Abstract
Background
In face of the Coronavirus Disease (COVID)-19 pandemic, best practice for mechanical ventilation in COVID-19 associated Acute Respiratory Distress Syndrome (ARDS) is intensely debated. Specifically, the rationale for high positive end-expiratory pressure (PEEP) and prone positioning in early COVID-19 ARDS has been questioned.
Methods
The first 23 consecutive patients with COVID-19 associated respiratory failure transferred to a single ICU were assessed. Eight were excluded: five were not invasively ventilated and three received veno-venous ECMO support. The remaining 15 were assessed over the first 15 days of mechanical ventilation. Best PEEP was defined by maximal oxygenation and was determined by structured decremental PEEP trials comprising the monitoring of oxygenation, airway pressures and trans-pulmonary pressures. In nine patients the impact of prone positioning on oxygenation was investigated. Additionally, the effects of high PEEP and prone positioning on pulmonary opacities in serial chest x-rays were determined by applying a semiquantitative scoring-system. This investigation is part of the prospective observational PA-COVID-19 study.
Findings
Patients responded to initiation of invasive high PEEP ventilation with markedly improved oxygenation, which was accompanied by reduced pulmonary opacities within 6 h of mechanical ventilation. Decremental PEEP trials confirmed the need for high PEEP (17.9 (SD ± 3.9) mbar) for optimal oxygenation, while driving pressures remained low. Prone positioning substantially increased oxygenation (p<0.01).
Interpretation
In early COVID-19 ARDS, substantial PEEP values were required for optimizing oxygenation. Pulmonary opacities resolved during mechanical ventilation with high PEEP suggesting recruitment of lung volume.
Funding
German Research Foundation, German Federal Ministry of Education and Research.
Research in context.
Evidence before this study
We searched PubMed without language restriction for studies published from database inception until August 15th, 2020, with the terms “SARS-CoV-2″ or ”COVID-19″ and “ARDS” or “mechanical ventilation” or “PEEP” or “prone positioning” or “respiratory failure” and found no relevant articles pertaining to COVID-19 ARDS. To our knowledge, there have been no reports on PEEP titration in conjunction with the assessment of radiographic changes and lung mechanics in early COVID-19 ARDS.
Added value of this study
We demonstrate that patients with early COVID-19 ARDS can benefit in terms of oxygenation from mechanical ventilation with high PEEP as well as from prone positioning.
Implications of all the available evidence
COVID-19 ARDS lung exhibits a remarkable high lung compliance but despite its unique nature we show here that COVID-19 ARDS patients benefit from high PEEP and respond well to prone positioning regarding oxygenation. Our findings provide evidence that may help guide intensivists in the treatment of early COVID-19 ARDS.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global health emergency. The progression to respiratory failure and the requirement for mechanical ventilation in some patients has pushed health care systems worldwide to or beyond their limits [1]. The burden of disease is still on a rising trajectory in most parts of the world [2].
Approximately 17 percent of patients with COVID-19 pneumonia require invasive mechanical ventilation [3,4]. While the COVID-19 phenotype has recently been proposed to be similar to high-altitude pulmonary edema [5] or to represent a novel class, [6] COVID-19 induced pneumonia often fulfills all criteria of the acute respiratory distress syndrome (ARDS) as defined by the Berlin definition [7]. In line with the classic pathology of ARDS, [8] these clinical findings are associated with a hyperinflammatory response in the lungs of COVID-19 patients evident as distinct inflammatory cell infiltration and diffuse alveolar damage [9].
A series of editorials and case reports recently proposed that principles of mechanical ventilation in early COVID-19 should deviate from classic ARDS [10], [11], [12], [13]. This suggestion is based on the notion that some COVID-19 ARDS patients (“L-type”) are characterized by low lung elastance, low lung weight, and low lung recruitability; and the ”H-type” are characterized by high lung elastance, high intrapulmonary right-to-left shunt, high lung weight, and high lung recruitability, typical for classic ARDS. Based on the l-type characteristics, the editorialists have argued for more liberal tidal volumes and against the use of high positive end-expiratory pressure (PEEP) in early COVID-19 ARDS. As well, the usefulness of prone positioning in early COVID-19 ARDS has been questioned. These editorials have generated considerable debate regarding optimal ventilatory strategies for COVID-19 ARDS.
In this prospective study we set out to address this debate by examining the effects of PEEP titration and prone positioning in the first cohort of early COVID-19 ARDS patients transferred to our ICU. We studied detailed physiological responses to PEEP maneuvers and prone position in these patients. Our findings suggest that patients with early COVID-19 ARDS do not differ in their response to high PEEP and prone positioning from classic ARDS, and should therefore be ventilated according to established ARDS principles and regimens.
2. Methods
2.1. Study design and oversight
Between March 15 and April 11, 2020 we enrolled the initial cohort of 23 consecutive patients with COVID-19 ARDS admitted to one of the three ICUs of the Charité - Universitätsmedizin Berlin ARDS/ECMO Center as part of the prospective, observational PA-COVID-19 trial on the pathophysiology and clinical course of COVID-19 (retrospectively registered in the German clinical trials register on May 13, 2020 (ID: DRKS00021688) [14]. The study was approved by the ethics committee of the Charité - Universitätsmedizin Berlin (EA2/066/20) and was performed according to the Declaration of Helsinki and Good Clinical Practice principles (ICH 1996). Written informed consent was obtained from all patients, or their legal representatives.
