Abstract
Cell models are promising tools for studying hereditary human neurodegenerative diseases. Neuronal derivatives of pluripotent stem cells provide the opportunity to investigate different stages of the neurodegeneration process. Therefore, easy and large-scale production of relevant cell types is a crucial barrier to overcome. In this work, we present an alternative protocol for iPSC differentiation into GABAergic medium spiny neurons (MSNs). The first stage involved dual-SMAD signalling inhibition through treatment with SB431542 and LDN193189, which results in the generation of neuroectodermal cells. Moreover, we used bFGF as a neuronal survival factor and dorsomorphin to inhibit BMP signalling. The combined treatment of dorsomorphin and SB431542 significantly enhanced neuronal induction, which was confirmed by the increased expression of the telencephalic-specific markers SOX1 and OTX2 as well as the forebrain marker PAX6. The next stage involved the derivation of actively proliferating MSN progenitor cells. An important feature of our protocol at this stage is the ability to perform prolonged cultivation of precursor cells at a high density without losing phenotypic properties. Moreover, the protocol enables multiple expansion steps (> 180 days cultivation) and cryopreservation of MSN progenitors. Therefore, this method allows quick production of a large number of neurons that are relevant for basic research, large-scale drug screening, and toxicological studies.
Electronic supplementary material
The online version of this article (10.1007/s10616-020-00406-7) contains supplementary material, which is available to authorized users.
Keywords: Induced pluripotent stem cells, Neuronal differentiation, Medium spiny neurons, Neuronal precursors
Introduction
GABAergic medium spiny neurons (MSNs) represent the major cell population of the striatum, which is the region that is most affected in Huntington’s disease. MSN loss leads to cognitive, psychiatric and motor dysfunctions, resulting in the death of the patient (Graybiel 2005; Knowlton et al. 1996). Currently, there are no effective ways to treat the disease due to insufficient understanding of the pathological mechanisms. Different animal models recapitulate only some of the relevant processes occurring in the human brain, while the molecular mechanisms of neurodegeneration remain unclear. Human induced pluripotent stem cell-derived MSNs can serve as an adequate model for studying molecular processes in affected cells. Thus, the development of an easily reproducible differentiation protocol is an important task.
There are different protocols for pluripotent stem cell differentiation into MSNs (Arber et al. 2015; Aubry et al. 2008; Hunt et al. 2017; Jeon et al. 2012; Ma et al. 2012; Mattis et al. 2012; Nekrasov et al. 2016). Early protocols are based on 3D cultivation of pluripotent cells, or they include a step of cocultivation with stromal cells, as well as the manual selection of neural rosettes, which is time-consuming (Aubry et al. 2008; Jeon et al. 2012; Kawasaki et al. 2000; Liu and Zhang 2011). In 2009, the first protocol for 2D differentiation of pluripotent stem cells into MSNs was developed using dual-SMAD signalling inhibition, which was achieved by treating cells with the small molecules SB431542 and LDN193189/Noggin for BMP/TGFβ inhibition and induction ventral telencephalic specification (Chambers et al. 2009). This method allowed the quick generation of a large number of synchronously differentiated cells in feeder-free conditions. Additional components for the transition of pluripotent cells into neuroectodermal (NE) cells are purmorphamine and dorsomorphin, which increase the efficiency of neuronal induction (Hunt et al. 2017; Ma et al. 2012; Nekrasov et al. 2016) and inhibit BMP signalling (Arber et al. 2015; Nekrasov et al. 2016).
Neural induction is followed by the proliferation of neuronal progenitor cells and their subsequent terminal differentiation into MSNs (Arber et al. 2015; Hunt et al. 2017; Ma et al. 2012). Activation of the TGFβ/BMP signalling pathway is important for terminal differentiation into MSNs, which makes this step common for all differentiation protocols. Activin A plays a key role in forebrain neurogenesis induction, promoting cell differentiation towards lateral ganglionic eminence (precursor of the ventral striatum) (Abdipranoto-Cowley et al. 2009; Arber et al. 2015; Hunt et al. 2017). Another important component is the brain-derived neurotrophic factor (BDNF), which is necessary for MSN survival (Arber et al. 2015; Aubry et al. 2008; Baquet et al. 2004; Hunt et al. 2017; Jeon et al. 2012; Ma et al. 2012; Mattis et al. 2012; Nekrasov et al. 2016; Xu et al. 2017; Zuccato and Cattaneo 2007). Ascorbic acid also plays an important role in terminal differentiation (Hunt et al. 2017; Xu et al. 2017). Some components of differentiation media differ; for example, Nekrasov et al. added Forskolin (Nekrasov et al. 2016), activating adenylyl cyclase and increasing cAMP levels in cells, while other authors directly added cAMP (Aubry et al. 2008; Hunt et al. 2017; Ma et al. 2012; Mattis et al. 2012; Xu et al. 2017). Here, we present a novel protocol for differentiating pluripotent stem cells into GABAergic striatal neurons. The three-step protocol includes dual SMAD inhibition, subsequent progenitor cell cultivation and terminal differentiation into neurons. Neuronal progenitors undergo successful long-term cultivation and cryopreservation, during which time they maintain stage-specific markers. This benefit of the protocol is that it facilitates obtaining a large number of target neurons for high-throughput drug screening.
Materials and methods
Ethical statements
This study was approved by the Scientific Ethics Committee of Research Institute of Medical Genetics, Tomsk NRMC (protocol number 106; 27th June 2017). Written informed consent was obtained from the couple. The animals were conducted in an SPF vivarium according to the Guidelines for Manipulations with Experimental Animals and approved by the Ethics Committee of The Federal Research Center Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences, Novosibirsk (permit No. 22.4 by 30.05.2014).
Cultivation of iPSCs
iPSC lines were cultivated on mitotically inactivated mouse embryonic fibroblast layer (feeder layer) in iPSC-medium (Knockout DMEM (KoDMEM, Life Technologies), 15% Knockout serum replacement (Life Technologies), 10 ng/ml rhFGF basic (bFGF, StemCell Technologies), 1 × NEAA (Lonza), 1× GlutaMAX-I (Life Technologies), 0.1 mM 2-mercaptoethanol (2-mce, Sigma), 1× penicillin–streptomycin (pen/strep, Lonza)). TrypLE Express (TrypLE, Life Technologies) was used for iPSC disaggregation. ROCK inhibitor (10 μM Y-27632; R&D systems) was added to the culture medium following passage for the subsequent 24 h.
