Abstract
Supplemental Digital Content is available in the text.
The care of geriatric patients is complex, and patients are often treated inappropriately based on their age, degree of frailty, or both. Therefore, it is imperative to evaluate frailty and identify areas of intervention before surgery is undertaken. In addition, patient goals should be taken into account from the start of a treatment plan, and functional recovery should be measured and addressed. This compendium presents data to help colon and rectal surgeons evaluate and treat geriatric patients. In addition, to help all surgeons function in a multidisciplinary, geriatric-friendly environment, a financial framework is included. The framework can be used to advocate for the hiring of specialists trained in caring for older adults.
INTRODUCTION
The 2019 American Society of Colon and Rectal Surgeons Annual Meeting featured the inaugural symposium on the care of geriatric colorectal surgical patients. Because extensive data were presented during the session, the speakers have compiled the key information necessary to initiate a geriatric surgery program.
In the United States, 56% of new colorectal cancer diagnoses are in patients >65 years of age.1 At the same time, an average 75-year-old man in good health has 18 years of life expectancy, whereas an 82 year old has 10. The presence of severe comorbidities decreases life expectancy to 6 and 2 years.2 Although there still exists lack of consensus about the definition of what age is considered the cutoff for geriatrics (65 vs 70 vs 75 years old), there is consensus that patients should not be treated based on their age alone.3 However, as surgeons we still struggle to appropriately treat geriatric patients, and especially those with cancer have been shown to receive inappropriate care, being either undertreated based on their chronological age or overtreated for their degree of frailty.4 This compendium will help surgeons evaluate for and optimize frailty and focus on outcomes that matter to patients. The final section will help surgeons build a business case to establish a geriatric multidisciplinary surgery program at their hospital.
GERIATRIC ASSESSMENT FOR THE COLORECTAL SURGEON
Frailty is a state of reduced tolerance to stress of surgery because of progressive and life-long decline in physiological systems.5 In a practical example, a fit and a frail patient may undergo the same surgery with very similar surgery and intraoperative characteristics, but a frail patient declines significantly after surgery and requires a very prolonged recovery period and high probability of dying in the first year, whereas the fit patient recovers very quickly from surgery, is discharged from the hospital shortly after surgery, and is able to resume all of his/her functional activity before surgery. As a result, assessing frailty before surgery is critical for surgical decision-making by surgeons, patients, and their caregivers.
The gold standard for assessing frailty is Geriatric Assessment (GA), a multidimensional assessment of patients, typically performed by geriatricians.6 However, a study showed that only a minority of surgeons collaborate with geriatricians.7 Moreover, the number of geriatricians is limited, with ≈7000 practicing geriatricians in the United States.8 As a result, it is crucial for surgeons to become familiar with components of the GA.
DOMAINS OF GA
Functional Assessment
One of the most important domains of GA is preoperative functional assessment. Assessing basic and instrumental activities of daily living9 provides an insight into an older patient’s ability to care for themselves. These following questions can be incorporated into routine assessment of older patients by the surgery team: Are you able to prepare meal for yourself? Who does the house cleaning? Do you take your medications on your own? Assessing gait speed and balance is an important objective assessment of patient’s functional status. A simple Timed Up and Go (TUG) test,10 in which you ask the patient to rise from their chair without using arms, walk 10 feet, turn and walk back to the chair, and sit down, takes <30 seconds to perform. A normal time is <20 seconds and a prolonged time has been shown to be predictive of surgical complications. In the Pre-operative Risk Estima-tion for Onco-geriatric Patients study, a prolonged TUG was associated with a 50% rate of major complications, whereas the rate of complications in the patients with a normal TUG was 13.6%.11 A prolonged TUG has a simple 3-item algorithm that may assist in distinguishing those with slow gait speed from those who walk faster.12 Supplemental Digital Content 1 (http://links.lww.com/DCR/B379) presents an algorithm for simple office-based frailty evaluation to trigger additional referrals. Supplemental Digital Content 2 (http://links.lww.com/DCR/B378) presents a summary of the common tests for frailty and the threshold for abnormal results.