2.2. Study population
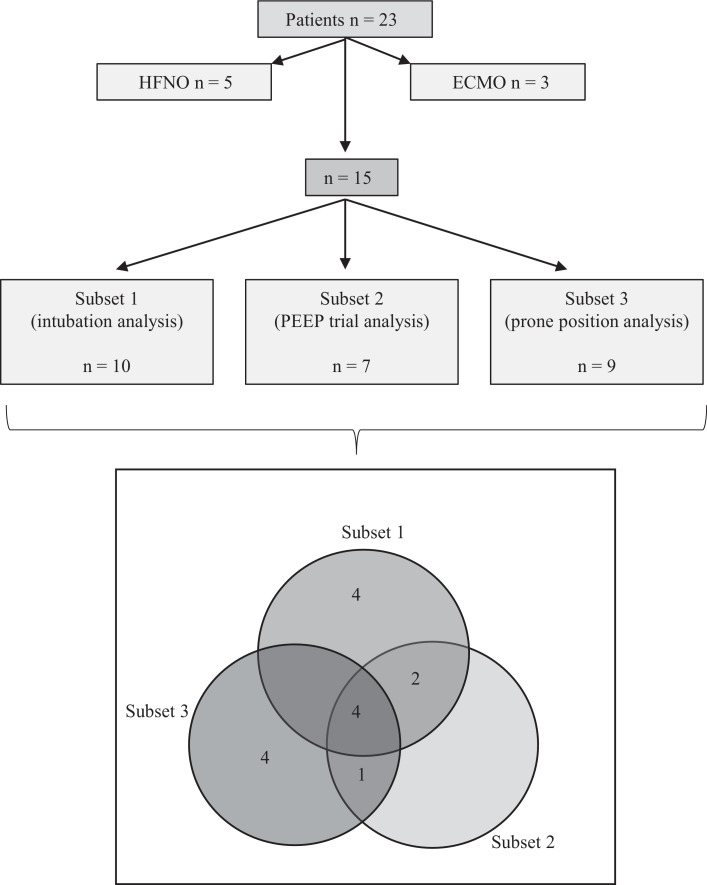
All 23 patients were referred to the ICU of a tertiary referral center with respiratory failure following positive polymerase chain reaction (PCR) SARS-CoV-2 infection. All 23 patients were included in the descriptive analysis of the cohort. For the analysis of the effect of mechanical ventilation with high PEEP on recruitment and oxygenation, PEEP titration and effect of prone positioning the three patients on veno-venous extracorporeal membrane oxygenation (vvECMO) were excluded as ECMO therapy was judged to be a significant bias in the analysis of oxygenation. Five patients were excluded as they were treated with high flow nasal oxygen, and did not receive mechanical ventilation. The remaining 15 mechanically ventilated patients were studied for up to 15 days following intubation. All ten patients intubated in our ICU were included in Subset 1 (intubation group) to assess the initial effects of invasive mechanical ventilation on oxygenation (four patients were intubated due to severe oxygenation failure, 6 were intubated due to severe oxygenation failure and concomitant high ventilatory effort). For seven of the 15 mechanically ventilated patients a full dataset of decremental PEEP trials was available and they were accordingly included in Subset 2 (PEEP trial group) to determine best PEEP. Nine of the 15 patients were place prone within the first 3.0 (SD ± 3.9) days following intubation and were included in Subset 3 (prone position group) to assess the effect of prone positioning. Fig. 1 details the assignment of all 23 patients. Of all patients, 15 patients were transferred from the emergency room or regular wards within the hospital of which ten were intubated in our ICU. Eight patients were transferred intubated to our ICU from regional secondary hospitals including the three vvECMO patients. Demographic and clinical characteristics of the patients at admission are listed in Table 1.
Fig. 1.
Study cohort flowchart. A, The first consecutive 23 COVID-19 patients treated on our ICU were enrolled to this study, investigating PEEP and prone positioning in mechanically ventilated patients. Eight patients were excluded from the assessment of the specific interventions as they received ECMO therapy (three patients, ECMO therapy would interfere with the analysis of oxygenation), or high-flow oxygen therapy but no mechanical ventilation (five patients that could not be asses regarding the effects of invasive mechanical ventilation, PEEP and prone positioning), resulting in 15 patients eligible for this study. Ten patients were intubated in our ICU (Subset 1, intubation group), while eight patients were transferred intubated to our ward. For seven of the 15 mechanically ventilated patients, a full dataset of decremental PEEP trials was available and they were accordingly included in Subset 2 (PEEP trial analysis) for determination of optimal PEEP. Nine of the fifteen patients were subject to prone positioning (Subset 3, prone position analysis). B, Illustration of the allocation of the 15 patients to each analysis.
Table 1.
Demographic and clinical characteristics of the patients.
