, but it does not differentiate between examples working “in the wild” and those that are done under laboratory conditions. So, while in theory, these approaches may well be useful, until they are evaluated within an applied context it is hard to quantify their level of usefulness, in fact, it may well be the case that AI-based tools actually inhibit effectiveness if they discreetly contain some level of bias, or replace the decision making capability of a more competent human actor.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M, Malik Y.S, Singh R, Michalak I, Sah R, Bonilla-Aldana D.K, Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Veterinary Quarterly. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rantsios A.T. Elsevier; 2016. Zoonoses. in: encyclopedia of food and health; pp. 645–653. [DOI] [Google Scholar]
- 4.Orbann C., Sattenspiel L., Miller E., Dimka J. Defining epidemics in computer simulation models: how do definitions influence conclusions? Epidemics. 2017;19:24–32. doi: 10.1016/j.epidem.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Orbann C., Sattenspiel L., Miller E., Dimka J. Defining epidemics in computer simulation models: how do definitions influence conclusions? Epidemics. 2017;19:24–32. doi: 10.1016/j.epidem.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Henderson D.A. Prometheus Books; New York: 2009. Smallpox — the death of a disease. [Google Scholar]
- 7.Spreeuwenberg P., Kroneman M., Paget J. Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic. Am. J. Epidemiol. 2018;187:2561–2567. doi: 10.1093/aje/kwy191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization: 10 facts on Rabies, https://www.who.int/features/factfiles/rabies/en/, last accessed 2020/06/30.
- 9.World Health Organization: HIV AIDS, https://www.who.int/news-room/fact-sheets/detail/hiv-aids, last accessed 2020/06/30.
- 10.Avšič-Županc T., Saksida A., Korva M. Hantavirus infections. Clinical Microbiology and Infection. 2019;21:e6–e16. doi: 10.1111/1469-0691.12291. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization: Dengue and severe dengue, https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue, last accessed 2020/06/30.
- 12.World Health Organization: Marburg virus disease, https://www.who.int/en/news-room/fact-sheets/detail/marburg-virus-disease, last accessed 2020/06/30.
- 13.Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M., Franco M.A., Greenberg H.B., O'Ryan M., Kang G., Desselberger U., Estes M.K. Rotavirus infection. Nature Reviews Disease Primers. 2017;3:17083. doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization: Ebola virus disease, https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease, last accessed 2020/06/30.
- 15.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. New England Journal of Medicine. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 16.John Hopkins University: COVID-19 Maps - John Hopkins Coronavirus Research Center, https://coronavirus.jhu.edu/map.html, last accessed 2020/07/18.
- 17.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. New England Journal of Medicine. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. 2020;7:1–19. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 20.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of Chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: a Report of 1014 Cases. Radiology. 2020;2019 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. ACM International Conference Proceeding Series. 2014 doi: 10.1145/2601248.2601268. [DOI] [Google Scholar]
- 26.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. International Journal of Environmental Research and Public Health. 2020;17:1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuite A.R., Ng V., Rees E., Fisman D. Estimation of COVID-19 outbreak size in Italy. The Lancet Infectious Diseases. 2020;20:537. doi: 10.1016/S1473-3099(20)30227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO: Coronavirus (COVID-19) events as they happen, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen, (2020).
- 33.Wang L., Gao Y.-.H., Lou L.-.L., Zhang G.-.J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. European Respiratory Journal. 2020;55 doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Organization, W.H.: Coronavirus Disease (COVID-19): situation Report - 199 (6 August 2020). (2020).
- 35.Sethy P.K., Behera S.K., Ratha P.K., Biswas P. Detection of coronavirus disease (COVID-19) based on deep features and support vector machine. International Journal of Mathematical, Engineering and Management Sciences. 2020;5:643–651. doi: 10.20944/preprints202003.0300.v1. [DOI] [Google Scholar]
- 36.Shi, F., Xia, L., Shan, F., Wu, D., Wei, Y., Yuan, H., Jiang, H., Gao, Y., Sui, H., Shen, D.: Large-Scale Screening of COVID-19 from Community Acquired Pneumonia using Infection Size-Aware Classification. (2020). [DOI] [PubMed]
