In a recently published article, Culbertson et al1 make the case for antibiotic irrigation at the time of breast augmentation. The abstract states that this method has “been proven to reduce capsular contracture by 10× over the past 20 years.” At the 2019 U.S. Food and Drug Administration (FDA) Hearing, Dr. Adams, a coauthor, testified that the 14-point plan, which includes antibiotic irrigation, has reduced the risk of capsular contracture from 50% to less than 1% over the last 30 years.2 However, manufacturer core studies do not support either statement.3 There has been no significant downward trend in capsular contracture rates (Fig. 1).
FIGURE 1.
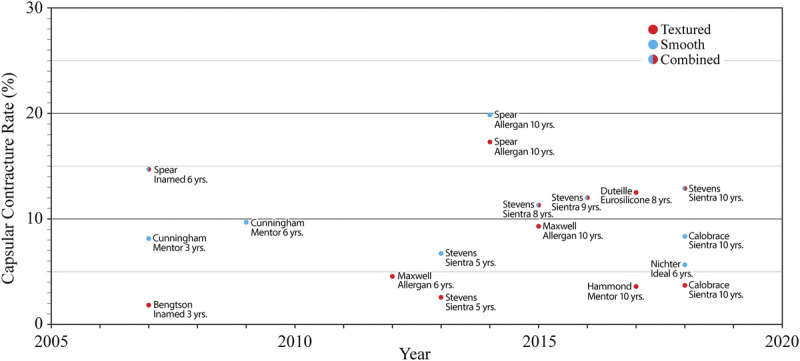
Grade III/IV capsular contracture rates for primary breast augmentation as reported in manufacturer core studies. There is no significant trend. Reprinted from Swanson3 with permission from Wolters Kluwer Health.
The authors state that the 14-point plan can also minimize the risk of Breast Implant-Associated Anaplastic Large-Cell Lymphoma (BIA-ALCL).1 At the 2019 FDA Hearing, Dr. Adams testified that this plan could even eliminate BIA-ALCL risk.2 A 2017 study by Adams et al4 is offered to support this claim. The findings of this study have been challenged.5 Presentations and publications by the authors during the “prospective” study period show that in fact the 8 contributors did not all follow at least 13 points as reported.5 A mean 11.7-year follow-up was claimed, although Dr. Adams shortened this period to 9 years in his FDA presentation.2 Regardless, such a long mean follow-up period is simply not credible for this group of patients.5 This point is well known to any investigator who has studied breast augmentation patients, who are not known for their willingness to return for long-term follow-up appointments, particularly if they have no complaints.6 In fact, an 11.7-year mean follow-up is not even mathematically possible for patients followed for 1 to 14 years. Moreover, the 14-point plan was published in 2013.7 There is no mention as to how the reported 2.2% capsular contracture rate was determined.4 Six of the 8 authors of this study were consultants to Allergan (Allergan plc, Dublin, Ireland).4 A retrospective study design allows selection bias in that only surgeons who were not known to have a case of BIA-ALCL were selected for inclusion.5 These important factual issues undermine the conclusion.5
Importantly, Culbertson et al deny any “potential” or “related” conflict of interest.1 Drs. Adams and Deva, both coauthors of the study, have previously acknowledged conflicts with Allergan and other implant companies.4 The authors may believe that money received from breast implant manufacturers does not constitute a relevant conflict of interest. That determination should be made by the reader. A representative for Allergan at the FDA hearing also referenced the 2017 study by Adams et al,4 claiming that following these 14 points eliminates the BIA-ALCL risk.2 Allergan even paid for the graphic demonstrating the 14 points.8 Blaming a faulty technique rather than acknowledging a problem with textured implants fits with the corporate narrative.3,5 Allergan has insisted on the safety of all of its implants as posing “no immediate risk.”9 Nevertheless, on July 24, 2019, Allergan issued a global recall of Biocell implants and tissue expanders10 in response to a request from the FDA.11
In his testimony to the FDA, Dr. Clemens emphasized the lack of scientific support for any of the 14 points.2 It is remarkable that these points do not include “avoid textured implants,” in view of the well-known fact, conceded by these authors,1 that implant surface texturing is exclusively linked to BIA-ALCL. Dr. Deva has not promoted the 14-point plan in his recent panel presentations.12,13 However, the 14 points still appear in journal publications.14,15 A “Best Practices” advisory for preventing BIA-ALCL, with the endorsement of the major plastic surgery professional societies, continues to recommend these steps and others, such as “limit unnecessary personnel in the operating room,” with no mention of abandoning textured devices.16
In support of an infectious etiology for BIA-ALCL, the authors reference a commonly cited study by Hu et al.17 The findings of this study are often misquoted. This study found 4.7 × 106 bacteria/mg in the BIA-ALCL samples (n = 21) versus 4.9 × 106 bacteria/mg in the nontumor capsule specimens from patients with capsular contracture (n = 62). The nontumor samples from patients with capsular contracture but without BIA-ALCL actually contained more bacteria than the BIA-ALCL samples, although probably not significantly. This study was limited by a lack of control specimens. A comparison of bacterial counts in 3 women, comparing the breast capsule affected by BIA-ALCL to the normal breast capsule, was too small to be meaningful.
