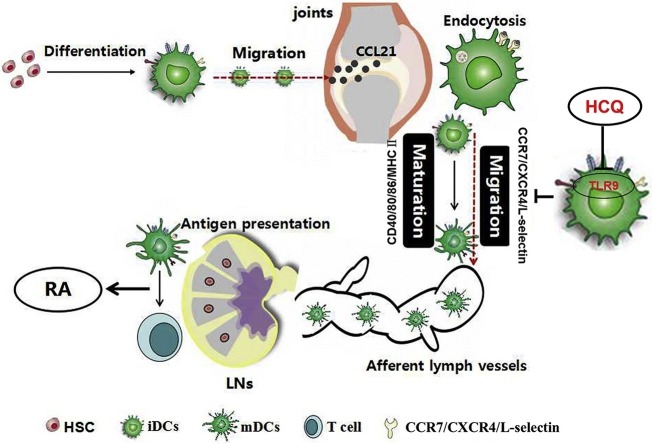
Graphical abstract
DCs act as sentinels for the immune system. DCs capture antigens locally and become mature, characterized by up-regulation of chemokine receptors (CCR7 and CXCR4), adhesion molecule (L-selectin), co-stimulator molecules (CD40, CD80 and CD86) and MHC II. Under high concentration of CCL21 in draining lymph nodes (LNs), mature DCs traffic to LNs via afferent lymph vessels and present antigens to T cells, resulting in the development of arthritis. HCQ impaires DC maturation via blocking TLR9 signaling, retrains DC migration to LNs, and prevents the initiation and progression of RA.
Keywords: Hydroxychloroquine, Rheumatoid arthritis, Dendritic cells, Toll like receptor 9
Highlights
-
•
HCQ efficiently inhibited DC phenotypic and functional maturation stimulated by serum from RA patients.
-
•
HCQ prevented progression of arthritis by inhibiting DC maturation and migration from peripheral blood to LNs.
-
•
HCQ inhibited CpG ODN 1826-activated BMDC maturation and migration.
-
•
The effect of HCQ on DCs was related to the block in TLR9 signaling.
-
•
The development of arthritis was impaired in TLR9−/− mice.
Abstract
Hydroxychloroquine (HCQ) is one of the most commonly prescribed immune-suppressants in treating rheumatoid arthritis (RA). Our previous research showed that HCQ suppressed RA development by inhibiting T follicular helper (Tfh) cells directly. Dendritic cells (DCs) serve as the link between innate and acquired immunity. Whether HCQ suppressed Tfh cell through DCs was not clear. In current study, we found that HCQ efficiently inhibited CD86, chemokine (C-X-C motif) receptor 4 (CXCR4) expression and interferon-α (IFN-α) secretion of healthy donor derived purified DCs stimulated by RA patient serum. To mimic RA, collagen-induced arthritis (CIA) mouse model was used and treated with HCQ daily for fifty-four days prior to sacrifice. We found HCQ inhibited DC maturation and migration to lymph nodes (LNs), manifested as down-regulated expression of CD40, CD80, CD86, MHCII (I-Aq) on LN DCs. In addition, HCQ reduced the level of chemokine receptor 7 (CCR7) and L-selectin on peripheral blood DCs and diminished percentage of LN DCs. Of note, HCQ only inhibited CpG ODN 1826-induced IL-12 secretion by bone marrow DCs (BMDCs) stimulated by various toll like receptor (TLR) agonists. Mechanistically, HCQ down-regulated the expression of TLR9 not only in healthy donor PBMC-derived DCs stimulated by RA patient serum, but also in LN DCs of CIA mice and CpG-activated BMDCs. Furthermore, arthritis scores in TLR9−/− mice were much lower than that in wild type mice with impaired maturity and migration capability of DCs. Collectively, activation of DCs contributes to the pathogenesis of RA and HCQ shows protective effects on RA by inhibition of DC activation via blocking TLR9.
1. Introduction
Dendritic cells (DCs) are powerful antigen-presenting cells and play a vital function in inducing and regulating of immune responses [[1], [2], [3]]. The central functions of DCs are their specialized capacities to sense and respond to the changes of the micro-environment; to acquire and process antigens and undergo a cellular differentiation program called as phenotypic and functional “maturation”, characterized by up-regulation of CD40, CD80, CD86, MHC class II molecule as well as inflammatory molecules; and then to traffic to T cell zones of lymphoid organs to activate immune cells and initiate adaptive immunity [[4], [5], [6]]. Therefore, DCs are a significant intermediary between innate and acquired immunity and thought to be crucial for triggering many types of autoimmune diseases, including psoriasis [7], rheumatoid arthritis (RA) [8] and systemic lupus erythematosus (SLE) [9].
Hydroxychloroquine (HCQ), an antimalarial drug, has been shown to have therapeutic effects in COVID-19 (corona virus disease, 2019) not only because of the antiviral effect [10], but the immune-modulatory function in diminishing the amount of pro-inflammatory factors [11,12]. In fact, HCQ is one of the most commonly prescribed immune-suppressants in treating rheumatic diseases [13]. Numerous studies have demonstrated that HCQ has therapeutic effects on RA [14,15] and several potential therapeutic mechanisms have been identified, including inhibition of autophagy [16,17], suppression of cell death [17], and down-regulation of platelet function [18]. In addition, our previous research showed that HCQ suppressed RA development by inhibition of T follicular helper (Tfh) cells ex vivo and in vivo [19]. DCs function as antigen-presenting cells and serve as the link between innate immunity and acquired immunity. In our previous research, HCQ was found to down-regulate Tfh cells directly, but whether HCQ suppressed Tfh cell through DCs was not clear. Growing evidences show that DCs play a key role in the occurrence and persistence of RA [9], and our previous experimental results demonstrated that modulation of DC functions may be an effective measure in treating RA [[20], [21], [22]]. Thus we explored whether one of the mechanisms responsible for the effects of HCQ in RA is related to the modulation of DC functions.
