Abstract
Introduction
This study sought to compare healthcare resource utilization (HCRU), costs, and workplace productivity among patients with depression, with and without overactive bladder (OAB).
Methods
This retrospective, case–control cohort analysis compares HCRU, costs, and workplace productivity among propensity score matched patients with depression and OAB (case cohort) and patients with depression without OAB (control cohort). Patients were aged 18 years or older, insured/on Medicare, and had diagnosed depression and an antidepressant medication claim pre index. First OAB-related event was index for cases; controls were assigned a proxy (study period 12 months). Comparisons of HCRU and costs and regression models assessed the relationship between OAB and costs. For the workplace productivity subset analyses cases and controls were balanced on baseline covariates for the short-term disability analyses but as they were unbalanced for the absentee analyses, multivariate regression analyses were used for this subset.
Results
The study criteria were met by 39,085 cases and 308,736 controls, from which, 37,997 patients were successfully matched 1:1 (mean age 55 years; 81% female). Most depression-related HCRU measures were similar across cohorts; however, outpatient visits, ER visits, and number of unique depression medications were significantly higher (all p < 0.05) among cases. Cases also had 13% higher total depression-related costs (p < 0.0001). Total mean (standard deviation [SD]) depression-related costs were $1796 ($4235) for cases versus $1597 ($3863) for controls (p < 0.0001). For workplace productivity (absentee data: cases [n = 686], controls [n = 642]; short-term disability data: cases [n = 4395], controls [n = 4433]) absentee outcomes were similar across cohorts. However, a higher percentage of cases used short-term disability benefits compared to controls (21.3% versus 16.9%; p < 0.0001) and cases experienced more case days (11.0 versus 8.6 mean days) and received higher mean payments than controls ($1226 versus $1033; p < 0.0001) in this subset.
Conclusions
OAB was associated with 13% higher depression-related costs and 4.4% more cases used short-term disability benefits.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01485-w) contains supplementary material, which is available to authorized users.
Keywords: Depression, Costs, Healthcare resource utilization, Major depressive disorder, OAB, Overactive bladder, Urology
Key Summary Points
| Why carry out this study? |
| Major depressive disorder is a serious, recurrent disorder affecting millions of Americans. Studies have linked depression and overactive bladder, another highly prevalent condition, but little is known about the specific impact of these conditions co-occurring. |
| This study sought to compare the healthcare costs, healthcare resource utilization (HCRU), and workplace productivity among patients with depression and co-occurring overactive bladder (OAB) and those with depression alone. |
| What was learned from the s.tudy? |
| This study found that OAB was associated with 13% higher depression-related costs and 4.4% more cases used short-term disability benefits. |
| Comorbidity of OAB on patients with depression impacted direct HCRU and healthcare costs and had some impact on indirect costs measured by short-term disability claims utilized in the workplace. |
| The observed impact of OAB on patients with depression indicates a challenge for the medical community in the management of comorbid patients across the healthcare system. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12853835.
Introduction
Major depressive disorder (MDD) is a serious, recurrent disorder affecting 17.3 million adults in the USA in 2017 [1]. The condition is linked to diminished role functioning and quality of life, medical morbidity and mortality, and is characterized by depressed mood, diminished interests, impaired cognition, and vegetative symptoms [2, 3]. It occurs most often in young adults 25–34 years of age and more frequently in women than men [4]. The economic burden of the condition is considerable; Greenberg et al. estimated the burden of MDD in the USA to be $210.5 billion in 2010 [5]. A substantial part of that figure is the result of comorbid conditions; findings from the same study indicated that 62% of the burden of MDD could be attributed to comorbidity [5].
Overactive bladder syndrome (OAB) is defined as urinary urgency, generally with frequency and nocturia with or without urgency urinary incontinence [6]. It is a condition that increases with age; prevalence estimates for the general population are 16.5% but rise to over 30% in those aged 65 years or older [7]. In addition, OAB has been found to affect twice as many women as men [8]. It has been previously shown that OAB is a cost multiplier when comorbid with conditions like dementia [9] and osteoporosis [10]. Several biological and epidemiological studies have shown an association between depression and urinary incontinence, a symptom of OAB [11, 12]. Studies of health-related quality of life (HRQoL) in OAB have reported participants expressing feelings of isolation, hopelessness, and depression resulting from anxiety over the symptoms of their condition, as well as feelings of depression resulting from exhaustion and fatigue as a result of sleep interrupted by nocturia [13, 14]. OAB affects more than 37 million adults in the USA [7, 15] and poses a substantial burden to the healthcare system, with a projected cost of $82.6 billion in 2020 [15]. The syndrome is an often overlooked comorbid condition among patients with depression, and patients with both depression and OAB may experience a compounding of the high cost of care associated with each separate condition [11].
An association between urinary incontinence and depression, especially among women, has been denoted in several studies, and outcomes related to OAB and depression have been explored independently within the literature [16]. Little is known, however, about the specific impact of co-occurring OAB among patients with depression. Given the prevalence of both conditions and the potential for poor outcomes for patients resulting from their comorbidity, a better understanding of how these conditions interact is needed. How costs and workplace productivity are affected by the conditions co-occurring can address part of that knowledge gap. This study characterizes the healthcare costs, healthcare resource utilization (HCRU), and workplace productivity among two cohorts of patients with depression, those with and those without OAB.
