Abstract
Objective:
Nonvariceal upper gastrointestinal bleeding (NUGIB) is a common cause of hospitalization and is associated with considerable mortality and morbidity. Octreotide has been shown to be an effective treatment in the control of variceal UGIB. Theoretically, octreotide could be effective in the treatment of other types of bleeding ulcers.
Methods:
This randomized, double-blind, placebo-controlled trial was carried out on patients with NVUGIB who had been admitted to two referral centers in Shiraz, Iran. Patients were randomized to two groups: Group A (n = 58) received octreotide and Group B (n = 58) received a placebo. Patients in both groups received pantoprazole 40 mg as an initial dose, then 40 mg every 12 h intravenously. In addition to the pantoprazole, patients in Group A received 100 μg octreotide subcutaneously every 8 h for 72 h or until they were discharged. Patients in Group B received pantoprazole and a placebo at the same dose schedule.
Findings:
There were no statistically significant differences between Groups A and B in terms of mortality (0 vs. 5.17%; P = 0.21,) rebleeding rate (5.17% vs. 1.72%; P = 0.5), blood transfusion requirement (1.65 ± 0.47 units vs. 1.70 ± 0.45 units; P = 0.45), length of hospital stay (1.96 ± 1.00 days vs. 1.65 ± 0.84 days; P = 0.44), and need for surgery (1.72% vs. 1.72%; P = 0.7).
Conclusion:
The results showed that use of subcutaneous octreotide as an adjuvant treatment did not have a beneficial effect on the treatment of NVUGIB.
KEYWORDS: Nonvariceal upper gastrointestinal bleeding, Octreotide, randomized controlled trial
INTRODUCTION
Acute upper gastrointestinal bleeding (UGIB) is a common cause of hospitalization. The incidence of hospitalization for acute UGIB varies from 50 to 150 hospital admissions per 100,000 population each year.[1,2,3,4] Acute UGIB is associated with high morbidity and mortality, as well as a significant use of health-care resources.[4] UGIB is more frequent among men than women, and increases with age.[4] Acute UGIB can be divided into variceal and nonvariceal types. Most UGIB are nonvariceal and are commonly caused by peptic gastroduodenal ulcers.[5] Other major causes of UGIB include mucosal tears of the esophagus or fundus (Mallory–Weiss tears), erosive gastritis/duodenitis, erosive esophagitis, and mass lesions (i.e., polyps or cancerous tumors). Duodenal ulcers (DUs) are more common than gastric ulcers (GUs), although the incidence of bleeding is the same in both types of ulcer. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) and antiplatelet drugs is a common risk factor for nonvariceal UGIB (NVUGIB).[6] Endoscopic gastroduodenal ulcers have been found in 15%–45% of patients who regularly take NSAIDs; GUs are about four times more common than DUs.[7]
One common treatment of NVUGIB involves proton-pump inhibitors (PPIs) with/without endoscopic intervention. PPIs are recommended for all patients with NVUGIB because they increase the gastric pH, which stabilizes the blood clotting process.[8] Although UGIB is a common medical problem, the overall mortality rate associated with this condition has not significantly changed over the past few decades and the rebleeding rate has remained at 20%.[9] Some cases of NVUGIB require surgery or radiologic intervention due to the failure of medical and endoscopic treatments. Therefore, improvements are still needed in the medical treatment of NVUGIB.[10]
Octreotide (synthetic peptide analog of somatostatin) reduces portal venous pressure, splanchnic blood flow, and gastrointestinal motility. It also decreases gastric mucosal blood flow and exerts profound inhibitory effects on several gastrointestinal functions, including the secretion of gastric acid, gastrin, and pepsin.[11] The inhibition of pepsin secretion can stabilize clots or fibrin plugs, which are readily digested by proteolytic activity. Theoretically, octreotide might offer an advantage over drugs that only inhibit gastric acid secretion, such as H2-receptor antagonists and PPIs. Peak serum concentrations of octreotide occur within 30 min after subcutaneous administration. The average bioavailability of a subcutaneous dose is 100%.[12]
Intravenous octreotide is part of the standard treatment of variceal UGIB, although its role in the management of NVUGIB is uncertain. One meta-analysis suggested that the intravenous administration of somatostatin or octreotide, which is a long-acting form of somatostatin, decreases the risk of rebleeding from peptic ulcers when compared with a placebo or an H2-receptor blocker. Somatostatin has been found to be more effective than pantoprazole in maintaining a high gastric pH during the first 12 h of infusion.[13] Because the majority of rebleeding occurs within 24 h after index bleeding, adding somatostatin or octreotide to PPIs might further promote hemostasis in patients who are at high risk of rebleeding. However, few studies have investigated somatostatin or octreotide as an adjuvant therapy, and some of the studies that have looked at this issue have had methodological limitations.[6,11,14,15]
The present study used a randomized, double-blind, placebo-controlled trial to assess the effectiveness of octreotide as an adjuvant therapy with standard PPI ± endoscopic therapy of NVUGIB.