2. Materials and methods
2.1. Participants & purification of PBMC-derived DCs
Twenty-nine patients with newly diagnosed RA according to the 1987 revised criteria of the American College of Rheumatology were recruited. Twenty healthy volunteers of similar ages served as healthy controls. Further details of RA patients were shown in Table 1 . It was supported by the Ethics Committee of Clinical Research, the Third Affiliated Hospital, Southern Medical University (No.201501002) and informed consent was obtained from all subjects. In order to compare the percentages of plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) in peripheral blood between RA patients and healthy donors, PBMCs were isolated, labeled with appropriate antibodies and then tested by flow cytometry. In order to detect the effect of serum from RA patients on DCs and the therapeutic effect of HCQ, DCs purified from peripheral blood of one healthy volunteer (donated 200 mL of peripheral blood due to the very low proportion of DCs in the blood) were treated with serum from RA patients (DAS28-ESR>5.1, n = 6) with or without HCQ, respectively.
Table 1.
Characteristics of RA patients studied.
| RA (n = 29) | |
|---|---|
| Age, years | 54.4 (27−77) |
| Female, n (%) | 26 (89.7 %) |
| RA activity, n (DAS28-ESR) | 5 (2.6−3.2) |
| 18 (3.2−5.1) | |
| 6 (>5.1) | |
| ESR (mm/h) | 49.1 (7−145) |
| WBC (*109/L) | 6.57 (2.8−11.47) |
| Hb (g/L) | 104.3 (40−136) |
| Plt (/μL) | 285.7 (133−498) |
| IgG (g/L) | 16.35 (6.72−42.66) |
| IgM (g/L) | 1.44 (0.62−4.98) |
| IgA (g/L) | 3.48 (0.45−4.92) |
| C3 (g/L) | 1.24 (0.7−1.75) |
| C4 (g/L) | 0.27 (0.09−0.45) |
| C1q (g/L) | 205.5 (103−283) |
| R-IgG (U/mL) | 66.2 (13.5−200.1) |
| R-IgM (U/mL) | 57.6 (9.4−211.6) |
| CCP (U/mL) | 67.8 (7.1−215.3) |
2.6−3.2, Low disease activity; 3.2−5.1, Moderate disease activity; >5.1, High disease activity.
2.2. Mice & cells
Male DBA/1 J (I-Aq) mice aged 6–8 weeks were applied to induce collagen-induced arthritis (CIA) mouse model and HCQ was administered per day for fifty-four days prior to sacrifice. The mice were divided into three groups randomly (healthy control group, CIA group and CIA + HCQ group, n = 6). Male C57BL/6 (I-Ab) wild type (WT) (n = 10) and TLR9-knockout (TLR9−/−) (n = 10) mice aged 8–10 weeks were also used for the mouse experiments. Bone marrow derived dendritic cells (BMDCs) used in vitro experiments were derived from bone marrow of C57BL/6 (I-Ab) wild type (WT) mice that were 6–8 weeks as described previously [23] and the specific methods can be found in supplementary material. The experiments on mice were carried out abide by the ethical standards of Sun Yat-sen University, Guangdong, China (No. L102012016060Z). There was no blinding about the mouse experiments.
2.3. Reagents & antibodies
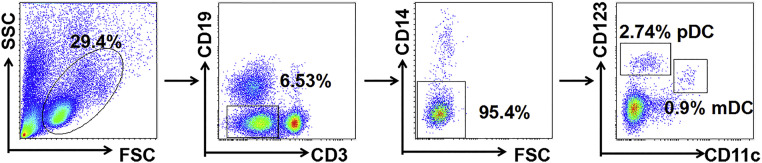
All flow antibodies were purchased from BD Biosciences and BioLegend of USA. And all ELISA kits were purchased from NEO Biosciences of China. EasySep™ Human Pan-DC Pre-Enrichment Kit for DC purification was purchased from STEMCELL of Canada and the purity of the DC up to 86.6 % in the research (Fig. S1). Immunization Grade Bovine Type II Collagen, immunization Grade Chick Type II Collage, Complete and incomplete Freund's adjuvants were purchased from Chondrex, USA. CpG ODN 1826 (tlrl-1826) and recombinant murine GM-CSF (AF-315-03) were purchased from InvivoGen and PeproTech of USA.
2.4. Induction of collagen-induced arthritis and treatment with HCQ
In order to mimic RA, the model of mouse collagen-induced arthritis (CIA) was used, as described elsewhere [24,25]. The incidence and clinical scores were daily assessed via a scoring system described elsewhere [26]. In the group treated with HCQ, mice were administrated by gavage with a dose of 80 mg/kg per day from day 0 to day 54 and then mice were sacrificed. In CIA model and health control group, mice received saline instead. See supplementary material for specific methods to induce CIA and scoring system.
2.5. Phenotype and chemokine receptor analyses
For analyzing the DC sub-populations in peripheral blood from patients with RA and healthy donors, DC phenotypes, chemokine receptors and TLR9 expressions in vivo and in vitro were detected by flow cytometry as described previously [27]. To detect the intracellular expression of TLR9, DCs were first fixed and cell membrane was punched, then labeled with antibodies and tested by flow cytometry.
2.6. Cytokine and chemokine analyses
To detect the cytokines and chemokines in vivo and in vitro, we collected the supernatants of healthy volunteer PBMC-derived DCs stimulated by RA patient serum and the supernatants of mouse BMDCs stimulated by CpG with or without HCQ, then the amount of TNF-α, IL-1β, IFN-α and CCL21 in samples from different experiments were detected with ELISA kits.
2.7. Chemotaxis assay
Migration of BMDCs was assessed using a 24-well BD falcon cell culture insert system. Firstly, BMDCs were purified using mouse CD11c+ kit. CCL21 (200 ng/mL) was placed in the bottom chamber and 100 μl cell suspension (2 × 105 cells) placed in the top well. After incubation for 3 h, the BMDCs migrating to the lower chamber were obtained and assayed by flow cytometry.
2.8. Statistical analysis
The data analysis was conducted by SPSS 21.0 and the figures were made by Graphpad Prism software 5.5. Nonparametric Mann-Whitney U test was applied to evaluate the arthritis score. One-way analysis of variance (ANOVA) was conducted to compare the difference of intergroup and LSD test was applied to compare the difference between groups. The P < 0.05 was thought to be significantly different. All data were expressed as mean ± SEM. Experiments in vitro were performed in triplicate for twice or three times independently.