Differentiation of iPSCs into the MSNs
Before differentiation
iPSCs were seeded on hESC-qualified Matrix (Matrigel-ESQ, BD Biosciences) treated plates and cultivated in Essential 8™ Medium (Essential 8, Life Technologies) for 2 passages and detached by 0.5 mM EDTA (Life Technologies). iPSCs were cultured until 70–80% confluency. All stages of differentiation were performed in 5% CO2, 37 °C and humidity atmosphere.
The first step: obtaining NE derivatives
When iPSCs confluent reached 70–80%, medium was changed to neuronal differentiation medium (NDM) (F12/DMEM:Neurobasal (Life Technologies) 2:1, 1x N2 Supplement (N2, Life Technologies), 100 ng/ml LDN193189 hydrochloride (LDN, Sigma), 8 µM SB431542 inhibitor (SB, StemRD), 2 µM dorsomorphin (Dors, Sigma), 4 ng/ml bFGF, 1× pen/strep). This day was referred to as day 0 of differentiation (0 day). The cells were cultured for 5 days with daily medium refreshing. Next 7 days the medium was replaced to NDM without SB and Dors (NDM-SD). Purmorphamin (0.6 µM, Stemgent) was added from 1 day till 12–13 days.
The second step: prolonged cultivation of pMSNs
At 12d the NE cells were disaggregated with Accutase Cell Dissociation Reagent (Accutase, Life Technologies) and seeded in the ratio 1:2 on Matrigel-ESQ in NDM-SD medium supplemented with ROCK. The next 2 days the medium was changed to 1:1 mixture of NDM-SD:NeuroB. The NeuroB medium consisted of Neurobasal, 1× B-27 Supplement (B-27, Life Technologies), 20 ng/ml recombinant human BDNF (BDNF, PeptoTech), 1.1 mM ascorbic acid (Sigma), 1× pen/strep. At 15 days the medium was completely changed to NeuroB and the cells were cultured in a dense monolayer, passaged every 7–10 days at a density of 2.5–3.25 × 105 cells/cm2. Medium for cryopreservation of pMSNs consisted of 10% DMSO (Sigma), 10% fetal bovine serum (Life Technologies) and 80% NeuroB medium.
The third step: terminal differentiation of cells into MSNs
The pMSNs were passaged using Accutase and seeded at a density of 2–3 × 104 cells/cm2 on Matrigel-ESQ or poli-D-lisin/laminin (Sigma) coated plates in NeuroBC medium (Neurobasal, 1× B-27, 1× pen/strep, 20 ng/ml BDNF, 1.1 mM ascorbic acid, 25 ng/ml recombinant human/murine/rat Activin A (Activin A, Peprotech), 10 ng/ml recombinant human CNTF (CNTF, BioLegend), 0.5 mM dbcAMP (Selleckchem)). The medium was refreshed every other day. MSNs were cultured and maturated for 10–20 days in the NeuroBC medium.
Cell analysis by flow cytometry
Cell suspension was fixed in 1% paraformaldehyde (Sigma). For transcription factors, cell membrane was permeabilized in 0.2% Triton X-100 (Sigma) for 10 min, washed 1 time with PBS (Life Technologies) and immunoprecipitated with the first antibodies diluted in 0.2% Triton X100 overnight at 4 °C. For cytoplasmic markers, cell membrane was permeabilized for 30 min using 0.1% Saponin (Sigma) and then cells were incubated with the first antibodies diluted in 0.1% Saponin overnight at 4 °C.
Flow cytometry was performed by the BD FACSCanto II (BD Biosciences) using the BD FACSDiva Software. All measurements were made in at least three replicas. The antibodies sets used are presented in the S1 Table in Supplementary Information.
Immunofluorescent analysis
Immunofluorescent staining was performed according to the previously described protocols (Grigor’eva et al. 2019; Medvedev et al. 2011) with modifications. Permeabilization of cells was carried out using 0.5% Triton X100 for 30 min to detect transcription factors. Incubation with primary antibodies was performed at 4 °C overnight. The list of antibodies is presented in S1 Table in Supplementary Information. The visualization of the preparations was performed using the Nikon eclipse Ti-E microscope (Japan) and NIS Advanced Research software.
RNA isolation, RT-PCR and Real-time PCR
RNA isolation and RT-PCR were performed according to the previously described methods (Grigor’eva et al. 2015; Medvedev et al. 2011). Quantitative PCR was carried out using the reaction mixture containing SYBR Green I (No. M-427, Syntol) on a Light Cycler 480II (Roche). Relative gene expression levels were analyzed using the Comparative CT Method (ΔΔCT Method). The primer sets used are presented in Table 1 and S2 Table in Supplementary Information.
Table 1.
List of primer sequences used for positional specification analysis
| Gene | Forward | Reverse |
|---|---|---|
| SYP | CAATGCCTGCCTGAACAAAG | GGGTCCTAAACTGTCCTCTCTA |
| CALB1 | CCGAACGGATCTTGCTCTTAT | ACTCCCTTATAGTGCACAGTTATT |
| ARPP21 | CTGGATGAAGAGGAGAAACTGG | CCTGCTCCTGACTTGGATTT |
| GAD1 | AAACCGTGCAATTCCTCCTG | GCAACTGGTGTGGGTGATGA |
| DRD1 | CAACCTGAACTCGCAGATGAA | CAGAGTCTCACCGTACCTTAGT |
| DRD2 | CACTCCTCTTCGGACTCAATAAC | GACAATGAAGGGCACGTAGAA |
| MAP2 | TTCGTTGTGTCGTGTTCTCA | AACCGAGGAAGCATTGATTG |
| FOXP2 | CCAAAGCATCACCACCAATAAC | CTGTCTCGTCTTGCACTTAGAA |
| HPRT House-keeping gene | GACTTTGCTTTCCTTGGTCAGG | AGTCTGGCTTATATCCAACACTTCG |
Cell proliferation and survival assays
For cell proliferation analysis pMSN-1L were plated on Matrigel-treated 48-well plate at the equal density of 3 × 105 cells/cm2. Cell counting was performed at the 3, 5, 7, 9 and 11 days after plating, 3 wells for every time point.
For survival assay pMSNs were thawed and stained by trypan blue (Thermo Fisher Scientific) and counted using Countess Automated Cell Counter (Thermo Fisher Scientific).
