Objectives:
While evidence has mounted regarding the short-term effectiveness of pharmacotherapy for opioid use disorder (OUD), little is known about longer-term psychosocial, economic, and health outcomes. We report herein 12-month outcomes for an observational study enrolling participants who had previously taken part in a long-acting buprenorphine subcutaneous injection (BUP-XR) trial for moderate to severe OUD.
Methods:
The RECOVER (Remission from Chronic Opioid Use: Studying Environmental and SocioEconomic Factors on Recovery; NCT03604861) study enrolled participants from 35 US community-based sites. Self-reported sustained opioid abstinence over 12 months and self-reported past-week abstinence at 3-, 6-, 9-, and 12-month visits were assessed. Multiple regression models assessed the association of BUP-XR duration with abstinence, controlling for potential confounders. Withdrawal, pain, health-related quality of life, depression, and employment at RECOVER baseline and 12-month visits were also compared to values collected before treatment in the BUP-XR trial.
Results:
Of 533 RECOVER participants, 425 completed the 12-month visit (average age 42 years; 66% male); 50.8% self-reported sustained 12-month and 68.0% past-week opioid abstinence. In multiple regressions, participants receiving 12-month versus ≤2-month BUP-XR treatment duration had significantly higher likelihood of sustained opioid abstinence (75.3% vs 24.1%; P = 0.001), with similar results for past-week self-reported abstinence over time. During RECOVER, participants had fewer withdrawal symptoms, lower pain, positive health-related quality of life, minimal depression, and higher employment versus pre-trial visit.
Conclusions:
RECOVER participants reported positive outcomes over the 12-month observational period, including high opioid abstinence and stable or improved humanistic outcomes. These findings provide insights into the long-term impact of pharmacotherapy in OUD recovery.
Keywords: buprenorphine, BUP-XR, opioid use disorder, patient-centered outcomes
In 2017, the United States (US) Department of Health and Human Services (HHS) declared the opioid epidemic a public health emergency. The first strategy recommended for fighting the opioid crisis by the HHS was improving access to treatment and recovery services (US Department of Health and Human Services, 2018). However, efforts to combat this emergency appear to focus more on the promotion of overdose-reversing medications and provision of tools to healthcare providers to limit unnecessary use of opioids for pain relief (Sharfstein and Olsen, 2019). Although these efforts may decrease the number of opioid-related overdoses and potentially avoid future cases of opioid use disorder (OUD), effective treatment of patients with OUD is paramount to saving patient lives (Sharfstein and Olsen, 2019).
The clinical efficacy of buprenorphine, methadone, and naltrexone for the treatment of OUD has been well characterized (Mattick et al., 2009; Schwartz et al., 2013; Mattick et al., 2014). More recently, buprenorphine extended-release subcutaneous injection (BUP-XR a.k.a. RBP-6000 or SUBLOCADE, Indivior Inc, North Chesterfield, VA) was shown to be a safe and effective OUD treatment option (Haight et al., 2019) that could improve the quality of life (Ling et al., 2019b) of treatment-seeking individuals with moderate or severe OUD.
Pharmacotherapy for OUD is underutilized (Leshner and Dzau, 2019) with estimates from the year 2019 suggesting that fewer than 35% of adults with OUD received OUD treatment in the past year (National Academies of Sciences, 2019). Although the beneficial effects of pharmacotherapy, including decreased opioid use, decreased opioid-related overdose deaths, decreased criminal activity, decreased infectious disease transmission, and increased social functioning, are clearly demonstrated (Mattick et al., 2009; Schwartz et al., 2013; Mattick et al., 2014), little is known about patient recovery following pharmacologic treatment of OUD. The objectives of this analysis were to describe 12-month outcomes from an observational study enrolling individuals who had previously taken part in a BUP-XR trial.
METHODS
Study Design and Setting
The RECOVER study (Remission from Chronic Opioid Use: Studying Environmental and SocioEconomic Factors on Recovery; NCT03604861)(Ling et al., 2019a) was a 24-month, observational study that assessed life changes in patients with OUD who had received up to 12 monthly BUP-XR injections as part of either a separate randomized clinical efficacy trial (NCT02357901) (Haight et al., 2019; Ling et al., 2019b) and/or a separate open-label safety (NCT02510014) study before entering RECOVER. Additional details of the study design and characteristics of patients enrolled in the RECOVER study have been previously published (Ling et al., 2019a). Data reported herein represent analyses through the 12-month visit, which was completed in March 2018.