| Demographic and clinical characteristics of the patients | |
|---|---|
| Characteristics | |
| Patients | n = 23 |
| Age (years) | 62·1 ± 14·1 |
| Age (range) | 26 - 81 |
| Sex - Male (%) | 73·9 |
| BMI, kg/m2 | 29·3 ± 4·8 |
| Mean duration of symptoms before admission (days) | 6·4 ± 3·2 |
| Mean duration of symptoms before intubation (days) | 7·6 ± 3·7 |
| Number of patients with high-flow nasal oxygen (no intubation) | 5 |
| Number of patients with ECMO | 3 |
| Hospital mortality rate (%) * | 29·2 |
| Hospital mortality rate of patients with high-flow nasal oxygen (%) | 20·0 |
| Hospital mortality rate of ECMO patients (%) | 0·0 |
| Length of Stay (days)* | 35·7 ± 32·2 |
| Length of Stay of patients with high-flow nasal oxygen (days) | 14·2 ± 5·8 |
| Length of Stay of ECMO patients (days) | 70·7 ± 36·9 |
| Percentage of all patients who received intermittently muscle relaxants (%) | 30·4 |
| Percentage of ECMO patients who received intermittently muscle relaxants (%) | 100·0 |
| Percentage of all patients who received intermittently norepinephrine and vasopressin (%) | 30·4 |
| Percentage of ECMO patients who received intermittently norepinephrine and vasopressin (%) | 66·7 |
| APACHE II | 18·0 ± 9·4 |
| SOFA all patients | 6·3 ± 4·9 |
| SOFA of patients with high-flow nasal oxygen (no intubation) | 2·2 ± 1·3 |
| SOFA of patients with ECMO | 11·0 ± 5·3 |
| COVID-19 symptoms - no. (%) | |
| Dyspnea | 19 (82·6) |
| Cough | 18 (78·3) |
| Sore throat | 3 (13) |
| Fever | 22 (95·7) |
| Myalgia | 9 (39·1) |
| Exhaustion | 13 (56·5) |
| Coexisting conditions - no. (%) | |
| COPD | 3 (13·0) |
| Asthma | 1 (4·3) |
| Other lung disease | 3 (13·0) |
| Pre-obesity | 10 (43·5) |
| Obesity | 8 (34·8) |
| Arterial hypertension | 18 (78·3) |
| Diabetes mellitus Type 2 | 9 (39·1) |
| Coronary heart disease | 2 (8·7) |
| HIV, transplantation or immuno-supressive medications | 2 (8·7) |
| Subset 1 (Intubation group) | |
| Patients | 10 |
| Age (years) | 70·4 ± 10·8 |
| Sex - Male (%) | 80 |
| BMI, kg/m2 | 28·4 ± 4·5 |
| Mean duration of symptoms before admission (days) | 7·2 ± 4·3 |
| Mean duration of symptoms before intubation (days) | 8·7 ± 4·2 |
| Hospital mortality rate (%) | 40·0 |
| Length of Stay (days)* | 38·3 ± 26·1 |
| Percentage of patients who received intermittently muscle relaxants (%) | 40·0 |
| Percentage of patients who received intermittently norepinephrine and vasopressin (%) | 30·0 |
| APACHE II | 17·8 ± 9·4 |
| SOFA | 3·9 ± 2·9 |
| Subset 2 (PEEP trial group) | |
| Patients | 7 |
| Age (years) | 61·4 ± 15·8 |
| Sex - Male (%) | 57·1 |
| BMI, kg/m2 | 30·0 ± 6·5 |
| Mean duration of symptoms before admission (days) | 5·7 ± 2·6 |
| Mean duration of symptoms before intubation (days) | 6·7 ± 2·7 |
| Hospital mortality rate (%) | 42·9 |
| Length of Stay (days) | 56·3 ± 32·9 |
| Percentage of patients who received intermittently muscle relaxants (%) | 28·6 |
| Percentage of patients who received intermittently norepinephrine and vasopressin (%) | 28·6 |
| APACHE II | 20·7 ± 8·8 |
| SOFA | 5·7 ± 5·2 |
| Respiratory rate/ min | 16·0 ± 2·6 |
| Subset 3 (Prone position group) | |
| Patients | 9 |
| Age (years) | 62·0 ± 14·2 |
| Sex - Male (%) | 66 |
| BMI, kg/m2 | 30·4 ± 6·5 |
| Mean duration of symptoms before admissions (days) | 6·7 ± 4·8 |
| Mean duration of symptoms before intubation (days) | 7·2 ± 4·8 |
| Hospital mortality rate (%) | 55·6 |
| Length of Stay (days)a | 50·4 ± 34·9 |
| Percentage of patients who received intermittently muscle relaxants (%) | 55·6 |
| Percentage of patients who received intermittently norepinephrine and vasopressin (%) | 44·4 |
| APACHE II | 26·2 ± 6·5 |
| SOFA | 7·4 ± 4·9 |
| Mean time to first prone positioning (days) | 3 ± 3·9 |
| Duration of each prone positioning (hours) | 15·4 ± 2·5 |
Values are means ± SD. COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; APACHE II, Acute Physiology And Chronic Health Evaluation II; SOFA, Sepsis-related organ failure assessment score. APACHE II and SOFA score were obtained at admissions day. #One patient is still hospitalized and thus excluded from the hospital mortality rate.
One patient is still hospitalized and excluded from hospital mortality rate and length of stay analyses.
2.3. Mechanical ventilation and decremental PEEP trials
The initial PEEP after intubation was determined using the ARDSnet high PEEP table [15]. In patients receiving low flow oxygen prior to intubation, the FiO2 necessary to maintain adequate oxygenation was calculated from the demand of supplemental oxygen [16]. In patients treated with high flow nasal oxygen (HFNO), the adjusted FiO2 was used. Notably high minute ventilation under supplemental oxygen even when delivered as HFNO may lower the effective FiO2. Further hyperventilation results in decreased alveolar CO2 and vice versa increased alveolar O2 which would improve oxygenation by raising the diffusion gradient for oxygen. In the current setting the estimated FiO2 could not be corrected for both opposing interrelations. In general, PEEP values 2 mbar higher than the value suggested by the higher PEEP / lower FiO2 table of the ARDS Network were initially applied after intubation (Supplementary Table S1) [15]. In patient 2, the initial PEEP was set lower as blood oxygenation increased quickly at this PEEP level after intubation. Patients were ventilated in either volume or pressure controlled mode with a tidal volume targeting 6 ml/kg predicted body weight. Respiratory rate was set to facilitate CO2 removal while preventing dynamic hyperinflation, and permissive hypercapnia was tolerated if necessary to adhere to lung protective ventilation. Assisted spontaneous breathing was allowed after PEEP titration, when considered safe by the attending physician. An esophageal catheter (, NutriVent™,Sidam Group, San Glacomo Roncole, Italy) was placed allowing to measure the esophageal pressure by connecting the balloon filled with 4 ml of air to the corresponding pressure port of the ventilator (Hamilton S1, Hamilton Medical, Bonaduz, Switzerland). Position of the balloon in the mid to lower third of the thorax was validated by typical cardiac oscillations, dynamic occlusion tests and by chest x-ray (CXR). Transpulmonary pressure at end-inspiration and end-expiration were calculated directly from simultaneously measured airway and esophagus pressures.
