Abstract
Simultaneous digestion and in situ biogas upgrading in high-pressure bioreactors will result in elevated CO2 partial pressure (pCO2). With the concomitant increase in dissolved CO2, microbial conversion processes may be affected beyond the impact of increased acidity. Elevated pCO2 was reported to affect the kinetics and thermodynamics of biochemical conversions because CO2 is an intermediate and end-product of the digestion process and modifies the carbonate equilibrium. Our results showed that increasing pCO2 from 0.3 to 8 bar in lab-scale batch reactors decreased the maximum substrate utilization rate (rsmax) for both syntrophic propionate and butyrate oxidation. These kinetic limitations are linked to an increased overall Gibbs free energy change (ΔGOverall) and a potential biochemical energy redistribution among syntrophic partners, which showed interdependence with hydrogen partial pressure (pH2). The bioenergetics analysis identified a moderate, direct impact of elevated pCO2 on propionate oxidation and a pH-mediated effect on butyrate oxidation. These constraints, combined with physiological limitations on growth exerted by increased acidity and inhibition due to higher concentrations of undissociated volatile fatty acids, help to explain the observed phenomena. Overall, this investigation sheds light on the role of elevated pCO2 in delicate biochemical syntrophic conversions by connecting kinetic, bioenergetic, and physiological effects.
Introduction
High-pressure anaerobic digestion (HPAD) has been proposed as a technology for in situ biogas upgrading,1−3 able to achieve a CH4 content >90%, after which the produced CH4 is in principle suitable for further direct use in, for example, (decentralized) gas grid injection or advanced industrial processes. HPAD takes advantage of the large difference in solubility between CH4 and CO2, which is most pronounced at high pressures in a digester equipped with a pressure valve for biogas release. However, by letting the pressure rise, the CH4 content increases in the headspace, whereas CO2 and other ionizable gases such as H2S dissolve in the liquid. Thus far, the effects of increased dissolved CO2 on the overall performance of the high-pressure system have hardly been studied beyond accumulating acidity.4 As far as the authors are aware, limited attention has been paid to its possible impact on metabolic conversion routes and degradation rates.
CO2 has multiple roles in biological systems such as electron acceptor, carbon donor, intermediate, and end-product of biochemical reactions, and contributes to the aquatic buffer system via the carbonate equilibrium.5 These multiple roles complicate studies searching for a mechanistic description of the response to increased CO2 partial pressure (pCO2) in natural and engineered environments, except for the bacteriostatic effects of high pCO2 applied for sterilization purposes at 40–300 bar and 20–50 °C. The bacteriostatic action leads to cytoplasm acidification, cell rupture, and inactivation of key enzymes and transport proteins.6−8 The impact of “moderate” pCO2 from 0.1 up to 10 bar is less comprehensively described and is mainly attributed to a decreased intracellular pH.9 However, pH reduction by itself does not explain the reduced microbial activity of denitrifying bacteria observed by Wan et al.10 because of dissolved CO2 concentrations up to 30,000 ppm. These authors proposed that elevated pCO2 caused direct inhibition of the carbon metabolism, electron transport chain, enzymatic activity, and substrate consumption at the expense of increased buffer concentration to prevent a pH drop.10,11
Research on the impact of moderate pCO2 on methanogenesis is limited to observations relevant to oil reservoirs. Operational conditions of 50 bar pressure, 10% pCO2, and temperature of 55 °C resulted in a shift from syntrophic acetate oxidation (SAO) to aceticlastic methanogenesis (AcM).12 The effects of CO2 supplementation at atmospheric pressure in anaerobic digesters (ADs) are better documented in literature; when accompanied by stoichiometric H2 provision, it enhances CH4 production because of promoted hydrogenotrophic methanogenesis (HyM).13 Also, exogenous CO2 can be indirectly converted to CH4 via homoacetogenesis coupled to AcM. This mechanism has been proposed to explain the increased CH4 production after CO2 direct injection in (a) pilot-scale AD treating food waste14,15 and (b) two-phase AD-treating sewage. The accompanying. The accompanying electron donor was not highlighted; nonetheless, this role could be performed by additional H2 coming from enhanced acidogenesis15 or after the release of other hydrolyzed material from cell lysis.16
Increased CO2 also induces changes in microbiome activity, diversity, community structure, and microbial interactions.8 The last one is of vital importance in ADs, which rely on syntrophy to overcome thermodynamic limitations for the conversion of intermediate compounds, namely propionate and butyrate.17,18 The accumulation of these intermediates correlates with reactor disturbance because of the increased organic loading rate, pH changes, and unpaired acidogenesis and methanogenesis.19 Since these conversions operate close to thermodynamic equilibrium, subtle variations in substrate/product concentrations and environmental conditions can modify the actual Gibbs free energy change (ΔGR1) of a specific pathway.20 The effects of elevated CO2 on syntrophic interactions have been studied in subsurface environments destined for geological carbon storage.21,22 Bioenergetic simulations have shown different outcomes on the ΔGR of the intermediate reactions: the energetic feasibility of substrate oxidation and aceticlastic methanogenic conversions decreased, whereas the contrary occurred for HyM.22,23 As a consequence of the apparent thermodynamic control exerted by pCO2, specific bacterial metabolisms might be promoted or inhibited.24
In our present work, we studied the impact of elevated pCO2 on the kinetics and bioenergetics of the syntrophic conversion of propionate and butyrate. It is hypothesized that an increase in the overall available Gibbs free energy for substrate conversion, because of increased pCO2, could provoke an imbalance in the energy share among syntrophic partners that might translate into kinetic limitations. A scenario analysis is proposed to understand the individual and combined effects of pCO2 and pH on the bioenergetics of syntrophic conversions. Furthermore, the relationship between bioenergetic and kinetic data is evaluated through a correlation analysis aiming to provide insight into the system response to changing available energy.
Materials and Methods
Experimental Setup and Reactor Operation
Five initial operational pCO2, that is, 0.3, 1, 3, 5, and 8 bar, were selected for the experimental treatments based on pH equilibrium calculations performed with the hydrogeochemical software PHREEQC (version 3, USGS). The application of an elevated buffer concentration of 100 mM as HCO3– in the system allowed to maintain circumneutral pH, despite the elevated pCO2. Batch experiments at 0.3 and 1 bar were carried out at atmospheric pressure in 250 mL Schott bottles sealed with rubber stoppers. In parallel, the elevated pressure experiments were performed in 200 mL stainless-steel pressure-resistant reactors (Nantong Vasia, China). The experiments were conducted at a liquid: gas ratio of 1.5:1 and inoculum/substrate ratio of 2:1 g COD g VSS–1. The liquid medium consisted of macronutrient and micronutrient stock solutions (6 and 0.6 mL L–1, respectively) prepared according to Lindeboom et al.1 and 1 g of COD L–1 of the substrates propionate or butyrate.