Cognitive Function
Patients who are cognitively impaired are at higher risk for postoperative delirium.13 Many instruments are available to assess cognitive function of older adults. The time to complete these assessments ranges from 2 to 3 minutes for Mini-Cog14 to >20 minutes for tests such as the Mini-Mental Status Examination.15 The Mini-Cog is a test that includes a 3-word recall and clock drawing. The patient must place the numbers correctly on the clock and set the time correctly to get full credit for the clock drawing. The test is scored out of 5 points. If the patient is able to recall all 3 words (3 points) and draw the clock with the appropriate time displayed (2 points), they receive a score of 5.
Polypharmacy
Always review a patient’s medication list. It gives you an idea on comorbid conditions that patient has, its severity (eg, taking 5 different blood pressure medications instead of just 1), and the possibility of medications interfering with the anesthesia and surgical procedure. When feasible, consult with a pharmacist for patients having excessive polypharmacy (eg, >10 medications).
Nutritional Assessment
With more emphasis on prehabilitation, assessing nutritional status of patients may allow for referral to a dietician for improvement in nutritional status of patients while the preoperative workup is completed.
Social Support
Frail patients are more likely to have functional decline after surgery and, as a result, they rely on their social support to provide care for them. Assessing social support of a patient before surgery will allow social worker and case manager involvement during the perioperative period. Consider asking whether the patient lives alone, if the spouse/partner/family member can take care of the patient during the perioperative period, how the patient came to the clinic, and whether they had any issues with transportation.
PREHABILITATION FOR THE COLORECTAL SURGERY PATIENT
Preventing the decline in older frail patients in anticipation of surgery should focus on restoration of function and increase of physiological reserve.16 Those who are more vulnerable to the surgical stress, like the malnourished, frail, with several comorbidities, need appropriate screening to minimize postoperative complications, prolonged hospitalization, disability, and risk of mortality.17,18 Therefore, preoperative intervention aimed at decreasing the risk of postoperative deconditioning can be a valuable strategy and can potentially lead to better outcomes and less social burden.19
The process enabling patients to withstand the stressor of surgery through augmenting preoperative functional capacity is termed prehabilitation (as opposed to rehabilitation, which is enhancing functional capacity after an injury or postsurgery).20 It begins in the preoperative period and is part of an integrated enhanced recovery after surgery program. The importance of patient education, self-efficacy, and empowerment cannot be understated. Treatment of coexisting medical conditions in the colorectal patient includes glycemic control, anemia correction, and smoking and alcohol cessation.
A structured and personalized exercise program is the central component of prehabilitation. The premise is that repeatedly exposing patients to the physiological stress of physical activity will prepare them for the surgery and better allow them to tolerate impairments.21 For optimal results, a presurgical exercise program should consist of both resistance and aerobic training and be supplemented by flexibility and balance exercises to favor weight loss, to reduce incidence of falls, and to increase range of motion in a number of joints22 (Table 2). Physical therapy services are typically covered in the preoperative period by Medicare and private insurance companies if an appropriate indication is provided. In the era of bundled care, the number of physical therapy visits postoperatively may be limited by the number of preoperative visits. Therefore, a social worker should be involved in the multidisciplinary care team to ensure that the patients are getting optimal therapy based on their individual plans.
TABLE 2.