PEEP trials were performed applying volume controlled ventilation with constant respiratory rate, FiO2 and tidal volume (VT; 6.4 (SD ± 0.5) ml/kg predicted bodyweight). After a stabilization period of at least 10 min, PEEP was reduced stepwise in 2 mbar decrements. Prior to each PEEP reduction, arterial blood gas analysis was performed and respiratory parameters (as detailed in Table 2 and Supplementary Table S2) were recorded including PaO2, PaCO2, end-inspiratory airway plateau airway pressure, end-inspiratory esophageal pressure, end-expiratory esophageal pressure and respiratory system compliance (CRS). Driving pressure (ΔP= end inspiratory airway plateau pressure - PEEP), inspiratory trans-pulmonary pressure (TPi= end-inspiratory airway plateau pressure – end-inspiratory esophagus pressure), end-expiratory trans-pulmonary pressure (TPexp= PEEP - end-expiratory esophageal pressure), trans-pulmonary driving pressure (ΔTP= (TPi-TPexp)), were calculated. Optimal PEEP was defined at the PEEP corresponding to the maximal PaO2/FiO2. Downward PEEP titration was stopped when PaO2 decreased. In general, under mechanical ventilation target oxygen saturation was 90–95%, in patients receiving vasopressor support a mean arterial pressure of 65–70 mmHg was adjusted. Lactate levels were measured at least 3 times a day and transthoracic echocardiography was regularly performed to monitor cardiac function particularly when high airway pressures were applied.
Table 2.
PEEP trials in early COVID-19 ARDS patients.
| Patient: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | SD | Median | Inter-quartile range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FiO2 prior-intubation | 1 | 0·7 | 0·8 | 0·7 | 0·75 | 0·7 | 0·8 | 0·8 | 0·1 | 0·75 | 0·1 |
| Initial PEEP at start of PEEP trial (mbar) | 24 | 13 | 24 | 18 | 20 | 18 | 24 | 20·1 | 3·9 | 20·0 | 6·0 |
| Results PEEP Trials: | |||||||||||
| Optimal PEEP (mbar) | 22.0 | 11.0 | 20.0 | 18.0 | 16.0 | 16.0 | 22.0 | 17·9 | 3·6 | 18·0 | 5·0 |
| VT (ml/ kg) | 6·3 | 6·1 | 7·0 | 6·7 | 5·5 | 7·2 | 6·4 | 6·4 | 0·5 | 6·4 | 0·53 |
| Respiratory rate (min−1) | 13.0 | 15.0 | 13.0 | 15.0 | 22.0 | 16.0 | 16.0 | 15·7 | 2·8 | 15·0 | 2·0 |
| Respiratory system compliance (CRS) (ml/cmH2O) | 59·6 | 45·0 | 79.0 | 64·8 | 30.0 | 41·9 | 60·1 | 54·3 | 15·2 | 59·6 | 19·0 |
| Driving pressure (ΔP) (mbar) | 8.0 | 9.0 | 7.0 | 11.0 | 17·2 | 11.0 | 9.0 | 10·3 | 3·1 | 9·0 | 2·5 |
| Trans-pulmonary driving pressure (ΔTP) (mbar) | 6·9 | 7·9 | 4·5 | 7·1 | 11·8 | 8·4 | 6·1 | 7·5 | 2·1 | 7·1 | 1·65 |
| End-expiratory trans-pulmon-ary pressure (TPexp) (mbar) | 3·1 | 0·3 | 0·8 | 10.0 | 10·8 | 2·6 | 7·9 | 5·1 | 4·1 | 3·1 | 7·25 |
| FiO2 | 0·5 | 0·4 | 0·3 | 0·35 | 0·5 | 0·6 | 0·35 | 0·4 | 0·1 | 0·4 | 0·15 |
| PaO2 (mmHg) | 85.0 | 85·0 | 71·0 | 65·3 | 81·7 | 79·6 | 88·7 | 79·5 | 7·8 | 81·7 | 9·7 |
| PaCO2 (mmHg) | 59.0 | 44·0 | 50·0 | 47·3 | 73·4 | 45·0 | 48·4 | 52·4 | 9·7 | 48·4 | 8·35 |
| PaO2/FiO2 ratio (mmHg) | 170·0 | 242·9 | 236·7 | 186·6 | 163·4 | 132·7 | 253·4 | 197·9 | 43·0 | 186·6 | 78·35 |
Results of PEEP trial shows the optimal PEEP as determined by decremental PEEP trial, and corresponding respiratory system mechanics and arterial blood gas analyses for all seven patients, as well as means, standard deviations (SD), median, and interquartile range.
2.4. Prone maneuver
Prone positioning was applied when PaO2/FiO2 ratio was <150 mmHg (n = 10). Patients remained prone on average 15.4 (SD ± 2.5) h for 6.2 (SD ± 3.4) consecutive days. The PaO2/FiO2 ratio, oxygenation-index (OI; OI = FiO2 * mean airway pressure/PaO2), and PEEP (with optimal PEEP levels set by decremental PEEP trials in supine and prone position, respectively) were assessed after 4 h in the supine position (SP) or 12 h prone. Mean values ± standard deviation (SD) were compared after the first prone positioning (first supine positioning vs. first prone positioning), after the first three prone maneuvers (first three supine positioning vs. first three prone positioning), and of all prone positions received (all supine positioning vs. all prone positioning).