- 37.Milletari F., Navab N., Ahmadi S.-.A. 2016 Fourth international conference on 3D vision (3DV) IEEE; 2016. V-Net: fully Convolutional Neural Networks for Volumetric Medical Image Segmentation; pp. 565–571. [DOI] [Google Scholar]
- 38.Toğaçar M., Ergen B., Cömert Z. COVID-19 detection using deep learning models to exploit Social Mimic Optimization and structured chest X-ray images using fuzzy color and stacking approaches. Computers in Biology and Medicine. 2020;121 doi: 10.1016/j.compbiomed.2020.103805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassanien A.E., Mahdy L.N., Ezzat K.A., Elmousalami H.H., Ella H.A. Automatic X-ray COVID-19 Lung Image Classification System based on Multi-Level Thresholding and Support Vector Machine. medRxiv. 2020 doi: 10.1101/2020.03.30.20047787. 03.30.20047787 (2020) [DOI] [Google Scholar]
- 40.Kumar R., Arora R., Bansal V., Sahayasheela V.J., Buckchash H., Imran J., Narayanan N., Pandian G.N., Raman B. Accurate Prediction of COVID-19 using Chest X-Ray Images through Deep Feature Learning model with SMOTE and Machine Learning Classifiers. medRxiv. 2020 doi: 10.1101/2020.04.13.20063461. 2020.04.13.20063461. [DOI] [Google Scholar]
- 41.Pereira R.M., Bertolini D., Teixeira L.O., Silla C.N., Costa Y.M.G. COVID-19 identification in chest X-ray images on flat and hierarchical classification scenarios. Comput Methods Programs Biomed. 2020;194 doi: 10.1016/j.cmpb.2020.105532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuncer T., Dogan S., Ozyurt F. An automated Residual Exemplar Local Binary Pattern and iterative ReliefF based corona detection method using lung X-ray image. Chemometrics and Intelligent Laboratory Systems. 2020;203 doi: 10.1016/j.chemolab.2020.104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardakani, A.A., Acharya, U.R.: Computed Tomography COVIDiag : a clinical CAD system to diagnose COVID-19 pneumonia based on CT findings. 1–24 (2020). [DOI] [PMC free article] [PubMed]
- 44.Al-karawi D., Al-Zaidi S., Polus N., Jassim S. Machine Learning Analysis of Chest CT Scan Images as a Complementary Digital Test of Coronavirus (COVID-19) Patients. medRxiv. 2020 doi: 10.1101/2020.04.13.20063479. 2020.04.13.20063479. [DOI] [Google Scholar]
- 45.Ozkaya, U., Ozturk, S., Barstugan, M.: Coronavirus (COVID-19) Classification using Deep Features Fusion and Ranking Technique. 1–13 (2020).
- 46.Barstugan, M., Ozkaya, U., Ozturk, S.: Coronavirus (COVID-19) Classification using CT Images by Machine Learning Methods. 1–10 (2020).
- 47.Brunese L., Mercaldo F., Reginelli A., Santone A. Explainable Deep Learning for Pulmonary Disease and Coronavirus COVID-19 Detection from X-rays. Comput Methods Programs Biomed. 2020;196 doi: 10.1016/j.cmpb.2020.105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apostolopoulos I.D., Mpesiana T.A. Covid-19: automatic detection from X-ray images utilizing transfer learning with convolutional neural networks. Physical and Engineering Sciences in Medicine. 2020;43:635–640. doi: 10.1007/s13246-020-00865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal A., Gianchandani N., Singh D., Kumar V., Kaur M. Classification of the COVID-19 infected patients using DenseNet201 based deep transfer learning. Journal of Biomolecular Structure and Dynamics. 2020;0:1–8. doi: 10.1080/07391102.2020.1788642. [DOI] [PubMed] [Google Scholar]
- 50.Xu X., Jiang X., Ma C., Du P., Li X., Lv S., Yu L., Ni Q., Chen Y., Su J., Lang G., Li Y., Zhao H., Liu J., Xu K., Ruan L., Sheng J., Qiu Y., Wu W., Liang T., Li L. A Deep Learning System to Screen Novel Coronavirus Disease 2019 Pneumonia. Engineering. 2020 doi: 10.1016/j.eng.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S. Drawing insights from COVID-19-infected patients using CT scan images and machine learning techniques: a study on 200 patients. Environmental Science and Pollution Research. 2020 doi: 10.1007/s11356-020-10133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin C., Chen W., Cao Y., Xu Z., Zhang X., Deng L., Zheng C., Zhou J., Shi H., Feng J. Development and Evaluation of an AI System for COVID-19 Diagnosis. medRxiv. 2020 doi: 10.1101/2020.03.20.20039834. 2020.03.20.20039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S., Zha Y., Li W., Wu Q., Li X., Niu M., Wang M., Qiu X., Li H., Yu H., Gong W., Bai Y., Li L., Zhu Y., Wang L., Tian J. A Fully Automatic Deep Learning System for COVID-19 Diagnostic and Prognostic Analysis. European Respiratory Journal. 2020 doi: 10.1183/13993003.00775-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X., He X., Zhao J., Zhang Y., Zhang S., Xie P. COVID-CT-Dataset: a CT Scan Dataset about COVID-19. ArXiv e-prints. 2020:1–11. [Google Scholar]
- 55.Pu J., Leader J., Bandos A., Shi J., Du P., Yu J. Any unique image biomarkers associated with COVID-19? Eur Radiol. 2020 doi: 10.1007/s00330-020-06956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, S., Georgescu, B., Xu, Z., Yoo, Y., Chabin, G., Chaganti, S., Grbic, S., Piat, S., Teixeira, B., Balachandran, A., RS, V., Re, T., Comaniciu, D.: 3D Tomographic Pattern Synthesis for Enhancing the Quantification of COVID-19. 1–23 (2020).