Prehabilitation program: exercises, nutrition, and relaxation exercises
| Aerobic exercise |
|---|
| _Start a slow walk to warm up for 5 min |
| _30 min minimum of aerobic activity (walking/biking) 3 times per week at moderate intensity (4–6 on the Borg Scale). If the participant finds the activity to be easier (2–3 on the Borg Scale), then, the walking pace or duration should be gradually increased. It is recommended not to surpass 7 to 8 on the Borg Scale. |
| Resistance exercise |
| _ All exercises are to be performed starting with 1 set of about 10 to 12 repetitions. Number of sets and repetitions gradually increase to 2 sets and 12 to 15 repetitions. |
| _ Use of a resistance band/handheld weights |
| - Push-ups (wall, modified, or full) |
| - Squats with the use of a chair |
| - Hamstring curls |
| - Calf raises |
| - Abdominal crunches (chair or floor) |
| _ Theraband/handheld weight exercises involve the following |
| - Chest exercise |
| - Deltoid lifts |
| - Bicep curls |
| - Triceps extension |
| Flexibility |
| _ Flexibility exercises are given for the following muscles (each exercise should be performed twice and held for a minimum of 20 s). |
| _ Chest |
| _ Biceps |
| _ Triceps |
| _ Quadriceps |
| _ Hamstring |
| _ Calf |
| Breathing/relaxation exercises |
| _ Abdominal breathing (15 min twice daily) |
| _ Use of relaxation CD (nature sounds and breathing instructions) |
Adapted from Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33:17–33. Reproduced with permission.22
The nutritional aspect of aging needs to receive attention in view of the strong relationship between the state of nutrition risk, malnutrition, and postoperative outcomes. In addition, anabolic resistance of elderly muscle to a physiological dose of amino acids impacts tissue protein synthesis. A dietary plan that includes sufficient high-quality proteins and energy per meal will provide sufficient essential amino acids, particularly leucine, which is needed to elicit muscle protein synthetic response and accretion of muscle protein.23 Medicare and many insurance companies limit nutrition consultations to patients with diabetes mellitus and chronic kidney disease. It can also be difficult to find dietician services in the outpatient setting, with the exception of cancer centers and bariatrics programs. Existing salaried staff in these programs may be used in a prehabilitation program if resources allow. Alternatively, staff dieticians can prepare educational materials as part of a prehabilitation program for all patients if individual consultation is not available. Dietary supplements such as Boost/Ensure are not covered by Medicare Part D, but coverage is possible by private insurance companies if a prescription is sent with an indication for use.
Although the optimal prehabilitation program is different based on a variety of factors for each patient, several authors have developed in-home prehabilitation models, and preliminary data have shown decreased length of stay, more likely discharge to home, and decreased costs.24,25 Some combination of office-based and home-based prehabilitation is likely the optimal program currently, but this is rapidly evolving and should be patient-centered.
MINIMALLY INVASIVE SURGERY AND ENHANCED RECOVERY PROTOCOLS: ARE THEY SAFE IN GERIATRIC PATIENTS?
Enhanced recovery programs have been shown to significantly decrease time to flatus, time to stool, and length of stay without an increase in complications, mortality, and readmission, which has also been shown to be true in geriatric patients.26,27 In addition, studies have shown decreased rates of delirium in patients on enhanced recovery protocols.28,29
Minimally invasive colorectal surgery has also been shown to be beneficial for geriatric patients and is associated with faster clinical postoperative progression, decreased perioperative complications, and shorter length of stay.30–33
FUNCTIONAL RECOVERY: WHAT TO MEASURE AND HOW?
Several of the postoperative surgical and oncologic outcomes that physicians have been measuring are the product of a deep misunderstanding of what geriatric patients really want. Thirty-day morbidity and mortality, length of stay, time to first flatus, and the cancer-centered overall survival or progression-free survival have long been considered the best indicators to define clinical and cancer control. Unfortunately, none of those have been shown to be primarily of interest for older patients, who are mainly focused on the chance of surviving surgery enduringly (90-day mortality) and returning to independence as soon as possible.34
Loss of independence, which may occur as a consequence of major colorectal surgery, is a devastating life change for older adults, and functional recovery has been shown to be of critical value in the older population.35,36 Functional recovery should not be confused with restoration of bowel function or time to oral food intake after surgery and is instead a multidimensional outcome. Functional recovery should be addressed regarding 2 main areas: organ-specific postoperative outcomes and individual ability to regain independence.
Many instruments have been proposed and offered to gain data about these particular domains. Currently the most convincing data in the literature about functional recovery are regarding the use of Activities of Daily Living score, the Mini-Cog, TUG/6-minute walking test, and sarcopenia (defined as decreased muscle mass and function).37,38 Early results of the Geriatric Oncology Surgical Assessment and Functional Recovery After Surgery (GOSAFE) study,39 the largest prospective study specifically focused on functional recovery in senior adults, have been published recently. The GOSAFE study showed how several domains of the preoperative evaluation that are often neglected by colorectal surgeons (eg, the cognitive status, ability to maintain a steady gait, and recent hospitalization) could be related to worse postoperative outcomes and prolonged loss of independence.
