Abstract
Aims:
This study compares the cardiovascular risks between users and non-users of SGLT2 inhibitors based on electronic medical record data from a large integrated delivery health system in South Louisiana.
Materials and methods:
Demographic, anthropometric, laboratory and medication prescription information for patients with type 2 diabetes who were new users of SGLT2 inhibitors either as initial treatments or as add-on treatments were obtained from electronic health records. Mediation analysis was performed in demonstrating the association of use of SGLT2 inhibitors and changes of metabolic risk factors with the risk of incident ischemic heart disease.
Results:
A total of 5,338 new users of SGLT2 inhibitors were matched with 13,821 non-users. During a mean follow up of 3.26 years, 2,302 incident cases of ischemic heart disease were defined. After adjusting for multiple confounding factors, patients using SGLT2 inhibitors had a lower risk of incident ischemic heart disease compared to patients not using SGLT2 inhibitors (hazard ratio [HR] 0.63; 95% confidence interval [CI] 0.54–0.73). Patients using SGLT2 inhibitors also had a lower risk of incident ischemic heart disease within 6 months (HR 0.36; 95% CI 0.25–0.44), 12 months (HR 0.40; 95% CI0.32–0.49), 24 months (HR 0.53; 95% CI 0.43–0.60) and 36 months (HR 0.65; 95% CI 0.54–0.73), respectively. Reductions in systolic blood pressure partly mediated lowering risk of ischemic heart diseases among patients using SGLT2 inhibitors.
Conclusions:
Evidence from real world data indicated the contribution of SGLT2 inhibitors to reducing risk of ischemic heart disease, as well as the benefits beyond glucose-lowering.
Introduction
Diabetes has imposed huge social and healthcare burdens worldwide, of which type 2 diabetes has accounted for the majority.1 Diabetes is associated with a significantly high risk of cardiovascular diseases.2 Lowering the risk of cardiovascular diseases is a big challenge. While metformin is usually the first-line therapy for patients with type 2 diabetes,3,4 researchers have long been studying the cardiovascular benefits of several second-line glucose lowering drugs including dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 (SGLT-2) inhibitors after the US Food Drug Administration (FDA)5 and the European Medicines Agency6 mandated cardiovascular outcomes trial (CVOT) for newer antidiabetic agents in 2008 and 2012, respectively. However, none of the DPP4 inhibitors have demonstrated superiority on cardiovascular outcomes in several large-scale trials such as SAVOR-TIMI7 and EXAMINE.8 GLP-1 receptor agonists in LEADER9 and SUSTAIN-6,10 and SGLT2 inhibitors in EMPA-REG11 and CANVAS12 showed superiority on major adverse cardiovascular events driven by a reduction in CV mortality, while DECLARE-TIMI13 demonstrated a lower risk in cardiovascular mortality and hospitalization for heart failure. Based on these results, patients with poor glycemic control (hemoglobin A1c over 7.5%) with metformin monotherapy are recommended to use GLP-1 receptor agonists or SGLT2 inhibitors as the first choice of add-on therapies.14
Evidence from clinical trials is robust. However, these trials may be limited to the generalization due to the inclusion criteria of their subjects rather than a real world population. Large-scale, well-designed studies of comparative effectiveness on cardiovascular outcomes for second-line glucose lowering drugs under real world settings are of great importance and interests. As the latest new class of glucose lowering drugs, SGLT2 inhibitors have garnered much attention. SGLT2 inhibitors can reduce body weight by 1–5 kg, reduce HbA1c levels by approximately 0.5–1.0% and also have a significant effect on blood pressure during 16–52 weeks of the treatment period.15–17 It is important to perform a study with a sufficient sample size, a long follow-up, and study samples at low to moderate risk of cardiovascular diseases to investigate the comparative effectiveness of SGLT2 inhibitors. The best way to conduct it would be using high-quality observational data. Furthermore, no real world studies have previously investigated the changes in clinical characteristics and the potential mechanisms underlying the findings. Therefore, in this study we compared the cardiovascular risks between users and non-users of SGLT2 inhibitors based on electronic medical record data from one large healthcare system in South Louisiana and investigate the potential mechanisms behind the cardiovascular benefits.
Materials and methods
Study participants
Data on patients with type 2 diabetes in the Louisiana Experiment Assessing Diabetes outcomes (LEAD) cohort study were obtained through the Research Action for Health Network (REACHnet), which has been described previously.18,19 The dataset included electronic health record data for the study cohort between January 1, 2013 and March 31, 2019. For the present study, data from one REACHnet partner health system (Ochsner Health System) were included in the analysis.
The definition of type 2 diabetes in the present study was formulated according to the SUPREME-DM20 criteria as follows: a) 1 or more of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes and Tenth Revision, Clinical Modification (ICD-10-CM) codes for type 2 diabetes associated with in-patient encounters; b) 2 or more ICD codes associated with out-patient encounters on different days within 2 years; c) combination of 2 or more of the following associated with out-patient encounters on different days within 2 years: 1) ICD codes associated with out-patient encounters; 2) fasting glucose level ≥ 126 mg/dl; 3) 2-hour glucose level ≥ 200 mg/dl; 4) random glucose ≥ 200 mg/dl; 5) HbA1c ≥ 6.5%; and 6) prescription for an antidiabetic medication. A total of 93,034 patients between the ages of 30 and 94 years were identified. After the exclusion of patients with a past history of ischemic heart disease prior to the entry of the baseline date (confirmed with ICD-9-CM codes 410–414.9, 429.2 and ICD-10-CM codes I20-I25.9) and those with incomplete data, the present study included 62,111 patients with type 2 diabetes (38,162 whites and 23,949 African Americans). Compared with patients with type 2 diabetes excluded from the present study, the patients included had similar ages (66.4±11.8 versus 66.3±12.0 years of age) with more African Americans (42.1% versus 36.0%) and slightly fewer men (46.5% versus 48.4%). The study and the analysis plan were approved by the Institutional Review Boards (Research Ethics Committee) of the Pennington Biomedical Research Center (2016–064-PBRC), Tulane University (906810), and Ochsner Health System (Ochsner acknowledged Tulane’s approval). We did not obtain informed consent from participants involved in our study because we used anonymized data compiled from electronic medical records.