The headspace of bottles and reactors was replaced with N2 gas (>99%) to ensure anaerobic conditions after filling. Then, the bottles were flushed with the corresponding gas mixture: 70:30% N2/CO2 for 0.3 bar pCO2 or >99% CO2 for 1 bar pCO2. Elevated pressure reactors were subjected to three consecutive pressurization-release cycles to ensure complete N2 replacement by CO2 (>99%) at the intended pressure. Temperature and agitation speed were controlled using an incubator shaker (Innova 44, Eppendorf, USA) set to 35 ± 1 °C and 110 ± 10 rpm. Pressure was online-monitored using digital sensors (B + B Thermo-Techniek, Germany) and a microcontroller (Arduino Uno, Italy). The experiments had a fixed duration of 14 days.
Inoculum Selection
Preliminary experiments of propionate anaerobic conversion under 1 bar pCO2 were conducted in triplicates using three mesophilic inocula collected from (A) sludge digester-treating excess sewage sludge, (B) UASB reactor-treating sugar beet wastewater, and (C) anaerobic membrane bioreactor-treating food industry wastewater. The three inocula were characterized in terms of physicochemical parameters (Supporting Information, Table S1), and inoculum C was selected for the experiments here described (Supporting Information, Figure S1).
Analyses
Experiments were carried out in triplicate incubation; however, because of the small working volume of the reactors (200 mL), a sampling strategy for liquid and gas samples was designed that enabled us to account for replicate variability, minimizing disturbance of the batch incubations (Supporting Information, Table S2). Headspace composition and volatile fatty acids (VFAs) were analyzed using gas chromatography (7890A GC system, Agilent Technologies, US). In the first one, gas samples (5 mL) taken two times per week at atmospheric pressure were measured via a thermal conductivity detector and directed through an HP-PLOT Molsieve GC column (30 m length × 0.53 mm inner diameter × 25 μm film thickness). Helium was used as the carrier gas at a constant flow of 10 mL min–1. The oven and detector were operated at 45 and 200 °C, respectively. In the second one, VFAs were determined according to Ghasimi et al.25 Total and soluble COD, total suspended solids, volatile suspended solids (VSS), and pH were measured at the beginning and end of the experiment according to Standard Methods.26
Estimation of Kinetic Parameters
The modified Gompertz equation27
| 1 |
where y represents the substrate concentration (mg L–1), λ is the lag phase (day), rsmax is the maximum substrate utilization rate (mg L–1 day–1), A is the maximum substrate concentration (mg L–1), and t is the time (days), was used to fit the data from the atmospheric and pressure experiments. The kinetic parameters were estimated using nonlinear minimization methods from the package nlstools in R (v3.6.1).28
Bioenergetic Calculations
ΔGR1, the actual Gibbs free energy change for the reactions, was calculated according to29
| 2 |
where ΔGR01 is the Gibbs free energy at pH 7 and 308.15 K, R is the gas constant (8.31 J K–1 mol–1), T is the temperature in kelvin, YSi is the stoichiometric coefficient of compound i, and aSi is the molar concentration of compound i. ΔGR01 was corrected for temperature using the Gibbs–Helmholtz equation.29 The values at standard conditions, ΔGR, were taken from Heijnen and Kleerebezem.30
Estimation of Potential Biochemical Energy Distribution in Syntrophic Oxidation of Propionate and Butyrate
The stoichiometry of the overall syntrophic reaction and the intermediate catabolic reactions is presented in Table 1. From the acetotrophic reactions, only AcM was included in the analysis because SAO was considered unlikely to occur under our experimental conditions and initial community composition (Supporting Information, Figure S2). The stoichiometric coefficients of AcM and HyM for each substrate correspond to the balance of the formed species during the oxidation.17 At the initially adjusted circumneutral pH, the dissolved inorganic carbon corresponds to H2CO3* and HCO3–. H2CO3 can be expressed in terms of pCO2 using Henry’s law with its proportionality constant (kH) corrected by temperature. The equations, as presented in Table 1, are deliberately written in terms of the H+ concentrations and pCO2 to illustrate the effect of these variables on the thermodynamic calculations.
Table 1. Stoichiometry of the Main Subreactions Related to Syntrophic Propionate and Butyrate Oxidation with Their Corresponding ΔGR01 (kJ mol–1) Calculated at Biochemical Standard Conditions of Temperature = 298.15 K, Concentration of Aqueous Reactants = 1 mol L–1, Pressure of Gaseous Reactants = 1 bar, and pH = 7.
| substrate | reaction | ΔGR01 (kJ mol–1) | |
|---|---|---|---|
| propionate | overall | C3H5O2– + H+ + 0.5H2O → 1.75CH4 + 1.25CO2 | –60.2 |
| oxidation (Pr-Ox) | C3H5O2– + 2H2O → C2H3O2– + 3H2 + CO2 | +73.7 | |
| AcM | C2H3O2– + H+ → CH4 + CO2 | –35.8 | |
| HyM | 3H2 + 0.75CO2 → 0.75CH4 + 1.5H2O | –98.0 | |
| butyrate | overall | C4H7O2– + H+ + H2O → 2.5CH4 + 1.5CO2 | –88.8 |
| oxidation (Bu-Ox) | C4H7O2– + 2H2O → 2C2H3O2– + H+ + 2H2 | +48.2 | |
| AcM | 2C2H3O2– + 2H+ → 2CH4 + 2CO2 | –71.6 | |
| HyM | 2H2 + 0.5CO2 → 0.5CH4 + H2O | –65.4 | |