METHODS
This study was designed according to the Consolidated Standards of Reporting Trials (CONSORT) checklist.[16] Male and female patients with acute UGIB were selected from the emergency departments of Namazi Hospital and Faghihi Hospital in Shiraz, Iran, in 2016–2017. Patients were enrolled in the study according to the inclusion and exclusion criteria. Patients were randomly divided into two groups using random allocation software: an intervention group (Group A) and an observation group (Group B).[17,18] The appropriate sample size was calculated using the mean comparison formula; the sample size considered the parameters related to the number of patients who needed blood transfusions according to the study by Nikolopoulou et al.[11]
This study was approved by the local Ethical Committee and the Research Council of Shiraz University of Medical Sciences, Shiraz, Iran (Ethics Committee: IR.SUMS.REC.1394.130). The study was registered in the Iranian Registry of Clinical Trials (IRCT: 2015050322066N).
Patients were included in the study if they were conscious, 18–80 years old, and presented with a complaint of hematemesis or tarry stools or melena during a rectal examination. Patients with Class 4 congestive heart failure, patients with end-stage renal disease on hemodialysis, pregnant or lactating women, patients with acute coronary events, patients with cirrhosis, patients who were taking an anticoagulant, and patients with coagulation disorders were excluded from the study. Patients were enrolled in the study after providing written consent. Each person was visited by a gastroenterologist, and all clinical information, such as presentation of bleeding (hematemesis, melena, or both), prior history of digestive disease, drug history, history of coagulation disorders, and demographic data, was recorded. According to the sampling method, the minimum number of patients in each group was 52. Allowing for the possibility that patients would withdraw or dropout of the study, there were considered seventy patients in each group.
Each patient was given an intravenous injection of 40 mg pantoprazole (Exir Pharmaceutical Company, Boroujerd, Iran) at the beginning of their treatment; thereafter, an intravenous dose of 40 mg pantoprazole was given every 12 h during their hospital stay. In addition, patients in Group A received 100 μg octreotide subcutaneously (Caspian Tamin Pharmaceutical Company, Rasht, Iran) before their endoscopy; thereafter, a subcutaneous dose of 100 μg octreotide was given every 8 h for 72 h or until they were discharged. Patients in Group B received a placebo at the same dose schedule used for octreotide.
All patients were given an esophagogastroduodenoscopy within 8 h of hemodynamic stabilization. Patients with GU or DUs with either active bleeding or a visible vessel were given a dual endoscopic treatment with an epinephrine injection followed by argon plasma coagulation. Blood clots were removed from the ulcers to determine the underlying pathology; patients received the dual endoscopic treatment if a visible vessel or active bleeding was found. A biopsy from the antrum was sent to the pathology lab to identify Helicobacter pylori infection. The decision to discharge a patient was based on hemodynamic stability, tolerance to diet, and the absence of rebleeding. Diet was initiated in patients with a clean base ulcer; if the patient was stable, they were discharged the same day. Patients who received endoscopic treatment were observed for 72 h in the hospital, whereas those with other types of ulcers were observed for a minimum of 24 h. Patients with bleeding varices in the esophagus or stomach were excluded from the study. Patients with endoscopic findings other than ulcers and varices, including Mallory–Weiss tears and Dieulafoy's lesions, received endoscopic intervention if there was active bleeding. Patients without active bleeding were observed for at least 12 h. Patients with rebleeding received a second endoscopic treatment; if the bleeding was not controlled, the patient was sent for surgery. Patients who developed complications from using octreotide at any time were excluded from the study; patients who wanted to withdraw from the study were also excluded. All patients were followed weekly for 1 month.
