Abstract
Artificial sweeteners (ASs) are popular for their characteristic property of providing sweetness with few or no calories. They are frequently consumed to minimize energy intake and to combat obesity and its related adverse health effects. However, since their introduction, concerns have been raised regarding their safety. Extensive research has designed a number of studies to evaluate potential adverse effects, the top among them being interference with glucose homeostasis. Numerous studies have tried to prove that AS may contribute to the development of metabolic diseases including obesity and type 2 diabetes (T2D). The matter remains controversial and a favorite topic of research. The purpose of this review was to identify and discuss the published articles that have examined the effects of AS consumption on glucose homeostasis and its association with T2D and obesity. It was observed that studies have failed to present concrete evidence to establish a link between AS consumption and glucose homeostasis, obesity, or T2D. Most studies have flaws in the study design resulting in haphazard claims with no follow-up studies to confirm reliability. It is concluded that while it is not possible to claim that ASs are metabolically inert, at the moment the haphazard evidence is not enough to link their use with glucose metabolism, obesity or T2D. There is a need to design cohort and case–control studies with reliable sample sizes to establish a cause–effect relationship or to exclude claims of safety problems.
Keywords: artificial sweeteners, glucose, diabetes, obesity
Introduction
Artificial sweeteners (ASs), or non-nutritive sweeteners, are food additives that provide a sweet flavor with zero or low calories; they are used in various products such as foodstuffs, beverages, drugs, and even toothpaste.1 They may be derived from plant extracts or manufactured by chemical synthesis. The first AS, benzoic sulfimide, was marketed in the USA in 1879, by Constantin Fahlberg and was commercialized as saccharin.2 Since then, the consumption of ASs has grown substantially, as reported by several studies in adults or children. A 2017 study in the USA showed that AS consumption is 41.4% among adults and 25.1% among children.3
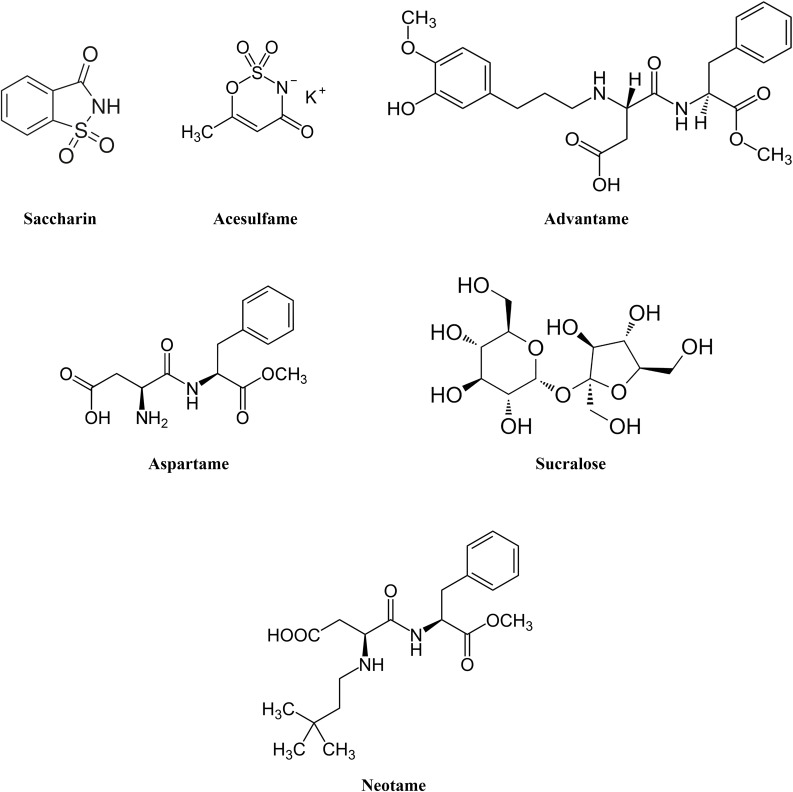
Currently, six ASs have been approved by the US Food and Drug Administration (FDA): saccharin, acesulfame, aspartame, neotame, sucralose, and advantame.4 In 2017, sucralose was the most widely AS consumed; it accounted for one-third of the global market, expected to be worth $2.8 billion by 2021.5 Figure 1 shows the chemical structures of the approved ASs.