Methods
Data Source
This study was conducted using data from the IBM MarketScan Commercial Claims and Encounters Database, the IBM MarketScan Medicare and Supplemental and Coordination of Benefits Database, and the MarketScan Health and Productivity Management Database. The Commercial database contains longitudinal medical and drug information, including paid amounts, for several million individuals (including spouses and dependents) across multiple employer-sponsored private health insurance plans. The Medicare supplemental database contains claims data for retirees with Medicare supplemental insurance through employers and includes approximately 3 million individuals annually. The MarketScan Health and Productivity database includes data on workplace absence, short-term disability, and worker’s compensation for a subset of enrollees in the Commercial database. MarketScan databases are Health Insurance Portability and Accountability Act (HIPAA) compliant. This study was conducted using anonymized patient data from MarketScan and as such review board approval and patient consent were not necessary.
Study Design/Search Strategy
This was a retrospective cohort analysis of HCRU, costs, and workplace productivity comparing a cohort of individuals with depression and OAB (the case cohort) to a propensity score matched cohort with depression but no OAB (the control cohort). Cohorts were constructed from a prevalent population of patients with depression (identified on the basis of medical and prescription claims for depression, Appendix 1 in the electronic supplementary material, ESM). The first diagnosis of OAB (or date of first prescription for an OAB medication) served as index for the case cohort (“OAB index date”). For patients with depression and without OAB, a proxy index date was constructed using the Harvey et al. [17] method selected randomly from within an April 1, 2012 to December 31, 2015 identification period. The 6-month period prior to the index date was the pre-index (baseline) period and the 12-month period following the index date was the post-index period. The full study period was October 1, 2011 to December 31, 2016.
Individuals in both cohorts were required to be at least 18 years of age with continuous insurance enrollment (in the pre-index through the post-index periods) and have a diagnosis of depression during an October 1, 2011 to December 31, 2015 identification period. Depression was identified on the basis of the presence of a depression diagnosis code on one inpatient claim or two outpatient claims AND one or more prescription claims for an antidepressant medication with first depression identification date (diagnosis or treatment) occurring prior to the index date. For the case cohort, an antidepressant medication prescription claim had to occur within 1 month prior or 1 month following the OAB index date; for the control cohort, within 1 month prior or 1 month following the proxy index date. Patients with OAB were identified using criteria similar to those of previously published studies of patients with OAB [9, 10]. Included were patients with the presence of at least one inpatient or two outpatient claims for an OAB diagnosis (International Classification of Diseases [ICD]-9 or ICD-10; Appendix 1 in ESM) or a prescription claim for a medication indicated almost exclusively to treat OAB (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, trospium, mirabegron; patients who may have been treated for other indications of these medications (i.e., neurogenic bladder) were excluded). A complete list of the ICD-9 and ICD-10 diagnosis codes and prescription drug codes associated with depression and OAB, respectively, is provided in Appendix 1 in ESM.
Patients were excluded if they had a diagnosis or procedure code indicative of pregnancy, malignant neoplasms (cancer), renal impairment, hepatic insufficiency, or organ transplantation during the pre- or post-index observation period or a diagnosis of neurogenic bladder, or physical trauma during pre-index. A diagnosis for bipolar depression, or psychosis, schizophrenia, other psychosis-related disorders, paranoid states, other mood disorders, drug-induced depression, Alzheimer’s disease, Parkinson’s disease, or dementia during the pre- or post-index observation period was also cause for exclusion, as was a long-term care or hospice stay in the pre-index period.
Measures
Depression-related HCRU measures included inpatient admissions (acute and non-acute), total bed days per patient, outpatient visits (excluding physician office and emergency room [ER] visits), physician office visits, and ER visits. Claims were considered depression-related if there was a primary diagnosis for MDD in the medical claim (Appendix 1 in ESM). Depression-related pharmacy utilization was defined as the total number of unique medications indicated for treatment of depression (Appendix 1 in ESM). Healthcare costs were captured as per patient costs over the 12-month post-index period and reported costs include depression-related total costs, total medical costs, and total pharmacy costs. Total depression-related medical costs included costs incurred during inpatient admissions (acute and non-acute), outpatient (excluding physician office and ER visits) physician, and ER visits. Depression-related pharmacy costs were calculated using outpatient pharmacy claims. For workplace productivity, absentee measures presented were total time absent from work as well as the proportion with each absence type (sick, leave, disability, recreational). For short-term disability, measures presented were total case days and total payments among all patients and for those using short-term disability.
Covariates and Other Measures
Demographic and enrollment characteristics of the patient population, including age, sex, geographic region, and health plan, were described during the pre-index period. Clinical characteristics described during this period included the Charlson Comorbidity Index, based on the Quan enhanced ICD-9 and ICD-10 set [18] and the number of Elixhauser comorbidities [18]. Baseline depression treatment characteristics described included types of treatments (selective serotonin reuptake inhibitor [SSRI], serotonin–norepinephrine reuptake Inhibitor [SNRI], atypical antidepressant, atypical antipsychotic, lithium, other antidepressants), cumulative days’ supply of any antidepressant drug, and total number of unique treatments for depression observed per patient. The full list of propensity score matching variables is indicated in Table 1.