As part of the RECOVER study, participants completed detailed self-administered assessments concerning illicit substance use, treatment for substance use disorder, and psychosocial measures at RECOVER enrollment and at 6 and 12 months post-enrollment. RECOVER study enrollment occurred at least 28 days after completing or discontinuing participation in the aforementioned BUP-XR trials. A shorter battery of assessments was conducted at 3 and 9 months after enrollment. Ten panel urine drug screens (UDS; T-cup Multi-Drug Urine Test) were conducted at all visits. An attempt to contact all actively enrolled RECOVER participants was made for all visits, regardless of whether participants had missed an earlier visit.
The RECOVER study was conducted in accordance with all applicable ethical and regulatory requirements. In accordance with national and local regulations, Institutional Review Board or Ethical Committee approval was obtained. Additionally, a Certificate of Confidentiality from the US government was granted to the RECOVER study to protect participants’ right to refuse requests for disclosure of assessment data. All participants provided informed consent before study participation. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (von Elm et al., 2007).
Treatment During RECOVER
As RECOVER was a prospective observational study, any treatment that participants received, including those for OUD, were not provided as part of the RECOVER study. Participants were asked to self-report whether they were receiving substance use disorder treatment at the 6- and 12-month visits. Some RECOVER participants (n = 146) elected to extend their BUP-XR treatment by 6 months after leaving the open-label safety study through participation in a BUP-XR open-label extension study (NCT02896296) that ran concurrently with RECOVER. Participants self-reporting OUD treatment at either the 6- or 12-month visit or enrolled in the open-label extension study concurrent with RECOVER were noted as receiving OUD treatment during RECOVER.
Study Outcomes
RECOVER 12-month analysis operationalized opioid abstinence as (1) self-reported sustained abstinence over the entire 12-month reporting period and (2) self-reported past week abstinence at each visit over 12 months.
Sustained 12-month abstinence was defined as no days of opioid use self-reported for the past 6 months (collected at 6 and 12 months) among those participants who had completed the 12-month visit. If the 6-month visit was missing, only self-report at 12 months was used. Self-reported opioid use included any misuse of prescription painkillers, methadone, or buprenorphine, or use of heroin, goofball (heroin + methamphetamine), or speedball (heroin + cocaine). While biologic assays such as UDS are useful for capturing drug use over short time spans (eg, 2–3 days for opioids), self-reported abstinence was used for the main definition in order to capture opioid use over longer time spans. In order to test the robustness of this definition, 2 sensitivity analyses were conducted. The first sensitivity analysis defined a participant as abstinent over the 12-month period if there was no opioid use on any self-reported measure (ie, past-week, past-month, and past 6 months) at 3, 6, 9, and 12 months. The second sensitivity analysis required participants to have a UDS that was negative for opioids in addition to no self-reported opioid use, as described in the first sensitivity analysis. If a positive UDS result was indicated for either opiates or oxycodone, the UDS was considered to indicate opioid use. In the case where the UDS result for any substance indicated that the test was invalid, the UDS was treated as missing.
Past-week, self-reported abstinence at each quarterly visit was defined as no self-reported opioid use in the past week. As a sensitivity analysis, participants were only considered abstinent if there were no days of self-reported opioid use and UDS results were negative for opioids. To understand the impact of missing data on observed results, sensitivity analyses were run in which the last observation was carried forward for all future missing visits, and a second analysis considered all missing visits as non-abstinent (ie, worst case imputation).
In addition to the aforementioned abstinence-related outcomes, outcomes collected at both the initial visit within the BUP-XR clinical trials (ie, before receiving treatment in the randomized efficacy or open-label safety studies) and within RECOVER were described. These included past-month self-reported abstinence, opiate abstinence according to UDS results, withdrawal symptoms (Subjective Opiate Withdrawal Scale [SOWS]), pain intensity (Brief Pain Inventory [BPI]), quality of life (36-Item Short Form Survey [SF-36] in efficacy and safety trials, 12-Item Short Form Survey [SF-12] in RECOVER), depression severity (Beck Depression Inventory [BDI-II]), and employment status.
Statistical Analysis
Primary abstinence outcomes were measured within the overall cohort and compared between BUP-XR treatment duration groups based on injections received within the randomized efficacy and open-label safety studies: 0- to 2-month BUP-XR (0–2m; ie, placebo or 1 or 2 BUP-XR injections), 3- to 5-month BUP-XR (3–5m; ie, 3–5 BUP-XR injections), 6- to 11-month BUP-XR (6–11m; ie, 6–11 BUP-XR injections), and 12-month BUP-XR (12m; ie, 12 BUP-XR injections).
