Abstract
Objective:
This study was performed to investigate whether levetiracetam should be preferred to carbamazepine as a treatment choice for benign childhood epilepsy with centro Temporal spikes (BCECTS), the most common partial epilepsy of childhood.
Methods:
This randomized clinical trial study included 92 children with rolandic epilepsy aged 4–12 years referred to the Pediatric Neurology Clinic at Imam Hossein Hospital, Isfahan, Iran, from April 2019 to January 2020. Patients were selected consecutively and randomly assigned to two study groups (levetiracetam and carbamazepine groups). Patients were followed and revisited every 2 months for 6 months after starting the medication. The frequency and duration of seizure attacks and drug side effects were recorded before treatment and in bi-monthly visits. Data were analyzed by SPSS software Version 24 using Mann–Whitney U- test and Friedman test.
Findings:
In our study, the seizure frequency decrease was not significantly different between the two groups; however, patients in both groups showed significantly lower seizure frequency in 2, 4, and 6 months of follow-up compared to starting time. After a follow-up for 6 months, one out of 47 (2.1%) patients using levetiracetam showed intolerance, resulting in changing the medication. In addition, two out of 48 (4.1%) patients in the carbamazepine group had skin rashes. No significant changes had been reported regarding the duration of seizure attacks in both groups after treatment.
Conclusion:
This study showed encouraging results for using levetiracetam, with acceptable results and fewer side effects for the treatment of children with BCECTS in Iran.
KEYWORDS: Efficacy, epilepsy, pediatrics, rolandic seizure
INTRODUCTION
Benign childhood epilepsy with centrotemporal spikes (BCECTS), also known as benign rolandic epilepsy, is generally considered the most common focal epilepsy of childhood and has a prevalence rate of 15% of children with seizures aged 1–15 years.[1,2,3] Saeed et al. already reported the prevalence of BCECTS of 7.44% in Iran.[4] The International League Against Epilepsy classified it as idiopathic localization-related epilepsy.[5] It is the most common age-related partial epilepsy with a peak time of onset of 9 years of age; it occurs in 15%–24% of normal children without any neurologic deficit,[6] although some coexisting conditions (e.g., learning disabilities and intellectual impairment) have been documented to occur with increased frequency in children with BCECTS.[7,8,9] Benign rolandic epilepsy is a sleep-related disorder which occurs focally with clonic movements of the mouth, increased salivary secretion, gurgling noises, and a strange sensation involving the tongue and progresses to secondary generalization. Most seizures involve the facial and oropharyngeal muscles.[6,10] Treatment is neglected because seizures are often infrequent, occur during sleep, and eliminate at higher ages. However, factors in favor of drug therapy include early age of onset, frequently recurring seizures, generalized seizures, and daytime seizures. One major disadvantage of using antiepileptic drugs for this population is the medication side effects. There is no drug preference as a selective first-line treatment for BCECTS.[6,11] According to the NICE guidelines, carbamazepine is an acceptable treatment for rolandic epilepsy. Levetiracetam has recently been used in the treatment of BCECTS. The Food and Drug Administration approved levetiracetam as a treatment for partial seizures in adults.[12,13,14] Levetiracetam is preferred due to its fewer side effects and acceptable efficacy in various studies. As there was no similar study in Iran, comparing the efficacy of these drugs on BCECTS treatment, we decided to design a study to evaluate the efficacy of these drugs and their possible side effects in patients with BCECTS. Our hypothesis is to assess whether levetiracetam effect is superior and its side effect is less than carbamazepine.