Figure 1.
Chemical structures of the approved artificial sweeteners.
ASs are designed as sugar substitutes to tackle obesity and its subsequent outcomes, including metabolic syndrome, diabetes, and cardiovascular diseases. Yet several concerns have been raised about the safety of these products. In fact, the adverse health effects associated with ASs remain open to discussion among researchers. For instance, the scientific report of the Dietary Guidelines Advisory Committee in 2015 discussed that added sugars should be minimized in the diet and substituted not with ASs, but rather with more healthy choices.6 A joint position statement from the American Heart Association and the American Diabetes Association also cautioned against AS intake and stated that there are limited data to conclude whether AS consumption to replace sugar sweeteners benefits body weight, energy balance, or metabolic risk factors.7 Meanwhile, Diabetes UK reported that there are adequate data to support that AS consumption improves postprandial blood glucose levels if consumed instead of sugars; moderate consumption of ASs to replace sugars can be a useful strategy to manage body weight.8 The purpose of this review is to summarize the available literature investigating the effects of AS consumption on glucose homeostasis and its association with T2D and obesity.
Glucose Homeostasis
Some clinical trials have observed the effects of ASs on glucose homeostasis. However, the results are contradictory and comparability among these trials is not feasible owing to variations in the sample size, type of AS, whether a placebo was included, exposure time, and outcomes assessed. For example, studies that evaluated the effect of sucralose consumption showed lower,9 higher,10 or no change in glucose levels.11 Likewise, GLP-1 levels were increased following sucralose intake in some studies,9,12 while other studies reported a reduction13 or no change.10 Aspartame consumption also showed inconsistent results, with lower14 or no change15 in plasma glucose levels. Other studies investigated the levels of appetite-regulating hormones, including ghrelin, cholecystokinin, and peptide YY, following AS consumption, and showed no significant changes.11,16
Overall, as provided in Table 1, the vast majority of clinical trials that have investigated the effects of AS intake on glycemic response observed no significant differences between AS consumption and placebo on various measures of glycemic response, including plasma glucose, insulin, HbA1c, and C-peptide. These findings are consistent with the fact that ASs provide few or no calories to the diet. Therefore, based on the available literature, the effect of ASs on glucose homeostasis cannot be established.
Table 1.
Clinical Trials Investigating the Effect of AS Consumption on Glucose Homoeostasis
| Authors and Year | Study Design | Population and Age | Parameters | Findings |
|---|---|---|---|---|
| Anton et al14 2010 |
Single-blind randomized crossover study | 19 participants with normal weight and 12 obese subjects (18–49 years) |
|
|
| Ma et al41 2010 |
Single-blind randomized crossover study | 10 healthy normal weight subjects (25–29 years) |
|
Intraduodenal infusion of sucralose did not change glucose intestinal absorption or GLP-1 secretion compared to control infusion |
| Brown et al42 2011 |
Double-blind randomized crossover study | 8 healthy normal weight women (19–24 years) |
|
No significant changes were described with sucralose consumption compared to water |
| Ford et al43 2011 |
Single-blind randomized crossover study | 8 healthy normal weight subjects (22–27 years) |
|
Sucralose consumption did not change any variables compared to water |
| Steinert et al44 2011 |
Randomized crossover study (no blinding) | 12 healthy normal weight subjects (22–24 years) |
|
No significant differences were observed in any of the parameters following consumption of aspartame, acesulfame-k, or sucralose compared to water |
| Wu et al45 2012 |
Single-blind randomized crossover study | 10 healthy obese subjects (25–33 years) |
|
Sucralose consumption had no effects on any parameters |
| Maersk et al16 2012 |
Randomized crossover study (no blinding) | 24 healthy obese subjects (20–50 years) |
|
Aspartame consumption did not change any variables |
| Brown et al11 2012 |
Randomized crossover study (no blinding) | 44 subjects divided into 3 groups: 25 healthy controls, 10 with T2D, and 9 with type 1 diabetes (12–25 years) |
|
|
| Stellingwerff et al46 2013 |
Double-blind randomized crossover study | 23 healthy normal weight men (22–36 years) |
|
Sucralose consumption immediately before exercise had no effects on glucose and insulin concentrations during exercise |
| Pepino et al10 2013 |