3. Results
3.1. The percentages of pDCs and mDCs were increased in the peripheral blood of patients with RA
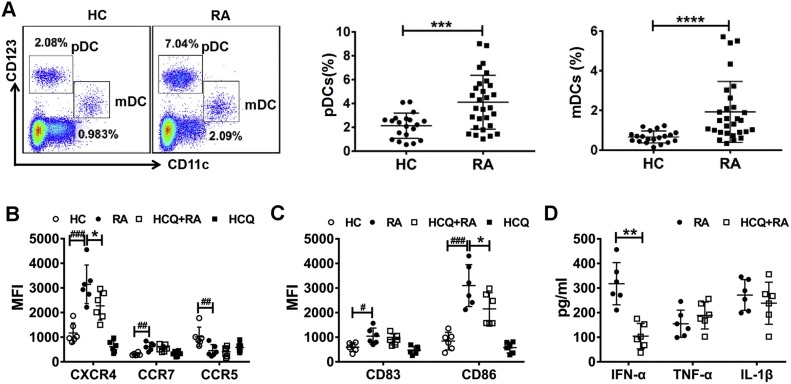
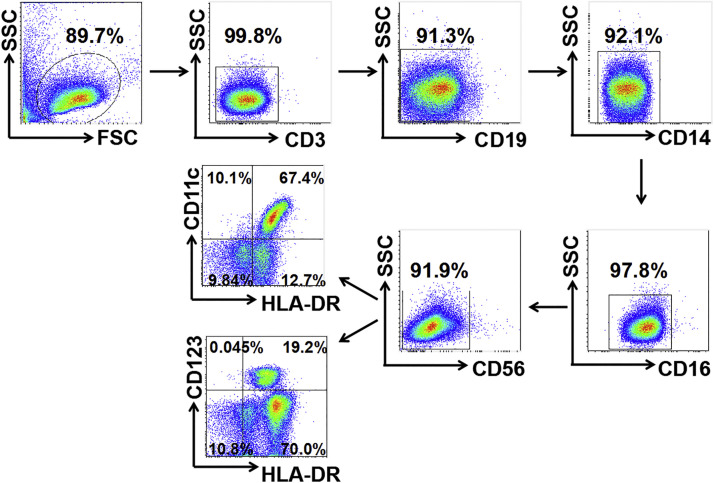
To analyze the distribution of human mDCs and pDCs in RA patients (Fig. S2), 29 newly diagnosed RA patients (89.7 % female, mean age 54.4 years) and 20 healthy controls (85 % female, mean age 51.6 years) were enrolled. PBMCs were obtained from the peripheral blood of RA patients and healthy volunteers. The frequency of pDCs (CD3-CD19-CD14-CD11c-CD123high) and mDCs (CD3-CD19-CD14-CD11c+CD123low) were tested by flow cytometry. The results showed that both pDCs and mDCs were increased in PBMCs from RA patients compared to healthy donors (P < 0.001) (Fig. 1 A).
Fig. 1.
HCQ inhibited DC activity stimulated by RA patient serum. (A) The percentage of pDCs and mDCs in peripheralblood of patients with RA (n = 29) was increased when compared to healthy donors (n = 20). *** P < 0.001, **** P < 0.0001: RA patient vs healthy control. (B-D) Purified DCs were obtained from PBMCs of a healthy donor and stimulated by serum of healthy controls (n = 6) and RA patients (DAS28-ESR>5.1, n = 6), respectively. Our data showed that the ability of migration and maturation of DCs stimulated by RA patient serum was enhanced, manifested as up-regulated expression of CXCR4, CCR7, decreased expression of CCR5 and increased level of CD83, CD86. HCQ prevented the activity of DCs by attenuating the expression of CXCR4, CD86 and IFN-α. #P < 0.05, ##P < 0.01, ###P < 0.001: DCs stimulated by RA patient serum vs DCs stimulated by healthy volunteer serum, * P < 0.05, ** P < 0.01: HCQ + DCs stimulated by RA patient serum vs DCs stimulated by RA patient serum. (HC, healthy control; HCQ, Hydroxychloroquine, MFI: mean fluorescence intensity).
3.2. HCQ efficiently inhibited DC phenotypic and functional maturation stimulated by serum from RA patients
The peripheral blood from one healthy donor was collected and PBMCs were isolated, then DCs were purified by EasyStep™ Human Pan-DC Pre-Enrichment Kit. Purified healthy donor PBMC derived-DCs were stimulated with the serum from healthy donors (HD) (n = 6) or RA patients (DAS28-ESR>5.1, n = 6) with or without HCQ treatment (25 μM) for 24 h. The cells were harvested and stained with the following antibodies: chemokine receptor 7 (CCR7), CCR5, chemokine (C-X-C motif) receptor 4 (CXCR4), CD83, and CD86. The results showed that the expression of CCR7, CXCR4, CD83 and CD86 were up-regulated on DCs stimulated by RA patient serum, as compared to that stimulated by HD serum. In contrast, CXCR5 expression was markedly decreased on DCs stimulated by RA patient serum. However, the up-regulation of CXCR4 and CD86 on DCs stimulated by RA patient serum was significantly inhibited by HCQ treatment (Fig. 1B, C). We also detected the cytokine production in the supernatants of purified DCs stimulated with RA patient serum with or without HCQ, and we found that HCQ inhibited the secretion of IFN-α, but not TNF-α and IL-1β (Fig. 1D).