METHODS
This study was a randomized controlled clinical trial on children with rolandic epilepsy aged 4–12 years referred to the Pediatric Neurology Clinic at Imam Hossein Hospital, Isfahan, Iran, from April 2019 to January 2020. After determining potential candidates, all aspects of the study were explained to the participants and their families. Written consent and oral assent were obtained from their parents and minors. All patient information, including name and address, was strictly confidential. The Research and Ethics Council of Isfahan University of Medical Sciences approved the study (Approval ID: IR.MUI.MED.REC.1398.349). Previous studies had already shown the efficacy of each drug used in the study; therefore, the opportunity for appropriate treatment was available to all patients, and treatment of them was not postponed due to participation in the study. The study has been submitted in the Iranian Registry of Clinical Trials (IRCT20190208042654N3). For calculating the data size, the rate of treatment response was assumed to be 50% for each of levetiracetam and carbamazepine to get the highest sample size. The least significant difference was defined as 30% as an expert opinion. Assuming the α = 0.05, β = 0.2, and 10% dropouts, the sample size was calculated 94 patients (47 for each group). Ninety-four children with BCECTS (clinical diagnosis and electroencephalography in favor of rolandic epilepsy) with the following inclusion criteria were recruited: should have more than two attacks per year, should have normal magnetic resonance imaging, and should be between 4 and 12 years old. Children under multidrug therapy, children with a history of severe side effects or drug reaction to levetiracetam or carbamazepine, inability of the patients to follow-up, and children/parents not having the consent to participate in the study were excluded from the study. In addition, in case of observing any severe drug reaction (such as severe side effects resulting in intolerance and skin rash) during the study, the drug was changed and he/she was excluded from the study. In this study started in winter 2018, patients were selected consecutively among children referred to the Pediatric Neurology Clinic of Imam Hossein Hospital if they met the inclusion criteria. After a complete explanation about the survey for parents and children, written informed consent from the guardian and oral assent from minors were obtained. Then, they were randomly assigned to the two study groups using SPSS-generated random numbers. This was an open-labeled clinical trial; patients and physicians and investigators were aware of groups. After getting a complete history and physical examination, the checklist had been filled with demographic information (age, gender, and weight), the number of attacks in recent 2 months, and the duration of attacks. Depending on their groups, patients were treated with levebel (levetiracetam) oral solution (100 mg/ml) at the initial dose of 25–30 mg/kg/day orally or tegretol (carbamazepine) syrup (20 mg/ml) at the initial dose of 15–20 mg/kg/day orally according to their groups. Patients were visited every 2 months by a pediatric neurologist at the Neurology Clinic of Imam Hossein Hospital for 6 months after starting the medication. The frequency and duration of seizure attacks and drug side effects were recorded during appointments using a predesigned checklist before treatment and every 2 months for 6 months thereafter. A patient was seizure-free when he/she did not experience any seizure for at least 1 month. Qualitative variables (gender) were reported as count and percentage, whereas quantitative variables (age and weight) were reported as mean and standard deviation. Fisher's exact test was used to evaluate the qualitative variables between the two groups. Independent t-test was used to examine the quantitative variables between the two groups. Nonparametric Mann–Whitney U-test was used when data were not distributed normally. Repeated-measure Friedman test was used to examine the number of attacks within the groups. Data were analyzed by SPSS software Version 24 (IBM SPSS Statistics for Windows, Version 24.0 Armonk, NY, USA: IBM Crop).