Electron microscopy
Cells cultured on plastic film (Agar Scientific, UK) were fixed in 2.5% glutaraldehyde solution in culture medium for 15 min and then in 0.1 M sodium cacodylate buffer, pH-7.3 for 1 h at RT. Samples were washed three times in buffer and postfixed in 1% OsO4 buffer solution with the addition of several pellets K3[Fe(CN)6] for 1 h (19). After three times washing in ddH2O, cells were incubated in 1% uranyl acetate water solution overnight at 4 °C. After dehydration with ethanol and acetone, samples were embedded in epoxide resin Agar-100 and polymerised for 2 days at 60 °C. Ultrathin sections were obtained with a diamond knife at Ultracut ultratome (Reichert) and analyzed in JEOL1400 microscope (JEOL, Japan) at 80 кB.
Electrophysiology
For patch clamp recordings, NeuroBC medium was gradually replaced over the course of 1 h by recording/perfusing solution, which contained the following (in mM): 140 NaCl, 2.8 KCl, 10 HEPES, 1 MgCl2*6H2O, 2 CaCl2*2H2O, 10 D-(+)-Glucose; pH 7.2–7.4 adjusted with NaOH. The solution was oxygenated with 95% O2/5% CO2, perfused through the recording chamber at ~ 1 ml/min and maintained at ~ 35 °C. Whole cell patch clamp recordings were held through borosilicate glass pipettes (~ 5 MΩ, P-1000, Sutter Instruments, fire-polished) filled with internal solution containing the following (in mM): 10 NaCl, 140 C6H11KO7, 10 HEPES, 1 MgCl2*6H2O; pH 7.2–7.4 adjusted with KOH. Cell viability was determined by ramp stimulus applied in voltage clamp mode yielding one to several current spikes in live and active cells. After that, background activity of the cell was recorded. To evaluate total cell currents incrementing depolarizing stimuli were applied in voltage clamp mode ranging from − 60 mV to + 60 mV with 10 mV increase per step. All electrophysiological experiments were carried out with Molecular Devices Multiclamp 700B amplifier and Axon Instruments Digidata 1440A digitizer; data was extracted, analysed and visualized using Clampfit software.
Statistical analysis
Represent the mean ± standard error of the mean (SEM). Comparisons between groups were assessed using a one-way ANOVA with Fisher’s LSD post hoc analysis. Differences were considered statistically significant when p < 0.05.
Results
iPSC generation
First, we generated iPSCs from human embryonic fibroblasts using episomal vectors that encode the following pluripotency factors: OCT4, KLF4, L-MYC, SOX2, LIN28 and Trp53 (see Online Resource) (Okita et al. 2011; Yu et al. 2009). Four iPSC lines (iMA-1T, iMA-1L, iMA-17L, and iMA-23L) demonstrated high proliferative activity and grew in tight flat colonies that were similar in appearance to human embryonic stem cells on a feeder layer of mouse embryonic fibroblasts. The iPSCs had a stable karyotype and expressed pluripotency markers (Fig. S1 and S2). Additionally, the cells differentiated into cell types from each of the three germ layers during spontaneous differentiation in vitro (Fig. S3) and in vivo (Fig. S4).
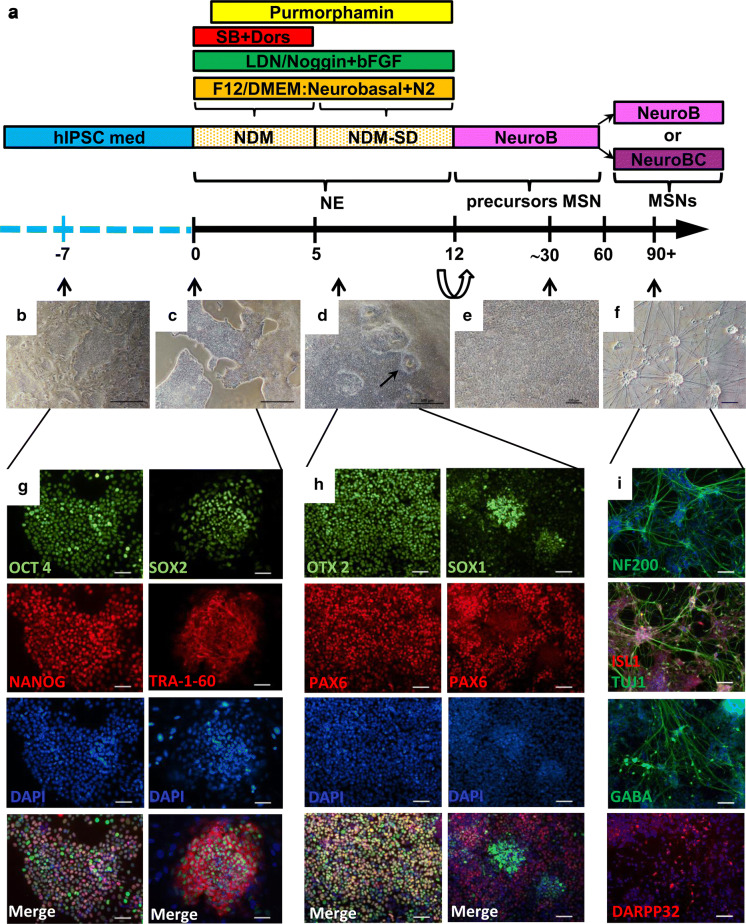
Our protocol was based on 2D cultivation of the cells in a dense monolayer. The differentiation media contained morphogens, which induced cells to specifically differentiate into striatal MSNs. The three main stages of the protocol are depicted in Fig. 1 which demonstrates the differentiation scheme (Fig. 1a), the cell morphology results (Fig. 1 b–f), and the immunofluorescent staining results (Fig. 1g–i).
Fig. 1.