2.5. Radiologic studies
CXR taken in supine position 5.9 h (SD± 1.8) after the respective prone positioning were evaluated independently by two board-certified radiologists. To assess changes in pulmonary opacification after intubation and prone positioning, respectively, a CXR scoring system was applied as described previously [17].
2.6. Statistics
To analyze the CXR scoring system results, a Wilcoxon signed-rank test was used to compare the changes in mean opacity. Otherwise analyses were performed using a Student`s paired, two-tailed, t-test. A p value <0.05 was considered to indicate statistical significance. The normal distribution of the data was verified by histogram visualization. As an additional sensitivity analysis we applied a non-parametric test, thereby relaxing the assumption of normality of the parameter and found no relevant changes in the results. Statistical analyses were performed with GraphPad 8 Prism.
2.7. Role of the founding source
The funders had no role in study design, data collection, analysis, or interpretation of the data, or in the writing of this study. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
3. Results
3.1. Invasive positive pressure ventilation improves oxygenation and reduces CXR opacities in early COVID-19 ARDS
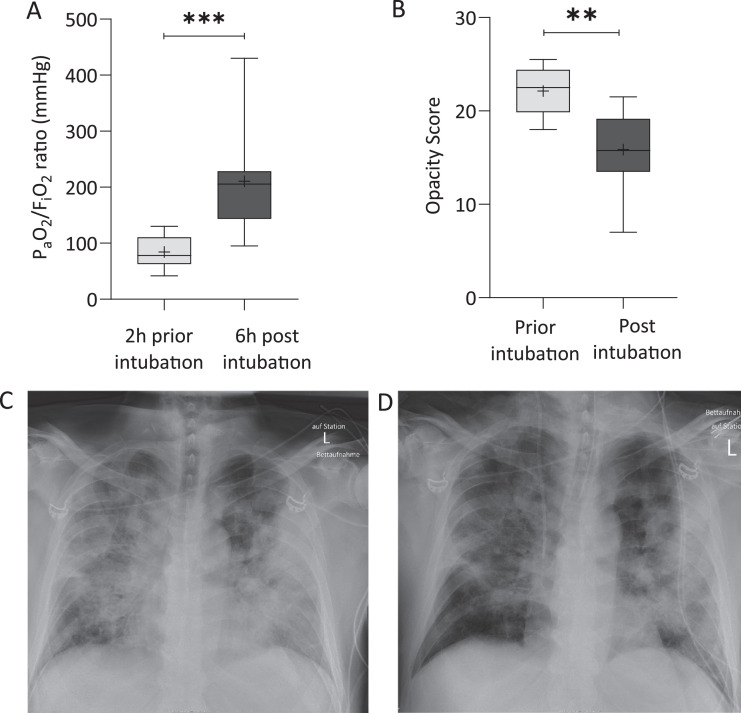
In Subset 1 (intubation group), the 10 consecutive patients (age: 70·4 (SD ± 10·8) years; 80% male) were referred to our ICU after being symptomatic for 7·2 (SD ± 2·3) days. Following an initial, unsuccessful treatment with HFNO, patients were intubated 8·7 (SD ± 4·2) days after onset of symptoms (Table 1). Respiratory rate and PaCO2 prior to intubation were 31 (SD ± 2·6) per min and 35·9 (SD ± 7·0) mmHg; FiO2 required to maintain peripheral arterial oxygen saturation (SaO2) between 88 and 92% was 0·8 (SD ± 0·12) (Supplementary Table S1). After intubation and initiation of invasive positive pressure ventilation the PaO2/FiO2 ratio increased from 84·3 (SD ± 28) mmHg to 210·7 (SD ± 86·6) mmHg within 6 h of mechanical ventilation (Fig. 2), and the FiO2 required for a SaO2 between 88% and 92% decreased to 0·4 (SD ± 0·11) (for detailed respiratory parameters please see Table S2). CXR images obtained before intubation and 3 to 16 h after invasive ventilation revealed a reduction in opacities (p = 0·002, Fig. 2). Paired CXR images prior and post intubation are provided for each patient in online Supplementary Figure S1.
Fig. 2.
Invasive positive pressure ventilation with high PEEP improves oxygenation and reduces opacities in chest x-rays. The left boxplots (A) present the PaO2/FiO2 ratio two hours prior and six hours post intubation from the ten patients of Subset 1. Scoring of pulmonary opacities was performed, showing a reduction in pulmonary opacity scores with positive pressure ventilation (B). Representative chest x-ray images of one patient obtained before intubation (C) and after onset of mechanical ventilation (D) are shown. Whiskers indicate the 5th and 95th percentile. The cross within the box marks the mean. A two-sided paired t-test (A), and a Wilcoxon signed-rank test (B) was performed. **p<0·01, ***p<0·001.
3.2. High PEEP is required for optimal oxygenation in early COVID-19 ARDS
In Subset 2 (PEEP trial group), decremental PEEP trials were performed in 7 ventilated patients to identify the optimal PEEP, defined as maximal PaO2/FiO2. PEEP trials were performed between 2 h and 60 h after initiation of mechanical ventilation. The optimal PEEP level was 17·9 (SD ± 3·6) mbar (Table 2), which was slightly above the corresponding recommended PEEP according to the ARDSnet high PEEP table (16 mbar for FiO2 of 0·4 – 0·5). Respiratory parameters and arterial blood gases for each step of the decremental PEEP trial and each individual patient are given in Supplementary Table S2.