Table 1.
Select baseline characteristics
| Pre-match | Post-match | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case N = 39,085 |
Control N = 308,736 |
p value* | SDIFF | Case N = 37,997 |
Control N = 37,997 |
p value* | SDIFF | |||||
| Demographic characteristics | ||||||||||||
| Age, years**, mean (SD) | 55.0 | (14.7) | 46.4 | (14.6) | < 0.0001 | 0.5883 | 54.7 | (14.6) | 55.0 | (14.3) | < 0.0001 | − 0.0253 |
| Sex, female**, n (%) | 31,807 | (81.4%) | 215,526 | (69.8%) | < 0.0001 | − 0.065 | 30,836 | (81.2%) | 31,284 | (82.3%) | < 0.0001 | − 0.0039 |
| Geographic region**, n (%) | ||||||||||||
| North Central | 9828 | (25.1%) | 73,135 | (23.7%) | < 0.0001 | 0.0848 | 9560 | (25.2%) | 9481 | (25.0%) | 0.6531 | 0.0114 |
| Northeast | 7598 | (19.4%) | 57,119 | (18.5%) | 7357 | (19.4%) | 7448 | (19.6%) | ||||
| South | 16,311 | (41.7%) | 127,107 | (41.2%) | 15,837 | (41.7%) | 15,927 | (41.9%) | ||||
| West | 5218 | (13.4%) | 50,123 | (16.2%) | 5115 | (13.5%) | 5024 | (13.2%) | ||||
| Unknown | 130 | (0.3%) | 1252 | (0.4%) | 128 | (0.3%) | 117 | (0.3%) | ||||
| Clinical characteristics, mean (SD) | ||||||||||||
| Baseline Quan-Charlson Comorbidity Index score | 0.26 | (0.66) | 0.12 | (0.44) | < 0.0001 | 0.252 | 0.24 | (0.62) | 0.22 | (0.59) | 0.0025 | 0.0209 |
| Baseline Elixhauser count | 1.0 | (1.3) | 0.6 | (0.9) | < 0.0001 | 0.2942 | 0.9 | (1.2) | 0.9 | (1.2) | <0.0001 | 0.0346 |
| Hypertension uncomplicated, n (%) | 6280 | (16.1%) | 27,209 | (8.8%) | < 0.0001 | 0.2211 | 5838 | (15.4%) | 5575 | (14.7%) | 0.0061 | 0.0194 |
| Diabetes uncomplicated, n (%) | 3321 | (8.5%) | 13,425 | (4.3%) | < 0.0001 | 0.1698 | 3096 | (8.1%) | 3023 | (8.0%) | 0.3197 | 0.0071 |
| Time from depression identification date to OAB index (or proxy index), days, mean (SD) | 567.3 | (358.9) | 458.1 | (328.2) | < 0.0001 | 0.3174 | 563.2 | (357.9) | 567.7 | (360.9) | 0.0634 | − 0.0128 |
SDIFF standardized difference
*p value calculated by chi-squared for categorical variables and t test or Wilcoxon test on the basis of the distribution of the variable (statistical significance is denoted by bold text)
**Variables included in propensity score model
Statistical Analysis
For HCRU analysis, propensity score matching was used to balance the case and control cohorts on a range of important baseline characteristics without the level of attrition that may result from an exact matching process on a large set of variables. Patients with depression and OAB (cases) and patients with depression without OAB (controls) were propensity score matched in a 1:1 ratio. Prior to matching, imbalance existed (standardized difference ≥ 0.1) between cohorts in 29 variables. The optimal propensity score model was selected on the basis of the model with the most balance observed between cohorts. Standardized differences were reported for all baseline covariates included in the propensity score model, both before and after matching.
Independent variables in the final selected propensity score model included patients’ age, sex, baseline clinical characteristics, health resource utilization, and other baseline characteristics that were important confounders with outcomes (see Table 1 for propensity score model variables). The analyses included comparisons of depression-related HCRU measures, costs, and productivity measures between the two cohorts. For the matched analyses, statistical tests accounted for matching and included Wilcoxon signed rank test for non-normally distributed continuous variables, paired t test for normally distributed continuous variables, and McNemar chi-square for categorical variables, unless otherwise noted.
Regression models (generalized linear models with gamma distribution accounting for matched pairs) were used to elicit the multiplicative impact of OAB status on total depression-related healthcare costs. These costs were assessed on the basis of the statistical significance of the ratio of costs for patients with OAB versus patients without OAB.
Within the subset with workplace absenteeism data, multivariate regression was conducted to address covariate imbalances between cohorts while for the short-term disability analysis no further covariate adjustment was conducted since cases and controls were observed to be balanced on baseline covariates. Negative binomial models were used to model number of absentee hours and logistic regression models were used to model binary outcomes (absent from work (yes/no); absence type: sick (yes/no), leave (yes/no), disability (yes/no), recreational (yes/no); short-term disability utilized (yes/no)). All data analyses for this study were conducted using SAS version 9.4 (SAS Institute, Cary, NC). The a priori alpha level for all inferential analyses was 0.05 and all statistical tests were two-tailed. Data were evaluated for violations of assumptions underlying the associated statistical tests as appropriate.