IPSCs differentiate into MSNs. a Scheme of the differentiation protocol. b Morphology of iPSC colonies on the feeder layer. c Morphology of iPSC colonies on Matrigel. d Monolayer of NE cells; neural rosette-like structures (black arrow). e pMSN morphology. f MSNs. g Immunofluorescent staining of iPSCs for pluripotency markers: NANOG and SOX2 (green signal), and OCT3/4 and TRA-1-60 (red signal). h Immunofluorescent staining of NE cells on the 8th day of differentiation: SOX1 and OTX2 (green signal), and PAX6 (red signal). i Immunofluorescent staining of terminally differentiated MSNs. b–f Phase contrast images. The nuclei were counterstained with DAPI (blue signal). The scale bars are as follows: b, c, d 500 µm, e–i 100 µm
First step of neuronal differentiation—neural induction
Three iPSC clones, iMA-1T, iMA-1L and iMA-17L, were chosen for differentiation experiments. iPSCs were cultured on mouse embryonic fibroblasts (Fig. 1b), and cells were plated on a Matrigel-ESQ matrix before differentiation (Fig. 1c and g). NE differentiation of iPSCs was induced by dual SMAD inhibition and neural conversion by adding SB431542 and LDN193189 to the growth medium (Chambers et al. 2009). Furthermore, to increase differentiation efficiency and to promote ventralization (El-Akabawy et al. 2011; Ma et al. 2012) we added purmorphamine to the growth medium. After day 12 (the last day of the first stage of differentiation), we observed formations of neural rosette-like structures (Fig. 1d), which are indicators of successful NE differentiation. At this stage cells expressed early NE markers, such as PAX6, SOX1 and OTX2 (Fig. 1h), and they had the potential to differentiate into diverse neuronal cell types (Li et al. 2005).
Second step of neuronal differentiation—proliferating neuronal progenitor cells
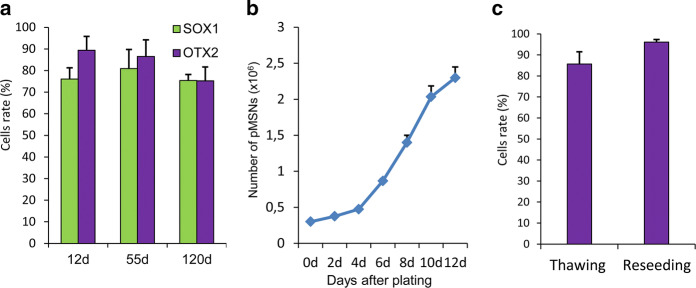
The next step of differentiation is the stage of neuronal progenitors of MSN (pMSN) (Fig. 2). At day 13, we reseeded cells on Matrigel-coated plates at a split ratio of 1:2, and we exchanged growth medium for NeuroB medium with brain-derived neurotrophic factor (BDNF) and ascorbic acid. These steps led to an intensive proliferation of OTX2- and SOX1-positive pMSNs (Fig. 2a). The expression level of the NE marker SOX1 in pMSNs varies insignificantly in the range of 89% at day 12 to 75% at day 120, and OTX2 expression was detected in approximately 75% of cells regardless of the differentiation day. Thus, we found that pMSNs have the ability to continuously grow in a dense monolayer (up to 2 × 106 cells per 1 cm2) and can undergo multiple passages for up to 180 days.
Fig. 2.
Proliferation and survival of pMSNs. a The number of OTX2- and SOX1-positive pMSNs on different days of differentiation. b Proliferation of pMSNs on the 85th day of differentiation after plating on Matrigel; cells were counted every other day. c Survival of pMSNs after reseeding and thawing
Proliferation intensity and cryopreservation of pMSN
To assess the possibility of large-scale production of MSNs, we analysed the proliferation intensity of the pMSNs at day 50 of differentiation. After seeding the cells at an equal density on Matrigel-treated plates, we calculated the number of pMSNs every other day for 12 days. It was found that the cells were able to multiply by more than 8 times during the 12-day experiment (Fig. 2b). Thus, an average doubling time of the pMSNs is 4 days.
PMSNs intensively proliferate and can be cryopreserved in the period from day 20 to day 180 of differentiation. To evaluate pMSN survival after thawing, we counted cells stained by trypan blue using an automatic cell counter. Living cells varied from 80 to 90%, which indicated good cell viability post-cryopreservation (Fig. 2c). Moreover, we evaluated pMSN viability after reseeding; cell survival was more than 95% (Fig. 2c). Thus, the cryopreservation and cultivation of precursor cells did not significantly affect cell viability, simplifying the differentiation procedure and enabling the generation of a large number of target neurons in a single step of terminal differentiation.
Third step—terminal differentiation
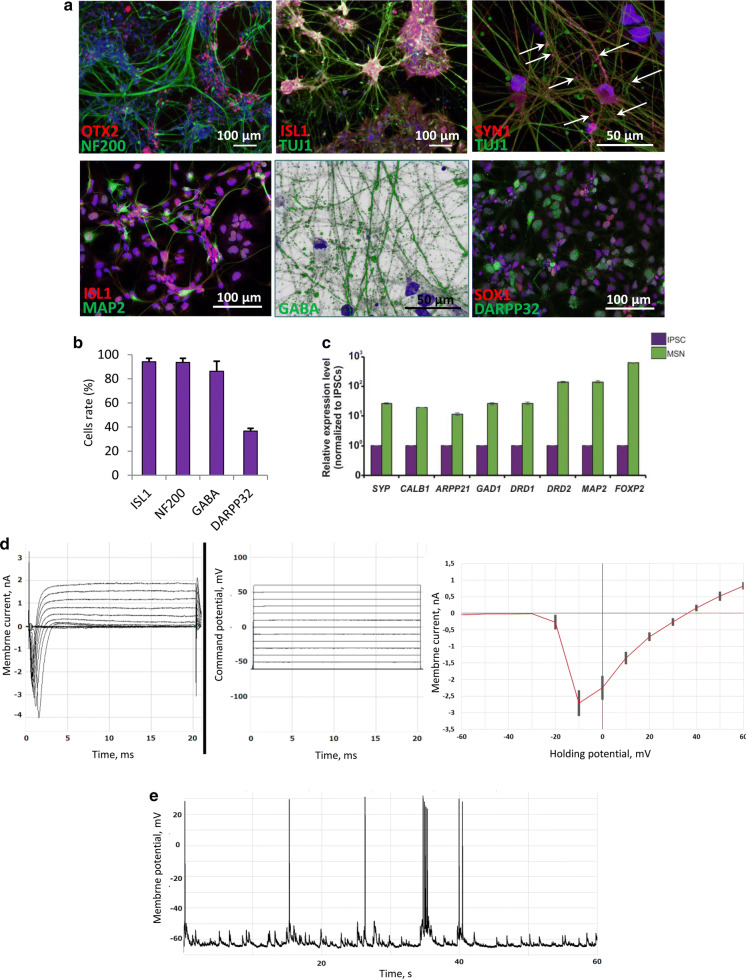
Terminal differentiation of pMSNs into MSNs was performed by reseeding of 1.5–2 × 104 cells per 1 cm2 on Matrigel-ESQ or poly-d-lysine/Laminin treated plates (Ma et al. 2008; Wang et al. 2015) in NeuroBC medium containing neurotrophic factors such as ciliary neurotrophic factor (CNTF), BDNF, Activin A and dibutyryl-cAMP (dbcAMP). We cultivated cells for 10–20 days in these conditions. We assessed the cells by immunostaining and FACS analysis and observed a high number that were positive for standard neuronal markers, such as TUJ1, NF200, and MAP2 as well as GABA, ISL1, and DARPP32, which are major MSN markers (Fig. 3a, b). Moreover, cells were positive for SYNAPSIN 1 (SYN1), which is responsible for the synapse formation between neurons. qRT-PCR showed the expression of the following MSN markers: ARPP21, CALB1, SYP, FOXP2, DRD1, and DRD2 (Fig. 3c).