Baseline measurements
The National Patient-Centered Clinical Research Network (PCORnet) common data model is a specification that defines a standard organization and representation of data for the PCORnet distributed research network.21 Patients’ data extracted from this common data model for the present study included date of birth, date of first diagnosis with diabetes, race, ethnicity, sex, encounter dates, weight, height, insurance type, body mass index (BMI), blood pressure, tobacco use, diagnoses of various diseases and dates of diagnoses, laboratory test dates, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), glycosylated hemoglobin (HbA1c), serum creatinine, urine albumin to creatinine ratio (ACR) and medication prescriptions including antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, antiplatelet drugs and anticoagulant drugs. For patients using SGLT2 inhibitors, the index rate is defined as the date of the first use of an SGLT2 inhibitor because SGLT2 inhibitors were commercially available since 2013 in the United States. Patients in the matched non-SGLT2 users group were not drug-naïve at baseline. However, as SGLT2 inhibitors were approved as second line therapies for patients with type 2 diabetes, they always appeared as an add-on therapy for these patients in the dataset. For patients in the matched non-SGLT2 users group (using other glucose-lowering drugs), the index date is the closest date of use of other glucose-lowering drugs in order to match the first use date of patients with an SGLT2 inhibitor. Baseline characteristics of the patients within three months after the index date of the first/chosen glucose-lowering drug are extracted from the PCORnet common data model. These data elements were collected starting from the baseline. Using smoking status reported at each clinical visit, we classified the patients into 3 groups: current smokers, ever smokers, and never smokers. The estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.22
Propensity score matching
Patients included in this study should have been using REACHnet for no less than 1 year and they should also attend regular check-ups or refill any prescriptions within one year before the end of study. Before the propensity score matching, patients with type 2 diabetes with initial or add on therapies of any SGLT2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin) were first extracted. Nearest neighbor matching was used as the matching algorithm. Caliper was set at 0.001 level. A propensity score was thus generated by a logistic regression model. Covariates for matching included age, race, sex, baseline levels of BMI, systolic blood pressure, HbA1c, LDL-c, HDL-c, triglycerides, eGFR, urine ACR, smoking, insurance type, anti-hypertensive medications, lipid-lowering medications, antiplatelet and anticoagulant medications. Based on the propensity score, patients on other glucose lowering drugs were matched in a 3:1 ratio with patients using SGLT2 inhibitors. Finally, 5338 users of SGLT2 inhibitors were matched with 13,821 non-users, composing a total of 19,159 patients. The baseline characteristics were well matched except for baseline HbA1c levels and baseline triglyceride levels (Table 1). Patients on SGLT2 inhibitors had higher baseline HbA1c levels and triglyceride levels than patients with other glucose lowering drugs. In addition, more non-users of SGLT2 inhibitors used antiplatelet and anticoagulant drugs than users. The prevalence of stroke, peripheral vascular disease, diabetic retinopathy and macroalbuminuria was a bit higher in users of SGLT2 inhibitors than that in non-users.
Table 1.
Baseline characteristics among patients with type 2 diabetes using different drugs
| SGLT2 inhibitors | P | ||
|---|---|---|---|
| Non-users | Users | ||
| Participants (n) | 13,821 | 5,338 | |
| Age (years) | 58.5±9.56 | 58.4±9.01 | 0.064 |
| Male (%) | 50.1 | 50.4 | 0.346 |
| Race (%) | 0.250 | ||
| African American | 35.6 | 35.1 | |
| White | 64.4 | 64.9 | |
| Body mass index (kg/m2) | 34.0±8.14 | 35.0±6.98 | 0.953 |
| Blood pressure (mmHg) | |||
| Systolic | 131±12.1 | 130±15.3 | 0.272 |
| Diastolic | 77.8±7.88 | 78.0±9.16 | 0.770 |
| Hemoglobin A1c (%) | 8.2±2.3 | 8.6±1.5 | <0.001 |
| Total cholesterol (mg/dL) | 169±38.2 | 173±39.1 | 0.899 |
| Low-density lipoprotein cholesterol (mg/dL) | 95.4±31.4 | 96.5±31.6 | 0.070 |
| High-density lipoprotein cholesterol (mg/dL) | 41.2±12.0 | 42.4±10.4 | 0.090 |
| Triglycerides (mg/dL) | 134±95.3 | 155±110.5 | <0.001 |
| Estimated GFR (mL/min/1.73 m2) | 79.3±6.23 | 79.4±4.71 | 0.307 |
| Urine albumin to creatinine ratio | 26.4 (7.4–108) | 29.1 (8.7–81.9) | 0.818 |
| Current smoker (%) | 6.5 | 7.3 | 0.950 |
| Insurance type (%) | 0.251 | ||
| Commercial/private | 56.9 | 58.1 | |
| Medicare | 32.8 | 33.2 | |
| Medicaid | 6.3 | 4.3 | |
| Self-pay | 2.6 | 2.5 | |
| Others | 1.4 | 1.9 | |
| Use of medications (%) | |||
| Metformin | 64.2 | 64.9 | 0.126 |
| Insulin | 34.2 | 34.4 | 0.865 |
| Sulfonylurea | 37.9 | 38.8 | 0.059 |
| DPP4 inhibitors | 29.3 | 29.1 | 0.154 |
| α-glucosidase inhibitors | 0.5 | 1.3 | <0.001 |
| GLP-1 agonists | 46.2 | 46.0 | 0.437 |
| Meglitinides | 1.0 | 2.0 | <0.001 |
| Thiazolidinediones | 3.0 | 6.9 | <0.001 |
| Lipid-lowering | 85.0 | 85.8 | 0.082 |
| Antihypertensive | 87.1 | 87.6 | 0.180 |
| Antiplatelet or anticoagulant | 23.2 | 17.8 | <0.001 |
| Presence of comorbidities at baseline (%) | |||
| Stroke | 4.2 | 4.9 | 0.024 |
| Peripheral vascular disease | 2.7 | 5.9 | <0.001 |
| Retinopathy | 1.7 | 7.9 | <0.001 |
| Macroalbuminuria | 3.1 | 3.6 | 0.074 |
DPP4: Dipeptidyl peptidase 4, GFR: Glomerular filtration rate, GLP-1: Glucagon-like peptide 1
Prospective follow-up
We created the follow-up database in electronic form by using the number assigned to each patient who visited the health system with a unique patient identifier. This analysis followed an on-treatment principle and we also assumed that all the patients followed intention-to-treat. The duration of follow-up for each cohort member (person-years) was tabulated from the first date when a documented prescription with SGLT2 inhibitors or other glucose-lowering drugs occurred to the index date of discontinuation of the index drug (defined as 6 months after the last refilled prescription), the date of diagnosis of the outcome, death of inpatients or March 31, 2019. The range of follow-up was from 0.27 to 6.24 years. Encounter types including ambulatory visits were considered as outpatient encounters, while encounter types including inpatient, emergency department, emergency admission to inpatient, institutional stay, observation stay and institutional consult were considered as either inpatient or emergency encounters.