Randomized crossover study (no blinding) | 17 healthy obese (34–36 years) |
|
|
| Olalde-Mendoza et al15 2013 |
Randomized study (no blinding) | 80 obese individuals with T2D (40–58 years) |
|
Diet soda consumption had no effects on capillary glucose concentrations |
| Bryant et al47 2014 |
Randomized crossover study (no blinding) | 10 normal weight subjects (18–24 years) |
|
Consumption of saccharine, aspartame, or acesulfame-k in combination with glucose did not change blood glucose concentrations compared to glucose alone |
| Temizkan et al9 2015 |
Single-blind randomized crossover design | 8 newly diagnosed type 2 diabetic patients (51.5±9.2 years) and 8 healthy obese subjects (45.0±4.1 years) |
|
|
| Boyle et al48 2016 |
Single-blind randomized crossover design | 40 healthy normal weight and obese subjects (50–65 years) |
|
Sucralose consumption had no effects on capillary or interstitial glucose concentrations |
| Dhillon et al49 2017 |
Single-blind randomized crossover design | 64 obese subjects (18–50 years) |
|
|
| Tey et al50 2018 |
Single-blind randomized crossover study | 32 healthy normal weight men (21–50 years) |
|
|
| Crézé et al51 2018 |
Randomized crossover study (no blinding) | 18 healthy normal weight subjects (age not provided) |
|
|
| Farhat et al52 2019 |
Single-blind randomized crossover study | 30 healthy normal weight and obese subjects (16–36 years) |
|
|
| Gómez-Arauz et al53 2019 |
Randomized placebo-controlled trial | 45 participants divided into 2 groups: 20 controls (21.55±2.18 years) and 25 who ingested sucralose (22±2.99 years) |
|
|
| Nichol et al54 2020 |
Randomized crossover study (no blinding) | 10 healthy normal weight and 11 healthy obese subjects (23–33 years) |
|
|
| Bueno-Hernández et al55 2020 |
Randomized double-blind placebo-controlled trial | 137 subjects divided into 3 groups: a) subjects receiving water as controls, b) subjects receiving 48 mg sucralose, and c) subjects receiving 96 mg sucralose |
|
Sucralose intake for 10 weeks resulted in:
|
Type 2 Diabetes
ASs are marketed to reduce the risk of metabolic disease, including T2D. Several reports have addressed the relationship between AS consumption and the development of T2D. A dose–response meta-analysis, published in 2020, included 13 cohort studies and investigated the association between artificially sweetened beverages and the risk of T2D; the median follow-up was 8.4 years. The results revealed a linear association between AS intake and T2D risk. The risk increased by 15% for each 250 mL/day increase in AS soft drink consumption. However, the study reported considerable heterogeneity and other unmeasurable confounders cannot be excluded. Thus, the results should not be taken for granted.17 In another systematic review, four cohorts were included from three observational studies that investigated the association between AS consumption in the form of soft drinks and risk of T2D. The review concluded that there was an increased risk of T2D when 330 mL of artificially sweetened soft drinks were consumed daily. However, the studies included were observational and significant heterogeneity was reported among the cohorts; therefore, any conclusion should be taken with caution.18 In addition, another systematic review analyzed the data from 17 cohorts and found a positive relationship between AS consumption and T2D incidence. However, the authors of this review reported that the evidence of their findings was insufficient and potential bias as well as heterogeneity among cohort studies existed.19
Observational studies investigating the relationship between AS consumption and the incidence of T2D showed inconsistent results. Four cohort studies reported a positive association between AS intake and the risk of T2D. Among them, a large cohort study conducted by Fagherazzi et al20 showed that the risk of T2D was significantly increased among women consuming ASs in the form of packets or tablets for more than 10 years compared to never or rare consumers, when adjusted for body mass index (BMI). However, adiposity cannot be excluded in this study as a confounding factor. Likewise, another study reported a significant positive association between AS intake and the incidence of T2D in women consuming over 600 mL of artificially sweetened soft drinks for 14 years, with a dose-dependent relationship. However, other independent risk factors for T2D in this study cannot be ruled out.21 A third study, carried out for 7 years, showed a 67% greater relative risk for T2D in individuals consuming at least one diet soda per day independent of primary measures of adiposity. However, the possibility of other confounding factors cannot be eliminated from this observational study.22 A fourth study investigated a limited number of Japanese men for 7 years and reported an increased risk of T2D among subjects who consumed one or more diet sodas daily. The results are not representative of the general population. Also, this study assessed the consumption of AS at the baseline examination and did not consider the possible changes during the 7 years of follow-up.23