HOW TO MAKE A BUSINESS CASE TO START A PROGRAM IN GERIATRIC SURGERY
Many hospitals lack the infrastructure for a geriatric surgery program, particularly for frailty assessment and prehabilitation. To make a business case for a hospital to hire a geriatrician, several factors can be considered.
STEPS TO MAKING BUSINESS CASE TO HOSPITAL ADMINISTRATION
Determine surgical volume by age and projection forgrowth (Fig. 1).
Collect data on complication rates, length of stay, and readmission rates.
Focus on decreased complications and length of stay after screening for and addressing frailty (Table 1).40
Determine the resources and their respective costs (including benefits) you would need for your program and any infrastructure requirements, such as office space.
FIGURE 1.
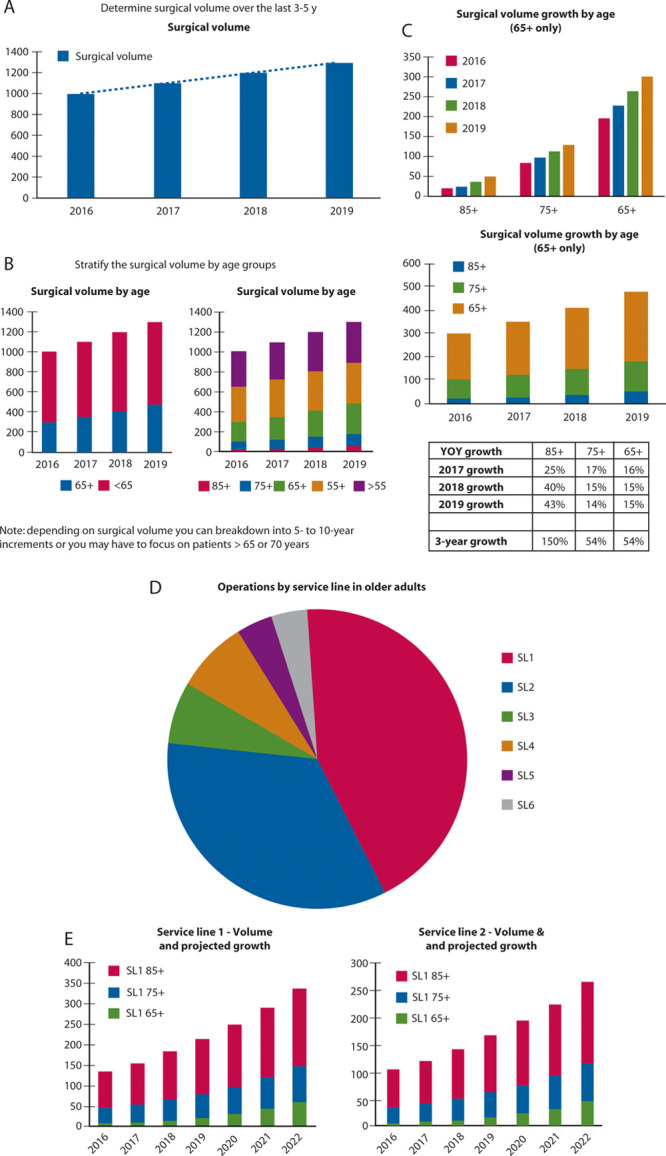
Estimation of surgical volume of older adults. A, Determine surgical volume over the last 3-5 years. B, Stratify the surgical volume by age groups. C, Determine growth of the older adult surgical volume. D, Identify procedures, service lines, or surgeons contributing to the surgical growth among older adults. E, Focus the business case on the highest volume + highest growth area. Forecast growth for next 3 years for these services lines. YOY = year over year.
TABLE 1.