3.3. Prone positioning improves oxygenation in early COVID-19 ARDS
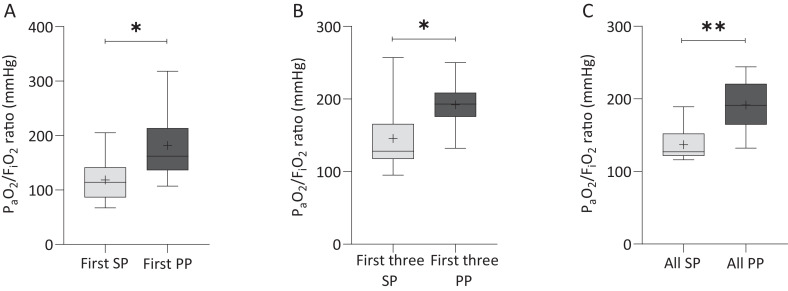
In Subset 3 (prone position group), 9 consecutive mechanically ventilated patients (62.0 (SD ± 14·2) years old, 66% male) were subjected to prone maneuvers. Patients had been symptomatic for 6·7 ± 4·8 days, were intubated 7·2 (SD ± 4·8) days after symptom onset, and had been ventilated for 3·0 (SD ± 3·9) days prior to first prone positioning (Table 1). In all analyses, PaO2/FiO2 ratio and OI were markedly improved in prone compared to the preceding supine position (p<0·01), while the optimal PEEP was slightly lower in prone positioning (p<0·05, Fig. 3, Supplementary Table S3). CXRs were obtained before and after the first prone maneuver in 7/9 patients, and before and after the second and third prone maneuver in 8/9 patients. CXR did not reveal a significant reduction in opacity score in response to prone positioning(after first prone positioning: p = 0·35, after third prone positioning: p = 0·37, Supplementary Fig. S2), suggesting that improved oxygenation did not primarily result from lung recruitment. CXR images of all patients before and after the first prone maneuver are provided in Supplementary Figure S2.
Fig. 3.
Prone positioning improves oxygenation. PaO2/FiO2 ratio was measured in nine patients subjected to prone positioning (Subset 3). Group data show PaO2/FiO2 ratio after the first prone positioning (PP) relative to the previous supine position (SP) (A), after the first three PP maneuvers vs. the three preceding SPs (B), and for all prone positions (C). Whiskers indicate the 5th and 95th percentile. The cross within the box marks the mean. A two-sided paired t-test was performed. *p<0·05, **p<0·01.
4. Discussion
In patients with early COVID-19 ARDS treated in our ICU, oxygenation improved markedly while radiographic pulmonary opacities decreased, after initiation of invasive mechanical ventilation. Decremental PEEP trials identified that high PEEP values of 18 (SD ± 4) mbar were required for optimal oxygenation. Individual optimal PEEP values were comparable to values suggested by the high ARDS network PEEP table [15]. Oxygenation increased reproducibly in response to repeated prone positioning as compared to supine positioning. Our findings suggest that patients with early COVID-19 ARDS do not differ in their response to high PEEP and prone positioning from classic ARDS, and should therefore be ventilated according to established ARDS principles and regimens.
Patients were referred to our ICU within 6.4 days (SD ± 3.2), and were intubated within 7.6 days (SD ± 3.7) after onset of symptoms. Regarding demographic characteristics and preexisting conditions the cohort was comparable to 1727 COVID-19 patients treated with mechanical ventilation on ICUs in Germany between 26. February 2020 and 19. April 2020 [18]. We did not use non-invasive ventilation, and patients were intubated if high flow nasal oxygen therapy was insufficient to ensure adequate oxygenation or if the patient remained tachypneic (respiratory rate >30 min−1) or hypocapnic despite being sufficiently oxygenated. Based on these characteristics, patients in this study can be considered to reflect cases of early COVID-19 ARDS.
A number of recent inter-related editorials have suggested that a subset of patients with COVID-19 induced ARDS have an unusual physiological phenotype (“L-type” phenotype), with low elastance, low lung weight, and low recruitability. Based on these physiological results the authors suggested that high levels of PEEP may be detrimental and that prone positioning is likely not indicated [11]. There is some question as to whether the respiratory mechanics of COVID-19 induced ARDS are indeed as heterogeneous or unusual as suggested in these editorials [10], [11], [12], [13]. Our case series does not directly address this particular question, as the number of patents we studied is quite small. However, we think this study is important because it directly addresses the conclusions of the editorialists with respect to what is the optimal ventilatory strategy for these patients.
The respiratory system mechanics of our patients are very close to those of Gattinoni et al. [10] with Crs of 54.3 (SD ± 15.2) ml/cmH2O at optimal PEEP, compared to 50.2 (SD ± 14.3) ml/cmH2O in their study. As such we were studying a group of patients with very similar mechanical characteristics to what they described. We carefully titrated PEEP in our patients to avoid potential adverse effects of high PEEP levels in lungs with rather low elastance. That notwithstanding, we identified that high PEEP levels were required for optimized oxygenation, while driving pressure remained within margins commonly considered safe. We did not detect PEEP related hemodynamic instabilities (i.e. no increase of norepinephrine demand or lactate levels). Our results are not in accord with the recommendation of Gattinoni et al. who suggested relatively low PEEP levels of 8–10 cm H2O [19]. In addition, they recommended that the prone positioning should only be used as a rescue maneuver as the lungs of these patients were “too good” for the prone positioning to be effective; we found that these patients responded to the prone with improved oxygenation. It is important to point out their recommendations were based on a theoretical assessment informed by baseline lung mechanics, and not on detailed measurements of the consequences of higher PEEP or prone positioning in these patients.