While some cohort studies and meta-analyses observed a positive relationship between AS consumption and the risk of T2D, other studies reported no association. Bhuphatiraju et al24 investigated the relationship between AS consumption and the incidence of T2D in 39,059 healthy professional men for 20 years. Although a positive association was observed with the development of T2D, in an age-adjusted analysis, the association was no longer significant when adjusted for BMI and total energy intake. These findings indicate that ASs were consumed to reduce weight and health conditions related to obesity, such as diabetes. Similarly, in a case cohort study of 15,384 subjects, there was no significant association between AS consumption and risk of T2D after adjustment for BMI and total energy intake.25 A summary of observational cohort studies investigating the association between AS consumption and risk of T2D is provided in Table 2.
Table 2.
Observational Cohort Studies Investigating the Association Between AS Consumption and Risk of Development of T2D
| Authors and Year | Number and Age of Participants | Follow-up Period | Main Outcomes |
|---|---|---|---|
| Palmer et al56 2008 | 43,960 women (21–69 years) |
4 years | No association was found between diet soft drink consumption and incidence of T2D |
| Nettleton et al22 2009 | 5011 adults (45–84 years) |
7 years | Daily diet soda intake was associated with a 67% higher risk of developing T2D |
| de Koning et al19 2011 | 40,389 male health professionals (40–75 years) |
20 years | Relationship of artificially sweetened beverages consumption and risk of T2D was observed in the age-adjusted analysis. However, in the multivariate-adjusted analysis no relationship was found |
| Bhupathiraju et al24 2013 | 74,749 female nurses (30–55 years) |
24 years | Significant association was observed between caffeine-free artificially sweetened soft drinks and incidence of T2D after multivariable adjustment for BMI and energy intake |
| Bhupathiraju et al24 2013 | 39,059 healthcare professional men (40–75 years) |
22 years | No association was found between caffeinated or non-caffeinated artificially sweetened beverage intake and risk of T2D after multivariable adjustment |
| The InterAct Consortium25 2013 | 34,234 adults (39–69 years) |
16 years | No relationship was observed between artificially sweetened beverage intake and the incidence of T2D after multivariable adjustment for BMI and energy intake |
| Fagherazzi et al21 2013 | 66,118 women (46–59 years) |
14 years | Significant association was described between high consumption of artificially sweetened beverages (>630 mL/week) and the development of T2D |
| Sakurai et al23 2014 | 2037 men (35–55 years) |
7 years | Daily consumption of diet soda was positively associated with increased risk of T2D after multivariable adjustment |
| O’Connor et al26 2015 | 24,653 adults (40–79 years) |
10.8 years | Significant association was found between artificially sweetened beverage consumption and incidence of T2D. Yet, after adjusting for adiposity (BMI and waist circumference) this became insignificant |
| Ma et al57 2016 | 1685 adults (43–61 years) |
14 years | No association was found between diet soda intake and increased prediabetes risk |
| Fagherazzi et al20 2017 | 61,440 women (46–59 years) |
18 years | Significant association was observed between AS consumption in packets or tablets and development of T2D after adjustment for BMI |
| Huang et al58 2017 | 64,850 women (50–79 years) |
8.4 years | Consumption of artificially sweetened beverages was associated with a 21% higher risk of developing T2D |
| Gardener et al59 2018 | 2019 adults (59–79 years) |
11 years | Strong positive association was found between diet soda intake and the development of T2D. Yet, after adjusting for BMI this became null |
| Jensen et al60 2020 | 1359 adults (25–60 years) |
8 years | No association was found between AS consumption and the risk of T2D |
A possible explanation for the relationship between AS consumption and risk of T2D that has been observed in some studies might be related to reverse causality. O’Connor et al,26 in a long-term large cohort study of 24,653 adults, concluded that the positive association between AS consumption and T2D may be an artifact of reverse causality. Subjects with higher BMI or a tendency for weight gain, already at risk of diabetes, consume AS products as a strategy to reduce calorie intake.