Business case for funding a geriatrician based on decreased complications, length of stay, and readmission rate
| Metric | Average costa | Current | Projected | Improvement (projected- current) | Volume | Savings (volume × improvement × average cost) |
|---|---|---|---|---|---|---|
| LOS | $2574 | 7 d | 6 d | 1 day | 300 | $772,200 |
| SSI | $28,219 | 1.5% | 1% | 0.50% | 300 | $42,329 |
| C diff | $17,260 | 1.5% | 1% | 0.50% | 300 | $25,890 |
| VAP | $47,238 | 1.5% | 1% | 0.50% | 300 | $70,857 |
| Readmit | $14,400 | 15% | 10% | 5% | 300 | $216,000 |
| Total | $1,127,276 |
Note that LOS, complication rates, and volume are all example values.
LOS = length of stay; SSI = surgical site infection; C diff = Clostridium difficile; VAP = ventilator-associated pneumonia; Readmit = readmission.
a Data show the average cost based on Agency for Healthcare Research and Quality estimates.40
Benefits include:
a. Number of billable appointments from the geriatrician * average reimbursement.
b. Savings from poor quality outcomes.
c. Expanded capacity from length-of-stay reduction.
d. Decreased readmissions.
e. Improved quality rankings.
f. Better patient experience.
CONCLUSIONS
The data included in the compendium along with the framework for the business case can help surgeons advocate to improve the care of their geriatric patients at their home institutions. In addition, finding a shared interest in caring for older patients can help to spark interest groups within surgical and multidisciplinary societies to advance this work and perform prospective, multi-institution studies. Together, we can improve the care of geriatric patients.
Supplementary Material
Footnotes
Funding/Support: None reported.
Financial Disclosure: None reported.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.dcrjournal.com).
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–193 [DOI] [PubMed] [Google Scholar]
- 2.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001; 285:2750–2756 [DOI] [PubMed] [Google Scholar]
- 3.Shahrokni A, Alexander K. The age of talking about age alone is over. Ann Surg Oncol. 2019; 26:12–14 [DOI] [PubMed] [Google Scholar]
- 4.Lawler M, Selby P, Aapro MS, Duffy S. Ageism in cancer care. BMJ. 2014; 348:g1614. [DOI] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015; 12:261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghignone F, van Leeuwen BL, Montroni I, et al. ; International Society of Geriatric Oncology (SIOG) Surgical Task Force. The assessment and management of older cancer patients: a SIOG surgical task force survey on surgeons’ attitudes. Eur J Surg Oncol. 2016; 42:297–302 [DOI] [PubMed] [Google Scholar]
- 8.Kottek A, Bates T, Spetz MJ. The Roles and Value of Geriatricians in Healthcare Teams: A Landscape Analysis. 2017. UCSF Health Workforce Research Center on Long-Term Care; Accessed October 15, 2019 https://healthworkforce.ucsf.edu/sites/healthworkforce.ucsf.edu/files/REPORT_Geriatricians_Lit_FINAL.pdf [Google Scholar]
- 9.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983; 31:721–727 [DOI] [PubMed] [Google Scholar]
- 10.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39:142–148 [DOI] [PubMed] [Google Scholar]
- 11.Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015; 41:844–851 [DOI] [PubMed] [Google Scholar]
- 12.Sasani K, Catanese HN, Ghods A, et al. Gait speed and survival of older surgical patient with cancer: prediction after machine learning. J Geriatr Oncol. 2019; 10:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007; 120:807–813 [DOI] [PubMed] [Google Scholar]
- 14.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000; 15:1021–1027 [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992; 40:922–935 [DOI] [PubMed] [Google Scholar]
- 16.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011; 27:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fearon KC, Jenkins JT, Carli F, Lassen K. Patient optimization for gastrointestinal cancer surgery. Br J Surg. 2013; 100:15–27 [DOI] [PubMed] [Google Scholar]
- 18.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004; 199:762–772 [DOI] [PubMed] [Google Scholar]
- 19.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005; 8:23–32 [DOI] [PubMed] [Google Scholar]