- 57.Mei X., Lee H.C., Diao K.yue, Huang M., Lin B., Liu C., Xie Z., Ma Y., Robson P.M., Chung M., Bernheim A., Mani V., Calcagno C., Li K., Li S., Shan H., Lv J., Zhao T., Xia J., Long Q., Steinberger S., Jacobi A., Deyer T., Luksza M., Liu F., Little B.P., Fayad Z.A., Yang Y. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nature Medicine. 2020 doi: 10.1038/s41591-020-0931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pathak Y., Shukla P.K., Tiwari A., Stalin S., Singh S. Deep Transfer Learning Based Classification Model for COVID-19 Disease. Irbm. 2020;1:1–6. doi: 10.1016/j.irbm.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang K., Liu X., Shen J., Li Z., Sang Y., Wu X. Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.045. 1423–33.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amyar A., Modzelewski R., Ruan S. Multi-task Deep Learning Based CT Imaging Analysis For COVID-19: classification and Segmentation. medRxiv. 2020 doi: 10.1101/2020.04.16.20064709. 2020.04.16.20064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bai H.X., Wang R., Xiong Z., Hsieh B., Chang K., Halsey K. AI Augmentation of Radiologist Performance in Distinguishing COVID-19 from Pneumonia of Other Etiology on Chest CT. Radiology. 2020;78 doi: 10.1148/radiol.2020201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butt C., Gill J., Chun D., Babu B.A. Deep learning system to screen coronavirus disease 2019 pneumonia. Applied Intelligence. 2020 doi: 10.1007/s10489-020-01714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akif Cifci M. Deep Learning Model for Diagnosis of Corona Virus Disease from CT Images. International Journal of Scientific & Engineering Research. 2020;11:273–278. [Google Scholar]
- 64.Singh D., Kumar V., Vaishali, Kaur M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution-based convolutional neural networks. European Journal of Clinical Microbiology & Infectious diseases : Official Publication of European Society of Clinical Microbiology. 2020 doi: 10.1007/s10096-020-03901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, S., Kang, B., Ma, J., Zeng, X., Xiao, M., Guo, J., Cai, M., Yang, J., Li, Y., Meng, X., Xu, B., MedRxiv, M.C.-, 2020, U., Cai, M., Yang, J., Li, Y., Meng, X., Xu, B.: a deep learning algorithm using CT images to screen for corona virus disease (COVID-19). medRxiv. 2020.02.14.20023028 (2020). doi: 10.1101/2020.02.14.20023028. [DOI] [PMC free article] [PubMed]
- 66.Farid A.A., Selim G.I., Awad H., Khater A. A Novel Approach of CT Images Feature Analysis and Prediction to Screen for Corona Virus Disease (COVID-19) International Journal of Scientific & Engineering Research. 2020;11 doi: 10.14299/ijser.2020.03.02. [DOI] [Google Scholar]
- 67.Fu M., Yi S.-.L., Zeng Y., Ye F., Li Y., Dong X., Ren Y.-.D., Luo L., Pan J.-.S., Zhang Q. Deep Learning-Based Recognizing COVID-19 and other Common Infectious Diseases of the Lung by Chest CT Scan Images. medRxiv. 2020 doi: 10.1101/2020.03.28.20046045. [DOI] [Google Scholar]
- 68.Jin S., Wang B., Xu H., Luo C., Wei L., Zhao W. AI-assisted CT imaging analysis for COVID-19 screening: building and deploying a medical AI system in four weeks. medRxiv. 2020 doi: 10.1101/2020.03.19.20039354. 03.19.20039354 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., Lu Y., Fang Z., Song Q., Cao K., Liu D., Wang G., Xu Q., Fang X., Zhang S., Xia J., Xia J. Artificial Intelligence Distinguishes COVID-19 from Community Acquired Pneumonia on Chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C., Deng X., Fu Q., Zhou Q., Feng J., Ma H., Liu W., Wang X. Deep Learning-based Detection for COVID-19 from Chest CT using Weak Label. medRxiv. 2020 doi: 10.1101/2020.03.12.20027185. 2020.03.12.20027185. [DOI] [Google Scholar]
- 71.Song Y., Zheng S., Li L., Zhang X., Zhang X., Huang Z., Chen J., Zhao H., Jie Y., Wang R., Chong Y., Shen J., Zha Y., Yang Y. Deep learning Enables Accurate Diagnosis of Novel Coronavirus (COVID-19) with CT images. medRxiv. 2020 doi: 10.1101/2020.02.23.20026930. 2020.02.23.20026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toraman S., Alakus T.B., Turkoglu I. Convolutional capsnet: a novel artificial neural network approach to detect COVID-19 disease from X-ray images using capsule networks. Chaos, Solitons & Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altan A., Karasu S. Recognition of COVID-19 disease from X-ray images by hybrid model consisting of 2D curvelet transform, chaotic salp swarm algorithm and deep learning technique. Chaos, Solitons and Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narayan Das N, Kumar N, Kaur M, Kumar V, Singh D. Automated Deep Transfer Learning-Based Approach for Detection of COVID-19 Infection in Chest X-rays. Irbm. 2020;1:1–6. doi: 10.1016/j.irbm.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, J., Xie, Y., Liao, Z., Pang, G., Verjans, J., Li, W., Sun, Z., He, J., Yi Li, Chunhua Shen, and Y.X.: Viral Pneumonia Screening on Chest X-ray Images Using Confidence-Aware Anomaly Detection. ArXiv e-prints. 1–11 (2020). [DOI] [PMC free article] [PubMed]
- 76.Das D., Santosh K.C., Pal U. Truncated inception net: COVID-19 outbreak screening using chest X-rays. Physical and Engineering Sciences in Medicine. 2020 doi: 10.1007/s13246-020-00888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asif S., Wenhui Y., Jin H., Tao Y., Jinhai S. Classification of COVID-19 from Chest X-ray images using Deep Convolutional Neural Networks. medRxiv. 2020 doi: 10.1101/2020.05.01.20088211. 05.01.20088211 (2020) [DOI] [Google Scholar]
- 78.Chowdhury, M.E.H., Rahman, T., Khandakar, A., Mazhar, R., Kadir, M.A., Mahbub, Z.Bin, Islam, K.R., Khan, M.S., Iqbal, A., Al-Emadi, N., Reaz, M.B.I., Islam, T.I.: Can AI help in screening Viral and COVID-19 pneumonia? XX, (2020).