3.3. HCQ treatment decreased LNs DCs in CIA model
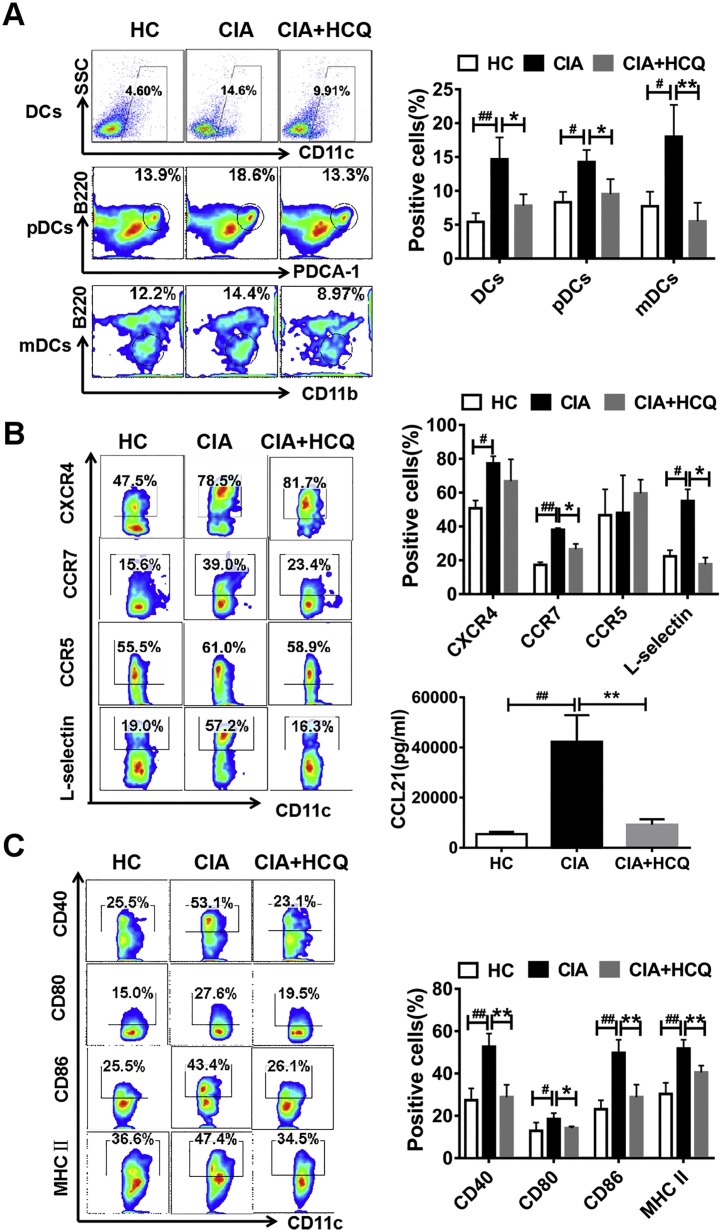
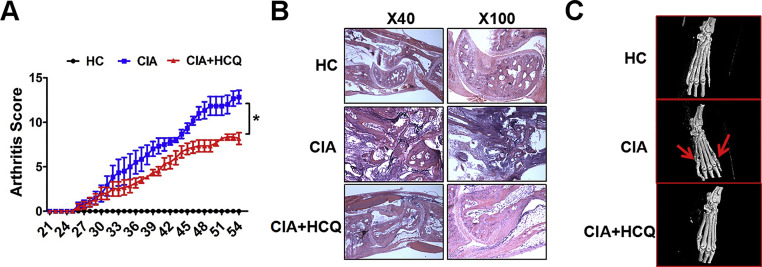
In our research, it was found that in CIA model, HCQ treatment showed reduced arthritis scores, relieved cartilage erosion and bone destruction (Fig. S3A–C), which was consistent with our previous findings [19]. Here, we determined whether HCQ affected the DC percentage in local LNs. We found that the percentage of DCs was extremely enhanced in LNs in CIA group, while this enhancement was dramatically inhibited after treating with HCQ (Fig. 2 A). Furthermore, we analyzed the different subsets of DCs in LNs and found that the percentage of both pDCs (CD11c+ PDCA+ B220+ DCs) and mDCs (CD11c+ CD11b+ B220- DCs) were dramatically decreased after HCQ treatment (Fig. 2A).
Fig. 2.
HCQ suppressed the development of CIA by restraining DC migration and maturation in vivo. (A) In LNs, the percentage of DCs including pDCs and mDCs increased in CIA group when compared to healthy control, and both of which were down-regulated after HCQ treatment (n = 6). (B, C) In contrast to healthy control, the migration ability of peripheralblood DCs and maturation of LN DCs were enhanced in CIA model, while HCQ repress the change by inhibiting the expression of CCR7, L-selectin, CCL21 and decreasing the level of CD40, CD80, CD86 and MHC II (n = 6). #P < 0.05, ##P < 0.01: CIA model vs healthy control mice. * P < 0.05, ** P < 0.01: HCQ-treated mice vs vehicle-treated mice.
3.4. HCQ treatment reduced the level of CCR7 and L-selectin on DCs in the peripheral blood and down-regulated the serum level of CCL21 in CIA mice
The above data for DC distribution may be explained by the inhibitory impact of HCQ on DC migration to LNs. It is well known that migration of DCs from peripheral blood to LNs is associated with chemokine and chemokine receptors. Thus, we determined the level of chemokine receptors on DCs of peripheral blood in different groups. We found that HCQ treatment markedly decreased the expression of CCR7 and L-selectin, but not CCR5 and CXCR4 on peripheral blood DCs (Fig. 2B). Meanwhile, the level of chemokine CCL21 in serum was reduced after HCQ treatment (Fig. 2B).
3.5. HCQ treatment suppressed the phenotypic activation of LN DCs in CIA mice
We collected LN cells and stained with cell membrane markers for DC maturation. We found that HCQ suppressed the level of CD40, CD80, CD86, and MHC II (I-Aq) on those DCs (Fig. 2C).