In conclusion, the reported studies assessing the risk of T2D reveal divergent results. Reverse causality and residual confounding factors such as adiposity were observed in most of the studies. Therefore, a final conclusion cannot be reached now, and further well-designed human studies, of long duration, evaluating the risk of diabetes are desperately needed.
Obesity
Various large cohort studies have described a positive dose-dependent relationship between AS consumption and increased BMI. In 2020, Qin et al,17 in a meta-analysis including six prospective cohort studies of 26,551 subjects, found that the risk of obesity increased by 21% for each 250 mL/day increase in AS soft drink consumption. Likewise, another meta-analysis including three cohort studies of 12,987 participants showed a pooled relative risk of obesity of 1.59% (95% CI 1.22–2.08) in subjects consuming artificially sweetened beverages.27 Azad et al28 conducted a meta-analysis of eight cohort studies and concluded that AS consumption was associated with a slightly positive increase in BMI, weight, waist circumference, and incidence of obesity. In a cohort study of 1454 subjects with a median follow-up of 10 years, AS consumers had 0.80 kg/m2 higher BMI, 2.5 cm greater waist circumference, and 53% higher incidence of abdominal obesity compared to non-consumers.29 In children, most of the observational studies demonstrated that AS intake is associated with increased weight.30 Moreover, Azad et al31 reported that the daily maternal consumption of AS during pregnancy was associated with a higher infant BMI and a two-fold greater risk of the infant being overweight at the age of 1 year.
Several proposed mechanisms in vitro and in vivo may describe the link between AS intake and obesity. One potential explanation derives from a hypothesis that the sensation of sweetness without calorie absorption may disturb brain metabolic signaling and appetite regulation.32 Absence of satiety can encourage more eating. Another mechanism is that AS consumption may activate intestinal and pancreatic sweet taste receptors stimulating GLP-1 and insulin release, as shown in in vitro studies.33 Alterations in the gut microbiota have also been described as a potential mechanism. Studies of rodent models showed that saccharin intake promotes weight gain and glucose intolerance by changing the physiology of the intestinal microbiota.34,35 Another important mechanism that should not be ignored is related to limitations in the study design. Residual confounding and reverse causality in these observational studies may elucidate the relationship between AS intake and obesity. Overweight or obese individuals tend to consume ASs to manage their weight. Likewise, obese people presenting with prediabetes or T2D start consuming ASs to improve their blood glucose control, which causes a false association between AS consumption and the increased risk of developing T2D. This mechanism is supported by the results of several interventional studies that showed no or even a beneficial effect of AS on body weight.
In a meta-analysis of 15 randomized controlled trials (RCTs), the results revealed significant benefits of AS consumption on body weight, BMI, fat mass, and waist circumference compared with sugar intake. The authors concluded that AS consumption as a replacement option for caloric sweeteners may help individuals cope with a weight management plan.36 Another meta-analysis of human RCTs of 4 weeks’ to 40 months’ duration found that AS consumption was associated with reduced body weight compared with a sugar sweetener or water consumption.37 Toews et al38 conducted a meta-analysis of RCTs with a study duration of 1 week or longer. The authors found no significant variations in body weight between adults consuming AS compared with those consuming caloric sweeteners or placebo. Subgroup analysis, by body weight level, reported that AS intake by obese or overweight subjects resulted in a reduction of 1.99 kg. However, no change was observed in adults of normal weight. In addition, BMI was 0.6 units lower in individuals consuming AS compared with sucrose consumers. In the meta-analysis mentioned earlier in this section by Azad et al,28 the included RCTs did not show a significant correlation between AS consumption and BMI or body weight. The authors concluded that the results from RCTs do not support the proposed benefits of AS for weight control.