Ischemic heart disease was defined as the primary outcome in the present comparative effectiveness analysis. ICD-9-CM and ICD-10-CM codes were used to identify the ischemic heart diseases events (ICD-9-CM codes 410–414.9, 429.2; ICD-10-CM codes I20-I25.9). The distributions of all ICD-9 and ICD-10 codes were: 410 (3.9%), 411 (2.0%), 412 (4.4%), 413 (2.8%), 414 (59.2%), 429.2 (1.2%), I20 (1.5%), I21 (2.9%), I22 (0.1%), I23 (0.1%), I24 (1.0%), I25 (20.9%). Other diagnosis codes included stroke (ICD-9-CM codes 430–436; ICD-10-CM codes I60-I66), peripheral vascular disease (ICD-9-CM codes 440.2–440.4 and 443; ICD-10-CM codes I70.2-I70.4 and I73), diabetic retinopathy (ICD-9-CM codes 250.5, 362.0; ICD-10-CM codes E11.3) and diabetic nephropathy (ICD-9-CM codes 250.4; ICD-10-CM codes E11.2). These diagnoses were recorded in the course of routine patient care by the patients’ treating clinicians. For safety analysis, hospitalization due to severe hypoglycemia was the outcome. We also used ICD-9-CM and ICD-10-CM to identify severe adverse events including hospitalizations due to severe hypoglycemia (ICD-9-CM codes 250.1, 251.1 and 251.2; ICD-10-CM codes E16.1 and E16.2), diabetic ketoacidosis (ICD-9-CM codes 250.10, 250.12 and ICD-10-CM codes E11.1) and acute kidney failure (ICD-9-CM codes 584.x and ICD-10-CM codes N17.x) in inpatient or emergency encounters.
Statistical analyses
All the analyses were performed following an on-treatment and intention-to-treat principle. The updated mean value of each anthropometric or lab measurement was calculated for each participant from baseline to each year of follow-up. For example, after 1 year, the updated mean was the average of the baseline and 1-year values, and after 3 years it was the average of baseline, 1-, 2-, and 3-year values. In the case of an event occurring during follow-up, the period for estimating the updated mean value was from baseline to the year before the event occurred. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for ischemic heart diseases within different follow-up periods (full follow-up, and ≤6, ≤12, ≤24, and ≤36 months) among patients using SGLT2 inhibitors compared to patients not using SGLT2 inhibitors (reference group). All analyses were adjusted for age, race, sex, body mass index, systolic blood pressure, HbA1c, LDL-c, HDL-c, triglycerides, estimated GFR, urine ACR, smoking, insurance type, hypoglycemia events, α-glucosidase inhibitors, meglitinides, thiazolidinediones, anti-hypertensive medications, lipid-lowering medications, antiplatelet, anticoagulant medications, history of stroke, peripheral vascular disease, diabetic retinopathy and diabetic nephropathy.
Per standard deviation (SD) changes of major ischemic heart diseases risk factors within 12 months, 24 months and 36 months including BMI, systolic blood pressure, HbA1c, triglyceride, LDL-c to HDL-c ratio and eGFR were calculated as the follow up values minus baseline values and then divided by the SD of the baseline values. The contributions of per SD changes of major ischemic heart diseases risk factors with the risk of ischemic heart diseases were also assessed by Cox models. Model 1 adjusted for age, race, sex, body mass index, systolic blood pressure, HbA1c, LDL-c to HDL-c ratio, triglycerides, estimated GFR, urine ACR, smoking, insurance type, hypoglycemia events, α-glucosidase inhibitors, meglitinides, thiazolidinediones, anti-hypertensive medications, lipid-lowering medications, antiplatelet, anticoagulant medications, history of stroke, peripheral vascular disease, diabetic retinopathy and diabetic nephropathy, other than the variable for analysis. Model 2 adjusted for variables in Model 1 plus baseline levels of body mass index, systolic blood pressure, hemoglobin A1c, triglyceride, LDL-c to HDL-c ratio, and estimated GFR.
Mediation analysis was used to quantify the contribution of one certain factor (independent variable) to the outcome adjusting for all confounding factors according to Baron and Kenny’s steps for mediation, which was first described in 1986.23 In step 1, we regressed the outcome on the independent variable to confirm that the independent variable was a significant predictor of the outcome. In step 2, we regressed the mediator on the independent variable to confirm that the independent variable was a significant predictor of the mediator. In step 3, we regressed the outcome on both the mediator and the independent variable to confirm that the mediator was a significant predictor of the outcome and the previously significant exposure in the first step was greatly reduced. Three β coefficients of the independent variable were thus generated and named β1, β2 and β3. The mediated proportion was calculated as (β1 - β3) / β1.
Three sensitivity analyses were performed. In sensitivity analysis 1, we included patients with prior metformin monotherapy for at least 3 months as well as with suboptimal glycemic control (recommended by American Diabetes Association as two HbA1c measurements between 7% and 9% within a 6-month period after the initial 3-month period or a single HbA1c measurement between 9% and 11% after the initial 3-month period). In sensitivity analysis 2, patients with hypertension or on anti-hypertensive medications were all excluded. In sensitivity analysis 3, patients with glucagon-like peptide-1 agonists were all excluded.
Statistical significance was considered to be P <0.05. All statistical analyses were performed by using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA). Propensity score matching was performed by using R statistical software version 3.2.0.