ΔGR1 for the reactions presented above can be affected by pCO2, pH, or by a combined interaction. The nature of the effect will depend on the role of the parameter in the catabolic reaction, meaning it acts as a reagent, product, or is not directly involved. As well, the magnitude of the effect might be amplified because of an initially less negative ΔGR. A scenario analysis was performed to understand the impact of changing pCO2 and pH on the ΔGR1 of the overall and intermediate catabolic reactions. The resulting calculations, subsequently, were used to estimate the change in the potential biochemical energy share. A summary of input parameters in each scenario (A, B, and C) is presented in Table S3, Supporting Information. The calculations were performed using a pH2 value of 1 × 10–5 bar, typical for ADs31 and at which syntrophic reactions become thermodynamically feasible.17
Statistical Analysis
Spearman’s rank-order correlation coefficient (rS) was calculated via the function rcorr() of the package “Hmisc” in R (v3.6.1),28 ordered using hierarchical clustering and plotted using the package “corrplot.”32
Results and Discussion
Effect of Elevated pCO2 on the Anaerobic Substrate Conversion and Metabolite Production Rate
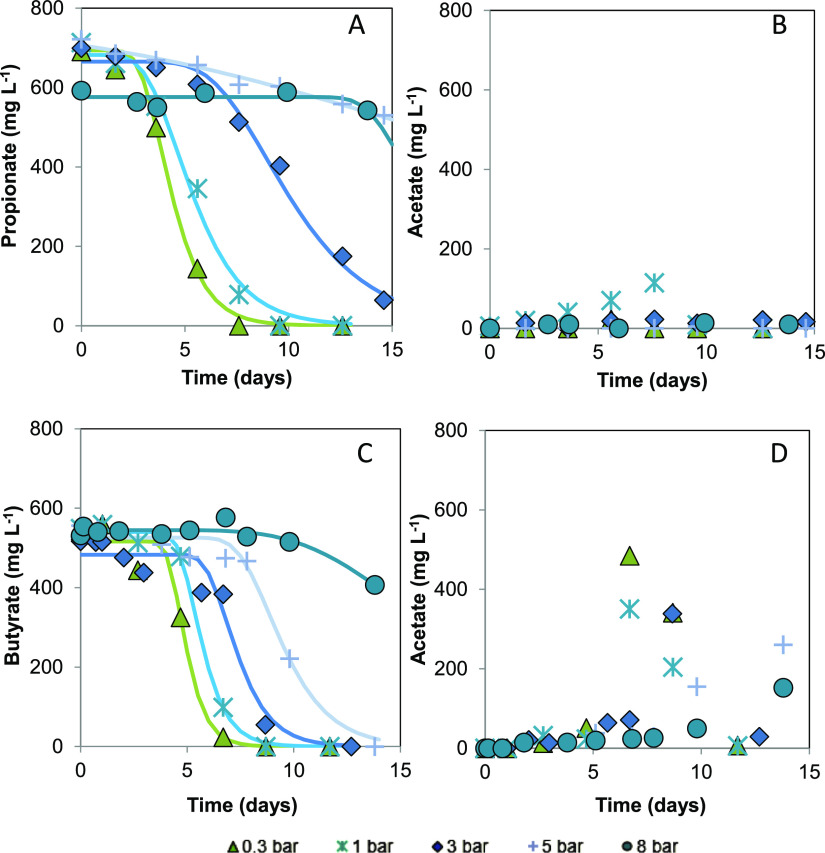
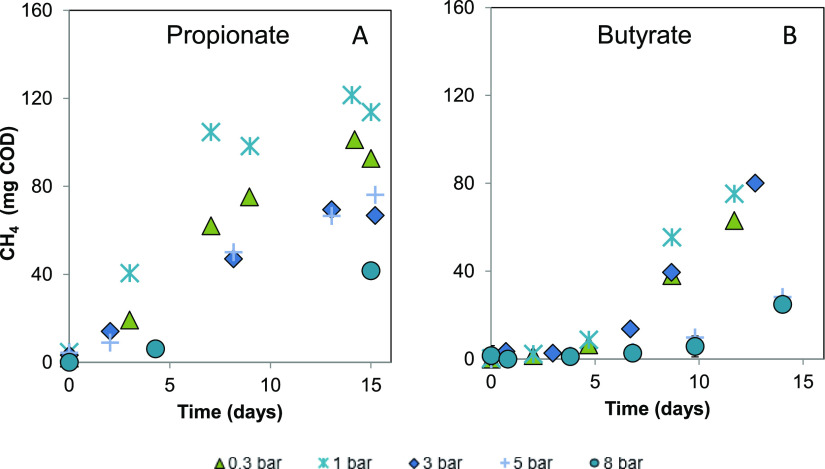
Subplots A and C, as presented in Figure 1, show the decrease in substrate conversion rates in the experimental treatments at increased pCO2 ranging from 0.3 to 8 bar during the 14 days. The reduction in rsmax was further quantified using the process parameters extracted from the data-fitting to the modified Gompertz equation, as presented in Table 2. Data from the 8 bar pCO2 experiment are not included because it was not possible to determine the kinetic parameters accurately. Increasing pCO2 from 0.3 to 5 bar led to a 93% reduction in rsmax for propionate, whereas for butyrate, the rsmax dropped by 57%. The calculated specific rsmax for propionate at 0.3 bar pCO2 is already in the low range of the values proposed in the literature: 150–292 mg propionate g VSS–1 day–1. In the case of butyrate, the specific rsmax at 0.3 bar pCO2 was 1 order of magnitude lower than the inferior boundary of the theoretical range: 3.9–10.9 g butyrate g VSS–1 day–1.33 For both cases, elevated pCO2 resulted in a concomitantly increase in the lag phase (λ), which is likely associated with inadequate levels of adaptation to operational conditions. A considerable effect on the production and consumption of acetate was not evident in the propionate experiment; however, for butyrate, a decrease in acetate production occurred (Figure 1B,D). Lower methane production was observed in the propionate experiment only at 8 bar pCO2 while it appeared already at 5 bar pCO2 for butyrate (Figure 2A,B).
Figure 1.
Evolution of substrate consumption and acetate production during mesophilic syntrophic substrate oxidation under 0.3, 1, 3, 5, and 8 bar initial pCO2. (A,B) correspond to the propionate and acetate concentration (mg L–1) for the propionate experiment, respectively. The concentrations shown in time points 0, 10, and 13 days represent the average of three sampled reactors with a relative standard deviation <16%. (C,D) correspond to the butyrate and acetate concentration (mg L–1) for the butyrate experiment, respectively. The concentrations presented in time points 0, 5, and 12 days represent the average of three sampled reactors with a relative standard deviation <18%. Data points represent experimental data. Continuous lines correspond to the simulated data using the modified Gompertz equation, the significance levels of which are presented in Table 2.
Table 2. Overview of the Kinetic Parameters Estimated Using the Modified Gompertz Equation for Propionate and Butyrate Oxidation at the Different Conditions of Initial pCO2: 0.3, 1, 3, and 5 bara,b.
| substrate | propionate | butyrate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| parameter | initial pCO2 (bar) | 0.3 | 1 | 3 | 5 | 0.3 | 1 | 3 | 5 |
| eq. pCO2 (bar) | 0.3 | 1 | 1.5 | 2 | 0.3 | 1 | 1.5 | 2.0 | |
| eq. pH | 7.4 | 6.9 | 6.4 | 6.2 | 7.4 | 6.9 | 6.4 | 6.2 | |
| A (mg L–1) | 667.9*** | 681.8*** | 664.8*** | 587.5** | 516.2*** | 540.1*** | 465.9*** | 525.9*** | |
| rsmax (mg L–1 day–1) | 223.9*** | 149.5** | 89.8*** | 14.4 ( ) | 291.2 ( ) | 238.9*** | 216.9* | 126.6** | |
| λ (day) | 3.3*** | 3.4** | 6.6*** | 4.7 ( ) | 4.3*** | 4.8*** | 6.3*** | 7.3*** | |
| specific rsmax (mg substrate g–1 VSS added day–1) | 117.2 | 78.3 | 46.9 | 7.5 | 138.7 | 113.8 | 103.3 | 60.3 | |
The measured equilibrium pCO2 and the calculated equilibrium pH are additionally provided.
Levels of significance of the parameter estimation: p-value ( ) < 0.1, * <0.05, ** <0.01, and *** <0.001.
Figure 2.
Evolution of methane production (mg COD) during mesophilic syntrophic substrate oxidation under 0.3, 1, 3, 5, and 8 bar initial pCO2. Data points represent experimental data. (A) Propionate experiment. Values presented in time points 0, 10, and 13 days represent the average of three sampled reactors with a relative standard deviation <14%. (B) Butyrate experiment. Values presented in time points 0, 5, and 12 days represent the average of three sampled reactors with a relative standard deviation <20%.