Therefore, unlike most prospective observational studies, the meta-analyses of RCT studies suggest that AS consumption has neutral or positive outcomes for weight management. However, methodological limitations in the included studies were apparent and have raised many questions about the outcomes and efficacy of ASs in weight management. Thus, no definite conclusions can be drawn about the effect on body weight of replacing natural sweeteners with ASs.
Future Studies
While the subject of ASs has generated extensive research, much about ASs has yet to be fully explored. The links between ASs and metabolic diseases remain unclear. At this stage, we are unable to ascertain whether AS consumption has beneficial or adverse effects on health outcomes. Most studies have flaws in study design, study population, sample size, control groups, managing the confounders, use of improper placebo, and interpretation of data. For example, experimental designs using sham feeding, acute exposure, and water as a placebo apparently give inconclusive results. Likewise, gas bubbles in carbonated drinks may act as a confounder in some study designs, resulting in a lack of consistent results among different groups. Thus, it is of paramount importance to design long-term cohort studies along the pattern of the Framingham Study to determine cause-and-effect relationships between AS consumption and the prevalence of different metabolic diseases. Likewise, case–control studies could be designed to establish cause-and-effect relationships in obese individuals presenting with T2D. In addition, future studies should attempt to explain the effects of different AS independently since ASs may differ in their physiological impacts. One study found that different ASs (saccharine, sucralose, and aspartame) had different effects on body weight over 3 months.39 Thus, the type and dose of AS used should be documented clearly in all studies.
Besides investigating the effects of AS intake on healthy individuals, research should also be directed toward diseased populations and other subgroups, such as pregnant women and their infants. Exposure of babies to ASs via breast milk has been postulated to have potential adverse outcomes in adult life.40 Further studies are needed to explore this matter in detail.
Conclusion
AS consumption is associated with obesity and T2D in observational cohort studies, but the findings are questionable since reverse causality and residual confounders cannot be excluded. A majority of interventional clinical trials observe neutral or even beneficial effects of AS when consumed in the context of a weight-loss program. Further well-designed studies that mimic real-life consumption are needed to explore the long-term specific effects of the different ASs available.
Based on the available data, it is not possible to claim that ASs are metabolically inert; however, current evidence is not sufficient to link their use with glucose metabolism, obesity, or T2D.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Gardener H, Elkind MS. Artificial sweeteners, real risks. Am Heart Assoc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remsen I. On the oxidation of substitution products of aromatic hydrocarbons. J Am Chem Soc. 1879;1(4):115–116. doi: 10.1021/ja02144a604 [DOI] [Google Scholar]
- 3.Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet. 2017;117(3):441–8. e2. doi: 10.1016/j.jand.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA. High-intensity sweeteners; 2014. Available from: https://www.fda.gov/food/food-additives-petitions/high-intensity-sweeteners. Accessed August17, 2020.
- 5.Busness Wire. Global food market-growth, trends, forecast for the period (2015-2020); 2017. Available from: https://www.businesswire.com/news/home/20170331005203/en/Global-Zero-Calorie-Sweetener-Market-Projected-Worth-USD. Accessed August17, 2020.