A recent microbiological study by Walker et al18 provides new information on this topic. Interestingly, these researchers identified no consistent differences in bacterial profiles (microbiomes) between patients with breasts affected by BIA-ALCL and contralateral control breasts and no significant difference in the relative abundance of Gram-negative bacteria, including Ralstonia pickettii, although the number of BIA-ALCL patients (n = 7) was small. A causal link to Ralstonia now seems fictitious.
To establish an infectious etiology, Koch's postulates must be satisfied. The first of Koch's postulates for an infectious etiology19 states that “the bacteria must be present in every case of the disease.” This standard has not been met for BIA-ALCL. In a recent case report, Dogan et al20 documented a case of ALK-negative, CD30-positive ALCL of the breast in a burn patient with no history of breast implants. The authors believe that this case supports the role of chronic inflammation in the development of ALCL in the breast. If this problem can develop in the absence of bacteria-laden biofilm, the role of bacteria as a causative agent is very much in question. There is still no precedent for bacteria causing a T-cell lymphoma.2,21 The laboratory finding that more bacteria are associated with textured breast implants22 speaks against bacteria as a cause of capsular contracture in view of the clinical observation that textured implants do not increase the capsular contracture rate.23,24
The value of antimicrobial pocket irrigation has been challenged.25 Bottles of Betadine (10% povidone-iodine) solution are not sterile.26 The warning, “for external use only,” appears on the bottles. Adams and Calobrace27 recently suggested that the inside of the bottle is sterile, if not the outside. Plastic surgeons may simply check a bottle of povidone-iodine to confirm that “nonsterile” is printed on the bottle.26 If a product does not state “sterile” on the label, it is nonsterile.28 Guidelines published in Annals of Surgery warn that povidone-iodine solution is ineffective in decontaminating wounds and has been shown to inhibit wound healing and/or increase wound infection.29 These guidelines recommend against its use.29 Fibrinogenic and proinflammatory antimicrobials that are used for pocket irrigation result in an almost 2.5-fold increased incidence of severe capsular contracture.30
“Optimizing” breast pocket irrigation1 suggests that antibiotic irrigation is effective in the first place. Individual publications referenced by the authors support antibiotic irrigation.31,32 However, this finding was no longer significant on multivariate analysis in 1 study,31 and the sample size was inadequate in the other.32 Three systematic reviews published recently, including a meta-analysis, find no benefit for antibiotic irrigation or Betadine irrigation.30,33,34 This author discontinued any form of antibiotic irrigation 20 years ago (using sterile saline instead) without a change in capsular contracture rate (6%) and without any cases of BIA-ALCL.25,35
Antimicrobial activity in vitro does not necessarily translate into clinical effectiveness in vivo. Many of the references provided by the authors relate to other types of surgery with sterile operative fields, such as orthopedic cases.1 Breast surgery is different in that the wound environment is clean-contaminated.25 Numerous commensal species are present, and it is impossible to eradicate them simply because the antibiotic solution cannot permeate all the breast tissue. In breast reconstruction at the time of mastectomy, all of the breast tissue is removed, yet these patients incur higher, not lower, capsular contracture rates.36 Moreover, it is not clear that eradicating commensal bacteria is beneficial, in that this practice may lead to an opportunistic infection.25
The 14-point plan includes a recommendation that for all future procedures that breach skin or mucosa, breast implant patients receive systemic antibiotics.4,7 Even if its advocates believe that antibiotic irrigation does not lead to antibiotic resistance,27 this practice in millions of implanted women can be expected to needlessly increase antibiotic resistance in the community.