- 20.Carli F, Silver JK, Feldman LS, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. 2017; 28:49–64 [DOI] [PubMed] [Google Scholar]
- 21.Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health. 2013; 13:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015; 33:17–33 [DOI] [PubMed] [Google Scholar]
- 23.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond). 2011; 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruns ERJ, Argillander TE, Schuijt HJ, et al. Fit4SurgeryTV at-home prehabilitation for frail older patients planned for colorectal cancer surgery: a pilot study. Am J Phys Med Rehabil. 2019; 98:399–406 [DOI] [PubMed] [Google Scholar]
- 25.Mouch CA, Kenney BC, Lorch S, et al. Statewide prehabilitation program and episode payment in Medicare beneficiaries. J Am Coll Surg. 2020; 230:306–313.e6 [DOI] [PubMed] [Google Scholar]
- 26.Lirosi MC, Tirelli F, Biondi A, et al. Enhanced recovery program for colorectal surgery: a focus on elderly patients over 75 years old. J Gastrointest Surg. 2019; 23:587–594 [DOI] [PubMed] [Google Scholar]
- 27.Braga M, Beretta L, Pecorelli N, et al. ; PeriOperative Italian Society Group. Enhanced recovery pathway in elderly patients undergoing colorectal surgery: is there an effect of increasing ages? Results from the perioperative Italian Society Registry. Updates Surg. 2018; 70:7–13 [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis. 2012; 14:1009–1013 [DOI] [PubMed] [Google Scholar]
- 29.Jia Y, Jin G, Guo S, et al. Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg. 2014; 399:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braga M, Pecorelli N, Scatizzi M, Borghi F, Missana G, Radrizzani D; PeriOperative Italian Society. Enhanced recovery program in high-risk patients undergoing colorectal surgery: results from the PeriOperative Italian Society Registry. World J Surg. 2017; 41:860–867 [DOI] [PubMed] [Google Scholar]
- 31.Person B, Cera SM, Sands DR, et al. Do elderly patients benefit from laparoscopic colorectal surgery?. Surg Endosc. 2008; 22:401–405 [DOI] [PubMed] [Google Scholar]
- 32.Hatakeyama T, Nakanishi M, Murayama Y, et al. Laparoscopic resection for colorectal cancer improves short-term outcomes in very elderly colorectal cancer patients. Surg Laparosc Endosc Percutan Tech. 2013; 23:532–535 [DOI] [PubMed] [Google Scholar]
- 33.Yap R, Oliva K, Wilkins S, McMurrick PJ. Colorectal cancer surgery in the very elderly: nonagenarians. Dis Colon Rectum. 2016; 59:501–507 [DOI] [PubMed] [Google Scholar]
- 34.Banks E, Byles JE, Gibson RE, et al. Is psychological distress in people living with cancer related to the fact of diagnosis, current treatment or level of disability? Findings from a large Australian study. Med J Aust. 2010; 193S5S62–S67 [DOI] [PubMed] [Google Scholar]
- 35.Montroni I, Ugolini G, Saur NM, et al. Personalized management of elderly patients with rectal cancer: expert recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commission on Cancer. Eur J Surg Oncol. 2018; 44:1685–1702 [DOI] [PubMed] [Google Scholar]
- 36.Suskind AM, Finlayson E. Focus on surgical outcomes that matter to older patients. JAMA Surg. 2016; 151:e161701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audisio RA, Pope D, et al. ; PACE participants. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008; 65:156–163 [DOI] [PubMed] [Google Scholar]
- 38.Pędziwiatr M, Pisarska M, Major P, et al. Laparoscopic colorectal cancer surgery combined with enhanced recovery after surgery protocol (ERAS) reduces the negative impact of sarcopenia on short-term outcomes. Eur J Surg Oncol. 2016; 42:779–787 [DOI] [PubMed] [Google Scholar]
- 39.Montroni I, Rostoft S, Spinelli A, et al. ; SIOG surgical task force/ESSO GOSAFE study group. GOSAFE - Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery: early analysis on 977 patients. J Geriatr Oncol. 2020; 11:244–255 [DOI] [PubMed] [Google Scholar]
- 40.Agency for Healthcare Research and Quality. Estimating the Additional Hospital Inpatient Cost and Mortality Associated With Selected Hospital-Acquired Conditions. AHRQ Publication No. 18-0011-EF. November 2017. Accessed November 2, 2019 https://www.ahrq.gov/hai/pfp/haccost2017.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