3.6. HCQ inhibited CpG-activated BMDC maturation and migration
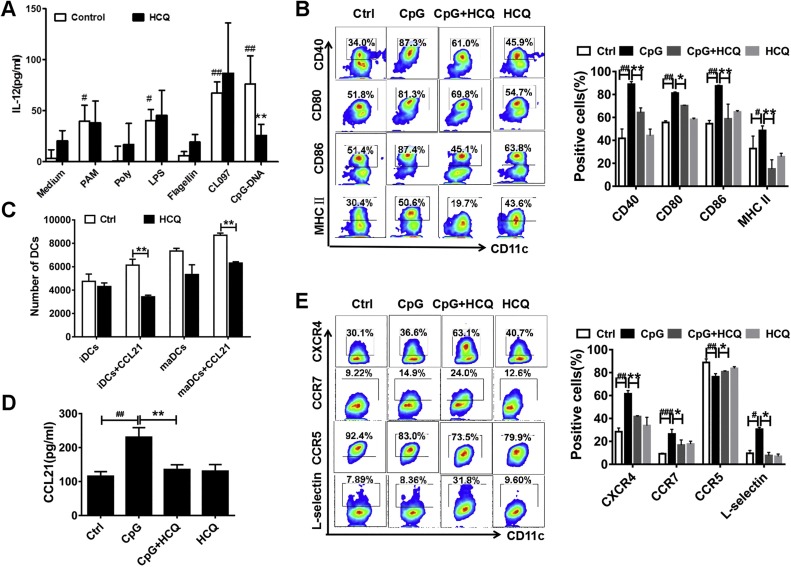
To elucidate the mechanisms by which HCQ inhibits DC functions. BMDCs derived from bone marrow of C57BL/6 (I-Ab) wild type (WT) mice were applied in vitro experiment. BMDCs were activated by various TLR agonists including Pam3CSK4 (TLR1/2), Poly (I: C) (TLR3), LPS (TLR4), Flagellin (TLR5), CL097 (TLR7/8) and CpG ODN 1826 (TLR9). IL-12 is mainly produced by activated inflammatory cells and DCs were found to be the first one to synthesize IL-12. However, we found CpG-induced IL-12 alone was inhibited by HCQ (Fig. 3 A). These data indicated that TLR9 might be a potential target of HCQ for RA treatment. Next, BMDC functions including maturation and migration were analyzed after stimulated with CpG (50 ng/mL) in the presence of HCQ (25 μM) or not for 24 h.
Fig. 3.
HCQ suppressed only CpG ODN 1826 induced BMDC activity but not other TLR stimulators. (A) HCQ attenuated IL-12 secretion only in CpG (the ligand of TLR9) induced BMDCs but not other TLR inducers including Pam3CSK4 (TLR1/2), Poly (I:C) (TLR3), LPS (TLR4), Flagellin (TLR5) and CL097 (TLR7/8). (B, E) HCQ prevented the migration and maturation of CpG activated BMDCs, reflected by decreased expression of CCR7, CXCR4, L-selectin, increased expression of CCR5 and diminished level of CD40, CD80, CD86, MHC II. (C) HCQ inhibited both immature DCs (iDCs) and CpG-stimulated mature DCs (maDCs) transportation/from transporting from the upper chamber to the lower chamber under the chemotactic effect of CCL21. (D) HCQ inhibited the CCL21 production of BMDCs activated by CpG. #P < 0.05, ##P < 0.01, ###P <0.001: CpG-activated BMDCs vs CpG-unactivated BMDCs (Ctrl); * P < 0.05, ** P < 0.01: HCQ + BMDCs stimulated by CpG vs BMDCs stimulated by CpG.
For phenotypic maturation, as shown in Fig. 3B, HCQ markedly suppressed the level of CD40, CD80, CD86, and MHC II (I-Ab) on BMDCs. To clarify the impact of HCQ on CpG-activated BMDC migration, chemotaxis experiment of purified BMDCs was carried out. The results showed that HCQ inhibited both immature DCs (iDCs) and CpG-stimulated mature DCs (maDCs) trafficking from the upper chamber to the lower chamber under the chemotactic attraction of CCL21 (Fig. 3C). We also noticed that HCQ inhibited the secretion of CCL21 in CpG-stimulated BMDCs (Fig. 3D). Additionally, chemokine receptors CCR7, CXCR4 and L-selectin were significantly increased in CpG stimulated BMDCs, while CCR5 was decreased. Of note, HCQ dramatically lowered the expression of CCR7, CXCR4 and L-selectin, and moderately enhanced CCR5 on CpG stimulated BMDCs (Fig. 3E).
3.7. HCQ inhibited DC function via TLR9
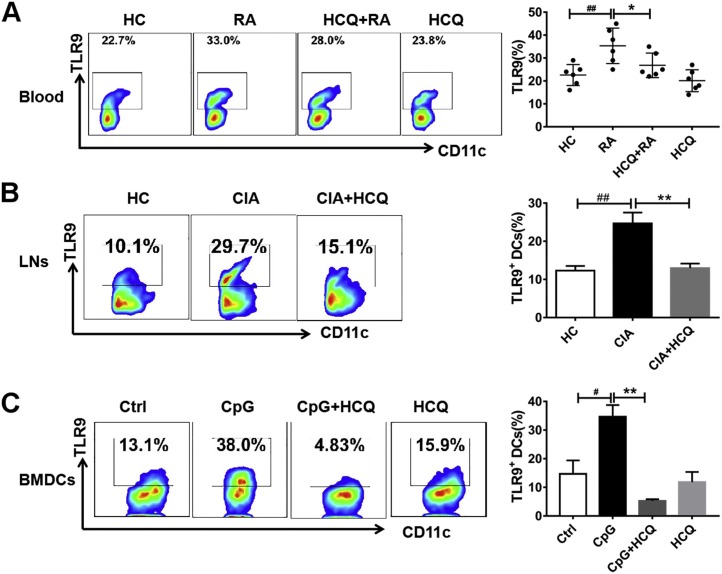
To better understand the role of TLR9 on DCs, the expression of TLR9 was detected in purified DCs from one healthy donor that was stimulated by serums from RA patients (DAS28-ESR>5.1, n = 6) or healthy donors (n = 6) in the presence of HCQ or not. The data showed that TLR9 expression was elevated in RA patient serum-stimulated DCs compared to HD serum-stimulated DCs and this effect was inhibited by HCQ (Fig. 4 A). Next, the level of TLR9 in LN DCs of the CIA mice was measured, and the results showed that it was increased in CIA group than that in healthy control group. Again, HCQ prevented the up-regulation of TLR9 as anticipated (Fig. 4B). Furthermore, we found higher TLR9 expression in CpG-activated BMDCs and HCQ dramatically inhibited the change (Fig. 4C).
Fig. 4.
HCQ prevented DC functions by TLR9. (A) Purified DCs were obtained from PBMC of a healthy donor and stimulated by serum of healthy controls (n = 6) and RA patients (DAS28-ESR>5.1, n = 6), respectively. The result showed that the level of TLR9 in RA patient serum stimulated DCs was enhanced and this effect was inhibited by HCQ. ##P < 0.01: DCs stimulated by RA patient serum vs DCs stimulated by healthy control serum, * P < 0.05: HCQ + DCs stimulated by RA patient serum vs DCs stimulated by RA patient serum. (B) In CIA mice, the level of TLR9 in LN DCs of CIA mice was elevated when compared to healthy control and this effect was inhibited by HCQ. ##P < 0.01: CIA model vs healthy control mice. ** P < 0.01: HCQ-treated mice vs vehicle-treated mice. (C) In BMDCs, the level of TLR9 in CpG-activated BMDCs was up-regulated and this was inhibited by HCQ. #P < 0.05: CpG-activated BMDCs vs CpG-inactivated BMDCs (Ctrl); ** P <0.01: HCQ + BMDCs stimulated by CpG compared to BMDCs stimulated by CpG.