Hansson and Molin first reported the adverse effects of pCO2 on the propionate and butyrate anaerobic conversion rate.34 These authors observed a decrease of 70% in the rsmax in propionate degradation when increasing pCO2 from 0.2 to 1 bar. The effect for butyrate was not significant, as opposed to our current work in which we identified an 18% reduction in rsmax at a comparable pCO2 increase. In a previously reported experiment, using suspended pressure-cultivated inoculum that originated from anaerobic granular sludge degrading propionate,5 it was shown that 5 bar pCO2 caused a 93% reduction in the rsmax. This value agrees with the calculations presented here (Table 2).
Effects of Elevated pCO2 on the ΔGOverall of Syntrophic Propionate and Butyrate Conversion and the Intermediate Biochemical Reactions
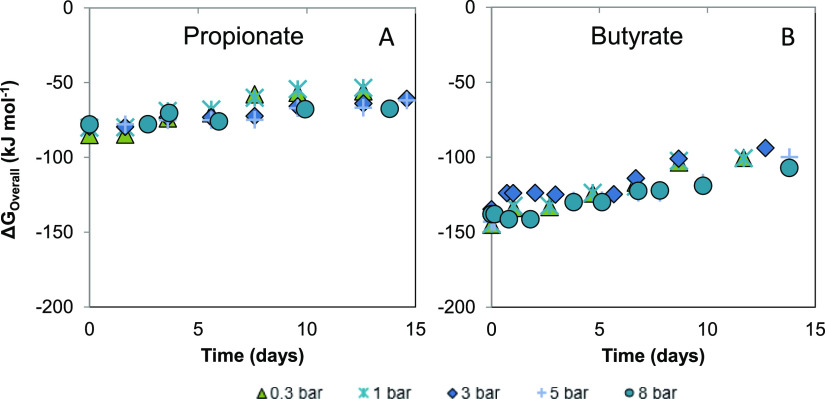
Figure 3 shows the effect of applied pCO2 on the overall available Gibbs free energy (ΔGOverall) during syntrophic propionate and butyrate conversion calculated using the actual concentrations of reactants during the atmospheric and pressure experiments and at pH2 = 1 × 10–5 bar. Results showed a less steep increasing trend over time for ΔGOverall from 1 bar pCO2 onward, indicating that the two syntrophic reactions became less energetically feasible because of decreased substrate consumption or product accumulation. At day 0, the ΔGOverall at 0.3 bar pCO2 for propionate oxidation was −85.0, compared to −145.0 kJ mol–1 for butyrate oxidation. At 8 bar pCO2, the ΔGOverall for propionate increased to −78.0 compared to −137.9 kJ mol–1 for butyrate. The calculated dissimilarity in the ΔGOverall of the reactions (≈40%) might have weakened the driving force to carry out propionate conversion at increased values of pCO2 at atmospheric and pressurized conditions. This observation relates well with what Kleerebezem and Stams18 proposed in their metabolic network analysis of syntrophic butyrate conversion, where they highlighted the possibility of a lowered specific reaction rate as a function of increased Gibbs free energy change of the catabolic reaction.
Figure 3.
Change in the overall available Gibbs free energy (ΔGOverall) during mesophilic syntrophic (A) propionate oxidation and (B) butyrate oxidation at 0.3, 1, 3, 5, and 8 bar initial pCO2 calculated with measured concentrations of reactants and products during the experimental period. Aqueous concentrations were used (in mol L–1), the partial pressure of gases (in bar), T = 35 °C, and a theoretical value of pH2 = 1 × 10–5 bar.
ΔGR1 responds to direct and indirect changes in biochemical reactions.35 A deliberate change in the concentration of one or more biochemical species is considered a direct intervention. A change in the concentration of the species induced by the modification of another operational parameter is an indirect intervention. The predominance of a direct or indirect effect of increased pCO2 on the ΔGOverall and intermediate biochemical reactions of syntrophic conversions has not been thoroughly elucidated in literature. We tried to gain further insight into the individual and combined effects of elevated pCO2 and pH on the bioenergetics using scenario analysis. By such analysis, possible bioenergetic limitations caused by an increase in the ΔGOverall value might be identified.
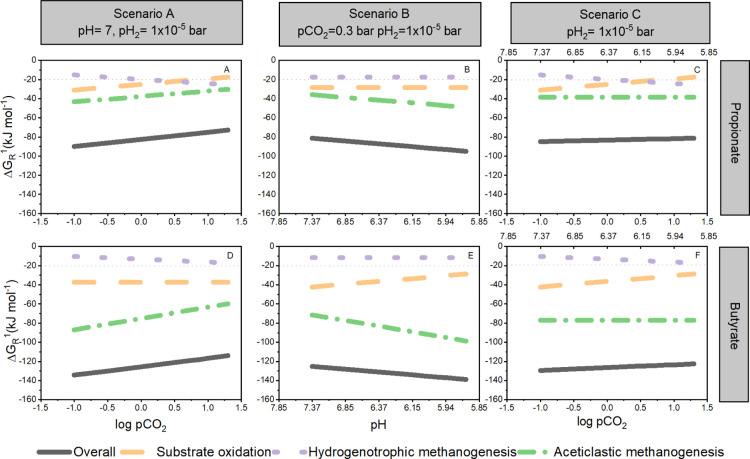
Figure 4 visualizes the change in the ΔGR1 value when the parameters pCO2 and pH are independently and concomitantly modified in syntrophic propionate and butyrate conversion. Lines represent the change in Gibbs free energy at increasing pCO2 or decreasing pH for the intermediate biochemical reactions: substrate oxidation (ΔGPr-Ox, ΔGBu-Ox), AcM (ΔGAcM), HyM (ΔGHyM), and for the overall reaction (ΔGOverall). An increase in the ΔGOverall in the subplots, as shown in Figure 4, means that less energy is available for all the subreactions, whereas a decrease implies that more energy is at hand. In scenario A, the ΔGOverall for the syntrophic conversion of propionate and butyrate was calculated for an initial pCO2 increasing from 0.1 to 20 bar to amplify the effect of elevated pCO2 in comparison to our experimental range (0.3–8 bar). An elevated pCO2 of 20 bar increased the ΔGOverall of propionate by 19% and butyrate by 15%, compared to 0.1 bar (A and D). In scenario B, ΔGOverall was calculated using the corresponding equilibrium pH values at pCO2 ranging between 0.1 and 20 bar and buffer concentration of 100 mM as HCO3–. A pH change from 7.9 to 5.5 caused the ΔGOverall to decrease by 14 and 10% for propionate and butyrate, respectively (B and E). In scenario C, ΔGOverall was calculated with pCO2 of scenario A and the pH values of scenario B. Under these conditions, there is a marginal increase in ΔGOverall for the conversion of both substrates (C and F).
Figure 4.