In this study, SPSS software (version 22, Co Ltd, Tokyo, Japan) was used to analyze the results. To investigate if the quantitative data had a normal distribution, the Kolmogorov–Smirnov test was used. In case of the rejection of the normality assumption, the Mann–Whitney and Chi-square tests were used to compare the quantitative and qualitative variables, respectively, between the groups. Spearman's rank correlation coefficient was used for testing the correlation between the quantitative variables. P < 0.05 was considered to be statistically significant. Data were plotted using GraphPad Prism (version 3, San Diego, CA, USA).
RESULTS
Figure 1 shows the trial profile and the patient flowchart based on the CONSORT statement.[16] A total of 140 patients with acute UGIB were screened and 116 were recruited to two groups. The demographic and clinical characteristics of the patients included age; sex; hemoglobin (Hb) level at admission; systolic blood pressure; alcohol consumption and smoking; NSAIDs and aspirin consumption; H. pylori (based on biopsy results); reason for GI bleeding; risk assessments of patients based on different scores according to Rockall, Blatchford, and AIMS65 scores [Table 1]; and the endoscopic results based on the Forrest classification [Table 2]. There were no differences in these characteristics between the two groups.
Figure 1.
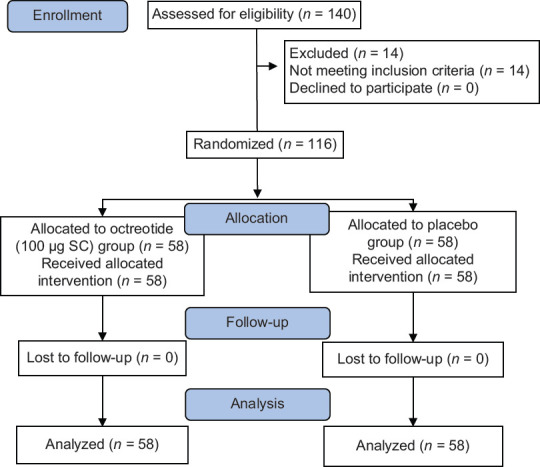
CONSORT flow diagram
Table 1.
Demographic and clinical baseline characteristics of patients
| Parameters | Octreotide group (n=58) | Placebo group (n=58) | P** |
|---|---|---|---|
| Age | 54.63±1.85 | 55.72±1.59 | 0.34 |
| Gender (male/female) | 38/20 | 38/20 | >0.5 |
| Hb at admission (g/dL) | 10.99±3.49 | 11.04±3.1 | 0.5 |
| Systolic BP (mmHg) | 114.08±14.54 | 112.65±13.28 | 0.13 |
| Alcohol abuse, n (%) | 2 (3.4) | 4 (6.9) | 0.34 |
| Smocking, n (%) | 8 (13.8) | 14 (24.1) | 0.11 |
| NSAID, n (%) | 23 (39.65) | 15 (25.86) | >0.5 |
| Aspirin, n (%) | 20 (34.48) | 19 (32.75) | >0.5 |
| Helicobacter pylori (Pathology positive), n (%) | 32 (55.17) | 39 (62.24) | 0.12 |
| Reason of GI bleeding | 0.98 | ||
| GU | 21 (36.2) | 20 (34.5) | |
| DU | 26 (44.8) | 27 (46.6) | |
| Esophagitis | 4 (6.9) | 4 (6.9) | |
| MWT | 4 (6.9) | 3 (5.2) | |
| EG/ED | 5 (8.6) | 2 (3.4) | |
| Classification of patients based on Rockall, Blatchford, and AIMS65 scores | |||
| Rockall score | 0.12 | ||
| ≤2 | 25 (43.1) | 38 (65.51) | |
| 3-7 | 32 (55.17) | 20 (34.48) | |
| ≥8 | 1 (1.72) | 0 | |
| Blatchford score | 0.61 | ||
| 0 | 9 (15.51) | 6 (10.34) | |
| 1-5 | 15 (25.86) | 21 (36.20) | |
| ≥6 | 33 (56.89) | 31 (53.44) | |
| AIMS65 score | 0.75 | ||
| ≤2 | 58 (100) | 56 (96.55) | |
| >2 | 0 | 2 (3.44) |
**Spearman’s statistical test. Hb=Hemoglobin, BP=Blood pressure, NSAID=Nonsteroidal anti-inflammatory drug, MWT=Mallory-Weiss tear, EG/ED=Erosive gastritis/Erosive doudenitis, GI=Gastrointestinal, GU=Gastric ulcer, DU=Duodenal ulcer
Table 2.