- 79.Khan A.I., Shah J.L., Bhat M.M. CoroNet: a deep neural network for detection and diagnosis of COVID-19 from chest x-ray images. Comput Methods Programs Biomed. 2020;196 doi: 10.1016/j.cmpb.2020.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahmud T., Rahman M.A., Fattah S.A. CovXNet: a multi-dilation convolutional neural network for automatic COVID-19 and other pneumonia detection from chest X-ray images with transferable multi-receptive feature optimization. Comput. Biol. Med. 2020;122 doi: 10.1016/j.compbiomed.2020.103869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajaraman S., Siegelman J., Alderson P.O., Folio L.S., Folio L.R., Antani S.K. Iteratively Pruned Deep Learning Ensembles for COVID-19 Detection in Chest X-rays. IEEE Access. 2020:1–11. doi: 10.1109/ACCESS.2020.3003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apostolopoulos I.D., Aznaouridis S.I., Tzani M.A. Extracting Possibly Representative COVID-19 Biomarkers from X-ray Images with Deep Learning Approach and Image Data Related to Pulmonary Diseases. J Med Biol Eng. 2020;40:462–469. doi: 10.1007/s40846-020-00529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahimzadeh M., Attar A. A modified deep convolutional neural network for detecting COVID-19 and pneumonia from chest X-ray images based on the concatenation of Xception and ResNet50V2. Informatics in Medicine Unlocked. 2020;19 doi: 10.1016/j.imu.2020.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajaraman S., Antani S. Weakly Labeled Data Augmentation for Deep Learning: a Study on COVID-19 Detection in Chest X-Rays. Diagnostics. 2020;10:358. doi: 10.3390/diagnostics10060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsiknakis N., Trivizakis E., Vassalou E., Papadakis G., Spandidos D., Tsatsakis A., Sánchez-García J., López-González R., Papanikolaou N., Karantanas A., Marias K. Interpretable artificial intelligence framework for COVID-19 screening on chest X-rays. Experimental and Therapeutic Medicine. 2020:727–735. doi: 10.3892/etm.2020.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, L., Wong, A.: COVID-Net: a Tailored Deep Convolutional Neural Network Design for Detection of COVID-19 Cases from Chest X-Ray Images. (2020). [DOI] [PMC free article] [PubMed]
- 87.Afshar, P., Heidarian, S., Naderkhani, F., Oikonomou, A., Plataniotis, K.N., Mohammadi, A.: COVID-CAPS: a Capsule Network-based Framework for Identification of COVID-19 cases from X-ray Images. ArXiv e-prints. 1–5 (2020). [DOI] [PMC free article] [PubMed]
- 88.Hall, L.O., Paul, R., Goldgof, D.B., Goldgof, G.M.: Finding Covid-19 from Chest X-rays using Deep Learning on a Small Dataset. 1–8 (2020).
- 89.Hammoudi, K., Benhabiles, H., Melkemi, M., Dornaika, F., Arganda-Carreras, I., Collard, D., Scherpereel, A.: Deep Learning on Chest X-ray Images to Detect and Evaluate Pneumonia Cases at the Era of COVID-19. 1–6 (2020). [DOI] [PMC free article] [PubMed]
- 90.Gueguim Kana E.B, Zebaze Kana M.G., Donfack Kana A.F., Azanfack Kenfack R.H. A web-based Diagnostic Tool for COVID-19 Using Machine Learning on Chest Radiographs (CXR) medRxiv. 2020 doi: 10.1101/2020.04.21.20063263. 04.21.20063263 (2020) [DOI] [Google Scholar]
- 91.Khalifa, N.E.M., Taha, M.H.N., Hassanien, A.E., Elghamrawy, S.: Detection of Coronavirus (COVID-19) Associated Pneumonia based on Generative Adversarial Networks and a Fine-Tuned Deep Transfer Learning Model using Chest X-ray Dataset. 1–15 (2020).
- 92.Li, X., Li, C., Zhu, D.: COVID-MobileXpert: on-Device COVID-19 Screening using Snapshots of Chest X-Ray. ArXiv e-prints. (2020).