Results
During a follow up of 62,559 person years, a total of 2,302 incident ischemic heart diseases events were recorded. The incidence rate of ischemic heart diseases was calculated as 3.68 events per 100 person-year. Users of SGLT2 inhibitors compared with non-users showed a significantly lower risk of ischemic heart diseases (multivariable-adjusted hazard ratio [HR] 0.63, 95% confidence interval [CI] 0.54 to 0.73) (Table 2). The multivariable-adjusted HRs of ischemic heart diseases within 6 months, 12 months, 24 months and 36 months among users of SGLT2 inhibitors compared with non-users were 0.36 (95% CI 0.25 to 0.44), 0.40 (95% CI 0.32 to 0.49), 0.53 (95% CI 0.43 to 0.60), and 0.65 (95% CI 0.54 to 0.73), respectively (Table 2), showing that patients with SGLT2 inhibitors would have the lowest risk of ischemic heart diseases within 6 months after starting the therapy.
Table 2.
Hazard ratios of ischemic heart diseases among patients using SGLT2 inhibitors compared to those using other glucose-lowering drugs
| SGLT2 inhibitors | ||
|---|---|---|
| Non-users | Users | |
| Full follow up | ||
| No. of patients | 13,821 | 5,338 |
| No. of cases | 1,976 | 326 |
| Person-years | 50,287 | 12,273 |
| Age-adjusted HR (95% CI) | 1.00 | 0.56 (0.50–0.64) |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 0.63 (0.54–0.73) |
| Follow up ≤6 months | ||
| No. of cases | 607 | 67 |
| Person-years | 5,836 | 2,231 |
| Age-adjusted HR (95% CI) | 1.00 | 0.32 (0.22–0.41) |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 0.36 (0.25–0.44) |
| Follow up ≤12 months | ||
| No. of cases | 904 | 117 |
| Person-years | 11,478 | 4,274 |
| Age-adjusted HR (95% CI) | 1.00 | 0.38 (0.31–0.47) |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 0.40 (0.32–0.49) |
| Follow up ≤24 months | ||
| No. of cases | 1,275 | 199 |
| Person-years | 22,282 | 7,671 |
| Age-adjusted HR (95% CI) | 1.00 | 0.47 (0.39–0.56) |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 0.53 (0.43–0.60) |
| Follow up ≤36 months | ||
| No. of cases | 1,552 | 273 |
| Person-years | 31,734 | 10,147 |
| Age-adjusted HR (95% CI) | 1.00 | 0.58 (0.50–0.64) |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 0.65 (0.54–0.73) |
Adjusted for age, race, sex, body mass index, systolic blood pressure, hemoglobin A1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated GFR, urine albumin to creatinine ratio, smoking, insurance type, hypoglycemia events, α-glucosidase inhibitors, meglitinides, thiazolidinediones, anti-hypertensive medications, lipid-lowering medications, antiplatelet, anticoagulant medications, history of stroke, peripheral vascular disease, diabetic retinopathy and diabetic nephropathy.
In order to confirm our findings, we performed several sensitivity analysis. For sensitivity analysis 1 (Supplementary Table S1), we only included patients with the prior metformin monotherapy for at least 3 months and patients with suboptimal glycemic control. Patients with an add-on therapy of SGLT2 inhibitors were matched in a 1:3 ratio with patients with an add-on therapy of other glucose lowering drugs. The total number of patients reduced to 1922 users of SGLT2 inhibitors matched with 5722 non-users. Similar results were found for all follow- up periods (multivariable-adjusted HR for full follow-up period 0.62 (95% CI 0.51–0.74); HR within 6 months 0.35 (95% CI 0.22–0.48); HR within 12 months 0.40 (95% CI 0.29–0.55); HR within 24 months 0.54 (95% CI 0.35–0.70); HR within 36 months 0.65 (95% CI 0.50–0.79)). For sensitivity analysis 2 (supplementary table S5), patients with hypertension or on anti-hypertensive medications were all excluded. Similar results were also found. For sensitivity analysis 3 (supplementary table S6), we excluded patients with glucagon-like peptide-1 agonists. Users of SGLT2 inhibitors still had a lower risk of ischemic heart diseases than non-users.
Further subgroup analyses also confirmed the findings among patients of different ages, races, sexes, BMI, HbA1c, eGFR, never and past or current smokers, and patients using lipid lowering, anti-hypertensive medications as well as antiplatelet or anticoagulant agents or not using (supplementary table S4). There were interactions among patients with different ages, sexes, eGFR subgroups, as well as using antiplatelet or anticoagulant drugs or not using.
Figure 1 shows the levels of major cardiovascular risk factors during the follow up. Users of SGLT2 inhibitors had significantly lower levels of blood pressure, BMI and LDL-c to HDL-c ratio and higher levels of eGFR at 6 months, 12 months, 24 months and 36 months compared with non-users (all P <0.05). We also compared the changes of these metabolic risk factors during the follow up (Figure 2). Users of SGLT2 inhibitors had much more reduction on HbA1c, blood pressure, BMI and triglyceride, while they had less reduction on LDL-c to HDL-c ratio (all P <0.05) compared with non-users. The change of eGFR at 36 months was also higher in users of SGLT2 inhibitors than that of non-users.
Figure 1.