Endoscopic result based on Forrest classification
| Parameters | Octreotide group | Placebo group | P** |
|---|---|---|---|
| GU, n (%) | 21 (36.2) | 20 (34.5) | |
| 1A | 2 (9.52) | 0 | 0.27 |
| 1B | 0 | 2 (7.40) | |
| 2A | 8 (38.09) | 4 (14.8) | |
| 2B | 0 | 0 | |
| 2C | 4 (19.04) | 3 (11.11) | |
| 3 | 7 (33.33) | 11 (40.74) | |
| DU, n (%) | 26 (44.8) | 27 (46.6) | |
| 1A | 1 (3.84) | 1 (3.70) | 0.5 |
| 1B | 0 | 0 | |
| 2A | 9 (34.61) | 6 (30) | |
| 2B | 0 | 0 | |
| 2C | 8 (33.76) | 6 (22.22) | |
| 3 | 8 (33.76) | 14 (51.85) |
**Spearman statistical test. DU=Duodenal ulcer, GU=Gastric ulcer
Table 3 shows the mean Hb levels of patients in both groups. There was no significant difference between the two groups in the Hb levels during admission and discharge. Twenty patients in Group A and 17 patients in Group B received blood transfusions [Table 3]; patients in Group A received an average of 1.65 ± 0.47 units, whereas patients in Group B received an average of 1.70 ± 0.45 units. Table 3 also shows the average length of hospital stay in the two groups. The mean hospital stay in Group A and Group B was 1.96 ± 1.00 days and 1.65 ± 0.84 days, respectively [Table 3 and Supporting Box 1].
Table 3.
Comparison of clinical outcomes difference between groups
| Parameters | Octreotide group (n=58) | Placebo group (n=58) | P** |
|---|---|---|---|
| Blood transfusion (mean±SEM) | 1.65±0.47 | 1.7±0.45 | 0.45 |
| Rebleeding during the first 3 days (%) | 3 (5.17) | 1 (1.72) | 0.5 |
| Hospital stay (day) | 1.96±1.00 | 1.65±0.84 | 0.44 |
| Surgery (%) | 1 (1.72) | 1 (1.72) | 0.77 |
| Mortality (%) | 0 | 3 (5.17) | 0.21 |
**Spearman statistical test. Hb=Hemoglobin, SEM=Standard error of means
About 5.17% of the patients in Group A and 1.72% of the patients in Group B had early-onset rebleeding within the first 3 days of admission [Table 3]. Despite receiving second endoscopic treatment, the bleeding in one patient from each group was not controlled and they were surgically treated [Table 3].
Three deaths (5.17%) occurred in Group B; there were no deaths in Group A. Two deaths occurred in the 1st week after index bleeding: one patient died due to acute myocardial infarction the day after discharge and one patient died due to unknown causes after leaving the hospital against medical advice. The third death occurred 14 days after discharge, which was due to sepsis.
The Rockall score correlated with the need for blood transfusion, the duration of hospital stay, the rate of rebleeding, and mortality [Table 4]. The Blatchford score significantly correlated with the risk of rebleeding, the need for blood transfusion, and the duration of hospital stay [Table 4]. The AIMS65 score had a significant correlation with mortality, the duration of hospital stay, and the need for blood transfusion [Table 4 and Supporting Box 2].
Table 4.