Effect of changing selected operational parameters on the ΔGR1 in the proposed scenarios for the syntrophic conversions. Scenario A—partial pressure of CO2 (pCO2) in propionate and butyrate conversion (A and D, respectively). Scenario B—pH in propionate and butyrate conversion (B and E, respectively). Scenario C—concomitant effect of pH and pCO2 on propionate and butyrate conversion (C and F, respectively). Lines represent the ΔGR for the intermediate biochemical reactions: dotted-purple (HyM—ΔGHyM), dashed-orange (oxidation of propionate—ΔGPr-Ox or butyrate—ΔGBu-Ox), short-dash-dotted green (AcM—ΔGAcM), and solid black (overall reaction—ΔGOverall). The experimental conditions (pH, pCO2, and pH2) that remained fixed during the calculation are included for reference in the upper part of the subplots. Values are presented as log pCO2 for data linearization purposes. Concentrations of liquid reactants (mol L–1) and gases (bar) correspond to the initial experimental conditions at T = 35 °C presented in the heading of Table S3, Supporting Information.
Concerning the intermediate reactions at 20 bar pCO2 in scenario A, ΔGPr-Ox increased by 44%, and ΔGBu-Ox remained constant because CO2 is not a reaction product. Regarding the methanogenic reactions, ΔGAcM increased by 30%, whereas ΔGHyM decreased by 40% for both substrates (A and D). The pH decrease to 5.5 in scenario B did not strongly affect the reactions where H+ ions are not produced, that is, ΔGPr-Ox and ΔGHyM. Contrastingly, ΔGBu-Ox increased by 32% and ΔGAcM decreased by 27 and 28% for the propionate- and butyrate-fed assays, respectively, suggesting enhanced energetical feasibility of this reaction (B and E). In scenario C, ΔGPr-Ox and ΔGBu-Ox changed analogously to scenario A. ΔGAcM remained the same in the entire pCO2 range, which could be attributed to the simultaneous variation of pCO2 annihilating the pH effects on the bioenergetics. The behavior of ΔGHyM resembled scenario A because of the absent effect of H+ production (C and F).
Scenario A highlighted the adverse effects of increased pCO2 on the bioenergetics of syntrophic reactions. In this regard, Jin and Kirk22 postulated that increasing pCO2 from 0 to 30 bar in simulated non-buffered and buffered aquifer systems made SAO and AcM less energetically feasible, whereas the contrary was calculated for HyM. Moreover, they proposed additional effects of elevated pCO2 on biochemical reactions because of induced changes in aqueous speciation, ionic strength, and in the reduction potential of redox couples such as H+/H2. Kato et al.21 found that increasing pCO2 from 0 to 1 bar strongly suppressed syntrophic activity in a model bacterial consortium for SAO, including the bacterium Thermacetogenium phaeum and the archaea Methanothermobacter thermautotrophicus and Methanosaeta thermophila. They established a 91% reduction in the rsmax of acetate, coincidently occurring when ΔGAc-Ox became higher than −20 kJ mol–1, which is considered the smallest quantum to sustain life.17 In our experiments, rsmax values decreased when pCO2 increased from 0.3 to 8 bar, and the most significant drop also occurred when, theoretically, ΔGPr-Ox was higher than −20 kJ mol–1 (Supporting Information, Table S4).
Scenario B showed that decreasing pH modifies the bioenergetics of syntrophic propionate and butyrate conversion in a different direction than elevated pCO2. Interestingly, pH can directly change the ΔGR1 when reactions produce or consume protons and indirectly as a result of modified chemical speciation.35,36 From the bioenergetics point of view, proton (H+)-consuming reactions, namely syntrophic oxidation and AcM (Table 1), could be promoted when decreasing pH inside a physiologically reasonable range. The more negative ΔGOverall value in this scenario indicates a potential increase in the driving force to carry out the syntrophic reaction. Nonetheless, this might be compromised by physiological limitations and enhanced toxicity effects37 observed at decreased pH levels, particularly in the case of methanogenic populations.38 In consequence, bioenergetics does not suffice to elucidate the detrimental effects observed on the syntrophic conversions if pH is considered as the main explanatory variable.
Elevated pCO2 as a Biochemical Steering Parameter
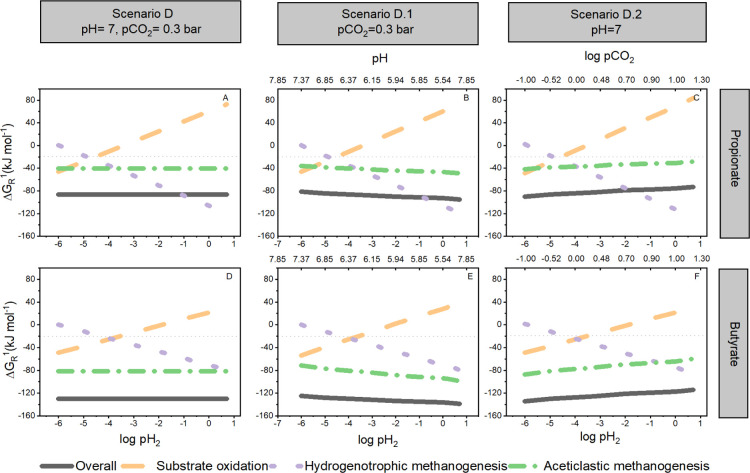
The distribution of available biochemical energy between the syntrophic partners is expected to change because of the direct and indirect effects of increasing pCO2 on ΔGR1 of the overall and intermediate reactions (Supporting Information, Figure S3). In our results, the biochemical energy allocation is proposed under conditions of fixed pH2. Under conditions of changing pH2, pH, and pCO2 (Figure 5, scenarios D, D.1, and D.2), a new thermodynamic equilibrium will be established, which can further modify the biochemical energy distribution among partners in syntrophic propionate and butyrate conversion. Values of pH2 lower than 6 × 10–4 bar will have a positive effect on reaction feasibility, whereas higher values will reduce the feasibility “niche.” The impact of increasing pH2 on the available Gibbs free energy has been previously discussed in the literature;39 nevertheless, its interaction with increased pCO2 and decreased pH, to the best of our knowledge, has not been thoroughly described. A correlation analysis with hierarchical clustering of bioenergetic and experimental data was performed in order to verify whether the highlighted trends of the scenario analysis were still valid at a varying pH2 (Supporting Information, Figure S4). Two theoretical values were chosen: a typical value for ADs at which syntrophic reactions are thermodynamically feasible (1 × 10–5 bar)31 and the lowest detection level of the used gas chromatograph (6 × 10–4 bar). A strong negative correlation was found between pCO2 and rsmax (rS = −0.82, p < 0.05) for both propionate and butyrate. Concerning the Gibbs free energy change, a strong negative correlation was encountered only between ΔGBu-Ox and pH (rS = −0.78, p < 0.05). ΔGAcM was strongly negatively correlated with ΔGHyM (rS = −0.87, p < 0.05), evidencing the role of increasing pCO2 and pH2 in modulating the feasibility of methanogenic reactions.
Figure 5.