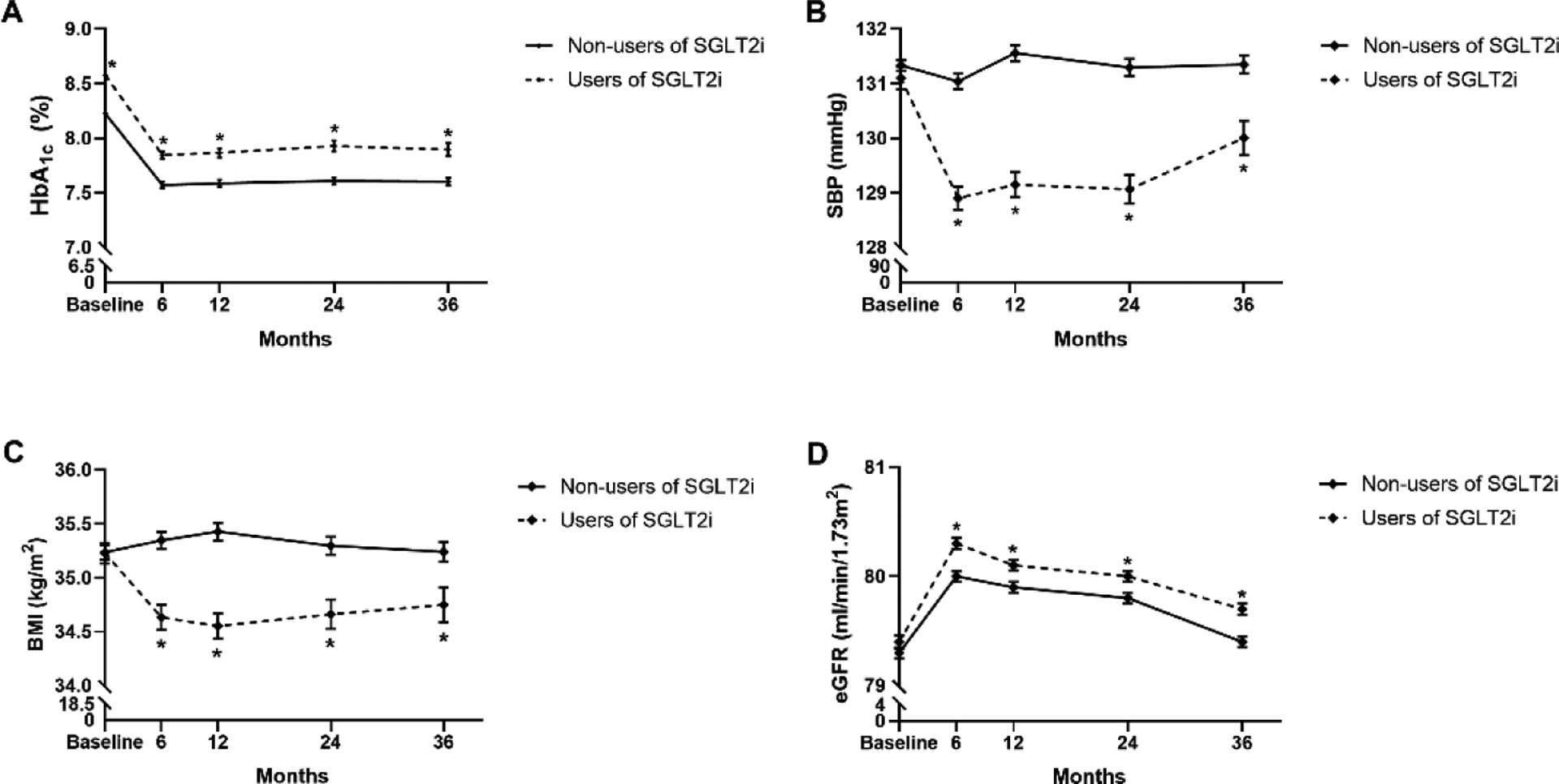
Updated mean values of metabolic risk factors in 6 months, 12months, 24 months and 36 months after initial of SGLT2 inhibitors compared to other glucose-lowering drugs, *P<0.05
Figure 2.
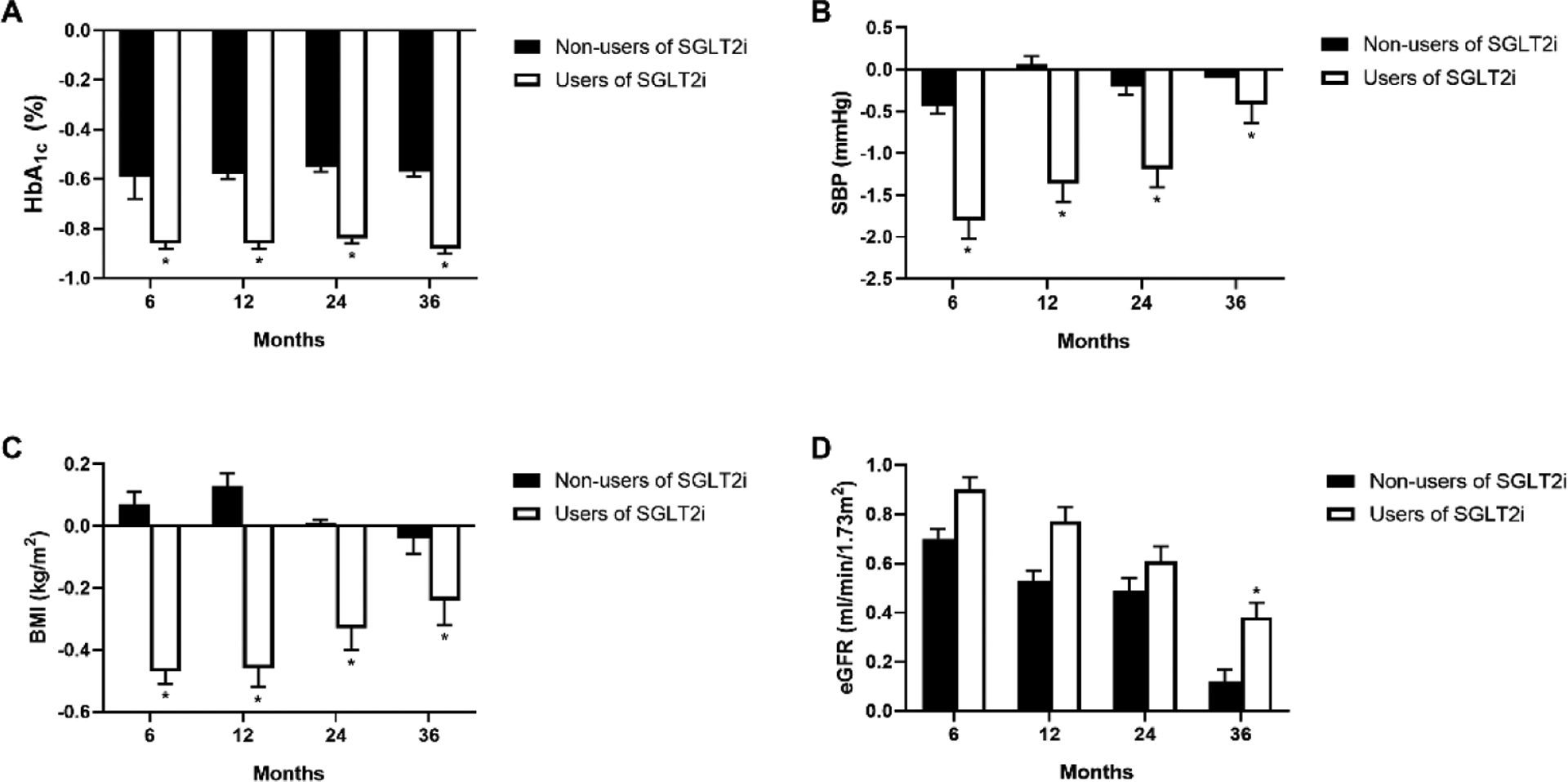
Changes of metabolic risk factors from baseline to 6 months, 12months, 24 months and 36 months after initial of SGLT2 inhibitors compared to other glucose-lowering drugs, *P<0.05
To investigate the contribution of per SD changes in major ischemic heart diseases risk factors including BMI, systolic blood pressure, HbA1c, triglyceride, LDL-c to HDL-c ratio and eGFR, we calculated the changes within different follow-up periods and then incorporated them into the Cox models (Table 3). For per SD change within 12 months, none of the risk factors showed a significant association with the risk of ischemic heart diseases. However, after additional adjustment for baseline levels, per SD change of systolic blood pressure was significantly associated with the risk of ischemic heart diseases in patients with SGLT2 inhibitors (HR 1.01, 95% CI 1.01–1.02). When per SD change within 24 months was considered, per SD change of LDL-c to HDL-c ratio further became significant in patients with other glucose lowering drugs after adjustments of baseline levels (HR 1.04, 95% CI 1.02–1.11), while only systolic blood pressure remained significant in users of SGLT2 inhibitors. Finally, when we analyzed the contribution of per SD change within 36 months, per SD increase of HbA1c (HR 1.17, 95CI 1.05–1.30), LDL-c to HDL-c ratio (HR 1.13, 95% 1.08–1.19 ) and per SD reduction of eGFR (HR 0.90, 95% CI 0.82–0.99) were significantly associated with the risk of ischemic heart diseases in non-users of SGLT2 inhibitors, while in users of SGLT2 inhibitors, per SD change of systolic blood pressure were the only independent contributor to ischemic heart diseases risk (HR 1.03, 95% CI 1.01–1.04). Remarkably, the contribution of per SD change of systolic blood pressure was found to be unique between users of SGLT2 inhibitors and non-users with a P for interaction <0.01.
Table 3.
Hazard ratios of ischemic heart disease according to each per SD change of major risk factors among patients with type 2 diabetes
| Non-users of SGLT2 inhibitors | Users of SGLT2 inhibitors | P for interaction | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| Changes within 12 months | |||||
| Body mass index | 0.95 (0.84–1.07) | 0.96 (0.82–1.11) | 0.87 (0.60–1.24) | 1.08 (0.64–1.67) | >0.25 |