Results
Baseline Characteristics
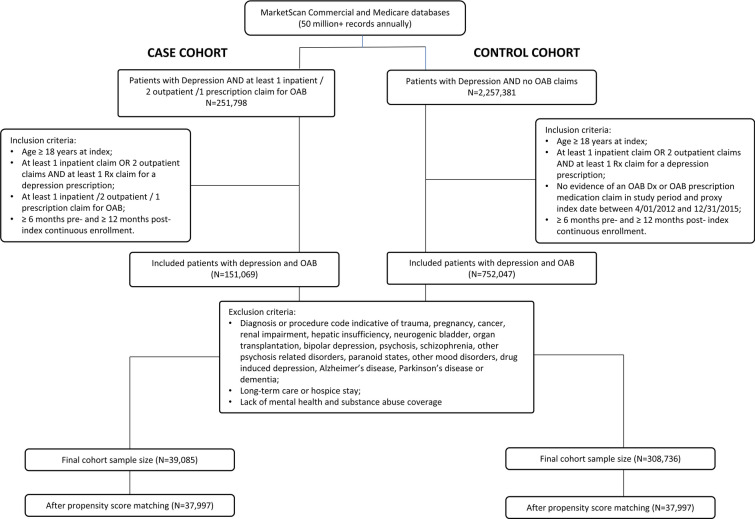
After application of the inclusion and exclusion criteria, 39,085 patients with depression and OAB (cases) and 308,736 patients with depression but without OAB (controls) were identified. After propensity score matching, each cohort comprised 37,997 patients (Fig. 1) and the standardized differences indicated no imbalance in observed baseline characteristics between the cohorts (all standardized differences < 0.1). Pre- and post-match baseline characteristics are presented in Table 1. The mean (SD) age of the case cohort was 54.7 (14.6) years and 81.2% were female; the mean (SD) age of the control cohort was 55.0 (14.3) years and 82.3% of the cohort were female. The most common comorbidities were uncomplicated hypertension (case cohort: 15.4%; control cohort: 14.7%; p = 0.0061) and uncomplicated diabetes (case cohort: 8.1%; control cohort: 8.0%; p = 0.3197). Further details of pre- and post-match baseline characteristics are detailed in Table 1.
Fig. 1.
Flow diagram describing the assembly of the study cohorts using the IBM MarketScan database
Depression-Related Healthcare Resource Utilization and Costs
During the 12-month post-index period, depression-related HCRU suggested a pattern of higher utilization for the case cohort in all categories in comparison to the control cohort and the differences, although slight (potentially due to the conservative definition used to identify depression-related events based on the primary diagnosis), were statistically significant for mean number of outpatient visits (excluding physician office and ER visits) per patient (p = 0.0101), the mean number of emergency room visits per patient (p = 0.002), and number of unique depression medications (< 0.0001; Table 2). Total mean depression-related costs for cases were 1.13 times the costs among controls (p < 0.0001) during the post-index period. Cases experienced higher depression-related costs in total healthcare costs and total medical and pharmacy costs, respectively, though differences were not significant in all subcategories (Table 2). For the case cohort total mean (SD) costs per patient were $1795.93 ($4235.02), while for controls they were $1596.85 ($3863.42; p < 0.0001); for medians, see Table 2. Pharmacy costs made up the majority of depression-related healthcare expenditure with mean (SD) costs per patient at $1315.14 [$2667.75] for the case cohort and $1135.45 ($2368.13; p < 0.0001) per patient for controls. Means and medians were substantially different across all categories.
Table 2.
Matched analysis of depression-related healthcare resource use and costs, 12-month post-index period
| Variable | Case Depression and OAB |
Control Depression without OAB |
p value | ||
|---|---|---|---|---|---|
| N = 37,997 | N = 37,997 | ||||
| Depression-related resource utilization measures | |||||
| Inpatient utilization, acute (binary), n (%) | 426 | (1.1%) | 400 | (1.1%) | 0.3645 |
| Inpatient admissions (acute), mean (SD) | 0.014 | (0.167) | 0.013 | (0.143) | 0.4013 |
| Total bed days per patient*, mean (SD) | 0.091 | (1.478) | 0.083 | (1.266) | 0.4328 |
| Inpatient utilization, non-acute (binary), n (%) | 23 | (0.1%) | 23 | (0.1%) | NS |
| Inpatient admissions (non-acute), mean (SD) | 0.001 | (0.030) | 0.001 | (0.030) | 0.9042 |
| Total bed days per patient*, mean (SD) | 0.004 | (0.210) | 0.003 | (0.139) | 0.4043 |
| Outpatient visits (excluding physician office and ER visits), mean (SD) | 1.520 | (5.706) | 1.416 | (5.510) | 0.0101 |