Effect of changing selected operational parameters on the ΔGR1 in the proposed scenarios for the syntrophic conversions. Scenario D—partial pressure of H2 (pH2) in propionate and butyrate (A and D, respectively). Scenario D.1—concomitant effect of pH and pH2 in propionate and butyrate (B and E, respectively). Scenario D.2—concomitant effect of pH2 and pCO2 in propionate and butyrate (C and F, respectively). Lines represent the ΔGR for the intermediate biochemical reactions: dotted-purple (HyM—ΔGHyM), dashed-orange (oxidation of propionate—ΔGPr-Ox or butyrate—ΔGBu-Ox), short-dash-dotted green (AcM—ΔGAcM), and solid black (overall reaction—ΔGOverall). The experimental conditions (pH, pCO2, and pH2) that remained fixed during the calculation are included for reference in the upper part of the subplots. Values are presented as log pCO2 and log pH2 for data linearization. Concentrations of liquid reactants (mol L–1) and gases (bar) correspond to the initial experimental conditions at T = 35 °C presented in the heading of Table S3, Supporting Information.
Response of Syntrophic Anaerobic Conversion at Elevated pCO2: Possible Physiological Effects
This study highlighted a possible relation between bioenergetic limitations and the observed kinetic effects occurring because of increased pCO2. However, additional limitations cannot be discarded. For example, in our experiments, the dissolution of CO2 from the headspace could decrease pH levels, irrespective of the applied high buffer concentration (100 mM HCO3–). Changes in pH disrupt cell homeostasis and impose limitations for growth, maintenance, and metabolic activity. In particular, syntrophic butyrate oxidizers (SBOs) and syntrophic propionate oxidizers (SPOs) demonstrate moderate growth at a pH lower than 6.540 and 6.0,41 respectively. The increased lag phases and limited conversion under elevated pCO2 could then be explained by the combination of pH effects on, for example, ΔGBu-Ox and physiological limitations affecting SBOs and SPOs at a different extent.
Also, the acidification of the fermentation medium modifies the equilibrium between undissociated and dissociated forms of the VFAs,42 further altering cell homeostasis. At the applied pCO2 of 8 bar and resulting equilibrium pH of 5.9, the concentrations of undissociated propionic acid (HPr) were slightly above inhibitory levels, that is, 20 mg L–1 HPr43 (Supporting Information, Table S5). The concentration of undissociated butyric acid (HBu) remained below 500 mg L–1 HBu,44 proposed in literature as inhibitory for growth in, for example, Clostridium acetobutylicum. Acetic acid concentrations (HAc) remained below indicative inhibitory levels in methanogenesis.45 However, the detrimental effects of elevated pCO2 in our experimental treatments were already seen at 1 bar pCO2. Consequently, increased undissociated VFA concentrations do not explain the observed phenomena.
At elevated pCO2, the equilibrium dissolved CO2 concentration in the liquid medium increased from 320 to 8,620 mg L–1 (Supporting Information, Table S5). These dissolved CO2 concentrations are in line with values reported by Wan et al.10 (3,000–30,000 ppm), which negatively impacted the nitrogen removal efficiency because of increased membrane permeability, thus inhibiting electron transport and protein expression.
Furthermore, Salek et al.46 showed that there is at least 1 order of magnitude difference in the kinetically controlled rate of physical reactions such as CO2 dissolution and biochemical reactions, such as production of VFAs. This, in turn, may affect the concentration of the various species that are responsible for the reactions used in the thermodynamic calculations, leading to disparities in the calculated and observed bioenergetic effects at specific time points. More accurate pH2 measurements in the low range, for example, <6 × 10–4 bar, are required to further validate the occurrence of the postulated effects on the feasibility of syntrophic reactions because of concomitant variation of pH2 and pH or pCO2. The possible role of other electron shuttles, whose appearance is favored by the presence of hydrogen and elevated pCO2, particularly formate, needs to be further addressed.47,48
Elevated pCO2 influences the kinetics and bioenergetics of the syntrophic conversion of propionate and butyrate. Based on this study, we propose that kinetic effects might appear as an evident sign of thermodynamic limitations, which is different for each compound. From detailed bioenergetic calculations, it was concluded that pCO2 increases the ΔGPr-Ox, induces pH changes that make ΔGBu-Ox more positive, and increases the ΔGOverall of the syntrophic conversion. The more positive ΔGOverall at elevated pCO2 likely induces a redistribution of the available biochemical energy among the syntrophic partners that, if unbalanced, will translate into kinetic constraints. However, the here discussed biochemical energy limitations could not fully explain the strong kinetic effects on the system at increasing pCO2. Presumably, the overall effects resulted from the concomitant impact of reduced thermodynamic feasibility, physiological effects associated with a lowered pH, and a minor detrimental impact of increased concentrations of undissociated VFAs. The observed kinetic and bioenergetic aftermath of elevated pCO2 exposure might confer potentials for steering metabolic pathways, if limitations are overcome. For instance, the use of acclimated inocula38 and energy-rich substrates such as sugars, proteins, or lipids could minimize the physiological impact of lowered pH and relieve bioenergetic limitations. Under such conditions, the steering potential of elevated pCO2 on biochemical pathways in mixed culture anaerobic conversions could be unraveled.
Acknowledgments
This research was funded by European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 676070 (SuPER-W). This communication reflects only author’s view, and the Research Executive Agency of the EU is not responsible for any use that may be made of the information it contains. Maria Gomez and Roberta Massini are acknowledged for their contributions to the experimental work.
Glossary
Nomenclature
- ΔGR0
Gibbs free energy change for reaction R at standard temperature and pressure (kJ mol–1)
- ΔGR01
Gibbs free energy change for reaction R corrected by biological pH reference value (pH = 7) (kJ mol–1)
- ΔGR1
Gibbs free energy change for reaction R corrected by actual operational conditions (kJ mol–1)
- ΔGOverall
Gibbs free energy change for the syntrophic reaction corrected by actual operational conditions (kJ mol–1)
- ΔGPr-Ox
Gibbs free energy change for propionate oxidation corrected by actual operational conditions (kJ mol–1)
- ΔGBu-Ox
Gibbs free energy change for butyrate oxidation corrected by actual operational conditions (kJ mol–1)
- ΔGAcM
Gibbs free energy change for aceticlastic methanogenesis corrected by actual operational conditions (kJ mol–1)
- ΔGHyM
Gibbs free energy change for hydrogenotrophic methanogenesis corrected by actual operational conditions (kJ mol–1)
- rS
Spearman’s correlation coefficient31
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c02022.