The fact that high PEEP may markedly improve oxygenation in lungs with a low elastance may seem puzzling. Despite their low elastance, patients commonly presented with a high proportion of non-aerated lung tissue as recently reported by Lieuwe and coworkers in 70 patients [20]. This finding is in line with improved oxygenation in our patients upon initiation of mechanical ventilation with high PEEP, in association with partial resolution of wide-spread pulmonary opacities. In our study optimizing oxygenation was the main goal to be achieved by PEEP titration. Setting PEEP to optimize oxygenation is rational as optimal oxygenation results from an optimized gas exchange area stabilized by high PEEP that implies an increased functional residual capacity which would result in minimized end-inspiratory lung strain, minimized cyclic opening and closing of alveoli - atelectrauma, and an ideally homogenously ventilated lung which altogether should be protective against ventilator-induced lung injury [21,22,23]. However, aiming solely on optimized oxygenation by setting the PEEP contains the risk of PEEP levels too high resulting in potentially injurious lung stress, which would promote ventilator-induced lung injury and hemodynamic deterioration. This may be avoided by rigorously limiting driving pressures and conscientious hemodynamic monitoring which we did during PEEP titration.
The observed marked improvement in oxygenation and recruitment of lung volume with high PEEP is in line with the study of van der Zee in which electric impedance tomography revealed that comparable high PEEP levels were needed to stabilize gas exchange area while hyperinflation of the lung was avoided [24]. Further the improvement in oxygenation is likely of clinical relevance, as indicated by previous work of Goligher and colleagues who demonstrated that a positive oxygenation response to increased PEEP is associated with a reduction of the probability of death in patients with severe ARDS [25]. Consistent with our findings, a recent retrospective analysis of a large COVID-19 ICU cohort from Northern Italy reported that ventilated patients require high PEEP levels for adequate oxygenation within the first 24 h after ICU admission [26].
Mechanistically, fluid accumulation may play an important role in the early disease stage in COVID-19 ARDS, as suggested by lung ultrasound, and opacities by CXR and CT scan [27]. Via functional loss of gas exchange area, increased diffusion distance, and ventilation-perfusion inhomogeneities, alveolar edema can contribute to severe hypoxemia in early COVID-19 ARDS, yet without necessarily increasing lung elastance as increased interalveolar strain may be counterbalanced by reduced surface tension [28]. The fact that high PEEP can re-aerate and thus functionally recruit fluid-filled alveoli in such a scenario [29] may in part explain the marked improvement in oxygenation in “L-type” lungs.
In contrast to the effects of mechanical ventilation per se, our finding that prone positioning markedly improved oxygenation did not appear to be related to lung recruitment as detectable by CXR. More homogeneous ventilation–perfusion matching and trans-pulmonary pressure distribution, regional changes in ventilation, as well as changes in chest wall mechanics may account for the oxygenation benefits of prone positioning in our patients [28,[30], [31], [32], [33]].
We analyzed CXR to assess lung recruitment. Compared to CT scans CXR have both shortcomings and advantages. CXR cannot dissect the exact underlying changes in the lungs as the CXR displays an anterior–posterior overlay image. Thus, further differentiation regarding radiographic phenotypes is not possible. Besides its shortcomings CXR was suitable to detect lung recruitment in our patients and may be considered of high value especially in the context of the current pandemic situation. (i) In daily routine CXR can be performed frequently; (ii) CXR demand less logistic and personal resources as compared to a CT scan. (iii) Further, intrahospital transportation to realize a CT scan for COVID-19 patients comprising the risk of contamination and infection of employees or other patients can be avoided by portable CXR machines. (iv) CXR are available comprehensively in most healthcare systems worldwide which is not the case for CT scans. This altogether underscores the value of our results for intensivists in nearly all health care systems especially in the current situation.
Our study has important limitations The number of investigated patients was relatively small. Importantly, however, due to the homogeneity of the studied cohort and the overall similar course of the early phase of COVID-19 related ARDS this small number was sufficient to address the key issue that high PEEP levels and the prone position in was effective in improving oxygenation in COVID-19 ARDS patients. As an initial observational study, focusing on the early phase of COVID 19 related ARDS, our study was not designed to assess outcome, and it is well known that improvements in oxygenation do not necessarily translate into decreased mortality. While we consider that our study provides important clues for mechanical ventilation in early COVID-19 ARDS, finally large multi-center randomized trials are necessary to determine the best ventilation strategies and their impact on outcome relevant parameters in this disease.
In summary, we demonstrate that patients with early COVID-19 ARDS can benefit in terms of oxygenation from mechanical ventilation with high PEEP as well as from prone positioning. Our findings provide evidence that may help guide intensivists in the treatment of early COVID-19 ARDS, and lend support to the pointed statement by Rice and Janz [34] that we should be cautious in withholding proven beneficial therapeutic strategies (e.g. PEEP and prone position) without evidence for patients with COVID-19 ARDS despite its remarkably unique nature.
Data sharing statement
Anonymised patients data that underlie the results (tables, figures) reported in this study are available upon reasonable request to the corresponding author.
Contributors
MM, AU, WMK, and HMR designed the design. MM, LBJ, AU, CG, FM, PP, AB, YL, FB, SW, and PK collected the data. MM, PP, FK, MD, FD, SWC, ASS, WMK, NS, and HMR were involved in data analysis. All authors were involved in the interpretation of the data and in the writing and critical review of the manuscript.
Declaration of Competing Interest
MD is European Society of Radiology (ESR) Research Chair (2019–2022) and the opinions expressed in this publication are the author's own and do not represent the view of ESR. MD gives lectures for Canon, and Guerbet, and holds hands-on cardiac CT courses (www.ct-kurs.de). He is Editor of Cardiac CT (Springer Nature). He has institutional research agreements with Siemens, General Electric, Philips, Canon, and has a patent on fractal analysis of perfusion imaging (jointly with Florian Michallek, PCT/EP2016/071551).