Relationship of different scores and rebleeding, mortality, the need for surgery, blood transfusion, and duration of hospitalization
| Score | Parameters | Rebleeding | Mortality | Need for surgery | Blood transfusion | Length of hospital stay |
|---|---|---|---|---|---|---|
| Rockall score | Spearman’s correlation score | 0.2 | 0.19 | −0.14 | 0.5 | 0.64 |
| CI | 1.41-1.43 | 2.621-2.688 | 0.673-0.739 | 0.690-0.757 | 0.842-0.916 | |
| Statistical significance | 0.009 | 0.037 | 0.13 | P<0.001 | P<0.001 | |
| Blatchford score | Spearman’s correlation score | 0.299 | 0.147 | −0.113 | 0.57 | 0.61 |
| CI | 2.38399-2.4091 | 2.3837-2.409 | 4.141-4.289 | 4.159-4.306 | 0.600-0.640 | |
| Statistical significance | 0.001 | 0.11 | 0.2 | P<0.001 | P<0.001 | |
| AIMES 65 score | Spearman’s correlation score | 0.095 | 0.22 | −0.066 | 0.36 | 0.31 |
| CI | 0.46074-0.4875 | 0.46055-0.4877 | 1.5609-1.4873 | 1.44-1.47 | 1.281-1.322 | |
| Statistical significance | 0.3 | 0.016 | 0.47 | P<0.001 | P<0.001 |
CI=Confidence interval
DISCUSSION
Despite a decrease in the incidence rate of peptic ulcers and an advance in the treatment of UGIB over the past few years, the prevalence of the condition and associated mortality are still high. These results can be partially attributed to the fact that UGIB mainly affects older patients who may also have other concurrent diseases, which are predictors of a poor prognosis. Currently, endoscopic treatment and PPIs are the treatment of choice for NVUGIB. However, despite treatment, there is a considerable risk of recurrent bleeding, which is associated with a high mortality.[19]
The success of endoscopic treatment in patients with UGIB is largely dependent on the skill of the endoscopist.[12] Although PPIs are now part of standard care, a recent study showed that the bolus and infusion of these drugs, even in high doses, might not be adequate for maintaining a stomach pH ≥6.[6] Thus, there is a search for adjuvant therapeutic methods that could work with endoscopic treatment and PPIs to improve the outcome in high-risk patients with UGIB.
In this study, we used subcutaneous octreotide as an adjuvant treatment with PPIs and endoscopic treatment for patients with NVUGIB. Somatostatin has been shown to be more effective than pantoprazole in maintaining a high gastric pH during the first 12 h after injection.[13] Theoretically, octreotide has powerful gastric antisecretory properties, which inhibit the basal and stimulated secretion of gastric acid and bile, as well as secretions from the pancreas. Somatostatin and octreotide inhibit acid secretion directly through cell wall inhibitory receptors and indirectly by reducing the release of gastrin. They also decrease the visceral blood flow and intestinal motility, which might have a stimulatory effect on the secretion of mucus.[20] The effects of somatostatin and octreotide might be beneficial for patients with NVUGIB, especially those with high-risk findings during endoscopy. However, while both drugs are used in the standard care of patients with variceal UGIB, clinical trials have reported contradictory results about their efficacy in patients with NVUGIB.[21,22]
In the present study, patients with NVUGIB were randomized to receive octreotide subcutaneously in addition to endoscopic treatment and PPIs. The results showed that octreotide provided no statistically significant benefit to patients in terms of blood transfusion requirement, rebleeding rate, need for surgery, length of hospital stay, and mortality (P ≥ 0.5).
In a meta-analysis, Imperiale and Birgisson[23] compared somatostatin or octreotide with H2 receptor antagonists or a placebo in the management of acute NVUGIB. They reported that somatostatin can control active bleeding and might be useful as an adjuvant therapy before endoscopy or when the endoscopy is either unsuccessful or unavailable.
In our study, three deaths occurred in the placebo group (5.1%), although this was not statistically significant in comparison with the intervention group (P = 0.2). It is notable that all of the deceased patients were elderly, did not experience rebleeding, and did not undergo surgery. Nikolopoulou et al.[11] found that the adjuvant use of octreotide in patients with NVUGIB had no statistically significant effect on mortality. In contrast with our study, they found that although the patients' age was a significant factor in predicting mortality, all deaths occurred in patients with coexisting diseases who underwent surgery. A similar report by Choi et al.[6] found that there was no benefit in using somatostatin as an adjuvant treatment in patients with NVUGIB; they reported that most of the deaths occurred in patients who experienced rebleeding.
In our study, one patient (1.72%) from each group underwent surgery. Octreotide did not have any beneficial effect on reducing the need for blood transfusions or decreasing the duration of hospital stays.