To control for differences between duration groups, weighted and adjusted models were applied. Inverse probability weights were calculated for the 3–5m, 6–11m, and 12m BUP-XR groups relative to the 0–2m BUP-XR group to balance pre-trial characteristics. Pre-trial characteristics used in the weighted analysis included: insurance status, BDI-II score ≥20, opiate UDS results, mean-weighted average BPI score, and mean-weighted total SOWS scores.
Multiple logistic regression models, including inverse probability weights, were used to assess the association of key patient treatment and demographic characteristics with abstinence outcomes including BUP-XR treatment duration, sex, age at baseline, race, education, marital status and housing stability at baseline, age of first nonmedical use of opioids, types of opioids used over lifetime (ie, heroin only, prescription opioid only, or heroin and prescription opioid use), OUD pharmacotherapy (ie, buprenorphine or methadone) use prior to enrollment in BUP-XR trial, community-level OUD treatment availability (ie, 2016 state-level naloxone composite score obtained through the 2016 to 2017 Prescription Drug Abuse Policy System [PDAPS], % of treatment centers in the state offering any outpatient pharmacotherapy per 2016 National Survey of Substance Abuse Treatment Service [N-SSATS]), and number of weeks between last BUP-XR injection during the randomized efficacy or open-label safety study and baseline RECOVER visit. To assess whether the association of BUP-XR treatment duration varied over the follow-up period, past-week abstinence models additionally included variables for the period of data collection (ie, 3, 6, 9, or 12 months) and the interaction between BUP-XR duration and survey collection period.
Patient-centered recovery outcomes were reported at the randomized efficacy and open-label safety trial screening visit, at RECOVER baseline, and at the RECOVER 12-month visit within the entire cohort to understand how these outcomes changed over time. For participants enrolled in both the randomized efficacy and open-label safety trials, the value obtained from the screening visit prior to the randomized efficacy trial was used, as this was prior to any BUP-XR exposure.
The data analysis for this paper was generated using SAS/STAT software, version 9.4, of the SAS System for Windows (SAS Institute Inc, Cary, NC).
RESULTS
Of the 533 RECOVER participants, 520 (98%) were eligible for survey completion (ie, not deceased or incarcerated) at 3 months, 518 (97%) at 6 months, 508 (95%) at 9 months, and 506 (95%) at 12 months. Assessment completion rates at each visit out of eligible participants were above 80% (82% [427/520] at 3 months, 86% [444/518] at 6 months, 81% [414/508] at 9 months, 84% [425/506] at 12 months), and UDS completion rates were almost as high (78% [407/520] at 3 months, 82% [425/518] at 6 months, 79% [402/508] at 9 months, 82% [416/506] at 12 months). A total of 339 participants completed all visits (64% of the entire sample). In total, there were 116 (22%) participants in the 0–2m BUP-XR duration group, 61 (11%) in the 3–5m BUP-XR duration group, 96 (18%) in the 6–11m BUP-XR duration group, and 260 (49%) in the 12m BUP-XR duration group based on BUP-XR use in the randomized efficacy and open-label safety studies.
Participant Characteristics
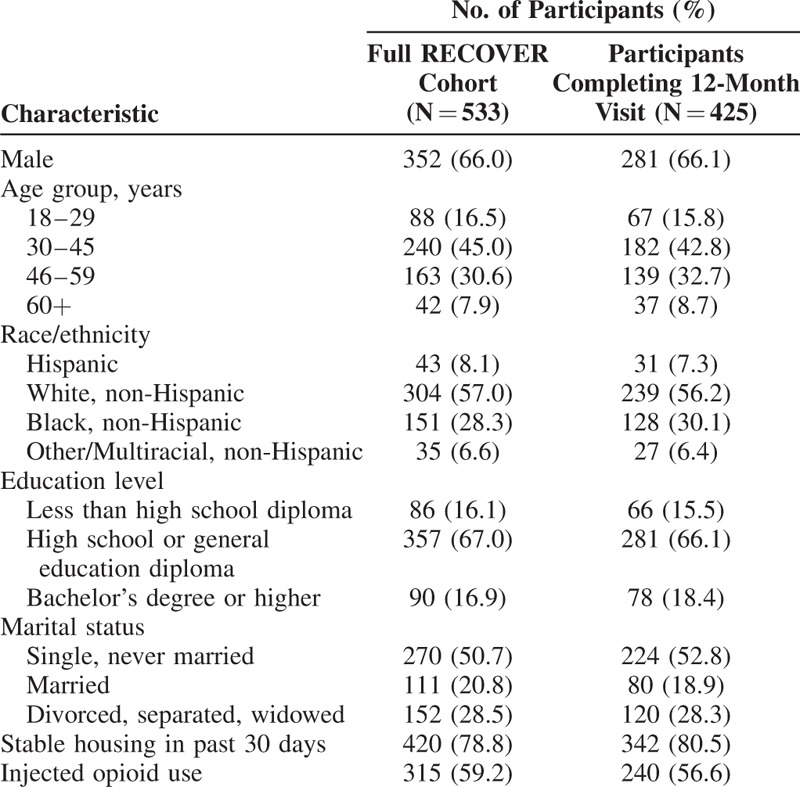
RECOVER participants were predominately male (66%), had a mean age at baseline of 42 years, and most had graduated from high school or had a general education diploma (67%; Table 1). Although the sample size completing the 12-month visit decreased to 425 participants, there was no evidence of systematic dropout based on observed participant characteristics at baseline.