Role of inoculum origin on pCO2 response; physicochemical characteristics of initial inocula; microbial community analysis, including Illumina sequencing protocol and absolute abundance results; sampling strategy; input parameters for the bioenergetics scenario analysis of syntrophic propionate and butyrate conversion; calculated values for ΔGPr-Ox in scenario A; theoretical share of ΔGOverall for each of the proposed scenarios for the syntrophic conversion of propionate and butyrate; correlogram at different pH2 values; and calculation of undissociated acids and carbonate equilibrium species for the anaerobic conversion experiments at 0.3, 1, 3, 5, and 8 bar pCO2 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lindeboom R. E. F.; Fermoso F. G.; Weijma J.; Zagt K.; van Lier J. B. Autogenerative High Pressure Digestion: Anaerobic Digestion and Biogas Upgrading in a Single Step Reactor System. Water Sci. Technol. 2011, 64, 647–653. 10.2166/wst.2011.664. [DOI] [PubMed] [Google Scholar]
- Lemmer A.; Chen Y.; Lindner J.; Wonneberger A. M.; Zielonka S.; Oechsner H.; Jungbluth T. Influence of Different Substrates on the Performance of a Two-Stage High Pressure Anaerobic Digestion System. Bioresour. Technol. 2015, 178, 313–318. 10.1016/j.biortech.2014.09.118. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu H.; Yan F.; Su D.; Wang Y.; Zhou H. High-Calorific Biogas Production from Anaerobic Digestion of Food Waste Using a Two-Phase Pressurized Biofilm (TPPB) System. Bioresour. Technol. 2017, 224, 56–62. 10.1016/j.biortech.2016.10.070. [DOI] [PubMed] [Google Scholar]
- Lindeboom R. E. F.; Ferrer I.; Weijma J.; van Lier J. B. Effect of Substrate and Cation Requirement on Anaerobic Volatile Fatty Acid Conversion Rates at Elevated Biogas Pressure. Bioresour. Technol. 2013, 150, 60–66. 10.1016/j.biortech.2013.09.100. [DOI] [PubMed] [Google Scholar]
- Lindeboom R. E. F.; Shin S. G.; Weijma J.; van Lier J. B.; Plugge C. M. Piezo-Tolerant Natural Gas-Producing Microbes under Accumulating pCO2. Biotechnol. Biofuels 2016, 9, 236. 10.1186/s13068-016-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilimbergo S.; Bertucco A. Non-Thermal Bacteria Inactivation with Dense CO2. Biotechnol. Bioeng. 2003, 84, 627–638. 10.1002/bit.10783. [DOI] [PubMed] [Google Scholar]
- Manzocco L.; Plazzotta S.; Spilimbergo S.; Nicoli M. C. Impact of High-Pressure Carbon Dioxide on Polyphenoloxidase Activity and Stability of Fresh Apple Juice. LWT--Food Sci. Technol. 2017, 85, 363–371. 10.1016/j.lwt.2016.11.052. [DOI] [Google Scholar]
- Yu T.; Chen Y. Effects of Elevated Carbon Dioxide on Environmental Microbes and Its Mechanisms: A Review. Sci. Total Environ. 2019, 655, 865–879. 10.1016/j.scitotenv.2018.11.301. [DOI] [PubMed] [Google Scholar]
- Watanabe T.; Furukawa S.; Kawarai T.; Wachi M.; Ogihara H.; Yamasaki M. Cytoplasmic Acidification May Occur in High- Pressure Carbon Dioxide-Treated Escherichia Coli. Biosci., Biotechnol., Biochem. 2007, 71, 2522–2526. 10.1271/bbb.70313. [DOI] [PubMed] [Google Scholar]
- Wan R.; Chen Y.; Zheng X.; Su Y.; Li M. Effect of CO2 on Microbial Denitrification via Inhibiting Electron Transport and Consumption. Environ. Sci. Technol. 2016, 50, 9915–9922. 10.1021/acs.est.5b05850. [DOI] [PubMed] [Google Scholar]
- Wan R.; Chen Y.; Zheng X.; Su Y.; Huang H. Effect of CO2 on NADH Production of Denitrifying Microbes via Inhibiting Carbon Source Transport and Its Metabolism. Sci. Total Environ. 2018, 627, 896–904. 10.1016/j.scitotenv.2018.01.315. [DOI] [PubMed] [Google Scholar]
- Mayumi D.; Dolfing J.; Sakata S.; Maeda H.; Miyagawa Y.; Ikarashi M.; Tamaki H.; Takeuchi M.; Nakatsu C. H.; Kamagata Y. Carbon Dioxide Concentration Dictates Alternative Methanogenic Pathways in Oil Reservoirs. Nat. Commun. 2013, 4, 1998. 10.1038/ncomms2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.; Xu S.; Florentino A. P.; Zhang L.; Yang Z.; Liu Y. Enhancing Blackwater Methane Production by Enriching Hydrogenotrophic Methanogens through Hydrogen Supplementation. Bioresour. Technol. 2019, 278, 481–485. 10.1016/j.biortech.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Bajón Fernández Y.; Soares A.; Vale P.; Koch K.; Masse A. L.; Cartmell E. Enhancing the Anaerobic Digestion Process through Carbon Dioxide Enrichment: Initial Insights into Mechanisms of Utilization. Environ. Technol. 2019, 40, 1744–1755. 10.1080/09593330.2019.1597173. [DOI] [PubMed] [Google Scholar]
- Bajón Fernández Y.; Green K.; Schuler K.; Soares A.; Vale P.; Alibardi L.; Cartmell E. Biological Carbon Dioxide Utilisation in Food Waste Anaerobic Digesters. Water Res. 2015, 87, 467–475. 10.1016/j.watres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Al-mashhadani M. K. H.; Wilkinson S. J.; Zimmerman W. B. Carbon Dioxide Rich Microbubble Acceleration of Biogas Production in Anaerobic Digestion. Chem. Eng. Sci. 2016, 156, 24–35. 10.1016/j.ces.2016.09.011. [DOI] [Google Scholar]
- Schink B. Energetics of Syntrophic Cooperation in Methanogenic Degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. 10.1128/.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem R.; Stams A. J. M. Kinetics of Syntrophic Cultures: A Theoretical Treatise on Butyrate Fermentation. Biotechnol. Bioeng. 2000, 67, 529–543. . [DOI] [PubMed] [Google Scholar]
- Henze M.Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing, 2008. [Google Scholar]
- Leng L.; Yang P.; Singh S.; Zhuang H.; Xu L.; Chen W.-H.; Dolfing J.; Li D.; Zhang Y.; Zeng H.; Chu W.; Lee P.-H. A Review on the Bioenergetics of Anaerobic Microbial Metabolism Close to the Thermodynamic Limits and Its Implications for Digestion Applications. Bioresour. Technol. 2018, 247, 1095–1106. 10.1016/j.biortech.2017.09.103. [DOI] [PubMed] [Google Scholar]
- Kato S.; Yoshida R.; Yamaguchi T.; Sato T.; Yumoto I.; Kamagata Y. The Effects of Elevated CO2 Concentration on Competitive Interaction between Aceticlastic and Syntrophic Methanogenesis in a Model Microbial Consortium. Front. Microbiol. 2014, 5, 575. 10.3389/fmicb.2014.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q.; Kirk M. F. Thermodynamic and Kinetic Response of Microbial Reactions to High CO2. Front. Microbiol. 2016, 7, 1696. 10.3389/fmicb.2016.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. F. Variation in Energy Available to Populations of Subsurface Anaerobes in Response to Geological Carbon Storage. Environ. Sci. Technol. 2011, 45, 6676–6682. 10.1021/es201279e. [DOI] [PubMed] [Google Scholar]
- Jin Q.; Bethke C. M. The Thermodynamics and Kinetics of Microbial Metabolism. Am. J. Sci. 2007, 307, 643–677. 10.2475/04.2007.01. [DOI] [Google Scholar]
- Ghasimi D. S. M.; Aboudi K.; de Kreuk M.; Zandvoort M. H.; van Lier J. B. Impact of Lignocellulosic-Waste Intermediates on Hydrolysis and Methanogenesis under Thermophilic and Mesophilic Conditions. Chem. Eng. J. 2016, 295, 181–191. 10.1016/j.cej.2016.03.045. [DOI] [Google Scholar]
- American Public Health Association . Standard Methods for the Examination of Water and Wastewater; American Public Health Association, 2005.