Fig. 3.
Characteristic of terminal differentiated MSNs. a Immunostaining of MSNs derived from 70-day pMSNs (58 days at pMSN stage and 12 days at the terminal stage) the following: OTX2 (red signal), NF200 (green signal), ISL1 (red signal), TUJ1 (green signal), SYN 1 (red signal), MAP2 (green signal), GABA (green signal), SOX1 (red signal), and DARPP32 (green signal). The nuclei were counterstained with DAPI (blue signal). The white arrows indicate SYN 1 point signals. b FACS analyses of ISL1+, NF200+, GABA+ and DARPP32+ cells. c Expression of MSN markers SYP, CALB, ARPP21, GAD1, DRD1, DRD2, MAP2, and FOXP2 measured by qRT-PCR (n = 3 per cell type; values for independent biological replicates shown as the mean ± SEM). d Total currents across cell membrane elicited by incrementing command potential protocol (from − 60 mV to + 60 mV in 10 mV increments) next to an I–V diagram with standard deviation plotted. e An example of the functional activity of neurons derived from iPSCs. Spontaneous action potentials recorded by the whole cell patch clamp method. Average rest potential value is − 62.4 mV
Via implementation of patch clamp technique cells were revealed (n = 15) to exhibit voltage-dependent membrane currents (Fig. 3d) as well as were capable of resting and action potentials generation (Fig. 3e).
Ultrastructural characteristics of cells during differentiation into MSNs
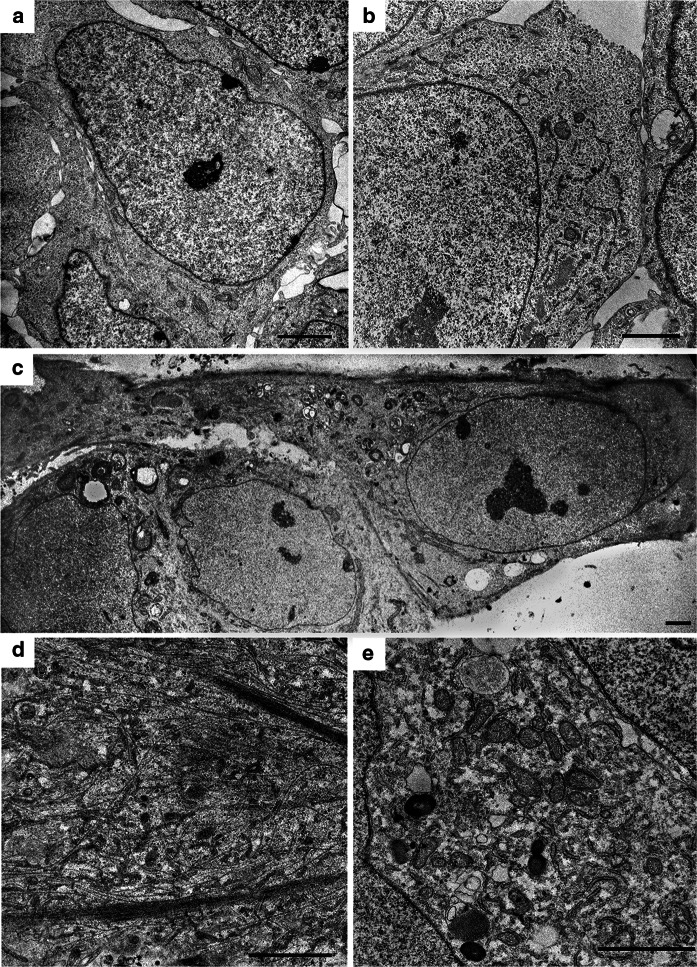
At every stage of differentiation from iPSCs to MSNs, we performed ultrastructural cell analysis. The analysis demonstrated dynamics in progressive morphological alterations of cells. Initially, we analysed iPSCs that were small and round, with large nuclei and few cytoplasm depleted of organells (Fig. 4a, b). Five days after the start of differentiation, the cells became larger and began to form short branches (processes); the cytoplasm became more enriched with organelles (Fig. 4c). At day 12–13 of differentiation (early precursor stage), we observed free and bundled neurofilaments present in cells (Fig. 4d). Moreover, there was an increase in the number of mitochondria, short cisterns of endoplasmic reticulum, numerous intermediate filaments and autolysosomes (Fig. 4e).
Fig. 4.