A claim that antibiotic wound irrigation can prevent a serious and sometimes deadly complication should be taken seriously. The claim that the 14 points can eliminate BIA-ALCL may have influenced the FDA panel in its original ruling37 to allow macrotextured implants to remain on the market.3 However, after 33 reported deaths and (at least) 573 BIA-ALCL cases globally, the FDA reconsidered.11 In retrospect, this decision seems obvious.
It is time to acknowledge that technique is not “critically important.”2 After all, BIA-ALCL was unknown prior to 1997.38 There is no evidence that sterile technique was suddenly and catastrophically compromised 2 decades ago. There is universal evidence that a faulty design is to blame, not substandard sterility.3 It is time for our societies to unendorse the 14-point plan and recommend against the continued use of textured breast implants.3
To make an informed decision regarding implant pocket antibiotic irrigation, we need to know which microbes, if any, are implicated in complications. Sorting out causation from correlation is notoriously difficult.18 Until we learn otherwise, an unproven practice that has possible risks is best avoided.25
Footnotes
Dr. Swanson is a plastic surgeon in private practice in Leawood, Kansas.
Conflict of interest and sources of funding: Dr. Swanson receives royalties from Springer Nature (Cham, Switzerland).
REFERENCES
- 1.Culbertson EJ Felder-Scott C Deva AK, et al. Optimizing breast pocket irrigation: the Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) era. Aesthet Surg J. Published 10 September 2019. [DOI] [PubMed] [Google Scholar]
- 2.Webcast General and Plastic Surgery Devices Panel Meeting. Day 1. http://fda.yorkcast.com/webcast/Play/a6baa43b37004ecab288779ac3a263bd1d. Accessed September 13, 2019.
- 3.Swanson E. Plastic surgeons defend textured breast implants at 2019 U.S. Food and Drug Administration hearing: why it is time to reconsider. Plast Reconstr Surg Glob Open. 2019;7:e2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams WP Jr. Culbertson EJ Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140:427–431. [DOI] [PubMed] [Google Scholar]
- 5.Swanson E. A 1-point plan to eliminate breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL). Ann Plast Surg. 2018;80:565–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwer DB Gibbons LM Magee L, et al. A prospective, multisite investigation of patient satisfaction and psychosocial status following cosmetic surgery. Aesthet Surg J. 2005;25:263–269. [DOI] [PubMed] [Google Scholar]
- 7.Deva AK, Adams WP, Jr., Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. [DOI] [PubMed] [Google Scholar]
- 8.14-Point Plan Checklist. http://saferbreastimplants.org/doctors/14-point-plan-checklist/. Accessed November 1, 2019.
- 9.Allergan suspends sales and withdraws supply of textured breast implants in European markets. https://www.allergan.com/news/news/thomson-reuters/allergan-suspends-sales-and-withdraws-supply-of-te. Accessed September 13, 2019.
- 10.Allergan Voluntarily Recalls BIOCELL® Textured Breast Implants and Tissue Expanders. https://www.allergan.com/news/news/thomson-reuters/allergan-voluntarily-recalls-biocell-textured-brea.aspx. Accessed September 13, 2019.
- 11.FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue expanders from market. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan. Accessed November 1, 2019.