| Systolic blood pressure | 0.96 (0.83–1.05) | 0.90 (0.70–1.11) | 1.16 (1.08–1.25) | 1.01 (1.01–1.02) | <0.01 |
| Hemoglobin A1c | 0.97 (0.89–1.05) | 1.11 (0.96–1.27) | 1.04 (0.78–1.31) | 0.65 (0.34–1.02) | >0.5 |
| Triglyceride | 1.04 (0.96–1.12) | 1.03 (0.92–1.16) | 1.00 (0.82–1.18) | 1.20 (0.89–1.52) | >0.25 |
| LDL-c/HDL-c | 1.03 (0.92–1.12) | 0.85 (0.74–1.01) | 0.98 (0.72–1.14) | 0.90 (0.62–1.20) | >0.25 |
| Estimated GFR | 1.02 (0.94–1.12) | 0.85 (0.78–0.94) | 0.93 (0.70–1.12) | 0.85 (0.54–1.07) | <0.05 |
| Changes within 24 months | |||||
| Body mass index | 0.92 (0.85–1.03) | 0.92 (0.80–1.05) | 0.90 (0.70–1.15) | 1.02 (0.73–1.45) | >0.25 |
| Systolic blood pressure | 0.93 (0.84–1.02) | 0.91 (0.83–1.40) | 1.18 (1.10–1.25) | 1.06 (1.01–1.10) | <0.01 |
| Hemoglobin A1c | 0.92 (0.86–1.01) | 1.04 (0.93–1.25) | 1.03 (0.82–1.24) | 0.72 (0.53–1.13) | >0.5 |
| Triglyceride | 1.03 (0.92–1.11) | 1.03 (0.94–1.16) | 0.92 (0.86–1.02) | 1.04 (0.90–1.36) | >0.1 |
| LDL-c/HDL-c | 1.07 (1.02–1.11) | 1.04 (1.02–1.11) | 1.05 (0.91–1.19) | 1.11 (0.86–1.36) | >0.25 |
| Estimated GFR | 1.03 (0.92–1.10) | 0.80 (0.74–0.86) | 1.02 (0.91–1.19) | 0.80 (0.58–1.12) | <0.05 |
| Changes within 36 months | |||||
| Body mass index | 0.92 (0.84–1.03) | 0.95 (0.81–1.09) | 0.90 (0.72–1.09) | 1.10 (0.81–1.40) | >0.25 |
| Systolic blood pressure | 0.94 (0.86–1.05) | 0.90 (0.86–1.05) | 1.10 (1.03–1.17) | 1.03 (1.01–1.04) | <0.01 |
| Hemoglobin A1c | 0.98 (0.90–1.05) | 1.17 (1.05–1.30) | 0.96 (0.85–1.19) | 0.90 (0.61–1.20) | >0.5 |
| Triglyceride | 1.03 (0.92–1.07) | 1.03 (0.90–1.12) | 0.97 (0.89–1.04) | 1.10 (0.92–1.35) | >0.25 |
| LDL-c/HDL-c | 1.16 (1.10–1.20) | 1.13 (1.08–1.19) | 1.07 (0.86–1.23) | 1.30 (0.99–1.64) | >0.25 |
| Estimated GFR | 1.12 (0.86–1.19) | 0.90 (0.82–0.99) | 1.11 (0.93–1.32) | 1.03 (0.83–1.24) | <0.05 |
GFR: Glomerular filtration rate; LDL-c: low-density lipoprotein cholesterol; HDL-c: high-density lipoprotein cholesterol
Model 1 adjusted for age, race, sex, smoking, insurance type, hypoglycemia events, α-glucosidase inhibitors, meglitinides, thiazolidinediones, anti-hypertensive medications, lipid-lowering medications, antiplatelet, anticoagulant medications, history of stroke, peripheral vascular disease, diabetic retinopathy and diabetic nephropathy and changes of body mass index, systolic blood pressure, hemoglobin A1c, triglyceride, LDL-c/HDL-c, and estimated GFR, other than the variable for analysis. Model 2 adjusted for variables in model 1 plus baseline levels of body mass index, systolic blood pressure, hemoglobin A1c, triglyceride, LDL-c/HDL-c, and estimated GFR.