| Physician office visits, mean (SD) | 0.951 | (2.433) | 0.959 | (2.894) | 0.6993 |
| Emergency room (ER) visits, binary, n (%) | 329 | (0.9%) | 255 | (0.7%) | 0.002 |
| Emergency room (ER) visits, mean (SD) | 0.009 | (0.112) | 0.007 | (0.094) | 0.0063 |
| Total number of unique depression medications, mean (SD) | 1.862 | (1.162) | 1.748 | (1.088) | < 0.0001 |
| Depression-related costs | |||||
| Total, mean (SD) | $1795.93 | ($435.02) | $1596.85 | ($3863.42) | <0.0001 |
| Median (IQR) | $451.24 | ($114.67, $1866.50) | $390.36 | ($101.93, $1583.24) | |
| Medical, mean (SD) | $480.80 | ($3100.58) | $461.40 | ($2847.53) | < 0.0001 |
| Median (IQR) | $0.00 | ($0.00, $186.10) | $0.00 | ($0.00, $175.70) | |
| Inpatient hospital admissions (acute), mean (SD) | $118.35 | ($2094.24) | $109.14 | ($1664.19) | 0.5 |
| Median (IQR) | $0.00 | ($0.00, $0.00) | $0.00 | ($0.00, $0.00) | |
| Inpatient hospital admissions (non-acute), mean (SD) | $4.90 | ($285.32) | $4.78 | ($230.67) | NS |
| Median (IQR) | $0.00 | ($0.00, $0.00) | $0.00 | ($0.00, $0.00) | |
| Outpatient visits (excluding physician office and ER visits), mean (SD) | $219.70 | ($1513.99) | $205.69 | ($1781.43) | < 0.0001 |
| Median (IQR) | $0.00 | ($0.00, $0.00) | $0.00 | ($0.00, $0.00) | |
| Physician office visits, mean (SD) | $129.66 | ($760.32) | $133.69 | ($707.26) | < 0.0001 |
| Median (IQR) | $0.00 | ($0.00, $89.43) | $0.00 | ($0.00, $83.64) | |
| Emergency room (ER) visits, mean (SD) | $8.19 | ($144.06) | $8.09 | ($172.47) | 0.0625 |
| Median (IQR) | $0.00 | ($0.00, $0.00) | $0.00 | ($0.00, $0.00) | |
| Pharmacy, mean (SD) | $1315.14 | ($2667.75) | $1135.45 | ($2368.13) | < 0.0001 |
| Median (IQR) | $283.34 | ($76.63, $1338.40) | $244.38 | ($67.94, $1063.64) | |
Mean healthcare resource utilization measures are captured as mean per patient utilization over the 12-month post-index period
p values are calculated using McNemar chi-squared for binary and t test/Wilcoxon tests for continuous measures (statistical significance is denoted by bold text)
Bed days = sum of length of stay of all hospitalizations during 12-month post-index period *among all patients
Costs measures are captured as per patient costs over the 12-month post-index period
Costs adjusted to 2016 US dollars (using the medical care component of the Consumer Price Index)
IQR interquartile range (25th percentile, 75th percentile), NS not specified
Workplace Productivity: Benefit Eligibility for Workplace Absences Sub-Cohort
The workplace productivity cohorts were a subset of the enrollees in the Commercial database. Baseline characteristics were assessed among the subset of cases and controls with data provided by employers on employee workplace absences. Cases (n = 686) and controls (n = 642) with available data on workplace absences were imbalanced (standardized differences ≥ 0.1) on baseline age, geographic region, some Elixhauser conditions, acute inpatient utilization, and total number of unique medications; these covariates were included in the multivariable regression analysis. The cases and controls in this subset did not differ on any of the workplace absentee outcomes. For cases and controls, time absent from work did not differ significantly either among all patients (unadjusted, p = 0.6051; adjusted, p = 0.5907) or among those with absences (unadjusted, p = 0.2456; adjusted, p = 0.1159). Neither were there significant differences in type of absence from work between cases and controls (p > 0.05) in unadjusted and adjusted analyses (see Table 3 for full results and p values).
Table 3.