TABLE 1.
RECOVER Baseline Sociodemographic Characteristics
Treatment During RECOVER
A total of 254 (47.7%) participants either self-reported receiving treatment for a substance use disorder or were participating in the open-label extension study (Table 2). The majority of these participants were receiving pharmacotherapy (207/254 [81%]), and nearly all who received pharmacotherapy (196/207 [95%]) were treated with buprenorphine. Approximately three-quarters of those treated with buprenorphine received BUP-XR through enrollment in the BUP-XR open-label extension study concurrent with RECOVER. Counseling, reported by approximately 10% of study participants (Table 2), was the most commonly reported non-pharmacologic treatment modality.
TABLE 2.
Treatments Received for Substance Use Disorder Between RECOVER Baseline and 12-month Visit
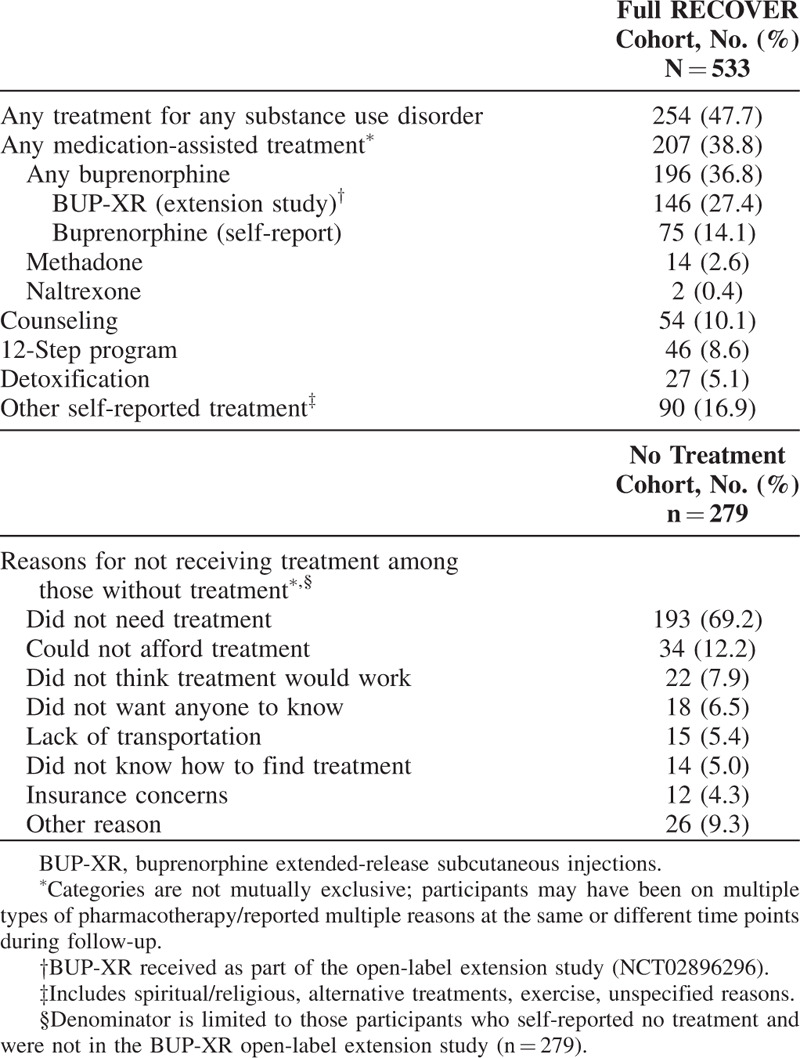
Among the 279 participants who did not receive any substance use disorder treatment (pharmacotherapy or other) during the entire 12-month follow-up period, 193 (69.2%) participants reported that they did not need further treatment (Table 2).