RESULTS
A total of 94 children (56 boys and 39 girls), aged between 4 and 12 years old, were included in our study. All of the selected families accepted (participation rate = 100%) to enter the study, but two of the children were excluded during the study due to the development of severe side effects. Mean seizure frequency before starting treatment was once during the last 2 months. Forty-six children were randomly assigned to each of the levetiracetam and carbamazepine groups. Levetiracetam group included 26 boys and 20 girls with a mean age of 8.7 ± 2.766 years, and the carbamazepine group included 28 boys and 18 girls with a mean age of 8.36 ± 2.250 years. There are no significant differences between the two groups regarding age, gender, and weight [Table 1]. After follow-up for 6 months, one out of 47 (2.1%) patients using levetiracetam and one out of 47 patients (2.1%) using carbamazepine showed severe drug reaction. Decreased appetite was the major severe drug reaction, which resulted in intolerance and changing the medication. The two patients, therefore, excluded from the study. In addition, two of the patients in the carbamazepine group (4.2%) had skin rashes. No other adverse side effects, including hematological, biochemical, or behavioral signs/symptoms, were reported in any children. In addition, none of the children exhibited worsening of their seizures and the physical examinations of children remained normal. No significant changes were reported in the duration of attacks after treatment in both groups. The seizure frequency trends in two groups during 2, 4, and 6 months after treatment are presented in Figure 1. No seizures continued after 6 months from initiation of treatment in both groups. In our study, although decrease in seizure frequency was not significantly different between the two groups (P for trend = 0.8897), patients in both groups showed significantly lower seizure frequency after 2, 4, and 6 months of follow-up compared to starting time (P < 0.001) [Table 2]. Approximately 75% and 100% of the patients in both groups were seizure-free after 2 and 4 months of treatment, respectively. Figure 2 shows the mean dosages of levetiracetam and carbamazepine and their associated seizure frequency along the time. The levetiracetam and carbamazepine dosages ranged from 27.07 to 31.57 mg/kg/daily and from 12.78 to 13.13 mg/kg/daily (differences due to rounding the amount of daily prescribed drug), respectively [Figure 2].
Table 1.
Demographic characteristics of participants (n=92)
| Levetiracetam | Carbamazepine | P | |
|---|---|---|---|
| Gender | 0.832* | ||
| Male | 26 | 28 | |
| Female | 20 | 18 | |
| Age (mean±SD) | 8.7±2.766 | 8.36±2.250 | 0.514† |
| Weight (mean±SD) | 28.45±9.306 | 28.72±8.754 | 0.882† |
*Fisher’s exact test was used, †Mann-Whitney U-test was used. SD=Standard deviation
Figure 1.
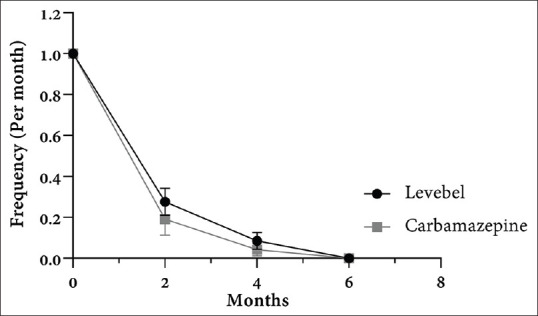
Changes in the frequency of seizures before and 2, 4, and 6 months after treatment. There is no significant difference between changes in seizure frequency between groups (P for trend = 0.8897). Data were shown as mean with standard error in each time point.
Table 2.
Comparison of frequency of seizures between two groups and along the time
| Frequency (/month) | Before treatment | After treatment |
P* | ||
|---|---|---|---|---|---|
| 2 months | 4 months | 6 months | |||
| Levetiracetam | 1 | 0.2766 | 0.0851 | 0 | <0.001 |
| Carbamazepine | 1 | 0.1915 | 0.0425 | 0 | <0.001 |
| P† | - | 0.408 | 0.404 | - | |
*Friedman repeated measure test was used. †Independent t-test was used
Figure 2.
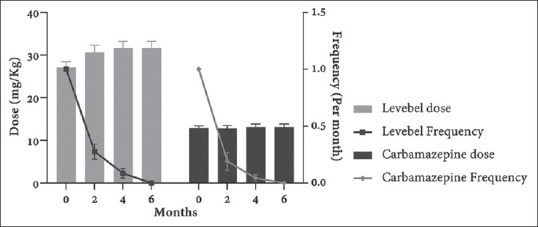
Changes in the average dose and associated frequency in two groups before and 2, 4, and 6 months after treatment. Data were shown as mean with standard error in each time point.