- 6.Millen BE, Abrams S, Adams-Campbell L, et al. The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr. 2016;7(3):438–444. doi: 10.3945/an.116.012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American diabetes Association. Circulation. 2012;126(4):509–519. doi: 10.1161/CIR.0b013e31825c42ee [DOI] [PubMed] [Google Scholar]
- 8.Dyson P, Twenefour D, Breen C, et al. Diabetes UK evidence‐based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35(5):541–547. doi: 10.1111/dme.13603 [DOI] [PubMed] [Google Scholar]
- 9.Temizkan S, Deyneli O, Yasar M, et al. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr. 2015;69(2):162–166. doi: 10.1038/ejcn.2014.208 [DOI] [PubMed] [Google Scholar]
- 10.Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36(9):2530–2535. doi: 10.2337/dc12-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35(5):959–964. doi: 10.2337/dc11-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohtsu Y, Nakagawa Y, Nagasawa M, Takeda S, Arakawa H, Kojima I. Diverse signaling systems activated by the sweet taste receptor in human GLP-1-secreting cells. Mol Cell Endocrinol. 2014;394(1–2):70–79. doi: 10.1016/j.mce.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Hall W, Millward D, Rogers P, Morgan L. Physiological mechanisms mediating aspartame-induced satiety. Physiol Behav. 2003;78(4–5):557–562. doi: 10.1016/S0031-9384(03)00034-9 [DOI] [PubMed] [Google Scholar]
- 14.Anton SD, Martin CK, Han H, et al. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55(1):37–43. doi: 10.1016/j.appet.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olalde-Mendoza L, Moreno-González YE. Modification of fasting blood glucose in adults with diabetes mellitus type 2 after regular soda and diet soda intake in the state of Queretaro, Mexico. Arch Latinoam Nutr. 2013;63(2):142–147. [PubMed] [Google Scholar]
- 16.Maersk M, Belza A, Holst JJ, et al. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr. 2012;66(4):523–529. doi: 10.1038/ejcn.2011.223 [DOI] [PubMed] [Google Scholar]
- 17.Qin P, Li Q, Zhao Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose–response meta-analysis of prospective cohort studies. Springer. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood D, Threapleton D, Evans C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Br J Nutr. 2014;112(5):725–734. doi: 10.1017/S0007114514001329 [DOI] [PubMed] [Google Scholar]
- 19.De Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–1327. doi: 10.3945/ajcn.110.007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagherazzi G, Gusto G, Affret A, et al. Chronic consumption of artificial sweetener in packets or tablets and type 2 diabetes risk: evidence from the E3N-European prospective investigation into cancer and nutrition study. Ann Nutr Metab. 2017;70(1):51–58. doi: 10.1159/000458769 [DOI] [PubMed] [Google Scholar]
- 21.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the etude epidémiologique auprès des femmes de la mutuelle générale de l’education nationale–European prospective investigation into cancer and nutrition cohort. Am J Clin Nutr. 2013;97(3):517–523. doi: 10.3945/ajcn.112.050997 [DOI] [PubMed] [Google Scholar]
- 22.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2009;32(4):688–694. doi: 10.2337/dc08-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai M, Nakamura K, Miura K, et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr. 2014;53(1):251–258. doi: 10.1007/s00394-013-0523-9 [DOI] [PubMed] [Google Scholar]
- 24.Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97(1):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.InterAct Consortium. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–1530. doi: 10.1007/s00125-013-2899-8 [DOI] [PubMed] [Google Scholar]
- 26.O’Connor L, Imamura F, Lentjes MA, Khaw K-T, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia. 2015;58(7):1474–1483. doi: 10.1007/s00125-015-3572-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruanpeng D, Thongprayoon C, Cheungpasitporn W, Harindhanavudhi T. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110(8):513–520. doi: 10.1093/qjmed/hcx068 [DOI] [PubMed] [Google Scholar]
- 28.Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189(28):E929–E39. doi: 10.1503/cmaj.161390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chia CW, Shardell M, Tanaka T, et al. Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: a cohort study. PLoS One. 2016;11(11):e0167241. doi: 10.1371/journal.pone.0167241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durán Agüero S, Angarita Davila L, Escobar Contreras M, Rojas Gomez D, de Assis Costa J. Noncaloric sweeteners in children: A controversial theme. Biomed Res Int. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azad MB, Sharma AK, de Souza RJ, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016;170(7):662–670. doi: 10.1001/jamapediatrics.2016.0301 [DOI] [PubMed] [Google Scholar]