Primary Abstinence Outcomes
Half (50.8%) of participants who completed the 12-month RECOVER visit had sustained abstinence over the entire 12-month observational period based on no self-reported opioid use within the past 6 months reported at both the 6- and 12-month visits. In sensitivity analyses when all self-reported evidence was used (i.e., no past-week, past-month, or past 6-month self-reported days of use at quarterly visits), the sustained abstinence rate was 45.2%. When additionally requiring all measured UDS results to be opioid negative, the sustained abstinence rate was 32.0%.
Within multiple regression analysis, longer BUP-XR treatment duration (6–11 m and 12 m BUP-XR groups) and female sex were associated with sustained 12-month abstinence, whereas previous use of pharmacotherapy for OUD and age 30 years and older were associated with non-abstinence (Table 3). After adjustment and weighting in multiple regression analyses, the likelihood of abstinence for the full 12 months of RECOVER was 75% for participants who had received 12m of BUP-XR treatment compared with 24% for participants who had received 0–2m of BUP-XR treatment (Fig. 1A).
TABLE 3.
Predictors of Sustained 12 Month and Past-week Abstinence
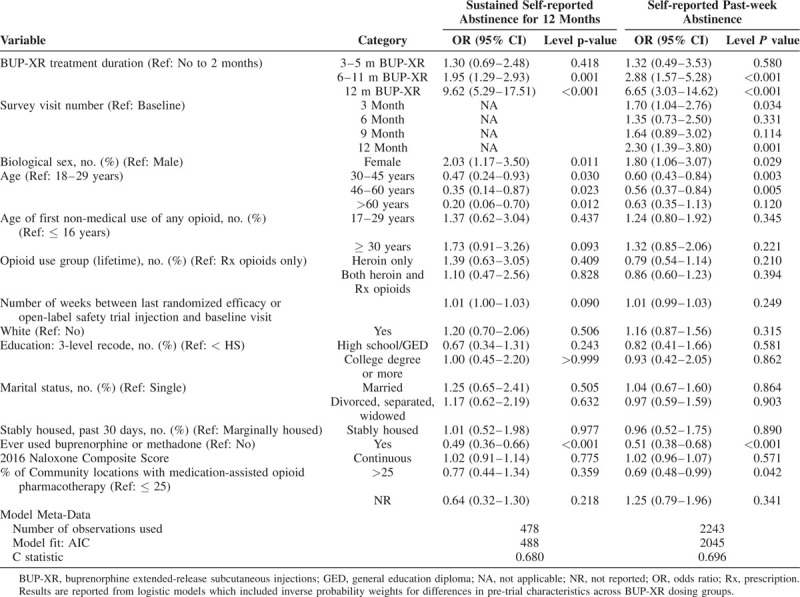
FIGURE 1.
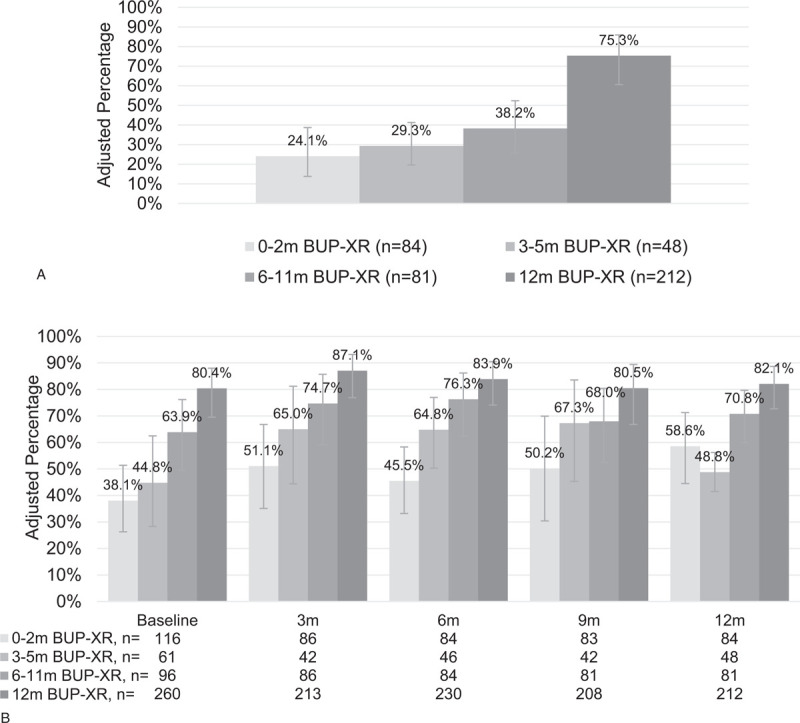
Predicted abstinence over time by within-trial BUP-XR treatment duration.a (A) Sustained self-reported abstinence for 12 months. (B) Self-reported past-week abstinence. BUP-XR, buprenorphine extended-release subcutaneous injections; m, months. aPredicted results from multiple regression results weighted for participant characteristics at the screening visit of their first clinical trial, including self-reported abstinence and controlling for demographics at baseline, illicit substance use history, treatment prior to trial, time from last randomized efficacy or open-label safety BUP-XR injection to RECOVER baseline, and select indicators from public health surveillance data systems.