DISCUSSION
We aimed to evaluate the efficacy of levetiracetam and carbamazepine and their possible side effects in patients with BCECTS in a randomized clinical trial in Iran. Kanemura et al. currently reported that levetiracetam was effective and well tolerated as monotherapy in pediatric patients with rolandic epilepsy, and it seemed to be superior to carbamazepine and valproic acid in its ability to suppress rolandic discharges in children; however, the trends of the efficacy of levetiracetam were not significantly higher.[15,16,17] Asadi-Pooya et al. reviewed the current literature and suggested that levetiracetam is probably a better choice compared with carbamazepine to be prescribed for children with rolandic epilepsy due to its better effect on cognitive problems, better tolerance, and fewer side effects in comparison with other antiepileptic drugs.[18,19] Moreover, it had already suggested that levetiracetam could even improve some cognitive skills.[20] In another study, both levetiracetam and oxcarbazepine were effective in controlling epilepsy in children with idiopathic rolandic convulsions during a 2-year follow-up.[2] In a study of 6–12-year-old children with partial seizures treated with levetiracetam, 45% of them had complete improvement after 7–12 months of follow-up.[21] In a case series of three children with BCECTS treated with levetiracetam, only one child experienced one seizure; otherwise, all the children were seizure-free.[1] This study achieved comparable results, in which all cases were seizure-free after six months. Maybe, this difference was due to cross-racial differences. Further, levetiracetam took a relatively short time to achieve therapeutic levels.[1] Figure 2 shows the association between administered dosages and seizure frequency [Figure 2]. The rates of seizure reduction were not significantly different between the two groups. Moreover, the rate of becoming seizure-free and the effectiveness of levetiracetam were similar to carbamazepine. The important advantage of levetiracetam in comparison with carbamazepine is the absence of severe side effects. Mcnally and Kossoff reported that an empiric 2–3-month trial of levetiracetam (20–40 mg/kg/day) could be offered to children with incidental rolandic spikes who had cognitive difficulties.[22] In another study, all of the children responded well to low-dose levetiracetam (mean dose: 22.7 ± 4.7; maximum dose: 37 mg/kg/day) without inducing severe side effects.[23] Pharmacokinetic studies revealed an increased clearance of levetiracetam in children (1.43 ± 0.36 ml/min/kg) compared to adults (1.13 ± 0.30 ml/min/kg), which may result in a higher daily maintenance dose in children.[1,13] Similarly, the mean dosage of levetiracetam in our study was 27.07 ± 9.28, and all of our participants had full response to the levetiracetam medication.
The ultimate goal of epilepsy treatment is to stop seizures, to have fewer adverse effects, and to achieve a better quality of life. In previous studies, the most common reported side effects were headache, infection, drowsiness, anorexia, and anxiety for levetiracetam and somnolence, dizziness, constipation, itching, poor concentration, and nausea and vomiting for carbamazepine.[24,25,26,27] In another study, sedation was the only mild, transient, and tolerable drug complication for levetiracetam.[1] Moreover, side effects such as rash, gastric pain, drowsiness, and cognitive impairment were well tolerated in the levetiracetam group, except for a mild and transient decreased appetite in two cases, which need to be discontinued in only one patient because of persisted headache and decreased appetite. In our study, there was just one case in the levetiracetam group with intolerance, which led to discontinuation and changing the medication. Unlike carbamazepine, levetiracetam did not cause weight gain and hair thinning.[1,28] The risk of skin rash in carbamazepine was 10%, whereas the risk of skin rash in levetiracetam was approximately close to the placebo.[29] We only had two cases (4.2%) showing skin rash in the carbamazepine group. Patients in the levetiracetam group did not develop any skin rash. However, similar study, in 2008, comparing levetiracetam with carbamazepine in children with partial epilepsy showed acceptable results from both drugs,[30] while another study showed overall better quality of life with levetiracetam treatment than carbamazepine that favors the use of levetiracetam instead of carbamazepine.[25]
One important limitation of this study is lacking serum drug levels, which are more reliable than weight-based dosages. In addition, shorter intervals and longer duration of follow-up visits may able us to make firmer conclusions. Moreover, we did not evaluate the cognitive and behavioral problems in our patients.