Analysis of workplace absentee benefit eligibility, 12-month post-index period
| Absenteeism | Case depression and OAB | Control depression without OAB | Unadjusted p value* |
Adjusted model ratio estimate^ Case/control |
95% confidence interval (adjusted ratio estimate) | Adjusted p value (from model) | |||
|---|---|---|---|---|---|---|---|---|---|
| N = 686 | N = 642 | Lower limit | Upper limit | ||||||
| Absent from work (yes), n (%) | 572 | (83.4%) | 543 | (84.6%) | 0.5523 | 0.903 | 0.668 | 1.22 | 0.5072 |
| Total time absent from work (number of hours), among all patients, mean (SD) | 298.02 | (279.19) | 285.75 | (257.54) | 0.6051 | 1.042 | 0.896 | 1.212 | 0.5907 |
| Median (IQR) | 266.70 | (134.00, 362.00) | 265.50 | (124.00, 354.00) | |||||
| Total time absent from work (number of hours), among patients with absences, mean (SD) | 357.41 | (268.78) | 337.85 | (246.59) | 0.2456 | 1.061 | 0.986 | 1.142 | 0.1159 |
| Median (IQR) | 293.90 | (220.52, 393.20) | 287.12 | (208.00, 382.50) | |||||
| Absence type, n (%) | |||||||||
| Sicka | 441 | (64.3%) | 425 | (66.2%) | 0.4644 | 0.905 | 0.712 | 1.152 | 0.4183 |
| Leaveb | 335 | (48.8%) | 313 | (48.8%) | 0.9768 | 1.036 | 0.828 | 1.298 | 0.755 |
| Disabilityc | 4 | (0.6%) | 4 | (0.6%) | 0.9251 | NS# | NS# | NS# | NS# |
| Recreationald | 567 | (82.7%) | 533 | (83.0%) | 0.8587 | 0.963 | 0.719 | 1.291 | 0.8011 |
*This is a subset of the matched population and cases to controls are not 1:1. Statistical tests do not account for matching. NS not specified, #Estimate and p value not specified because model unable to converge as a result of small counts for outcome
Model adjusted for age, geographic region, number of unique medications at baseline, acute inpatient hospitalization at baseline, and Elixhauser comorbidities
aSick—incidental illness
bLeave—includes bereavement, military leave, and jury duty
cDisability—occupational and non-occupational
dRecreational—includes vacation
p values are calculated using chi-square and Wilcoxon rank sum for unadjusted analysis
^Ratio estimate = odds ratio for categorical variables and mean to mean ratio for continuous variables
Workplace Productivity: Benefit Eligibility for Short-Term Disability Sub-Cohort
Baseline characteristics were assessed among cases and controls for those with data provided for benefit eligibility for short-term disability. Cases (n = 4395) and controls (n = 4433) with available data on short-term disability benefit utilization were balanced (standardized differences < 0.1) on all assessed baseline covariates and thus no further covariate adjustment was conducted. Among individuals with eligibility for workplace short-term disability benefit, significantly more cases than controls used this benefit during the post-index period (21.1% of cases versus 16.7% of controls; p < 0.0001). Among all eligible patients, cases experienced more case days (days away from work due to short-term disability; 11.0 versus 8.6 mean days) and received higher payments for short-term disability than controls ($1226 versus $1033 mean total payments; p < 0.0001). However, among cases and controls who utilized their short-term disability benefits neither case days nor total payments for short-term disability differed statistically (p > 0.05) (Table 4).
Table 4.
Analysis of short-term disability benefit eligibility, 12-month post-index period
| Short-term disability | Case Depression and OAB |
Control Depression without OAB |
p value* | Ratio estimate^ Case/control |
95% confidence Interval (ratio estimate) | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| N = 4395 | N = 4433 | Lower limit | Upper limit | ||||||
| Short-term disability (yes), n (%) | 926 | (21.1%) | 742 | (16.7%) | < 0.0001 | 1.328 | 1.193 | 1.478 | < 0.0001 |
| Total short-term disability—case days, among all patients, mean (SD) | 11.0 | (31.1) | 8.6 | (26.9) | < 0.0001 | 1.279 | 1.130 | 1.450 | 0.0001 |
| Median (IQR) | 0.0 | (0.0, 0.0) | 0.0 | (0.0, 0.0) | |||||
| Total short-term disability—case days, among patients using short-term disability, mean (SD) | 51.7 | (49.3) | 51.0 | (45.9) | 0.7883 | 1.014 | 0.927 | 1.109 | 0.7782 |
| Median (IQR) | 36.0 | (19.0, 65.0) | 36.0 | (17.0, 70.0) | |||||
| Total payments, among all patients, mean (SD) | $1226.15 | ($4472.90) | $1033.47 | ($4187.31) | < 0.0001 | 1.186 | 1.011 | 1.397 | 0.0379 |
| Median (IQR) | $0.00 | ($0.00, $0.00) | $0.00 | ($0.00, $0.00) | |||||
| Total payments, among patients using short-term disability, mean (SD) | $544.80 | ($822.01) | $6267.29 | ($8579.24) | 0.8097 | 0.933 | 0.816 | 1.068 | 0.3139 |
| Median (IQR) | $3156.83 | ($685.94, $7,429.64) | $3071.29 | ($458.47, $8831.95) | |||||
p values are calculated using chi-square and Wilcoxon rank sum (statistical significance is denoted by bold text)
*This is a subset of the matched population and cases to controls are not 1:1. Statistical tests do not account for matching
NS not specified: ratios computed by the method of EC Fieller, Suppl to J.R. Statist. Soc, 7, 1–64
^Ratio estimate = odds ratio for categorical variables and mean to mean ratio for continuous variables; p value for ratio measures computed by Altman D, Bland J. How to obtain the p value from a confidence interval. BMJ 2011; 343
Discussion
This study evaluated the HCRU, healthcare costs, and workplace productivity of patients with depression and OAB with a matched set of patients with depression and without OAB. Depression-related healthcare costs were higher among cases than controls in the 12-month post-index period with cases experiencing 1.13 times the total depression-related healthcare costs of controls. In the specific resource use categories, depression-related HCRU tended to be higher for cases than controls; however, statistical significance in HCRU was observed with regard to outpatient services, emergency room services, and number of unique depression medications. Cases used more outpatient and emergency room services and received more unique depression medications than controls. The analysis of workplace productivity found that time absent from work or type of absence from work did not differ statistically between cases and controls with available data on workplace absenteeism. Among all cases and controls eligible for short-term disability benefits, there was an observed difference in the percentage of cases (21.1%) and controls (16. 7%) with a claim for short-term disability, as well as days away from work and total payments for short-term disability during the post-index period.