The limitations of this study included the use of subcutaneous octreotide rather than intravenous octreotide due to the lower cost and ease of administration. It may be possible that a high dose of intravenous octreotide would have a beneficial effect on NVUGIB. Furthermore, we used this drug for all patients with NVUGIB regardless of the severity of the condition. Our study was not designed to show if octreotide had a beneficial effect in patients with severe bleeding. In addition, we did not use objective measurements to determine the effect of octreotide on intragastric pH or splanchnic circulation.
Despite the progress in the management of patients with NVUGIB, it is essential that the mortality and need for surgery be reduced. This is especially necessary in elderly patients, those with severe bleeding, and those with comorbidities. Our study showed that subcutaneous octreotide given as an adjuvant therapy with intravenous pantoprazole and endoscopic treatment did not have any beneficial effects on the mortality, rebleeding rate, need for surgery, length of hospital stay, and blood transfusion requirement in selected patients with NVUGIB. Future studies using intravenous octreotide or octreotide in high-risk patients with severe bleeding might be warranted.
AUTHORS' CONTRIBUTION
All the authors have significantly contributed to the work. Masoud Abrishami and Payam Peyman designed and performed the experiments, derived the models, and analyzed the data. Marziyeh Zare assisted as measurement team and helped carry out the data. Masoud Abrishami, Payam Peymani, and Marziyeh Zare. wrote the manuscript in consultation with Kamran B. Lankarani.
Financial support and sponsorship
This work was supported by grants from the Vice-Chancellor of Research and Health Policy Research Center, Shiraz University of Medical Sciences (Grant Number: 94-01-49-9292).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Mr. H. Argasi at the Research Consultation Center of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
SUPPORTING BOXES: Supporting Box 1
It should be noted that in this study, vomiting fresh blood, hypotensive shock (systolic pressure ≤90 mmHg or heart rate ≥110 bpm) with melena after initial stability and treatment, decreased hemoglobin levels by more than 2 g/dl, or hematocrit reduction of more than 6% within 24 h after a transfusion that results in hemoglobin levels of 10 or less, were considered as criteria for re-hemorrhage and the need for re-endoscopy.
Supporting Box 2
In the Rockall Scale, a score of 0 to 11 points is awarded, with a score of 0 to 2 indicating good prognosis, a score of 3 to 7 indicating increased risk of mortality and rebleeding, and a score of >8 is a sign of high mortality. In this study, one of the patients in the group receiving octreotide had score above 8.
Classification based on AIMS65 score ≤2 is associated with low mortality, but with score >2, mortality is increased. Frequency of patients based on AIMS65 score is summarized in Table 1. In the octreotide group, all patients scored ≤2, while in the control group, 96.5% of the patients scored ≤2 and 3.44% of patients had score >2.
REFERENCES
- 1.Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. 2008;67:422–9. doi: 10.1016/j.gie.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Theocharis GJ, Thomopoulos KC, Sakellaropoulos G, Katsakoulis E, Nikolopoulou V. Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol. 2008;42:128–33. doi: 10.1097/01.mcg.0000248004.73075.ad. [DOI] [PubMed] [Google Scholar]
- 3.Loperfido S, Baldo V, Piovesana E, Bellina L, Rossi K, Groppo M, et al. Changing trends in acute upper-GI bleeding: A population-based study. Gastrointest Endosc. 2009;70:212–24. doi: 10.1016/j.gie.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 4.Sharifian A, Tavakoli E, Ashtari S, Zali MR. Endoscopic findings in upper gastrointestinal bleeding patients at Tehran's Taleghani hospital, Iran. Govaresh. 2016;21:260–5. [Google Scholar]