Ultrastructural transformation of the cells during differentiation. a An overview of a pluripotent cell containing a large nucleus surrounded by a narrow layer of cytoplasm. b Part of a pluripotent cell at higher magnification showing the following: mitochondria, short ER cisternae and cytoplasm with organelles. c An overview of cells on the 5th day of differentiation: triangular cells were observed with an emerging branch and cytoplasm filled with organelles (on the right). d A branch fragment of the cell after a first stage of differentiation (12–13 days) with free-lying and bundled neurofilaments. e A part of the cytoplasm on day 12–13 with a high density of mitochondria, ER cisterns, and autolysosomes. The scale bar is 2 µm
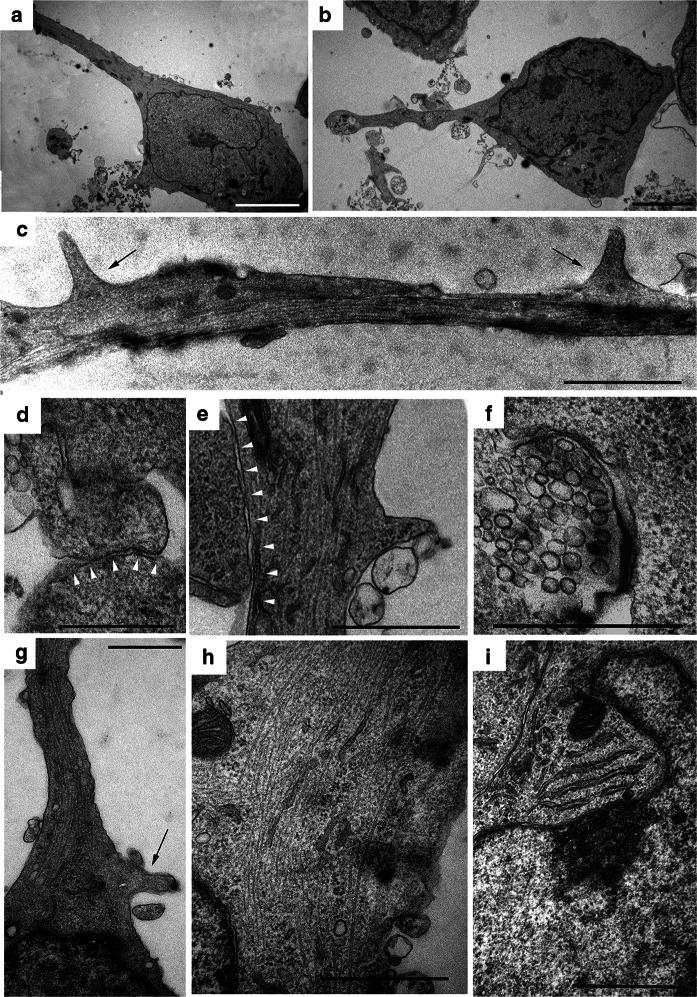
Finally, we analysed the ultrastructure of MSNs. Cells exhibited a morphology specific to mature neurons (Fig. 5). MSNs also featured smaller sizes and elongated shapes with long branches (Fig. 5a–c and g) that formed synapses with synaptic vesicles (Fig. 5d–f). We also observed neurofilaments (Fig. 5h), Nissl bodies (Fig. 5i) and numerous spines at dendrites and near the synapses, all of which are specific for MSNs (Fig. 5c, e and g).
Fig. 5.
Ultrastructural organization of spiny neurons derived from iPSCs. a, b An overview of neurons: the long branches are visible. c Two spines (black arrows) on the axon. d, e Synaptic connection between two different neurons (the synaptic cleft is marked by white arrowheads). f Synaptic vesicles in the terminal bulb. g A fragment of a neuron with a spine in the axon formation zone (in the region of the axon hillock). h Neurofilaments in a neuron. i Rough ER cisterns form Nissl bodies in the cytoplasm of a neuron. The scale bars are as follows: a–c 5 µm, d–i 1 µm
Discussion
Animal models and patient-derived post-mortem tissues are usually used in studies of Huntington’s disease. As an alternative approach, iPSC-derived medium spiny neurons can be created. Therefore, the development of an efficient and reproducible iPSC differentiation protocol is an actual problem. Although there are some different protocols for iPSC differentiation into MSNs (Arber et al. 2015; Aubry et al. 2008; Hunt et al. 2017; Jeon et al. 2012; Ma et al. 2012; Mattis et al. 2012; Nekrasov et al. 2016), the opportunity for scalability and cryopreservation of large-scale amounts of cells at the MSN progenitor stage has never been reported. We believe that our protocol has several advantages. First, it lacks a stage of co-cultivation with mitotically inactivated murine bone marrow-derived stromal feeder cells MS5. Another advantage is absence of the laborious stage of manual collection of rosette-like structures. In this study, we developed a new three-step protocol for human iPSC differentiation into striatal MSNs that allows prolonged cultivation and cryopreservation of MSN progenitors. We showed that cryopreservation of pMSN does not affect their viability and allows us to differentiate pMSN into terminal neurons in one step after thawing. This greatly simplifies the experiment.
The first stage of iPSC differentiation was treatment with dual SMAD inhibition, which is a common part of many protocols. The dual SMAD inhibition prevented cell differentiation towards meso- and endodermal cell types (Chambers et al. 2009). The inhibition was performed by adding SB431542 and LDN193189, which suppressed the Activin/TGFβ and BMP pathways, respectively (Elkabetz et al. 2008; Lee et al. 2007). The inhibition of these pathways leads to the formation of neuroepithelial cells. Immunofluorescence staining and flow cytometry showed the expression of NE markers, such as PAX6 and SOX1, in NE cells on day 8 (Fig. 1h). In addition, we observed the formation of neural rosette-like structures, which recapitulates neural tube formation in vitro (Fig. 1d) (Wilson and Stice 2006). We also detected alterations in the cell morphology by electron microscopy (Fig. 5).
During embryo development, the sonic hedgehog (SHH) concentration gradient in the dorsoventral direction promotes the induction of individual cell specifications in the neural tube (Ericson et al. 1995), and the exact concentration of SHH is critical for the correct induction of cell specification in forebrain cells (Ma et al. 2012). Thus, further regional cell specification was induced by SHH pathway activation using purmorphamine (an agonist of the SHH signalling pathway). Moreover, we used bFGF as a neuronal survival factor (Gage et al. 1995), and dorsomorphin for additional BMP signalling inhibition. We confirmed the co-expression of the forebrain markers OTX2, PAX6 and SOX1 in NE cells by immunostaining (Fig. 1h).
At the next stage of differentiation, we obtained MSN progenitor cells by replating NE cells and removing inhibitors and morphogens. We cultivated the progenitors in medium supplemented only with BDNF and ascorbic acid. Ascorbic acid plays an important role in the formation and maintenance of the neuronal microenvironment, enhances the expression of genes involved in neurogenesis, and has a neuroprotective effect (Haramoto et al. 2008). BDNF increases the survival rate of progenitors and MSNs by activating anti-apoptotic pathways (Alcalá-Barraza et al. 2010). The actively proliferating progenitors were able to undergo many passages (more than 20 passages) and could be cultured at a high density (up to 2 × 106 cells/cm2) without loss of marker expression (Fig. 2a). Additionally, MSN progenitors successfully underwent cryopreservation (Fig. 2c). All of these results allows great simplification of the large-scale production of MSN populations that is required for high-throughput drug screening and toxicological studies.