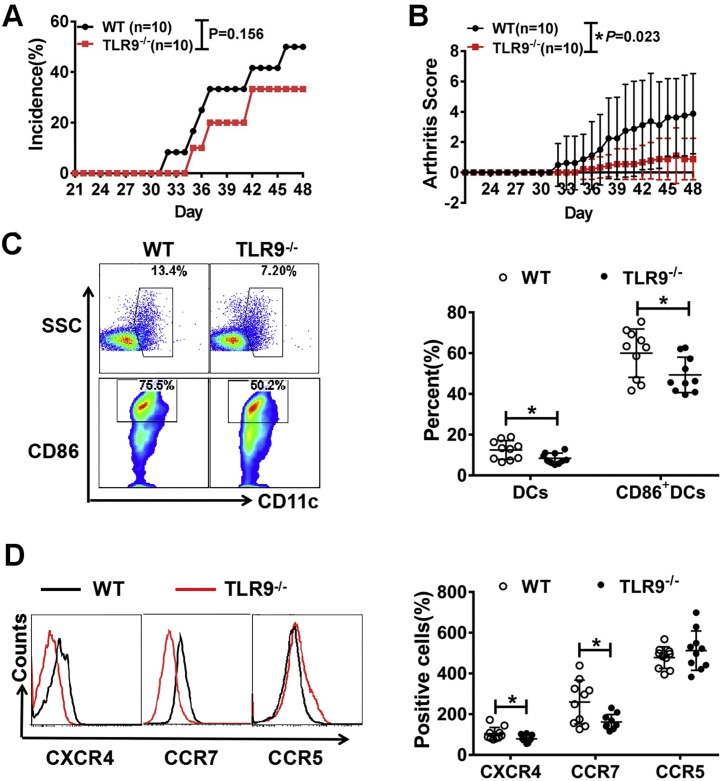
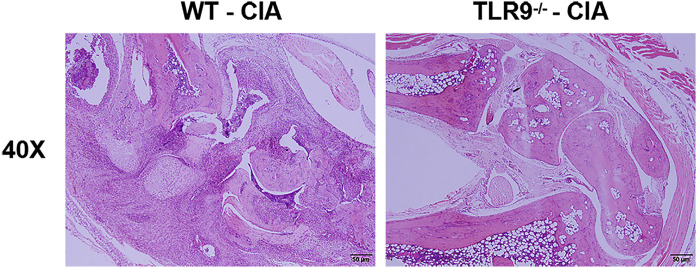
3.8. Amelioration of collagen-induced arthritis in TLR9−/− mice
To investigate whether TLR9 is involved in the modulation of DC in RA, wild-type (WT) and TLR9 knockout (TLR9−/−) mice were used to induce CIA model. Although there was no significant difference of the incidence of collagen-induced arthritis between TLR9−/− mice and WT mice (Fig. 5 A), TLR9−/− mice showed lower arthritis score (Fig. 5B) and alleviated synovial destruction and inflammatory cell infiltration (Fig. S4) than WT mice. Further we investigate the DC population in local LNs of TLR9−/− and WT mice and found that DCs were significantly diminished in LNs of TLR9−/− mice compared to WT mice (Fig. 5C). The co-stimulatory molecule CD86 was also decreased on LN DCs of TLR9−/− mice (Fig. 5C). Moreover, the expressions of CCR7 and CXCR4 on peripheral blood DCs were markedly decreased in TLR9−/− mice (Fig. 5D). These results suggested that knock out of TLR9 had a protective effect on arthritis by inhibiting DC maturation and migration.
Fig. 5.
The severity of CIA was attenuated in TLR9 knockout mice. Here we induced CIA model in C57BL/6 WT mice (n = 10) and TLR9−/− mice (n = 10). (A, B) In contrast to WT CIA mice, the arthritis incidence and score were decreased in TLR9−/− CIA mice. (C) In LNs, the percentage and the maturation of DCs in TLR9−/− CIA mice was down-regulated compared to WT CIA mice because the expression of CD86 was inhibited. (D) In contrast to WT CIA mice, the migration ability of peripheralblood DCs in TLR9−/− CIA mice was impaired with lowed level of CCR7 and CXCR4. * P < 0.01: TLR9−/− CIA mice VS WT CIA mice.
4. Discussion
Chloroquine (CQ) and hydrochloroquine (HCQ) have been used to treat autoimmune diseases for over 70 years. Accumulating evidences have shown the effectiveness of HCQ in the treatment of RA, while evidence regarding its mechanisms is limited. Dendritic cells (DCs) function as vital antigen-presenting cells, which play a crucial part in the occurrence and persistence of RA. Therefore, we determined to investigate whether HCQ had the potential to inhibit the immune response of RA by affecting the phenotypes or behaviors of DCs, and to further elucidate the related mechanisms.
In our research, DCs were found to be activated in RA patients and CIA mice, and HCQ inhibited the functions of DCs and prevented the arising and developing of RA. The mechanism might be associated to blocking the effect of TLR9, as confirmed by following findings: (1) HCQ suppressed the up-regulation of TLR9 and the migration and maturation of RA serum-stimulated DCs by inhibiting the expression of CXCR4, CD86 and the secretion of IFN-α in vitro; (2) HCQ decreased the level of TLR9 in LN DCs and inhibited DC maturation and migration in CIA mice, manifested by the down-regulation of the percentage of DCs (including pDCs and mDCs) and declined expression of CD40, CD80, CD86 and MHC II in LNs of CIA model; (3) HCQ specifically inhibited the TLR9 agonist CpG ODN 1826, but not other TLR agonists, induced IL-12 secretion in BMDCs; (4) compared to WT mice, the arthritis score was lower and the percentage and maturation of DCs in LNs were diminished in TLR9−/− mice.