- 5.Masoodi M, Saberifiroozi M. Etiology and outcome of acute gastrointestinal bleeding in Iran: A review article. Middle East J Dig Dis. 2012;4:193–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Choi CW, Kang DH, Kim HW, Park SB, Park KT, Kim GH, et al. Somatostatin adjunctive therapy for non-variceal upper gastrointestinal rebleeding after endoscopic therapy. World J Gastroenterol. 2011;17:3441–7. doi: 10.3748/wjg.v17.i29.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostom A, Muir K, Dubé C, Jolicoeur E, Boucher M, Joyce J, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: A Cochrane collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28. 828.e1–5. doi: 10.1016/j.cgh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis of proton pump inhibitor therapy in peptic ulcer bleeding. BMJ. 2005;330:568. doi: 10.1136/bmj.38356.641134.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamsen S, Bendix J, Kallehave F, Moesgaard F, Nilsson T, Wille-Jørgensen P. Clinical practice and evidence in endoscopic treatment of bleeding peptic gastroduodenal ulcer. Scand J Gastroenterol. 2007;42:318–23. doi: 10.1080/00365520600880989. [DOI] [PubMed] [Google Scholar]
- 10.Ayantunde AA. Current opinions in bleeding peptic ulcer disease. J Gastroint Dig Syst. 2014;4:2. [Google Scholar]
- 11.Nikolopoulou VN, Thomopoulos KC, Katsakoulis EC, Vasilopoulos AG, Margaritis VG, Vagianos CE. The effect of octreotide as an adjunct treatment in active nonvariceal upper gastrointestinal bleeding. J Clin Gastroenterol. 2004;38:243–7. doi: 10.1097/00004836-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Riha HM, Wilkinson R, Twilla J, Harris LJ, Jr, Kimmons LA, Kocak M, et al. Octreotide added to a proton pump inhibitor versus a proton pump inhibitor alone in nonvariceal upper-gastrointestinal bleeds. Ann Pharmacother. 2019;53:794–800. doi: 10.1177/1060028019833696. [DOI] [PubMed] [Google Scholar]
- 13.Avgerinos A, Sgouros S, Viazis N, Vlachogiannakos J, Papaxoinis K, Bergele C, et al. Somatostatin inhibits gastric acid secretion more effectively than pantoprazole in patients with peptic ulcer bleeding: A prospective, randomized, placebo-controlled trial. Scand J Gastroenterol. 2005;40:515–22. doi: 10.1080/00365520510015458. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Lee YS, Koh BS, Kim W, Lim KS. Does adding somatostatin to proton pump inhibitor improve the outcome of peptic ulcer bleeding? Journal of the Korean Critical Care Medicine. 2008;23:75–8. [Google Scholar]
- 15.Yazdi J, Taslimi R, Anjarani S, Khavaran K. Assessing the effect of octreotide in non-varicose upper gastrointestinal bleeding in emergency department. Annals of military and health sciences research. 2012;10:232–7. [Google Scholar]
- 16.CONSORT Transparent Reporting of Trials, 2010. 2019. [Last accessed on 2019 Aug 15]. Available from: http://www.consort-statement.org .
- 17.Peymani P, Ghavami S, Yeganeh B, Tabrizi R, Sabour S, Geramizadeh B, et al. Effect of chloroquine on some clinical and biochemical parameters in non-response chronic hepatitis C virus infection patients: Pilot clinical trial. Acta Biomed. 2016;87:46–53. [PubMed] [Google Scholar]
- 18.Peymani P, Yeganeh B, Sabour S, Geramizadeh B, Fattahi MR, Keyvani H, et al. New use of an old drug: Chloroquine reduces viral and ALT levels in HCV non-responders (a randomized, triple-blind, placebo-controlled pilot trial) Can J Physiol Pharmacol. 2016;94:613–9. doi: 10.1139/cjpp-2015-0507. [DOI] [PubMed] [Google Scholar]
- 19.Adler A, Miller-Roll T, Bradenstein R, Block C, Mendelson B, Parizade M, et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: The dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis. 2015;83:21–4. doi: 10.1016/j.diagmicrobio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. doi: 10.1136/bmj.l536. [DOI] [PubMed] [Google Scholar]
- 21.Riha HM, Wilkinson R, Twilla J, Harris LJ, Jr, Kimmons LA, Kocak M, et al. Octreotide added to a proton pump inhibitor versus a proton pump inhibitor alone in nonvariceal upper-gastrointestinal bleeds. Ann Pharmacother. 2019;53:794–800. doi: 10.1177/1060028019833696. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Deng F. clinical effects of pantoprazole and octreotide in upper gastrointestinal hemorrhage. Indian J Pharm Educ Res. 2019;53:330–3. [Google Scholar]
- 23.Imperiale TF, Birgisson S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Ann Intern Med. 1997;127:1062–71. doi: 10.7326/0003-4819-127-12-199712150-00002. [DOI] [PubMed] [Google Scholar]