- 93.Loey M., Smarandache F., Khalifa N.E.M. Within the lack of chest COVID-19 X-ray dataset: a novel detection model based on GAN and deep transfer learning. Symmetry. 2020;12 doi: 10.3390/SYM12040651. [DOI] [Google Scholar]
- 94.Mukherjee, H., Ghosh, S., Dhar, A., Obaidullah, S.: Shallow Convolutional Neural Network for COVID-19 Outbreak Screening using Chest. (2020). doi: 10.36227/techrxiv.12156522.v1. [DOI] [PMC free article] [PubMed]
- 95.Ozturk T., Talo M., Yildirim E.A., Baloglu U.B., Yildirim O., Rajendra Acharya U. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020;121 doi: 10.1016/j.compbiomed.2020.103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ucar F., Korkmaz D. COVIDiagnosis-Net: deep Bayes-SqueezeNet based diagnosis of the coronavirus disease 2019 (COVID-19) from X-ray images. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abbas A., Abdelsamea M.M., Gaber M.M. Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network. medRxiv. 2020 doi: 10.1101/2020.03.30.20047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farooq, M., Hafeez, A.: COVID-ResNet: a Deep Learning Framework for Screening of COVID19 from Radiographs. ArXiv e-prints. (2020).
- 99.Ghoshal, B., Tucker, A.: Estimating Uncertainty and Interpretability in Deep Learning for Coronavirus (COVID-19) Detection. 1–14 (2020).
- 100.Hemdan, E.E.-D., Shouman, M.A., Karar, M.E.: COVIDX-Net: a Framework of Deep Learning Classifiers to Diagnose COVID-19 in X-Ray Images. (2020).
- 101.Narin, A., Kaya, C., Pamuk, Z.: Automatic Detection of Coronavirus Disease (COVID-19) Using X-ray Images and Deep Convolutional Neural Networks. (2020). [DOI] [PMC free article] [PubMed]
- 102.Sedik A., Iliyasu A.M., Abd El-Rahiem B, Abdel Samea M.E., Abdel-Raheem A, Hammad M. Deploying Machine and Deep Learning Models for Efficient Data-Augmented Detection of COVID-19 Infections. Viruses. 2020;12:769. doi: 10.3390/v12070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Punn, N.S., Agarwal, S.: Automated diagnosis of COVID-19 with limited posteroanterior chest X-ray images using fine-tuned deep neural networks. ArXiv e-prints. (2020). [DOI] [PMC free article] [PubMed]
- 104.Hwang E.J., Kim H., Yoon S.H., Goo J.M., Park C.M. Implementation of a Deep Learning-Based Computer-Aided Detection System for the Interpretation of Chest Radiographys in Patients Suspected for COVID-19. Korean J Radiol. 2020;21:1–11. doi: 10.3348/kjr.2020.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El Asnaoui K, Chawki Y. Using X-ray images and deep learning for automated detection of coronavirus disease. Journal of Biomolecular Structure and Dynamics. 2020;0:1–12. doi: 10.1080/07391102.2020.1767212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alom, M.Z., Rahman, M.M.S., Nasrin, M.S., Taha, T.M., Asari, V.K.: COVID_MTNet: COVID-19 Detection with Multi-Task Deep Learning Approaches. (2020).
- 107.Razzak, I., Naz, S., Rehman, A., Khan, A., Zaib, A.: Improving Coronavirus (COVID-19) Diagnosis using Deep Transfer Learning. medRxiv. 1–16 (2020). doi: 10.1101/2020.04.11.20054643. [DOI]
- 108.Maghdid, H.S., Asaad, A.T., Ghafoor, K.Z., Sadiq, A.S., Khan, M.K.: Diagnosing COVID-19 Pneumonia from X-Ray and CT Images using Deep Learning and Transfer Learning Algorithms. ArXiv e-prints. 1–8 (2020).
- 109.Batista A.F., de M., Miraglia J.L., Donato T.H.R., Filho A.D.P.C. COVID-19 diagnosis prediction in emergency care patients: a machine learning approach. medRxiv. 2020 doi: 10.1101/2020.04.04.20052092. 04.04.20052092 (2020) [DOI] [Google Scholar]
- 110.Brinati D., Campagner A., Ferrari D., Locatelli M., Banfi G., Cabitza F. Detection of COVID-19 Infection from Routine Blood Exams with Machine Learning: a Feasibility Study. J Med Syst. 2020;44:135. doi: 10.1007/s10916-020-01597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang H.S., Vasovic L.V., Steel P., Chadburn A., Hou Y., Racine-Brzostek S.E., Cushing M., Loda M., Kaushal R., Zhao Z., Wang F. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. medRxiv. 2020 doi: 10.1101/2020.06.17.20133892. 06.17.20133892 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khanday A.M.U.D., Rabani S.T., Khan Q.R., Rouf N., Mohi Ud Din M. Machine learning based approaches for detecting COVID-19 using clinical text data. International Journal of Information Technology. 2020 doi: 10.1007/s41870-020-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J., Zhang P., Zhang L., Meng W., Li J., Tong C., Li Y., Cai J., Yang Z., Zhu J., Zhao M., Huang H., Xie X., Li S. Rapid and accurate identification of COVID-19 infection through machine learning based on clinical available blood test results. medRxiv. 2020 doi: 10.1101/2020.04.02.20051136. 04.02.20051136 (2020) [DOI] [Google Scholar]
- 114.Wang, Y., Hu, M., Li, Q., Zhang, X.-.P., Zhai, G., Yao, N.: Abnormal respiratory patterns classifier may contribute to large-scale screening of people infected with COVID-19 in an accurate and unobtrusive manner. (2020).