The last stage is terminal differentiation into MSNs, which is achieved by adding a cocktail of inhibitors and neurotrophic growth factors in the MSN progenitor medium. The medium contained Activin A [an activator of the TGFβ/BMP signalling pathway that promotes differentiation into DARPP32-positive neurons (Abdipranoto-Cowley et al. 2009; Arber et al. 2015; Hunt et al. 2017)], dibutyrylcyclic AMP [dbcAMP, an analogue of cAMP that significantly increases the neuronal output (López-Toledano et al. 2004; Mena et al. 1995; Sharma et al. 1990; Tojima et al. 2003; Zahir et al. 2009)], and CNTF [an inhibitor of pro-apoptotic signalling (Alcalá-Barraza et al. 2010)]. We found that over 90% of the cells were GABA, ISL1 and NF200 positive. ISL1 and GABA expression, indicates that the cells are derivatives of lateral ganglionic eminence, which is the precursor of ventral striatum (Skogh et al. 2003). We found increased expression of MAP2 (a common marker of mature neurons); SYP, which encodes synaptophysin presynaptic marker; and GAD1, encoding glutamate decarboxylase 1, which synthesizes the MSN mediator GABA (Fig. 3c). In addition, we found high expression levels of the main striatal markers, such as ARPP21 (which plays a central role in the integration of key signals of neurotransmitters to the MSNs through regulation of calmodulin-dependent kinase I and protein phosphatase-2B), CALB1 (calbindin, which is one of the main calcium-binding and buffer proteins and plays an important role in preventing the death of neurons, as well as in maintaining calcium homeostasis), FOXP2 (which is a transcription factor involved in the positive regulation of the differentiation of MSNs from the lateral ganglionic eminence cells and in the negative regulation of the formation of interneurons from the cells of the dorsal medial ganglionic eminence through interaction with the SHH signalling pathway), and DRD1 and DRD2, which are the main types of dopamine receptors in the striatum and, accordingly, divide the striatal MSN population into two subpopulations—D1- and D2-MSNs (Fig. 3c).
MSNs are tightly involved with vast array of functions including motor control, habit formation and motivated behavior (Chuhma et al. 2011). One of the most distinct and crucial features behind neuronal operation is the capability to generate and maintain potentials. AP generation requires chain of specific ion channels to be developed as well as finely tuned ion homeostasis system to be functional. The cells obtained demonstrated the ability to both maintain resting potential and generate APs. These processes are mediated by voltage sensitive ion channels complex activity, demonstrated in voltage clamp recordings.
Moreover, we first performed a step-by-step ultrastructural analysis of the cells during all differentiation processes. We found a gradual formation of neuron progenitors and then neurons from iPSCs. MSNs had round shape nucleus with a few indentations surrounded by cytoplasm containing Nissl bodies, neurofilaments and long processes with spines, which are typical for MSNs (Fig. 5). The ultrastructural analysis expands the understanding of subtle changes occurring during in vitro differentiation that reproduce the development of nervous tissue during in vivo embryogenesis. Electron microscopy is also indispensable for studying ultrastructural defects associated with different stages of neurodegenerative disease development (Morozova et al. 2018).
Conclusion
In this study, we established a protocol for efficient stepwise iPSC differentiation into GABAergic striatal neurons that enables long-term cultivation of neuronal precursors and multifold increases in MSN output. We confirmed that differentiated cells expressed general neuronal markers and MSN-specific markers and demonstrated specific electrophysiological activities. In addition, for the first time, we investigated the ultrastructural organization of the cells at every differentiation step. We believe that MSNs obtained by our protocol can serve as an adequate in vitro model for studying Huntington’s disease and other forms of neurodegeneration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The study was supported by the Russian Science Foundation (Project No 16-15-10128). The microscopy was conducted at the Joint Access Center for Microscopy of Biological Objects with the Siberian Branch of the Russian Academy of Sciences. We thank S. I. Bayborodin for the technical assistance. Teratoma test is implemented using the equipment of the Center for Genetic Resources of Laboratory Animals at ICG SB RAS, supported by the Ministry of Education and Science of Russia (Unique identifier of the project RFMEFI62117X0015). Biology material was provided by Research Institute of Medical Genetics, Tomsk NRMC.
Abbreviations
- BDNF
Brain derived neurotrophic factor
- iPSCs
Induced pluripotent stem cells
- MSN
Medium spiny neuron
- NDM
Neuronal differentiation medium
- NE
Neuroectodermal
- pMSN
Precursor of medium spiny neuron
Author contribution statement
Suren M. Zakian, Elena V. Grigor’eva, Igor N. Lebedev and Anastasia A. Malakhova conceived and designed the experiments. Elena V. Grigor’eva and Aizhan Surumbayeva reprogrammed and differentiated the cells. Elena V. Grigor’eva, Sophia V. Pavlova and Anastasia A. Malakhova performed immunofluorescent analysis and cell analysis by flow cytometry. Tuyana B. Malankhanova performed transfection experiments, RNA isolation, RT-PCR and qRT-PCR. Julia M. Minina performed cytogenetic analysis. Elena A. Kizilova performed teratoma assay. Lyubov A. Suldina, Ksenia N. Morozova and Elena Kiseleva performed electron microscopy. Evgeny D. Sorokoumov performed electrophysiology. Elena V. Grigor’eva, Tuyana B. Malankhanova and Anastasia A. Malakhova wrote the main manuscript text. All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elena V. Grigor’eva and Tuyana B. Malankhanova contributed equally to this work.
Contributor Information
Elena V. Grigor’eva, Email: evlena@bionet.nsc.ru
Tuyana B. Malankhanova, Email: tbmalankhanova@gmail.com
Aizhan Surumbayeva, Email: a.surumbayeva@mail.ru.
Sophia V. Pavlova, Email: spav@bionet.nsc.ru
Julia M. Minina, Email: minina_jul@bionet.nsc.ru
Elena A. Kizilova, Email: pinus@bionet.nsc.ru
Lyubov A. Suldina, Email: suldinalubov@gmail.com
Ksenia N. Morozova, Email: morozova.kn@gmail.com
Elena Kiseleva, Email: ekiseleva11@gmail.com.