The present study found that comorbid OAB increases costs in patients with depression. While the effects of comorbid OAB on HCRU, costs, and work productivity in this specific population have not been examined in the literature previously, some studies have assessed the converse relationship of depression in an OAB population. They found that a substantial portion (36%) of patients with OAB have comorbid depression and associated depression-related HCRU and costs [19, 20]. It is clear that there is an interrelationship between these two conditions given the frequency of comorbidity and the evidence suggests that comorbid OAB leads to increases in costs. The depression-related costs reported here for the control cohort are in line with previous cost of illness studies of depression [21]. The 12-month, total median depression-related costs reported here for the control cohort are higher than the 24-month, total mental-health-related costs reported in a study by Ivanova et al. for the MDD control cohort in their study ($390 versus $481). Some of the difference can be accounted for by a difference in sample selection criteria. Unlike the present study, a prescription for any antidepressant was not required for study participation and thus the median drug cost reported by Ivanova et al. [22] was $0. Costs were similar to those reported by Schultz et al.; they report mean total medical and pharmaceutical cost ranging from $1274 to $2319 among patients with MDD [23]. Both were database studies though not MarketScan and both covered time periods earlier than the present study. The finding that comorbid OAB increases depression-related costs echoes the findings of the effects of comorbid OAB with other conditions. A recent study of HCRU and costs in a population with dementia and comorbid OAB also noted the comorbidity was associated with higher disease-specific costs [24]. Finally, while this study did not attempt to quantify the effect of anticholinergic burden on the outcomes herein, it should be noted that both OAB and depression are frequently treated with anticholinergics [25, 26]. Studies have linked long-term anticholinergic use with dementia [27] and falls and fractures [28] and have noted concomitant increases in costs and resource use [29]. As such, it is possible that the increase in costs identified here could, in some measure, be the result of anticholinergic burden, particularly if patients are using drugs of that class to treat both conditions.
A strength of this study is its generalizability. The study population captures a representative sample of US patients with employer-sponsored health insurance and the results can be generalized to a similar population of patients with employer-sponsored health insurance with a diagnosis and treatment for depression (with and without OAB). Depression-related HCRU and costs were higher among cases compared to controls. As such, there is evidence to suggest that patients with depression and OAB experience additional HCRU and healthcare costs associated with depression-related medical or pharmacy claims when compared to matched patients with depression and without OAB. This suggests that additional HCRU and costs among patients with OAB and depression are not directly related to OAB management. Study limitations pertain to the definitions used and assumptions made during the analysis. HCRU and costs were allocated to care settings on the basis of a hierarchical classification of claims which may have misclassified some of the services to the incorrect setting. Other limitations are a function of the data source and primarily relate to the assessment of comorbidities and conditions using diagnosis codes on medical claims, particularly where coding is undertaken by non-clinicians. In addition, administrative claims data are collected for billing, rather than research, purposes and this too may cause misclassification as coding may be driven by reimbursement (rather than clinical) factors. To the extent that claims were miscoded or under-coded, there was a possibility of measurement error and misallocation of some depression-related HCRU and costs herein. Bearing in mind this potential for misclassification in disease-specific costs, all-cause costs were also calculated and were found to follow the same trajectory as the depression-related costs. As with any retrospective study, the findings are limited by the availability of and type of data, and duration of follow-up of patients, within the databases. In particular, information on severity of the conditions was not available. Given the sampling frame for the study (those with commercial or Medicare Supplemental plans), the study findings may not be reflective of outcomes or treatment for individuals who have other types of insurance or those without insurance. Additionally, only outpatient dispensations covered by commercial or Medicare Supplemental insurance appear in the database; and no data were available on patterns of actual prescription usage. Regarding the workplace productivity analyses, it should be noted as a measure of confounding adjustment when imbalance was observed, it was determined that regression analysis could be used to adjust for covariates in this population.
Conclusions
Comorbidity of OAB in patients with depression impacted direct HCRU and healthcare costs and had some impact on indirect costs measured by short-term disability claims utilized in the workplace. The observed impact of OAB on patients with depression indicates a challenge for the medical community in the management of comorbid patients across the healthcare system. A closer assessment of the specific types of depression-related healthcare sought in this population may be warranted.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The present study was initiated by Astellas Pharma Global Development, Inc., and funding for the conduct of this study was provided by Astellas Pharma Global Development, Inc. The study sponsor also paid for this journal’s Rapid Service and Open Access Fees.
Editorial Assistance
We would like to thank Elizabeth Badillo for drafting, reviewing, and editing this manuscript. Elizabeth Badillo is an employee of Broadstreet HEOR, which received payment from Astellas for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Aki Shiozawa, Daniel Ng, Matthias Stoelzel, and John Hairston are employees of Astellas. Rupali Fuldeore is currently an employee of Astellas but was an employee of Xcenda at the time of study completion. Sari Hopson is an employee of Xcenda, which received a research contract to conduct this study with and on behalf of Astellas.
Compliance with Ethics Guidelines
MarketScan databases are HIPAA compliant. This study was conducted using anonymized patient data from MarketScan and as such review board approval and patient consent were not necessary.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12853835.