- Do H.; Lim J.; Shin S. G.; Wu Y.-J.; Ahn J.-H.; Hwang S. Simultaneous Effect of Temperature, Cyanide and Ammonia-Oxidizing Bacteria Concentrations on Ammonia Oxidation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1331–1338. 10.1007/s10295-008-0415-9. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing, 2019. https://www.r-project.org/.
- Kleerebezem R.; Van Loosdrecht M. C. M. A Generalized Method for Thermodynamic State Analysis of Environmental Systems. Crit. Rev. Environ. Sci. Technol. 2010, 40, 1–54. 10.1080/10643380802000974. [DOI] [Google Scholar]
- Heijnen J. J.; Kleerebezem R.. Bioenergetics of Microbial Growth. Encyclopedia of Industrial Biotechnology; Wiley, 2010; pp 1–66. [Google Scholar]
- Patón M.; Rodríguez J. A Compilation and Bioenergetic Evaluation of Syntrophic Microbial Growth Yields in Anaerobic Digestion. Water Res. 2019, 159, 176–183. 10.1016/j.watres.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Wei T.; Simko V.. R Package “corrplot”: Visualization of a Correlation Matrix, version 0.84, 2017. https://github.com/taiyun/corrplot.
- van Lier J. B.; Mahmoud N.; Zeeman G.. Anaerobic Wastewater Treatment. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing, 2008; pp 401–442. [Google Scholar]
- Hansson G.; Molin N. End Product Inhibition in Methane Fermentations: Effects of Carbon Dioxide and Methane on Methanogenic Bacteria Utilizing Acetate. Eur. J. Appl. Microbiol. Biotechnol. 1981, 13, 236–241. 10.1007/bf00500105. [DOI] [Google Scholar]
- Jin Q.; Kirk M. F. pH as a Primary Control in Environmental Microbiology: 2. Kinetic Perspective. Front. Environ. Sci. 2018, 6, 101. 10.3389/fenvs.2018.00101. [DOI] [Google Scholar]
- Bethke C. M.; Sanford R. A.; Kirk M. F.; Jin Q.; Flynn T. M. The Thermodynamic Ladder in Geomicrobiology. Am. J. Sci. 2011, 311, 183–210. 10.2475/03.2011.01. [DOI] [Google Scholar]
- Ali S.; Hua B.; Huang J. J.; Droste R. L.; Zhou Q.; Zhao W.; Chen L. Effect of Different Initial Low pH Conditions on Biogas Production, Composition, and Shift in the Aceticlastic Methanogenic Population. Bioresour. Technol. 2019, 289, 121579. 10.1016/j.biortech.2019.121579. [DOI] [PubMed] [Google Scholar]
- Mao C.; Feng Y.; Wang X.; Ren G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renewable Sustainable Energy Rev. 2015, 45, 540–555. 10.1016/j.rser.2015.02.032. [DOI] [Google Scholar]
- De Kok S.; Meijer J.; Van Loosdrecht M. C. M.; Kleerebezem R. Impact of Dissolved Hydrogen Partial Pressure on Mixed Culture Fermentations. Appl. Microbiol. Biotechnol. 2013, 97, 2617–2625. 10.1007/s00253-012-4400-x. [DOI] [PubMed] [Google Scholar]
- Balk M.; Altinbas M.; Rijpstra W. I. C.; Sinninghe Damste J. S.; Stams A. J. M. Desulfatirhabdium Butyrativorans Gen. Nov., Sp. Nov., a Butyrate-Oxidizing, Sulfate-Reducing Bacterium Isolated from an Anaerobic Bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. 10.1099/ijs.0.65396-0. [DOI] [PubMed] [Google Scholar]
- Li J.; Ban Q.; Zhang L.; Jha A. K. Syntrophic Propionate Degradation in Anaerobic Digestion: A Review. Int. J. Agric. Biol. 2012, 14, 843–850. [Google Scholar]
- Xiao K.; Zhou Y.; Guo C.; Maspolim Y.; Ng W. J. Impact of Undissociated Volatile Fatty Acids on Acidogenesis in a Two-Phase Anaerobic System. J. Environ. Sci. 2016, 42, 196–201. 10.1016/j.jes.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Maillacheruvu K. Y.; Parkin G. F.; Ma K. Y. Kinetics of Growth, Substrate Utilization and Sulfide Toxicity for Propionate, Acetate, and Hydrogen Utilizers in Anaerobic Systems. Water Environ. Res. 1996, 68, 1099–1106. 10.2175/106143096x128126. [DOI] [Google Scholar]
- Monot F. d.; Engasser J.-M.; Petitdemange H. Influence of pH and Undissociated Butyric Acid on the Production of Acetone and Butanol in Batch Cultures of Clostridium Acetobutylicum. Appl. Microbiol. Biotechnol. 1984, 19, 422–426. 10.1007/bf00454381. [DOI] [Google Scholar]
- Xiao K. K.; Guo C. H.; Zhou Y.; Maspolim Y.; Wang J. Y.; Ng W. J. Acetic Acid Inhibition on Methanogens in a Two-Phase Anaerobic Process. Biochem. Eng. J. 2013, 75, 1–7. 10.1016/j.bej.2013.03.011. [DOI] [Google Scholar]
- Salek S. S.; van Turnhout A. G.; Kleerebezem R.; van Loosdrecht M. C. M. pH Control in Biological Systems Using Calcium Carbonate. Biotechnol. Bioeng. 2015, 112, 905–913. 10.1002/bit.25506. [DOI] [PubMed] [Google Scholar]
- Oswald F.; Stoll I. K.; Zwick M.; Herbig S.; Sauer J.; Boukis N.; Neumann A. Formic Acid Formation by Clostridium Ljungdahlii at Elevated Pressures of Carbon Dioxide and Hydrogen. Front. Bioeng. Biotechnol. 2018, 6, 6. 10.3389/fbioe.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger M.; Brown F.; Gabrielli W.; Sargent F. Efficient Hydrogen-Dependent Carbon Dioxide Reduction by Escherichia Coli. Curr. Biol. 2018, 28, 140–145. 10.1016/j.cub.2017.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.