When looking at quarterly abstinence within the full cohort, past-week, self-reported opioid abstinence was 62.7% at RECOVER baseline, 73.8% at 3 months, 70.3% at 6 months, 68.4% at 9 months, and 66.4% at 12 months. Abstinence based on combined past-week self-report and UDS ranged from 52.7% to 62.3% across visits (Supplemental Table 1); concordance between UDS and self-report was above 77% across all visits and showed that self-reports yielded consistently higher estimates of abstinence than UDS (Supplemental Table 2). As may be expected, worst case imputation led to a decrease in abstinence, ranging from 62.7% at baseline to 52.9% at 12 months when all missing visits were assumed to be non-abstinent; for last value carried forward, abstinence ranged from 62.7% at baseline to 70.0% at the 3-month visit (Supplemental Table 1).
Similar to sustained 12-month abstinence, key variables associated with self-reported, past-week abstinence over time were longer BUP-XR treatment duration (6–11m and 12-m BUP-XR vs. 0–2m), 3- and 12-month survey visits versus RECOVER baseline visit, and female sex. Age 30 to 45 and 46 to 60 years versus 18 to 29 years and prior use of pharmacotherapy for substance use disorder were associated with a higher likelihood of being non-abstinent. After adjustment and weighting, multiple regression analyses demonstrated that longer BUP-XR treatment duration was associated with a higher likelihood of self-reported, past-week abstinence (Fig. 1B).
RECOVER Patient-centered Outcomes Over Time
Within the full RECOVER cohort, over 50% of participants self-reported past-month abstinence at both RECOVER baseline and at the 12-month visit. This also was consistent with UDS results (Table 4). Withdrawal symptoms and pain scores, on average, were both lower during RECOVER as compared to the pre-trial visit.
TABLE 4.
Key Endpoints at Pre-Trial Screening, RECOVER Baseline, and RECOVER 12-Month Visit
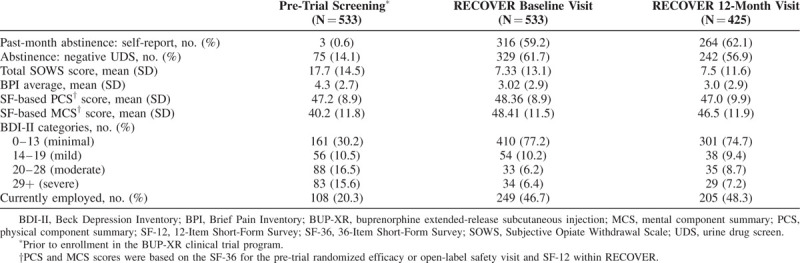
Limited difference was observed in SF-36/SF-12 physical component summary scores between the pre-trial, RECOVER baseline, and RECOVER 12-month visits; values stayed just under the norm-referenced value of 50, which is representative of the general US population. Mental component summary scores increased from the pre-trial to RECOVER baseline visit, with some of this change retained at the 12-month visit.
The proportion of participants reporting no to minimal depression (vs more severe depression) increased from 30.2% at the randomized efficacy or open-label safety trial screening visit to over 70% at RECOVER baseline and 12-month visits. Employment rates during the first 12 months of RECOVER also doubled from those measured at the randomized efficacy or open-label safety trial screening visit.