- 12.Panel: BIA-ALCL—What You Need to Know. Meeting of the American Society for Aesthetic Plastic Surgery. New Orleans, LA: May 16–21, 2019. [Google Scholar]
- 13.Panel: ALCL BIA A Quest for Understanding. Meeting of the Australian Society of Plastic Surgeons. Melbourne Australia: May 30–June 1, 2019. [Google Scholar]
- 14.Wan D, Rohrich RJ. Modern primary breast augmentation: best recommendations for best results. Plast Reconstr Surg. 2018;142:933e–946e. [DOI] [PubMed] [Google Scholar]
- 15.Jewell ML Fickas B Jewell H, et al. Implant surface options and biofilm mitigation strategies. Plast Reconstr Surg. 2019;144(1S):13S–20S. [DOI] [PubMed] [Google Scholar]
- 16.“Best Practices” Breast Implant Associated ALCL https://www.surgery.org/sites/default/files/BEST%20PRACTICES_1.pdf. Accessed November 1, 2019.
- 17.Hu H Johani K Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. [DOI] [PubMed] [Google Scholar]
- 18.Walker JN Hanson BM Pinknes CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9:10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medical definition of Koch's postulates. http://www.medicinenet.com/script/main/art.asp?articlekey=7105. Accessed November 1, 2019.
- 20.Dogan ZA Miranda RN Iyer S, et al. Anaplastic large cell lymphoma of the breast arising in a burn cicatrix. Aesthet Surg J. Published 9 September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner S. Pathogenesis from the cell origin, what biomarkers reveal. Presented at: 1st World Consensus Conference on BIA-ALCL. Rome, Italy: October 6, 2019. Available at: https://www.youtube.com/watch?v=YxPFayQsjUo&t=14536s. Accessed October 30, 2019. [Google Scholar]
- 22.Jacombs A Tahir S Hu H, et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133:471e–480e. [DOI] [PubMed] [Google Scholar]
- 23.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. [DOI] [PubMed] [Google Scholar]
- 24.Wong CH Samuel M Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118:1224–1236. [DOI] [PubMed] [Google Scholar]
- 25.Swanson E. The questionable role of antibiotic irrigation in breast augmentation. Plast Reconstr Surg. 2019;144:249–252. [DOI] [PubMed] [Google Scholar]
- 26.Avrio Health Products Betadine (povidone-iodine). Available at: https://betadine.com/medical-professionals/betadine-solution/. Accessed November 1, 2019.
- 27.Adams WP, Jr., Calobrace MB. Discussion: the questionable role of antibiotic irrigation in breast augmentation. Plast Reconstr Surg. 2019;144:253–257. [DOI] [PubMed] [Google Scholar]
- 28.Questions and Answers: FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection. https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-fda-requests-label-changes-and-single-use-packaging-some-over-counter-topical Accessed November 1, 2019. [PubMed]
- 29.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011;253:1082–1093. [DOI] [PubMed] [Google Scholar]
- 30.Drinane JJ Chowdhry T Pham T-H, et al. Examining the role of antimicrobial irrigation and capsular contracture: a systematic review and meta-analysis. Ann Plast Surg. 2017;79:107–114. [DOI] [PubMed] [Google Scholar]
- 31.Blount AL Martin MD Lineberry KD, et al. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet Surg J. 2013;33:516–521. [DOI] [PubMed] [Google Scholar]
- 32.Giordano S Peltoniemi H Lilius P, et al. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33:675–680. [DOI] [PubMed] [Google Scholar]
- 33.Horsnell JD, Searle AE, Harris PA. Intra-operative techniques to reduce the risk of capsular contracture in patients undergoing aesthetic breast augmentation—a review. Surgeon. 2017;15:282–289. [DOI] [PubMed] [Google Scholar]
- 34.Samargandi OA Joukhadar N Al Youha S, et al. Antibiotic irrigation of pocket for implant-based breast augmentation to prevent capsular contracture: a systematic review. Plast Surg. 2018;26:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson E. Open capsulotomy: an effective but overlooked treatment for capsular contracture after breast augmentation. Plast Reconstr Surg Glob Open. 2016;4:e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araco A Caruso R Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819. [DOI] [PubMed] [Google Scholar]
- 37.FDA Statement https://www.fda.gov/news-events/press-announcements/statement-fda-principal-deputy-commissioner-amy-abernethy-md-phd-and-jeff-shuren-md-jd-director-fdas. Accessed November 1, 2019.
- 38.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. [DOI] [PubMed] [Google Scholar]