TLR signaling in DCs are vital for orchestrating immune responses [28,29]. The ligation of TLRs selectively activates many kinds of signaling pathways, and results in the maturation of DCs [30,31]. TLR9 is localized in lysosomes and functions to recognize nucleic acids, especially DNA molecules containing unmethylated CpG-containing motifs. Early findings demonstrated that TLR9 was required for DCs to detect microbial nucleic acids during infection [32]. Later, TLR9 was found as a vital molecule for DCs to recognize and process nucleic acids molecules, which plays a pivotal function in the development of autoimmune diseases such as SLE [33] and psoriasis [34]. In this study, we found the expression of CXCR4, CCR7, CD83, 86 and IFN-α were up-regulated in RA serum-stimulated DCs and this effect was inhibited by HCQ, indicating that some substances in RA serum induced DC activation were blocked by HCQ. As TLR9 is a cytosolic DNA sensor, we hypothesized cell-free DNA in serum of RA patients, which is largely attributed to the excessive DNA released by numerous dead cells or incomplete clearance of apoptotic cells [35,36], enters into the cytoplasm when binding to antimicrobial peptide LL37, auto-antibodies or HMGB1 and triggers TLR9 and then induces activation of DCs in patient [37].
The results of DC percentage in peripheral blood of RA patients remain controversial. We observed an increase in the percentage of pDCs and mDCs in RA patients, consistent with previous reports [38,39]. However, other study showed decreased of DCs in peripheral blood of RA [40]. It was reported that pre-DCs are expanded in the bone marrow of RA patients, the increase of pre-DCs in the bone marrow shall lead to the increase of DCs in the peripheral blood, which supports our results [41].
The limitations of this study include: (1) whether CpG 1826 ODN could induce activation of BMDCs derived from TLR9−/− mice were not determined and we speculate the activation might be partially inhibited because there are other DNA receptors such as cGAS and AIM2 [42]; (2) the exact mechanism(s) of HCQ inhibition on TLR9 is not clear. One result suggested that structural modification of DNA by HCQ prevented DNA binding to TLR9 [43] and more evidence is warranted; (3) for in vivo study, it would be better to use mice whose TLR9 knockout was specifically in DCs but not systematically.
In summary, HCQ prevents the development of RA by suppressing DC activation via blocking TLR9, which provides a novel theoretical implications for HCQ in the treatment of RA. Recently, COVID-19 is found with cytokine release syndrome in the late stage, one possible reason is that a large amounts of DNA was released in the process of cell death induced by corona virus, which activates the immune system through TLR9, AIM2 or cGAS and produces a storm of inflammatory factors. HCQ has been shown to have therapeutic effects in some patients, but its mechanism is not clear. We supposed that the reasons are as follows: (1) the immune regulation function of HCQ in suppressing the secretion of pro-inflammatory factors [11,12], which can be explained by our results that HCQ diminished the levels of pro-inflammatory factors including IFN-α, TNF-α, IL-1β and IL-12 by blocking TLR9; (2) however, as mentioned above, HCQ could only blocking TLR9, so it just partially reduces the production of inflammatory cytokines. If HCQ could combine with cGAS inhibitor aspirin or AIM2 blocker, the treatment effect may be better; (3) although HCQ has been reported to have antiviral effect, most studies were done in vitro [10,44], the antiviral activity has not been proven in any virus in any model in vivo; (4) our research found that the effect of HCQ on the arthritis incidence and score of CIA was not very strong and knockout TLR9 only reduced the arthritis score but not the incidence in vivo. Therefore, we speculate that HCQ may not be used to prevent SARS-CoV-2 infection and asymptomatic when virus is replicating, may only be effective for patients with mild symptoms, but not for severe cases with inflammatory cytokine storm and multiple organ damage.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (Grant Nos. 81471613, 81601434).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopha.2020.110848.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Anderson D.A., Dutertre C., Ginhoux F., Murphy K.M. Genetic models of human and mouse dendritic cell development and function, Nature reviews. Immunology. 2020 doi: 10.1038/s41577-020-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutt S.L., Chopin M. Transcriptional networks driving dendritic cell differentiation and function. Immunity. 2020;52(6):942–956. doi: 10.1016/j.immuni.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Waisman A., Lukas D., Clausen B.E., Yogev N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2017;39:153–163. doi: 10.1007/s00281-016-0583-z. [DOI] [PubMed] [Google Scholar]
- 4.Shortman K. Dendritic cell development: a personal historical perspective. Mol. Immunol. 2020;119:64–68. doi: 10.1016/j.molimm.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Puhr S., Lee J., Zvezdova E., Zhou Y.J., Liu K. Dendritic cell development—history, advances, and open questions. Semin. Immunol. 2015;27:388–396. doi: 10.1016/j.smim.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mildner A., Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Terhorst D., Chelbi R., Wohn C., Malosse C., Tamoutounour S., Jorquera A., Bajenoff M., Dalod M., Malissen B., Henri S. Dynamics and transcriptomics of skin dendritic cells and macrophages in an imiquimod-induced, biphasic mouse model of psoriasis. J. Immunol. 2015;195:4953–4961. doi: 10.4049/jimmunol.1500551. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M.S., Bae Y. Dendritic cell-based immunotherapy for rheumatoid arthritis: from bench to bedside. Immune Netw. 2016;16(1):44. doi: 10.4110/in.2016.16.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieker J., Tel J., Pieterse E., Thielen A., Rother N., Bakker M., Fransen J., Dijkman H.B.P.M., Berden J.H., de Vries J.M., Hilbrands L.B., van der Vlag J. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 2016;68(2):462–472. doi: 10.1002/art.39417. [DOI] [PubMed] [Google Scholar]
- 10.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok C.C., Penn H.J., Chan K.L., Tse S.M., Langman L.J., Jannetto P.J. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res. 2016;68:1295–1302. doi: 10.1002/acr.22837. [DOI] [PubMed] [Google Scholar]