- 32.Sylvetsky AC, Rother KI. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity. 2018;26(4):635–640. doi: 10.1002/oby.22139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang H-J, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci. 2007;104(38):15069–15074. doi: 10.1073/pnas.0706890104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–186. doi: 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 35.Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6(2):149–155. doi: 10.1080/19490976.2015.1017700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100(3):765–777. doi: 10.3945/ajcn.113.082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers PJ, Hogenkamp PS, De Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes. 2016;40(3):381–394. doi: 10.1038/ijo.2015.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toews I, Lohner S, de Gaudry DK, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148(8):650–657. [DOI] [PubMed] [Google Scholar]
- 40.Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78(16):1029–1032. doi: 10.1080/15287394.2015.1053646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Chang J, Checklin HL, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104(6):803–806. doi: 10.1017/S0007114510001327 [DOI] [PubMed] [Google Scholar]
- 42.Brown AW, Brown MMB, Onken KL, Beitz DC. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr Res. 2011;31(12):882–888. [DOI] [PubMed] [Google Scholar]
- 43.Ford H, Peters V, Martin N, et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65(4):508–513. doi: 10.1038/ejcn.2010.291 [DOI] [PubMed] [Google Scholar]
- 44.Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011;105(9):1320–1328. [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Zhao BR, Bound MJ, et al. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr. 2012;95(1):78–83. doi: 10.3945/ajcn.111.021543 [DOI] [PubMed] [Google Scholar]
- 46.Stellingwerff T, Godin J-P, Beaumont M, et al. Effects of pre-exercise sucralose ingestion on carbohydrate oxidation during exercise. Int J Sport Nutr Exerc Metab. 2013;23(6):584–592. [DOI] [PubMed] [Google Scholar]
- 47.Bryant CE, Wasse LK, Astbury N, Nandra G, McLaughlin JT. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68(5):629–631. doi: 10.1038/ejcn.2014.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle NB, Lawton CL, Allen R, Croden F, Smith K, Dye L. No effects of ingesting or rinsing sucrose on depleted self-control performance. Physiol Behav. 2016;154:151–160. doi: 10.1016/j.physbeh.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 49.Dhillon J, Lee JY, Mattes RD. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol Behav. 2017;181:100–109. doi: 10.1016/j.physbeh.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tey SL, Salleh N, Henry CJ, Forde CG. Effects of consuming preloads with different energy density and taste quality on energy intake and postprandial blood glucose. Nutrients. 2018;10(2):161. doi: 10.3390/nu10020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crézé C, Candal L, Cros J, et al. The impact of caloric and non-caloric sweeteners on food intake and brain responses to food: a randomized crossover controlled trial in healthy humans. Nutrients. 2018;10(5):615. doi: 10.3390/nu10050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farhat G, Berset V, Moore L. Effects of stevia extract on postprandial glucose response, satiety and energy intake: a three-arm crossover trial. Nutrients. 2019;11(12):3036. doi: 10.3390/nu11123036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gómez-Arauz AY, Bueno-Hernández N, Palomera LF, et al. A single 48 mg sucralose sip unbalances monocyte subpopulations and stimulates insulin secretion in healthy young adults. J Immunol Res. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichol AD, Salame C, Rother KI, Pepino MY. Effects of sucralose ingestion versus sucralose taste on metabolic responses to an oral glucose tolerance test in participants with normal weight and obesity: a randomized crossover trial. Nutrients. 2020;12(1):29. doi: 10.3390/nu12010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bueno-Hernández N, Esquivel-Velázquez M, Alcántara-Suárez R, et al. Chronic sucralose consumption induces elevation of serum insulin in young healthy adults: a randomized, double blind, controlled trial. Nutr J. 2020;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma J, Jacques PF, Meigs JB, et al. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J Nutr. 2016;146(12):2544–2550. doi: 10.3945/jn.116.234047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang M, Quddus A, Stinson L, et al. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: the prospective women’s health initiative observational study. Am J Clin Nutr. 2017;106(2):614–622. doi: 10.3945/ajcn.116.145391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardener H, Moon YP, Rundek T, Elkind MS, Sacco RL. Diet Soda and sugar-sweetened soda consumption in relation to incident diabetes in the Northern Manhattan study. Curr Dev Nutr. 2018;2(5):nzy008. doi: 10.1093/cdn/nzy008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen PN, Howard BV, Best LG, et al. Associations of diet soda and non-caloric artificial sweetener use with markers of glucose and insulin homeostasis and incident diabetes: the strong heart family study. Eur J Clin Nutr. 2020;74(2):322–327. doi: 10.1038/s41430-019-0461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]