However, our preliminary data suggest that levetiracetam could be an acceptable choice for the monotherapy of BCECTS, especially in the Iranian population with high efficacy and without severe side effects.
This study showed encouraging results for using levetiracetam, with acceptable results and fewer side effects, instead of carbamazepine for the treatment of children with BCECTS in Iran.
AUTHORS' CONTRIBUTION
Parisa Ahadi and Jafar Nasiri contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Parisa Ahadi, Jafar Nasiri, Toktam Mosavian, and Vahid Mansouri. The first draft of the manuscript was written by Vahid Mansouri, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We should thank the pediatric neurology department for their support and every patient and their families for their patience. This clinical trial, supported by Isfahan University of Medical Sciences (Project Number: 398460), has been submitted in the Iranian Registry of Clinical Trials (IRCT20190208042654N3).
REFERENCES
- 1.Bello-Espinosa LE, Roberts SL. Levetiracetam for benign epilepsy of childhood with centrotemporal spikes-three cases. Seizure. 2003;12:157–9. doi: 10.1016/s1059-1311(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 2.Coppola G, Franzoni E, Verrotti A, Garone C, Sarajlija J, Operto FF, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): An open-label, parallel group trial. Brain Dev. 2007;29:281–4. doi: 10.1016/j.braindev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Duchowney MS. Benign childhood partial seizures and related epileptic syndromes. Neurology. 2000;54:1713. doi: 10.1136/pmj.76.892.127a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeed M, Muhammad A, Shabbir N, Qamar SA. Is benign childhood epilepsy with centrotemporal spikes always benign? Iran J Child Neurol. 2014;8:38. [PMC free article] [PubMed] [Google Scholar]
- 5.Manford M, Hart YM, Sander JW, Shorvon SD. The National General Practice study of Epilepsy. The syndromic classification of the International League against Epilepsy applied to epilepsy in a general population. Arch Neurol. 1992;49:801–8. doi: 10.1001/archneur.1992.00530320025008. [DOI] [PubMed] [Google Scholar]
- 6.Verrotti A, Latini G, Trotta D, Giannuzzi R, Cutarella R, Morgese G, et al. Typical and atypical rolandic epilepsy in childhood: A follow-up study. Pediatr Neurol. 2002;26:26–9. doi: 10.1016/s0887-8994(01)00353-8. [DOI] [PubMed] [Google Scholar]
- 7.Yung AW, Park YD, Cohen MJ, Garrison TN. Cognitive and behavioral problems in children with centrotemporal spikes. Pediatr Neurol. 2000;23:391–5. doi: 10.1016/s0887-8994(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 8.Verrotti A, Filippini M, Matricardi S, Agostinelli MF, Gobbi G. Memory impairment and benign epilepsy with centrotemporal spike (BECTS): A growing suspicion. Brain Cogn. 2014;84:123–31. doi: 10.1016/j.bandc.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Filippini M, Boni A, Giannotta M, Gobbi G. Neuropsychological development in children belonging to BECTS spectrum: Long-term effect of epileptiform activity. Epilepsy Behav. 2013;28:504–11. doi: 10.1016/j.yebeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Larsson PG, Bakke KA, Bjørnæs H, Heminghyt E, Rytter E, Brager-Larsen L, et al. The effect of levetiracetam on focal nocturnal epileptiform activity during sleep – A placebo-controlled double-blind cross-over study. Epilepsy Behav. 2012;24:44–8. doi: 10.1016/j.yebeh.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Tacke M, Borggraefe I, Gerstl L, Heinen F, Vill K, Bonfert M, et al. Effects of levetiracetam and sulthiame on EEG in benign epilepsy with centrotemporal spikes: A randomized controlled trial. Seizure. 2018;56:115–20. doi: 10.1016/j.seizure.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: A multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia. 2000;41:1276–83. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 13.Cereghino JJ, Biton V, Abou-Khalil B, Dreifuss F, Gauer LJ, Leppik I. Levetiracetam for partial seizures: Results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–42. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- 14.Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P, Group EL. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. Epilepsia. 2000;41:1179–86. doi: 10.1111/j.1528-1157.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanemura H, Sano F, Ohyama T, Aihara M. Efficacy of levetiracetam for reducing rolandic discharges in comparison with carbamazepine and valproate sodium in rolandic epilepsy. Seizure. 2018;62:79–83. doi: 10.1016/j.seizure.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Kanemura H, Sano F, Ohyama T, Sugita K, Aihara M. Effect of levetiracetam monotherapy in nonlesional focal childhood epilepsy. Neuropediatrics. 2018;49:135–41. doi: 10.1055/s-0037-1613680. [DOI] [PubMed] [Google Scholar]
- 17.Romoli M, Mazzocchetti P, D'Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, et al. Valproic acid and epilepsy: From molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019;17:926–46. doi: 10.2174/1570159X17666181227165722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadi-Pooya AA, Forouzesh M, Eidi H, Mirzaghafour SE. Levetiracetam versus carbamazepine in treatment of rolandic epilepsy. Epilepsy Behav. 2019;94:1–8. doi: 10.1016/j.yebeh.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Gerstl L, Willimsky E, Rémi C, Noachtar S, Borggräfe I, Tacke M. Treatment of BECTS: A systematic review of seizure freedom rates. Neuropediatrics. 2019;50:GNP–PO17. doi: 10.1097/WNF.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 20.Operto FF, Pastorino GM, Mazza R, Roccella M, Carotenuto M, Margari L, et al. Cognitive profile in BECTS treated with levetiracetam: A 2-year follow-up. Epilepsy Behav. 2019;97:187–91. doi: 10.1016/j.yebeh.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 21.Elberry AA, Felemban RK, Hareeri RH, Kurdi SM. Efficacy and safety of levetiracetam in pediatric epilepsy. Saudi Pharm J. 2012;20:81–4. doi: 10.1016/j.jsps.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally MA, Kossoff EH. Incidental rolandic spikes: Long-term outcomes and impact of treatment. Epilepsy Behav. 2015;43:135–8. doi: 10.1016/j.yebeh.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Xiao F, An D, Deng H, Chen S, Ren J, Zhou D. Evaluation of levetiracetam and valproic acid as low-dose monotherapies for children with typical benign childhood epilepsy with centrotemporal spikes (BECTS) Seizure. 2014;23:756–61. doi: 10.1016/j.seizure.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42:1574–9. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 25.Suresh SH, Chakraborty A, Virupakshaiah A, Kumar N. Efficacy and safety of levetiracetam and carbamazepine as monotherapy in partial seizures. Epilepsy Res Treat. 2015;2015:415082. doi: 10.1155/2015/415082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banu SH, Jahan M, Koli UK, Ferdousi S, Khan NZ, Neville B. Side effects of phenobarbital and carbamazepine in childhood epilepsy: Randomised controlled trial. BMJ. 2007;334:1207. doi: 10.1136/bmj.39022.436389.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormier J, Chu CJ. Safety and efficacy of levetiracetam for the treatment of partial onset seizures in children from one month of age. Neuropsychiatr Dis Treat. 2013;9:295–306. doi: 10.2147/NDT.S30224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French J. Use of levetiracetam in special populations. Epilepsia. 2001;42(Suppl 4):40–3. [PubMed] [Google Scholar]
- 29.Konishi T, Naganuma Y, Hongo K, Murakami M, Yamatani M, Okada T. Carbamazepine-induced skin rash in children with epilepsy. Eur J Pediatr. 1993;152:605–8. doi: 10.1007/BF01954091. [DOI] [PubMed] [Google Scholar]
- 30.Perry S, Holt P, Benatar M. Levetiracetam versus carbamazepine monotherapy for partial epilepsy in children less than 16 years of age. J Child Neurol. 2008;23:515–9. doi: 10.1177/0883073807309784. [DOI] [PubMed] [Google Scholar]