DISCUSSION
Within the RECOVER 12-month observational period, the majority of participants self-reported continuous opioid abstinence. Similar to the 18-month post-randomization results from POATS (Prescription Opioid Addiction Treatment Study) (Potter et al., 2015), which included up to 24 weeks of treatment with buprenorphine / naloxone ± counseling and enrolled adults with opioid dependence, we found continued or sustained benefits of buprenorphine treatment during the 12-month RECOVER follow-up period. Past-month self-reported abstinence was 50% in both RECOVER and POATS.
As recognized by the American Society of Addiction Medicine's definition of addiction adopted in 2019 (American Society of Addiction Medicine, 2019), addiction is inclusive of a person's environment and life experience—elements that were important facets of RECOVER study assessments. This study found that RECOVER participants reported either improved or maintained low levels of withdrawal symptoms, low pain levels, improved or maintained positive health-related quality of life outcomes, minimal depression, and higher employment rates as compared to the values observed prior to entering the BUP-XR randomized efficacy or open-label safety trials. Given that the RECOVER cohort only included previous participants of the BUP-XR trials by design, the generalizability of these results to individuals outside of a clinical trial treatment setting warrants further study.
Within the RECOVER cohort, results from multivariate models controlling for a number of potential confounders or effect modifiers showed that receiving a longer BUP-XR treatment duration within the randomized efficacy trial and/or open-label safety study was associated with a statistically significantly higher odds ratio for opioid abstinence in the first 12 months of the RECOVER study. This should also be interpreted in the context of almost 40% of the sample receiving further pharmacotherapy for OUD during the RECOVER period, which also may have affected abstinence. In particular, an important consideration when evaluating these results is that nearly half of those in the 12-month duration group continued to receive BUP-XR through the open-label extension study, which contributed to the 12-month abstinence rates reported for this group. Future analyses using the 24-month RECOVER data will provide a better opportunity to differentiate between patients receiving 12 months versus 13 to 18 months of BUP-XR and re-index patients to the end of BUP-XR exposure.
Innovative treatment strategies, such as passive-compliance formulations (eg, sustained-release injectable depots and implants), are needed for the treatment of OUD (US Food and Drug Administration, 2018). The US Food and Drug Administration notes that these sustained-release buprenorphine products not only provide effective treatment, but also can decrease misuse, abuse, and accidental exposure to buprenorphine as compared to self-administered formulations (US Food and Drug Administration, 2019). Results from a qualitative interview study of people taking daily oral methadone or buprenorphine treatment or heroin found that participants value access to buprenorphine formulations with less frequent administration requirements (Neale et al., 2019). Participants felt that longer duration products may benefit people wanting to avoid drug-thinking behaviors and drug-using associates or evade the stigma of a substance use disorder, and people who desire “normality” and “recovery.” The findings reported herein corroborate the long-term positive impact of pharmacotherapy on outcomes; it is reasonable to assume that with lower craving for/preoccupation with opioids, participants could dedicate time and energy to this return to “normality.”
For the main sustained and past-week abstinence definitions, we relied on self-reported data. Although UDS data were also collected at each 3-month follow-up, they only capture drug use over a limited time span of a few days. Therefore, participant self-reports are the only form of response that fully covered abstinence for the entire follow-up period. In order to improve the robustness of reporting, we also performed sensitivity analyses incorporating the UDS results for the primary abstinence endpoint. Of note, concordance was high between self-report and UDS results. Sensitivity analysis using the combination of self-report and UDS identified more opioid use since UDS can only detect opioids for a limited number of days after use. In addition, the sensitivity analysis around sustained response using all self-reports demonstrated that there may be a recall bias in asking participants to self-report their use and treatment over a full 6-month period, in that participants may not have recalled all instances of use.
Limitations
While there was no OUD treatment given to participants as part of the RECOVER study, knowledge of being within the RECOVER study and being asked to complete a survey quarterly may have modified behavior (ie, Hawthorne effect (McCarney et al., 2007)). During participation in the BUP-XR clinical program prior to RECOVER, duration of BUP-XR treatment was based on participants’ voluntary continuation in the clinical trial and participants were not randomized to treatment duration groups. Therefore, it is possible that there might be characteristics related to duration of treatment that might have an impact on abstinence and other outcomes. As a result, possible residual confounding might exist when examining the association between treatment duration groups and outcomes despite attempts to minimize by weighting the duration groups to reflect similar pre-trial characteristics and adjusting for several covariates, particularly in the case of unobserved characteristics. Differences in abstinence across BUP-XR duration groups may be attributable also to OUD pharmacotherapy that occurred during the RECOVER follow-up period which warrants further investigation. Fentanyl was not collected within the UDS and was not explicitly asked about within patient surveys (although misuse of prescription painkillers was captured and could conceivably include fentanyl). As noted above, generalizability of these results may be limited because all RECOVER participants had previously participated within a BUP-XR trial.