Eugeny D. Sorokoumov, Email: eg.sorokoumov@gmail.com
Igor N. Lebedev, Email: igor.lebedev@medgenetics.ru
Suren M. Zakian, Email: zakian@bionet.nsc.ru
Anastasia A. Malakhova, Email: amal@bionet.nsc.ru
References
- Abdipranoto-Cowley A, Park JS, Croucher D, Danie J, Henshall S, Galbraith S, Mervin K, Vissel B. Activin A is essential for neurogenesis following neurodegeneration. Stem Cells. 2009;27:1330–1346. doi: 10.1002/stem.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18:179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodríguez TA, Rosser AE, Dunnett SB, Li M. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Akabawy G, Medina LM, Jeffries A, Price J, Modo M. Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the hedgehog pathway. Stem Cells Dev. 2011;20:1873–1887. doi: 10.1089/scd.2010.0282. [DOI] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Jessell TM, Edlund T. Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int J Dev Biol. 1995;39:809–816. [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grigor’eva EV, Shevchenko AI, Medvedev SP, Mazurok NA, Zhelezova AI, Zakian SM. Induced pluripotent stem cells of Microtus levis x Microtus arvalis vole hybrids: conditions necessary for their generation and self-renewal. Acta Naturae. 2015;7:56–69. doi: 10.32607/20758251-2015-7-4-56-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigor’eva EV, Malankhanova TB, Surumbayeva A, Minina JM, Morozov VV, Abramycheva NY, Illarioshkin SN, Malakhova AA, Zakian SM. Generation of induced pluripotent stem cell line, ICGi007-A, by reprogramming peripheral blood mononuclear cells from a patient with Huntington’s disease. Stem Cell Res. 2019 doi: 10.1016/j.scr.2018.101382. [DOI] [PubMed] [Google Scholar]
- Haramoto M, Tatemoto H, Muto N. Essential role of ascorbic acid in neural differentiation and development: high levels of ascorbic acid 2-glucoside effectively enhance nerve growth factor-induced neurite formation and elongation in PC12 Cells. J Health Sci. 2008;54:43–49. doi: 10.1248/jhs.54.43. [DOI] [Google Scholar]
- Hunt CPJ, Pouton CW, Haynes JM. Characterising the developmental profile of hESC-derived medium spiny neuron progenitors and assessing mature neuron function using a CRISPR-generated human DARPP-32WT/eGFP-AMP reporter line. Neurochem Int. 2017;106:3–13. doi: 10.1016/j.neuint.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Jeon I, Lee N, Li JY, Park IH, Park KS, Moon J, Shim SH, Choi C, Chang DJ, Kwon J, Oh SH, Shin DA, Kim HS, Do JT, Lee DR, Kim M, Kang KS, Daley GQ, Brundin P, Song J. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells. 2012;30:2054–2062. doi: 10.1002/stem.1135. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang S. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Toledano MA, Redondo C, Lobo MV, Reimers D, Herranz AS, Paíno CL, Bazán E. Tyrosine hydroxylase induction by basic fibroblast growth factor and cyclic AMP analogs in striatal neural stem cells: role of ERK1/ERK2 mitogen-activated protein kinase and protein kinase C. J Histochem Cytochem. 2004;52:1177–1189. doi: 10.1369/jhc.3A6244.2004. [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis VB, Svendsen SP, Ebert A, Svendsen CN, et al. Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev SP, Grigor’eva EV, Shevchenko AI, Malakhova AA, Dementyeva EV, Shilov AA, Pokushalov EA, Zaidman AM, Aleksandrova MA, Plotnikov EY, Sukhikh GT, Zakian SM. Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem Cells Dev. 2011;20:1099–1112. doi: 10.1089/scd.2010.0249. [DOI] [PubMed] [Google Scholar]
- Mena MA, Casarejos MJ, Bonin A, Ramos JA, García Yébenes J. Effects of dibutyryl cyclic AMP and retinoic acid on the differentiation of dopamine neurons: prevention of cell death by dibutyryl cyclic AMP. J Neurochem. 1995;65:2612–2620. doi: 10.1046/j.1471-4159.1995.65062612.x. [DOI] [PubMed] [Google Scholar]
- Morozova KN, Suldina LA, Malankhanova TB, Grigoreva EV, Zakian SM, Kiseleva E, Malakhova AA. Introducing an expanded CAG tract into the huntingtin gene causes a wide spectrum of ultrastructural defects in cultured human cells. PLoS ONE. 2018;13:e0204735. doi: 10.1371/journal.pone.0204735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov ED, Vigont VA, Klyushnikov SA, Lebedeva OS, Vassina EM, Bogomazova AN, Chestkov IV, Semashko TA, Kiseleva E, Suldina LA, Bobrovsky PA, Zimina OA, Ryazantseva MA, Skopin AY, Illarioshkin SN, Kaznacheyeva EV, Lagarkova MA, Kiselev SL. Manifestation of Huntington’s disease pathology in human induced pluripotent stem cell-derived neurons. Mol Neurodegene. 2016;11:1–15. doi: 10.1186/s13024-016-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hansen J, Notte M. Effects of NGF and dibutyryl cAMP on neuronal differentiation of embryonal carcinoma cells. Int J Dev Neurosci. 1990;8:33–45. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Skogh C, Parmar M, Campbell K. The differentiation potential of precursor cells from the mouse lateral ganglionic eminence is restricted by in vitro expansion. Neuroscience. 2003;120:379–385. doi: 10.1016/s0306-4522(03)00427-5. [DOI] [PubMed] [Google Scholar]
- Tojima T, Kobayashi S, Ito E. Dual role of cyclic AMP-dependent protein kinase in neuritogenesis and synaptogenesis during neuronal differentiation. J Neurosci Res. 2003;74:829–837. doi: 10.1002/jnr.10754. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo X, Leighton J. Extracellular matrix and integrins in embryonic stem cell differentiation Biochem Insights. 2015;8:15–21. doi: 10.4137/BCI.S30377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PG, Stice SS. Development and differentiation of neural rosettes derived from human embryonic stem cells. Stem Cell Rev. 2006;2:67–77. doi: 10.1385/SCR:2:1:67. [DOI] [PubMed] [Google Scholar]
- Xu X, Tay Y, Sim B, Yoon SI, Huang Y, Ooi J, Utami KH, Ziaei A, Ng B, Radulescu C, Low D, Ng AYJ, Loh M, Venkatesh B, Ginhoux F, Augustine GJ, Pouladi MA. Reversal of phenotypic abnormalities by CRISPR/Cas9-mediated gene correction in Huntington disease patient-derived induced pluripotent stem cells. Stem Cell Reports. 2017;8:619–633. doi: 10.1016/j.stemcr.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–802. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir T, Chen YF, MacDonald JF, Leipzig N, Tator CH, Shoichet MS. Neural stem/progenitor cells differentiate in vitro to neurons by the combined action of dibutyryl cAMP and interferon-gamma. Stem Cells Dev. 2009;18:1423–1432. doi: 10.1089/scd.2008.0412. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.