Finally, mediation analysis was used to quantify the contribution of per SD change of systolic blood pressure to the risk of ischemic heart diseases (Figure S1). The β1 of use of SGLT2 inhibitors for the risk of ischemic heart diseases was −0.56 (P<0.001) without per SD change of systolic blood pressure in the model. The β2 of use of SGLT2 inhibitors for per SD change of systolic blood pressure was 0.11 (P<0.001). The β3 of use of SGLT2 inhibitors for per SD change of systolic blood pressure was −0.49 (P<0.001) after controlling for per SD change of systolic blood pressure. The mediated proportion was 11.3% accordingly.
Discussion
In this analysis using data from a real world healthcare delivery system, we demonstrated a significantly lower risk of ischemic heart diseases among patients with type 2 diabetes using SGLT2 inhibitors compared to those not using SGLT2 inhibitors. Patients with type 2 diabetes using SGLT2 inhibitors could have significant reductions in HbA1c as 0.2%, systolic blood pressure as 1.0 mmHg, BMI as 0.5 kg/m2, and triglyceride as 9.5 mg/dl compared to those not using SGLT2 inhibitors during the first 6 months of the treatment, while the reduction of diastolic blood pressure seemed to be stable. Furthermore, the mediation analysis indicated that use of SGLT2 inhibitors was shown to reduce the development of ischemic heart diseases partly through a reduction of systolic blood pressure.
The benefits of SGLT2 inhibitors on major adverse cardiovascular events and cardiovascular mortality among patients with type 2 diabetes have already been demonstrated in randomized clinical trials.11–13 However, comparisons to placebos were used in all these trials, which could not address the comparative effectiveness and safety relative to other glucose lowering drugs. In this case, observational datasets from the real world healthcare are important in addressing these questions. Several large-scale national or multinational real world studies from Europe24–26 and Asia27,28 have shown that use of SGLT2 inhibitors was associated with reduced risk of cardiovascular disease and cardiovascular mortality compared with use of other glucose lowering drugs in patients with type 2 diabetes, however, these studies did not assess the major reasons for these benefits. Very few real world studies from the United States29–31 have investigated the comparative effectiveness of SGLT2 inhibitors on outcomes including heart failure and lower limb amputation, and several other studies only investigated the effectiveness of canagliflozin on short term outcomes.32–35 To our knowledge, this is the first real world study that has investigated the comparative effectiveness of SGLT2 inhibitors on ischemic heart diseases risk between users and non-users in the United States. Our findings are consistent with the results from clinical trials in patients with type 2 diabetes. In addition, a mediation effect of reduction of systolic blood pressure between use of SGLT2 inhibitors and the low risk of ischemic heart diseases was also confirmed in our analysis. Per SD reduction of systolic blood pressure could account for a 12.6% reduction of ischemic heart diseases risk. Our findings were also consistent with the recommendations newly updated by European Society of Cardiology (ESC) in collaboration with European Association for the Study of Diabetes (EASD) that SGLT2 inhibitors are recommended in patients with type 2 diabetes and cardiovascular disease, or at very high or high cardiovascular risk.36
In our subgroup analysis, compared to patients with other glucose lowering drugs, we found a consistent lower risk of ischemic heart diseases in patients with SGLT2 inhibitors at different ages, races, sexes, BMI, HbA1c, eGFR, never and past or current smokers, and patients using lipid lowering, anti-hypertensive medications as well as antiplatelet or anticoagulant agents or not using. These findings indicated that patients on SGLT2 inhibitors have a lower risk of ischemic heart diseases independent of the factors for stratification. We also examined sex and race differences in comparative effectiveness, which none of the studies from Europe and Asia investigated. A significant interaction between sexes was noticed while there was no interaction between whites and African Americans. Men could obtain more benefits from SGLT2 inhibitors than women. Older patients with poorer renal function at baseline could obtain more benefits from SGLT2 inhibitors than younger patients, while use of antiplatelet or anticoagulant drugs would slightly compromise the effectiveness of SGLT2 inhibitors.
The mechanism underlying the cardiovascular benefits of SGLT2 inhibitors has drawn much attention. However, little is known about potential mechanisms from clinical trials. Our findings support the hypothesis of the insulin-independent glucose lowering effect of SGLT2 inhibitors through increased urinary excretion of glucose.37 SGLT2 inhibitors can also increase fractional excretion of sodium, thus causing a modest diuretic effect.38 This is why a significant reduction of blood pressure was found in patients with SGLT2 inhibitors. This effect beyond glucose lowering actually contributed to the relatively low risk of ischemic heart diseases. Animal models also showed that SGLT2 inhibitors can improve oxidative stress and endothelial function.39 SGLT2 inhibitors also have a great impact on the neurohormonal modulation and regulation of inflammation.37 All these contributions together may finally help improve the outcomes in patients using SGLT2 inhibitors.
A major strength of this study is the mediation analysis that is usually presented in clinical trials. The adherence to the follow up visits of patients in one healthcare system in our analysis allowed us to look into the changes in clinical characteristics and study the potential mechanisms underlying our findings. The relatively rich clinical data and numerous events also make the results robust. The data we used were derived from administrative databases, avoiding the problem of differential recall bias. Data in this study were extracted from one partner of REACHnet, which minimizes the influence of low accessibility of health care. There are also several limitations in this study. First, the sample size of patients with SGLT2 inhibitors was relatively small compared to the sample size of other real world studies. Meanwhile, patients from Louisiana cannot fully represent the population in the United States as the proportion of African Americans is a bit higher than that in other states. Second, the propensity score matching was suboptimal on some covariates. In our opinion, it is reasonable in clinical practice that patients with an initial or add-on therapy of SGLT2 inhibitors may have poorer glycemic control and more comorbidities than other patients. In addition, some socioeconomic variables were missing in the EMR data including education level, family income, etc. A dose-response effect of SGLT2 inhibitors on risk of ischemic heart diseases was not analyzed. The incidence rates of ischemic heart diseases were high, thus we were unable to perform a chart review to validate all ischemic heart disease events that occurred. Finally, our analyses adjusted for some confounding factors, however, unmeasured factors such as duration of diabetes, family history of diabetes, other related chronic diseases, dietary factors and physical activity could not be evaluated.