- 115.Cho K., van Merrienboer B., Gulcehre C., Bahdanau D., Bougares F., Schwenk H., Bengio Y. Proceedings of the 2014 Conference on Empirical Methods in Natural Language Processing (EMNLP) Association for Computational Linguistics; Stroudsburg, PA, USA: 2014. Learning Phrase Representations using RNN Encoder–Decoder for Statistical Machine Translation; pp. 1724–1734. [DOI] [Google Scholar]
- 116.Imran A., Posokhova I., Qureshi H.N., Masood U., Riaz S., Ali K., John C.N., Hussain I., Nabeel M. AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an app. Informatics in Medicine Unlocked. 2020 doi: 10.1016/j.imu.2020.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown C., Chauhan J., Grammenos A., Han J., Hasthanasombat A., Spathis D., Xia T., Cicuta P., Mascolo C. 26th SIGKDD Conference on Knowledge Discovery and Data Mining. 2020. Exploring Automatic Diagnosis of COVID-19 from Crowdsourced Respiratory Sound Data. [DOI] [Google Scholar]
- 118.Jiang Z., Hu M., Gao Z., Fan L., Dai R., Pan Y., Tang W., Zhai G., Lu Y. Detection of Respiratory Infections using RGB-infrared sensors on Portable Device. IEEE Sensors Journal. 2020;XX:1. doi: 10.1109/JSEN.2020.3004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bai X., Fang C., Zhou Y., Bai S., Liu Z., Xia L., Chen Q., Xu Y., Xia T., Gong S., Xie X., Song D., Du R., Zhou C., Chen C., Nie D., Qin L., Chen W. Predicting COVID-19 Malignant Progression with AI Techniques. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3557984. [DOI] [Google Scholar]
- 120.Albahri O.S., Al-Obaidi J.R., Zaidan A.A., Albahri A.S., Zaidan B.B., Salih M.M. Helping doctors hasten COVID-19 treatment: towards a rescue framework for the transfusion of best convalescent plasma to the most critical patients based on biological requirements via ml and novel MCDM methods. Comput Methods Programs Biomed. 2020;196 doi: 10.1016/j.cmpb.2020.105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X., Liu Z. Early prediction of mortality risk among severe COVID-19 patients using machine learning. medRxiv. 2020 doi: 10.1101/2020.04.13.20064329. 2020.04.13.20064329. [DOI] [Google Scholar]
- 122.Zhu J., Shen B., Abbasi A., Hoshmand-Kochi M., Li H., Duong T.Q. Deep transfer learning artificial intelligence accurately stages COVID-19 lung disease severity on portable chest radiographs. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0236621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malki Z., Atlam E.S., Hassanien A.E., Dagnew G., Elhosseini M.A., Gad I. Association between weather data and COVID-19 pandemic predicting mortality rate: machine learning approaches. Chaos, Solitons and Fractals. 2020;138 doi: 10.1016/j.chaos.2020.110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iwendi C., Bashir A.K., Peshkar A., Chatterjee J.M., Pasupuleti S., Mishra R. COVID-19 Patient Health Prediction Using Boosted Random Forest Algorithm. Front Public Health. 2020;8:1–9. doi: 10.3389/fpubh.2020.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matos J., Paparo F., Mussetto I., Bacigalupo L., Veneziano A., Perugin Bernardi S, Biscaldi E, Melani E, Antonucci G, Cremonesi P, Lattuada M, Pilotto A, Pontali E, Rollandi G.A. Evaluation of novel coronavirus disease (COVID-19) using quantitative lung CT and clinical data: prediction of short-term outcome. European Radiology Experimental. 2020;4 doi: 10.1186/s41747-020-00167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hassanien A.E., Salam A., Darwish A., Hassanien A.E., Salama A., Darwsih A. Artificial Intelligence Approach to Predict the COVID-19 Patient ’ s Recovery Artificial Intelligence Approach to Predict the COVID-19 Patient ’ s Recovery. EasyChair. 2020;3223 [Google Scholar]
- 127.Zhang N., Zhang R., Yao H., Xu H., Duan M., Xie T., Pan J., Peng E., Huang J., Zhang Y., Xu X., Zhou F., Wang G. Severity Detection For the Coronavirus Disease 2019 (COVID-19) Patients Using a Machine Learning Model Based on the Blood and Urine Tests. SSRN Electronic Journal. 2019. 2020 doi: 10.2139/ssrn.3564426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qi X., Jiang Z., YU Q., Shao C., Zhang H., Yue H. Machine learning-based CT radiomics model for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: a multicenter study. medRxiv. 2020 doi: 10.1101/2020.02.29.20029603. 2020.02.29.20029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarkar J., Chakrabarti P. A Machine Learning Model Reveals Older Age and Delayed Hospitalization as Predictors of Mortality in Patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.25.20043331. 03.25.20043331 (2020) [DOI] [Google Scholar]
- 130.Tang, Z., Zhao, W., Xie, X., Zhong, Z., Shi, F., Liu, J., Shen, D.: Severity Assessment of Coronavirus Disease 2019 (COVID-19) Using Quantitative Features from Chest CT Images. 2019, 1–18 (2020).