References
- 1.The National Institute of Mental Health. Major depression–prevalence of major depressive episode among adults. 2019. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed 8 May 2020.
- 2.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nature Rev Disease Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 3.Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(1):90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari AJ, Charlson FJ, Norman RE, et al. The epidemiological modelling of major depressive disorder: application for the global burden of disease study 2010. PLoS One. 2013;8(7):e69637. doi: 10.1371/journal.pone.0069637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 6.Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60(5 Suppl 1):7–12. doi: 10.1016/S0090-4295(02)01784-3. [DOI] [PubMed] [Google Scholar]
- 7.Stewart W, Van Rooyen J, Cundiff G, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 8.Onukwugha E, Zuckerman IH, McNally D, Coyne KS, Vats V, Mullins CD. The total economic burden of overactive bladder in the United States: a disease-specific approach. Am J Managed Care. 2009;15(4 Suppl):S90–S97. [PubMed] [Google Scholar]
- 9.Caplan EO, Abbass IM, Suehs BT, Ng DB, Gooch K, van Amerongen D. Impact of coexisting overactive bladder in medicare patients with dementia on clinical and economic outcomes. Am J Alzheimers Dis Other Demen. 2019;2019:1533317519841164. [DOI] [PMC free article] [PubMed]
- 10.Caplan EO, Abbass IM, Suehs BT, et al. Impact of coexisting overactive bladder in Medicare patients with osteoporosis. Arch Gerontol Geriatr. 2018;75:44–50. doi: 10.1016/j.archger.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy J Hum Pharmacol Drug Therapy. 2005;25(4):511–519. doi: 10.1592/phco.25.4.511.61033. [DOI] [PubMed] [Google Scholar]
- 12.Lai HH, Shen B, Rawal A, Vetter J. The relationship between depression and overactive bladder/urinary incontinence symptoms in the clinical OAB population. BMC Urol. 2016;16(1):60. doi: 10.1186/s12894-016-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–96. doi: 10.1016/j.urology.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Nicolson P, Kopp Z, Chapple CR, Kelleher C. It’s just the worry about not being able to control it! A qualitative study of living with overactive bladder. Br J Health Psychol. 2008;13(Pt 2):343–359. doi: 10.1348/135910707X187786. [DOI] [PubMed] [Google Scholar]
- 15.Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526–532. doi: 10.1016/j.urology.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 16.Dugan E, Cohen SJ, Bland DR, et al. The association of depressive symptoms and urinary incontinence among older adults. J Am Geriatr Soc. 2000;48(4):413–416. doi: 10.1111/j.1532-5415.2000.tb04699.x. [DOI] [PubMed] [Google Scholar]
- 17.Harvey R, Jankus DD, Mosley D. Random assignment of proxy event dates to unexposed controls in observational studies: an automated technique using SAS. Presented at the annual Midwest SAS Users Group, Minneapolis, MN. 2012.
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Yehoshua A, Chancellor M, Vasavada S, et al. Health resource utilization and cost for patients with incontinent overactive bladder treated with anticholinergics. J Manag Care Spec Pharm. 2016;22(4):406–413. doi: 10.18553/jmcp.2016.22.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin DE, Mungapen L, Milsom I, Kopp Z, Reeves P, Kelleher C. The economic impact of overactive bladder syndrome in six Western countries. BJU Int. 2009;103(2):202–209. doi: 10.1111/j.1464-410X.2008.08036.x. [DOI] [PubMed] [Google Scholar]
- 21.Luppa M, Heinrich S, Angermeyer MC, Konig HH, Riedel-Heller SG. Cost-of-illness studies of depression: a systematic review. J Affect Disord. 2007;98(1–2):29–43. doi: 10.1016/j.jad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Ivanova JI, Birnbaum HG, Kidolezi Y, Subramanian G, Khan SA, Stensland MD. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26(10):2475–2484. doi: 10.1185/03007995.2010.517716. [DOI] [PubMed] [Google Scholar]
- 23.Schultz J, Joish V. Costs associated with changes in antidepressant treatment in a managed care population with major depressive disorder. Psychiatric Serv. 2009;60(12):1604–1611. doi: 10.1176/ps.2009.60.12.1604. [DOI] [PubMed] [Google Scholar]
- 24.Caplan EO, Abbass IM, Suehs BT, Ng DB, Gooch K, van Amerongen D. Impact of coexisting overactive bladder in medicare patients with dementia on clinical and economic outcomes. Am J Alzheimers Dis Other Demen. 2019;34(7–8):492–499. doi: 10.1177/1533317519841164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6S):2455–2463. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong C. APA releases guidelines on treatment of patients with major depressive disorder. Am Fam Physician. 2011;83(10):1219. [Google Scholar]
- 27.Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315. doi: 10.1136/bmj.k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo SM, Gooch K, Schermer C, et al. Association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder: US-based retrospective cohort study. BMJ Open. 2019;9(5):e026391. doi: 10.1136/bmjopen-2018-026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano-Ortega G, Schermer CR, Walker DR, et al. Fall/fracture-related healthcare costs and their association with cumulative anticholinergic burden in people with overactive bladder. Pharmacoecon Open. 2020;2020:1–11. doi: 10.1007/s41669-020-00215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.