CONCLUSIONS
Participants in the RECOVER study achieved and retained positive effects over 12 months for abstinence, psychosocial, and employment outcomes. By measuring a multitude of endpoints longitudinally, the RECOVER study allows for understanding the role of continuity of care, monitoring participant recovery in a 12- to 24-month time frame post BUP-XR trial participation, and providing further insights into the underlying causes of relapse. Additionally, information obtained from the RECOVER cohort can be used to understand the role of novel pharmacologic treatments in supporting recovery. Future analyses of the RECOVER data will provide deeper insights into patient-centered endpoints including cravings and withdrawal, physical and mental health, employment and productivity, and crime at 24 months of follow-up.
Supplementary Material
Supplementary Material
Acknowledgments
The authors wish to thank all participants and investigators who took part in the RECOVER study. The authors wish to thank Mike Mills for his programming support. Mr Mills had full access to all study data and takes responsibility for data integrity and accuracy of the data analysis. We also wish to thank Nick Peiper for his insight and feedback on the community level datasets, Frank Mierzwa and the field research team for data collection, and Beth Lesher for medical writing assistance.
Footnotes
WL is a consultant for Indivior Inc, Alkermes, Camurus/Braeburn, Opiant, and Titan Pharmaceuticals. VM is a clinical investigator for BUP-XR clinical trials and a consultant for Indivior Inc. VRN, SML, HC, and CH are employees and own stock in Indivior Inc. NAR was an employee of Indivior Inc when this work was developed. APA is an employee of RTI International and consultant for Indivior Inc. VA was an employee of RTI International and consultant for Indivior Inc when this work was developed. CTS and CJ are employees of Pharmerit International and consultants for Indivior Inc. The RECOVER study was sponsored by Indivior Inc.
REFERENCES
- American Society of Addiction Medicine. Definition of Addiction. Available at: https://www.asam.org/resources/definition-of-addiction. Accessed November 15, 2019. [Google Scholar]
- Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2019; 393:778–790. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Dzau VJ. Medication-based treatment to address opioid use disorder. JAMA 2019; 321:2071–2072. [DOI] [PubMed] [Google Scholar]
- Ling W, Nadipelli VR, Ronquest NA, et al. Remission from chronic opioid use-studying environmental and socio-economic factors on recovery (RECOVER): study design and participant characteristics. Contemp Clin Trials 2019; 76:93–103. [DOI] [PubMed] [Google Scholar]
- Ling W, Nadipelli VR, Solem CT, et al. Patient-centered outcomes in participants of a buprenorphine monthly depot (BUP-XR) double-blind, placebo-controlled, multicenter, phase 3 study. J Addict Med 2019; 13:442-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009. CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014. CD002207. [DOI] [PubMed] [Google Scholar]
- McCarney R, Warner J, Iliffe S, et al. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol 2007; 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences E. and Medicine. Medications for Opioid Use Disorder Save Lives. Washington, DC: National Academies Press; 2019. [PubMed] [Google Scholar]
- Neale J, Tompkins CNE, Strang J. Prolonged-release opioid agonist therapy: qualitative study exploring patients’ views of 1-week, 1-month, and 6-month buprenorphine formulations. Harm Reduct J 2019; 16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Dreifuss JA, Marino EN, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. J Subst Abuse Treat 2015; 48:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health 2013; 103:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfstein JM, Olsen Y. Making amends for the opioid epidemic. JAMA 2019; 321:1446–1447. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. HHS Acting Secretary declares public health emergency to address national opioid crisis. Available at: https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Accessed May 11, 2018. [Google Scholar]
- US Food and Drug Administration. FDA in Brief: FDA finalizes new policy to encourage widespread innovation and development of new buprenorphine treatment for opioid use disorder. February 06, 2019. Available at: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-finalizes-new-policy-encourage-widespread-innovation-and-development-new-buprenorphine. Accessed May 2, 2019. [Google Scholar]
- US Food and Drug Administration. Opioid dependence: developing depot buprenorphine products for treatment, guidance for industry. In: Silver Spring, MD: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