- 131.Yan L., Zhang H.-.T., Goncalves J., Xiao Y., Wang M., Guo Y., Sun C., Tang X., Jin L., Zhang M., Huang X., Xiao Y., Cao H., Chen Y., Ren T., Wang F., Xiao Y., Huang S., Tan X., Huang N., Jiao B., Zhang Y., Luo A., Mombaerts L., Jin J., Cao Z., Li S., Xu H., Yuan Y. A machine learning-based model for survival prediction in patients with severe COVID-19 infection. medRxiv. 2020 doi: 10.1101/2020.02.27.20028027. 2020.02.27.20028027. [DOI] [Google Scholar]
- 132.Kavadi M.D.P., Patan D.R., Ramachandran D.M., Gandomi A.H. Partial Derivative Nonlinear Global Pandemic Machine Learning Prediction Of Covid 19. Chaos, Solitons & Fractals. 2020 doi: 10.1016/j.chaos.2020.110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carrillo-Larco R.M., Castillo-Cara M. Using country-level variables to classify countries according to the number of confirmed COVID-19 cases: an unsupervised machine learning approach. Wellcome Open Research. 2020;5:56. doi: 10.12688/wellcomeopenres.15819.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hu Z., Ge Q., Li S., Boerwinkle E., Jin L., Xiong M. Forecasting and Evaluating Multiple Interventions for COVID-19 Worldwide. Frontiers in Artificial Intelligence. 2020;3:1–11. doi: 10.3389/frai.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sameni, R.: Mathematical Modeling of Epidemic Diseases; A Case Study of the COVID-19 Coronavirus. (2020).
- 136.Paul S.K., Jana S., Bhaumik P. A multivariate spatiotemporal spread model of COVID-19 using ensemble of ConvLSTM networks. medRxiv. 2020 doi: 10.1101/2020.04.17.20069898. 04.17.20069898 (2020) [DOI] [Google Scholar]
- 137.Singh S., Parmar K.S., Makkhan S.J.S., Kaur J., Peshoria S., Kumar J. Study of ARIMA and least square support vector machine (LS-SVM) models for the prediction of SARS-CoV-2 confirmed cases in the most affected countries. Chaos, Solitons and Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeroual A., Harrou F., Dairi A., Sun Y. Deep learning methods for forecasting COVID-19 time-Series data: a Comparative study. Chaos, Solitons and Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang P., Zheng X., Li J., Zhu B. Prediction of epidemic trends in COVID-19 with logistic model and machine learning technics. Chaos, Solitons and Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farooq J., Bazaz M.A. A Novel Adaptive Deep Learning Model of Covid-19 with focus on mortality reduction strategies. Chaos, Solitons & Fractals. 2020;138 doi: 10.1016/j.chaos.2020.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yadav M., Perumal M., Srinivas M. Analysis on novel coronavirus (COVID-19) using machine learning methods. Chaos, Solitons and Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Melin P., Monica J.C., Sanchez D., Castillo O. Analysis of Spatial Spread Relationships of Coronavirus (COVID-19) Pandemic in the World using Self Organizing Maps. Chaos, Solitons and Fractals. 2020;138 doi: 10.1016/j.chaos.2020.109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tuli S., Tuli S., Tuli R., Gill S.S. Predicting the growth and trend of COVID-19 pandemic using machine learning and cloud computing. Internet of Things. 2020;11 doi: 10.1016/j.iot.2020.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ayyoubzadeh S.M., Ayyoubzadeh S.M., Zahedi H., Ahmadi M., R Niakan Kalhori S. Predicting COVID-19 Incidence Through Analysis of Google Trends Data in Iran: data Mining and Deep Learning Pilot Study. JMIR Public Health and Surveillance. 2020;6:e18828. doi: 10.2196/18828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chakraborty T., Ghosh I. Real-time forecasts and risk assessment of novel coronavirus (COVID-19) cases: a data-driven analysis. Chaos, Solitons and Fractals. 2020;135 doi: 10.1016/j.chaos.2020.109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dandekar, R., Barbastathis, G.: Neural Network aided quarantine control model estimation of COVID spread in Wuhan, China. 2020, 1–13 (2020).
- 147.Fong S.J., Li G., Dey N., Crespo R.G., Herrera-Viedma E. Composite Monte Carlo decision making under high uncertainty of novel coronavirus epidemic using hybridized deep learning and fuzzy rule induction. Applied Soft Computing Journal. 2020;93 doi: 10.1016/j.asoc.2020.106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gupta, R., Pandey, G., Chaudhary, P., Pal, S.K.: SEIR and Regression Model based COVID-19 outbreak predictions in India. 1–10 (2020). doi: 10.1101/2020.04.01.20049825. [DOI]
- 149.Hartono, P.: Generating Similarity Map for COVID-19 Transmission Dynamics with Topological Autoencoder. (2020).