- 14.Joo K., Park W., Kwon S.R., Lim M.J., Jung K.H. Improvement of vitiligo in a patient with rheumatoid arthritis after hydroxychloroquine treatment. Int. J. Rheum. Dis. 2015;18:679–680. doi: 10.1111/1756-185X.12442. [DOI] [PubMed] [Google Scholar]
- 15.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 16.Zhitomirsky B., Yunaev A., Kreiserman R., Kaplan A., Stark M., Assaraf Y.G. Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang T., Lv L., Pan M., Wen Y., Wang B., Li Z., Wu M., Wang F., Crowley S.D., Liu B. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achuthan S., Ahluwalia J., Shafiq N., Bhalla A., Pareek A., Chandurkar N., Malhotra S. Hydroxychloroquine’s efficacy as an antiplatelet agent study in healthy volunteers. J. Cardiovasc. Pharm. T. 2014;20:174–180. doi: 10.1177/1074248414546324. [DOI] [PubMed] [Google Scholar]
- 19.Han J., Zhou Q., Li X., He J., Han Y., Jie H., He Y., Sun E. Novel function of hydroxychloroquine: down regulation of T follicular helper cells in collagen-induced arthritis. Biomed. Pharmacother. 2018;97:838–843. doi: 10.1016/j.biopha.2017.10.132. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Li X., Zhou Q., Jie H., Lao X., Han J., He J., Liu X., Gu D., He Y., Sun E. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J. Immunol. 2015;195:4126–4135. doi: 10.4049/jimmunol.1401842. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Han Y., Zhou Q., Jie H., He Y., Han J., He J., Jiang Y., Sun E. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 2016;20:170–180. doi: 10.1111/jcmm.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J., Li X., Zhuang J., Han J., Luo G., Yang F., Sun Y., Liao P., Han Y., He Y., Shi H., Sun E. Blocking matrix metalloproteinase-9 abrogates collagen-induced arthritis via inhibiting dendritic cell migration. J. Immunol. 2018;201:3514–3523. doi: 10.4049/jimmunol.1800412. [DOI] [PubMed] [Google Scholar]
- 23.Lutz M.B., Kukutsch N., Ogilvie A.L.J., Rößner S., Koch F., Romani N., Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Brand D.D., Latham K.A., Rosloniec E.F. Collagen-induced arthritis. Nat. Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 25.Inglis J.J., Simelyte E., McCann F.E., Criado G., Williams R.O. Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 26.Ruchatz H., Leung B.P., Wei X.Q., McInnes I.B., Liew F.Y. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 1998;160:5654. [PubMed] [Google Scholar]
- 27.Gustafsson K., Karlsson M., Andersson L., Holmdahl R. Structures on the I-A molecule predisposing for susceptibility to type II collagen-induced autoimmune arthritis. Eur. J. Immunol. 1990;20:2127–2131. doi: 10.1002/eji.1830200935. [DOI] [PubMed] [Google Scholar]
- 28.Zanoni I., Granucci F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J. Mol. Med. 2010;88(9):873–880. doi: 10.1007/s00109-010-0638-x. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Wang M., Sun W., Cai F., Geng S., Su X., Shi Y. PU.1 serves a critical role in the innate defense against Aspergillus fumigatus via dendritic cell-associated C-type lectin receptor-1 and toll-like receptors-2 and 4 in THP-1-derived macrophages. Mol. Med. Rep. 2017;15(6):4084–4092. doi: 10.3892/mmr.2017.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaisho T., Tanaka T. Turning NF-κB and IRFs on and off in DC. Trends Immunol. 2008;29:329–336. doi: 10.1016/j.it.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Zaru R., Ronkina N., Gaestel M., Arthur J.S.C., Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat. Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- 32.Krug A., French A.R., Barchet W., Fischer J.A.A., Dzionek A., Pingel J.T., Orihuela M.M., Akira S., Yokoyama W.M., Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-García E.A., Zavala-Cerna M.G., Lujano-Benítez A.V., Sánchez-Hernández P.E., Martín-Márquez B.T., Sandoval-García F., Vázquez-Del Mercado M. Potential chronotherapeutic optimization of antimalarials in systemic lupus erythematosus: is toll-like receptor 9 expression dependent on the circadian cycle in humans? Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lande R., Gregorio J., Facchinetti V., Chatterjee B., Wang Y., Homey B., Cao W., Wang Y., Su B., Nestle F.O., Zal T., Mellman I., Schröder J., Liu Y., Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 35.Dong C., Liu Y., Sun C., Liang H., Dai L., Shen J., Wei S., Guo S., Leong K.W., Chen Y., Wei L., Liu L. Identification of specific joint-inflammatogenic cell-free DNA molecules from synovial fluids of patients with rheumatoid arthritis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelal I.T., Zakaria M.A., Sharaf D.M., Elakad G.M. Levels of plasma cell-free DNA and its correlation with disease activity in rheumatoid arthritis and systemic lupus erythematosus patients. Egypt. Rheumatol. 2016;38:295–300. [Google Scholar]
- 37.Gilliet M., Cao W., Liu Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 38.Estrada-Capetillo L., Hernández-Castro B., Monsiváis-Urenda A., Alvarez-Quiroga C., Layseca-Espinosa E., Abud-Mendoza C., Baranda L., Urzainqui A., Sánchez-Madrid F., González-Amaro R. Induction of Th17 lymphocytes and treg cells by monocyte-derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin. Dev. Immunol. 2013;2013:1–9. doi: 10.1155/2013/584303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu M.B., Langridge W.H.R. The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol. Int. 2017;37:1043–1051. doi: 10.1007/s00296-017-3671-z. [DOI] [PubMed] [Google Scholar]
- 40.Ramwadhdoebe T.H., Ramos M.I., Maijer K.I., van Lienden K.P., Maas M., Gerlag D.M., Tak P.P., Lebre M.C., van Baarsen L.G.M. Myeloid dendritic cells are enriched in lymph node tissue of early rheumatoid arthritis patients but not in at risk individuals. Cells. 2019;8:756. doi: 10.3390/cells8070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirohata S., Yanagida T., Tomita T., Yoshikawa H. Increased generation of pre-plasmacytoid dendritic cells in bone marrow of rheumatoid arthritis. Mod. Rheumatol. 2013;24:443–447. doi: 10.3109/14397595.2013.843759. [DOI] [PubMed] [Google Scholar]
- 42.Hornung V., Latz E. Intracellular DNA recognition. Nat. Rev. Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 43.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 44.Ooi E.E., Chew J.S.W., Loh J.P., Chua R.C.S. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006;3:39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.