All other authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We thank Jasmin Lienau for editing the article.
Funding
This study was supported by the Clinical Study Center of Berlin Institute of Health, Charité – Universitätsmedizin Berlin.
MM is supported by the “Charité Digital Clinician Scientist for Future Driven Medicine” program of the Berlin Institute of Health (BIH). WMK is supported by grants-in-aid from the Ministry of Education and Research BMBF (Sympath) and the German Research Foundation DFG (SFB TR-84, KU1218/9-1, KU1218/11-1).
NS is supported by CAPNETZ (FKZ 01KI07145), CAPSyS (01ZX1304B, 01ZX1604B),
PROGRESS - Pneumonia Research Network on Genetic Resistance and Susceptibility for the Evolution of Severe Sepsis (Ministry of Education and Research BMBF 01KI07114, DZL: 82DZLJ19A1).
MD is supported by the EU (603266-2), DFG (DE 1361/14-1, DE 1361/18-1, BIOQIC GRK 2260/1, Radiomics DE 1361/19-1 (428222922), 20-1 (428223139) in SPP 2177/1), Berlin University Alliance (GC_SC_PC 27), Berlin Institute of Health (Digital Health Accelerator).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100579.
Appendix. Supplementary materials
References
- 1.Mareiniss D.P. The impending storm: COVID-19, pandemics and our overwhelmed emergency departments. Am J Emerg Med. 2020;38(6):1293–1294. doi: 10.1016/j.ajem.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) Dashboard.
- 3.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31189-2. published online May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solaimanzadeh I. Acetazolamide, nifedipine and phosphodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19) Cureus. 2020;12(3):e7343. doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer S.L., Sharp W.W., Weir E.K. Differentiating COVID-19 pneumonia from Acute Respiratory Distress Syndrome (ARDS) and High Altitude Pulmonary Edema (HAPE): therapeutic Implications. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047915. published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Carsana L., Aurelio S., Nasr A. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. 2020 doi: 10.1101/2020.04.19.20054262. April 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. published online Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. published online Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. published online Apr 24. [DOI] [PubMed] [Google Scholar]
- 14.Kurth F., Roennefarth M., Thibeault C. Studying the pathophysiology of coronavirus disease 2019 - a protocol for the Berlin prospective COVID-19 patient cohort (Pa- COVID-19) medRxiv. 2020 doi: 10.1101/2020.05.06.20092833. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower R.G., Lanken P.N., MacIntyre N. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 16.Peruzzi W.T., Shapiro B.A. Respiratory Care. In: Murray MJ, Coursin DB, Pearl RG, Prough DS, editors. Critical Care Medicine: Perioperative Management. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 428–446. [Google Scholar]
- 17.Saraya T., Watanabe T., Tsukahara Y. The correlation between chest X-ray scores and the clinical findings in children and adults with mycoplasma pneumoniae pneumonia. Intern Med. 2017;56(21):2845–2849. doi: 10.2169/internalmedicine.8500-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karagiannidis C., Mostert C., Hentschker C. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 20.Bos L.D., Paulus F., Vlaar A.P.J., Beenen L.F.M., Schultz M.J. Subphenotyping ARDS in COVID-19 patients: consequences for ventilator management. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202004-376RL. published online May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L., Carlesso E., Caironi P. Stress and strain within the lung. Curr Opin Crit Care. 2012;18(1):42–47. doi: 10.1097/MCC.0b013e32834f17d9. [DOI] [PubMed] [Google Scholar]
- 22.Tonetti T., Vasques F., Rapetti F. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med. 2017;5(14):286. doi: 10.21037/atm.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremblay L., Valenza F., Ribeiro S.P., Li J., Slutsky A.S. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zee P., Somhorst P., Endeman H., Gommers D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(2):280–284. doi: 10.1164/rccm.202003-0816LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goligher E.C., Kavanagh B.P., Rubenfeld G.D. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014;190(1):70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 26.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Q.Y., Wang X.T., Zhang L.N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman C.E., Lederer D.J., Bhattacharya J. Micromechanics of alveolar edema. Am J Respir Cell Mol Biol. 2011;44(1):34–39. doi: 10.1165/rcmb.2009-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grune J., Tabuchi A., Kuebler W.M. Alveolar dynamics during mechanical ventilation in the healthy and injured lung. Intensive Care Med Exp. 2019;7(Suppl 1):34. doi: 10.1186/s40635-019-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappert D., Rossaint R., Slama K., Gruning T., Falke K.J. Influence of positioning on ventilation–perfusion relationships in severe adult respiratory distress syndrome. Chest. 1994;106(5):1511–1516. doi: 10.1378/chest.106.5.1511. [DOI] [PubMed] [Google Scholar]
- 31.Albert R.K., Leasa D., Sanderson M., Robertson H.T., Hlastala M.P. The prone position improves arterial oxygenation and reduces shunt in oleic-acid-induced acute lung injury. Am Rev Respir Dis. 1987;135(3):628–633. doi: 10.1164/arrd.1987.135.3.628. [DOI] [PubMed] [Google Scholar]
- 32.Pelosi P., Tubiolo D., Mascheroni D. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157(2):387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 33.Zarantonello F., Andreatta G., Sella N., Navalesi P. Prone position and lung ventilation/perfusion matching in acute respiratory failure due to COVID-19. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0775IM. published online May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice T.W., Janz D.R. In defense of evidence-based medicine for the treatment of COVID-19 ARDS. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202004-325IP. published online Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.