In conclusion, we demonstrated a significantly lower risk of ischemic heart diseases associated with the use of SGLT2 inhibitors compared to no use of SGLT2 inhibitors among patients with type 2 diabetes in one real world study. Use of SGLT2 inhibitors was also significantly associated with reductions of several metabolic risk factors. The contribution of reductions of systolic blood pressure beyond glucose lowering could partly explain the benefits to the risk of ischemic heart diseases from SGLT2 inhibitors.
Supplementary Material
Acknowledgments
The LEAD Study would like to acknowledge the contributions of our partners. The success of this study depended on their ongoing support and expertise. These partners include Ochsner Health System and the Ochsner Patient Research Advisory Board; Tulane Medical Center; University Medical Center New Orleans; Research Action for Health Network (REACHnet, a PCORnet Clinical Research Network) and their multi-stakeholder Diabetes Advisory Groups; Pennington Biomedical Research Center; Blue Cross and Blue Shield of Louisiana; and our patient and community partners Patricia Dominick, Catherine Glover, and Peggy Malone.
Sources of Funding: This work was supported by a Patient-Centered Outcomes Research (PCORI) cooperative agreement (NEN-1508-32257) as part of Natural Experiments for Translation in Diabetes 2.0 (NEXT-D2) and a grant from the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health. All statements in this manuscript, including findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or Methodology Committee.
Footnotes
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1.Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England). 2016;387(10027):1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun X, Du T. Trends in cardiovascular risk factors among U.S. men and women with and without diabetes, 1988–2014. BMC public health. 2017;17(1):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Executive summary: Standards of medical care in diabetes--2013. Diabetes care. 2013;36 Suppl 1:S4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: diabetes mellitus - evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [online]. 2008. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf (Accessed: August 2 2019).
- 6.European Medicine Agency, Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus [online]. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/02/WC500073570.pdf (Accessed: August 2 2019).
- 7.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine. 2013;369(14):1317–1326. [DOI] [PubMed] [Google Scholar]
- 8.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England journal of medicine. 2013;369(14):1327–1335. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 12.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 13.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 14.Garber AJ, Abrahamson MJ, Barzilay JI, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2019 EXECUTIVE SUMMARY. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2019;25(1):69–100. [DOI] [PubMed] [Google Scholar]
- 15.Mearns ES, Saulsberry WJ, White CM, et al. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in Type 2 diabetes: a network meta-analysis. Diabetic medicine : a journal of the British Diabetic Association. 2015;32(12):1530–1540. [DOI] [PubMed] [Google Scholar]
- 16.Kalra S Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2014;5(2):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cefalu WT, Riddle MC. SGLT2 inhibitors: the latest “new kids on the block”! Diabetes care. 2015;38(3):352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, Shi L, Nauman E, et al. Inverse Association Between HDL (High-Density Lipoprotein) Cholesterol and Stroke Risk Among Patients With Type 2 Diabetes Mellitus. Stroke. 2019;50(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y, Shi L, Nauman E, et al. Race and sex differences in rates of diabetic complications. J Diabetes. 2019;11(6):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathak RD, Schroeder EB, Seaquist ER, et al. Severe Hypoglycemia Requiring Medical Intervention in a Large Cohort of Adults With Diabetes Receiving Care in U.S. Integrated Health Care Delivery Systems: 2005–2011. Diabetes care. 2016;39(3):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 24.Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. The lancet Diabetes & endocrinology. 2017;5(9):709–717. [DOI] [PubMed] [Google Scholar]
- 25.Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes, obesity & metabolism. 2018;20(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norhammar A, Bodegard J, Nystrom T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: A nationwide observational study. Diabetes, obesity & metabolism. 2019;21(5):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study. Journal of the American College of Cardiology. 2018;71(23):2628–2639. [DOI] [PubMed] [Google Scholar]
- 28.Ha KH, Kim B, Choi H, Kim DJ, Kim HC. Cardiovascular events associated with second-line anti-diabetes treatments: analysis of real-world Korean data. Diabetic medicine : a journal of the British Diabetic Association. 2017;34(9):1235–1243. [DOI] [PubMed] [Google Scholar]
- 29.Chang HY, Singh S, Mansour O, Baksh S, Alexander GC. Association Between Sodium-Glucose Cotransporter 2 Inhibitors and Lower Extremity Amputation Among Patients With Type 2 Diabetes. JAMA internal medicine. 2018;178(9):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold SV, Inzucchi SE, Tang F, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: An NCDR(R) Research to Practice project. European journal of preventive cardiology. 2017;24(15):1637–1645. [DOI] [PubMed] [Google Scholar]
- 31.Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes, obesity & metabolism. 2018;20(11):2585–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buysman EK, Chow W, Henk HJ, Rupnow MF. Characteristics and outcomes of patients with type 2 diabetes mellitus treated with canagliflozin: a real-world analysis. BMC endocrine disorders. 2015;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buysman EK, Chow W, Henk HJ, Rupnow MF. Characteristics and short-term outcomes of patients with type 2 diabetes mellitus treated with canagliflozin in a real-world setting. Current medical research and opinion. 2015;31(1):137–143. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JF, Parsa R, Bailey R. Real world clinical outcomes and patient characteristics for canagliflozin treated patients in a specialty diabetes clinic. Current medical research and opinion. 2017;33(1):77–84. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre P, Pilon D, Robitaille MN, et al. Real-world glycemic, blood pressure, and weight control in patients with type 2 diabetes mellitus treated with canagliflozin-an electronic health-record-based study. Current medical research and opinion. 2016;32(6):1151–1159. [DOI] [PubMed] [Google Scholar]
- 36.Grant PJ, Cosentino F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019;40(39):3215–3217. [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes & vascular disease research. 2015;12(2):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–e324. [DOI] [PubMed] [Google Scholar]
- 39.Oelze M, Kroller-Schon S, Welschof P, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PloS one. 2014;9(11):e112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