- 150.Ibrahim M.R., Haworth J., Lipani A., Aslam N., Cheng T., Christie N. Variational-LSTM Autoencoder to forecast the spread of coronavirus across the globe. medRxiv. 2020 doi: 10.1101/2020.04.20.20070938. 2020.04.20.20070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kafieh R., Arian R., Saeedizadeh N., Minaee S., amini zahra, Kumar Yadav S. COVID-19 in Iran: a Deeper Look Into The Future. medRxiv. 2020 doi: 10.1101/2020.04.24.20078477. 2020.04.24.20078477. [DOI] [Google Scholar]
- 152.Kolozsvari L.R., Berczes T., Hajdu A., Gesztelyi R., Tiba A., Varga I., Szollosi G.J., Harsanyi S., Garboczy S., Zsuga J. Predicting the epidemic curve of the coronavirus (SARS-CoV-2) disease (COVID-19) using artificial intelligence. medRxiv. 2020 doi: 10.1101/2020.04.17.20069666. 2020.04.17.20069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu, D., Clemente, L., Poirier, C., Ding, X., Chinazzi, M., Davis, J.T., Vespignani, A., Santillana, M.: A machine learning methodology for real-time forecasting of the 2019-2020 COVID-19 outbreak using Internet searches, news alerts, and estimates from mechanistic models. (2020).
- 154.Tomar A., Gupta N. Prediction for the spread of COVID-19 in India and effectiveness of preventive measures. Science of the Total Environment. 2020;728 doi: 10.1016/j.scitotenv.2020.138762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou X., Hong N., Ma Y., He J., Jiang H., Liu C., Shan G., Su L., Zhu W., Long Y. Forecasting the Worldwide Spread of COVID-19 based on Logistic Model and SEIR Model. medRxiv. 2020 doi: 10.1101/2020.03.26.20044289. 03.26.20044289 (2020) [DOI] [Google Scholar]
- 156.Fang Y., Nie Y., Penny M. Transmission dynamics of the COVID‐19 outbreak and effectiveness of government interventions: a data‐driven analysis. J. Med. Virol. 2020;92:645–659. doi: 10.1002/jmv.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang C.-.J., Chen Y.-.H., Ma Y., Kuo P.-.H. Multiple-Input Deep Convolutional Neural Network Model for COVID-19 Forecasting in China. medRxiv. 2020 doi: 10.1101/2020.03.23.20041608. 2020.03.23.20041608. [DOI] [Google Scholar]
- 158.Yang Z., Zeng Z., Wang K., Wong S.S., Liang W., Zanin M. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J Thorac Dis. 2020;12:165–174. doi: 10.21037/jtd.2020.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ong E., Wang H., Wong M.U., Seetharaman M., Valdez N., He Y. Vaxign-ML: supervised machine learning reverse vaccinology model for improved prediction of bacterial protective antigens. Bioinformatics. 2020;36:3185–3191. doi: 10.1093/bioinformatics/btaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He Y., Xiang Z., Mobley H.L.T. Vaxign: the First Web-Based Vaccine Design Program for Reverse Vaccinology and Applications for Vaccine Development. Journal of Biomedicine and Biotechnology. 2010. 2010:1–15. doi: 10.1155/2010/297505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ong E., Wong M.U., Huffman A., He Y. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front Immunol. 2020;11:1–13. doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen T., Guestrin C. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. ACM; New York, NY, USA: 2016. XGBoost: a Scalable Tree Boosting System; pp. 785–794. [DOI] [Google Scholar]
- 163.Magar, R., Yadav, P., Farimani, A.B.: Potential Neutralizing Antibodies Discovered for Novel Corona Virus Using Machine Learning. 1–25 (2020). doi: 10.1101/2020.03.14.992156. [DOI] [PMC free article] [PubMed]
- 164.Patankar, S.: Deep learning-based computational drug discovery to inhibit the RNA Dependent RNA Polymerase: application to SARS-CoV and COVID-19. 1–17 (2020). doi: 10.31219/osf.io/6kpbg. [DOI]
- 165.Alex Z., Bogdan Z., Alexander Z., Vladimir A., Victor T., Quentin V., Dmitry S., B., Daniil P., Rim S., Andrey F., Michael B., Steve M., Edgardo L., Deborah B., Keita F., Yen-Chu L., Shih-Hsien H., Hsuan-Jen L, Alex A., Yan I. Potential Non-Covalent SARS-CoV-2 3C-like Protease Inhibitors Designed Using Generative Deep Learning Approaches and Reviewed by Human Medicinal Chemist in Virtual Reality. chemRxiv. 2020 doi: 10.26434/chemrxiv.12301457.v1. [DOI] [Google Scholar]
- 166.Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Metsky H.C., Freije C.A., Kosoko-Thoroddsen T.-S.F., Sabeti P.C., Myhrvold C. CRISPR-based surveillance for COVID-19 using genomically-comprehensive machine learning design. bioRxiv. 2020 doi: 10.1101/2020.02.26.967026. 02.26.967026 (2020) [DOI] [Google Scholar]
- 168.Zhavoronkov A., Aladinskiy V.A., Zhebrak A., Zagribelnyy B., Terentiev V., Bezrukov D.S., Polykovskiy D., Shayakhmetov R., Filimonov A., Orekhov P., Yan Y., Popova O., Vanhaelen Q., Aliper A., Ivanenkov Y.A. Potential 2019-nCoV 3C-like protease inhibitors designed using generative deep learning approaches DNA double-strand break repair in mammalian cells. View project Matrix-isolated systems modeling View project. 2020 doi: 10.13140/RG.2.2.29899.54569. [DOI] [Google Scholar]