Abstract
Mast cells (MCs) are granulated, immune cells of the myeloid lineage that are present in connective tissues. Apart from their classical role in allergies, MCs also mediate various inflammatory responses due to the nature of their secretory products. They are involved in important physiological and pathophysiological responses related to inflammation, chronic wounds, and autoimmune diseases. There are also indications that MCs are associated with diabetes and its complications. MCs and MC-derived mediators participate in all wound healing stages and are involved in the pathogenesis of non-healing, chronic diabetic foot ulcers (DFUs). More specifically, recent work has shown increased degranulation of skin MCs in human diabetes and diabetic mice, which is associated with impaired wound healing. Furthermore, MC stabilization, either systemic or local at the skin level, improves wound healing in diabetic mice. Understanding the precise role of MCs in wound progression and healing processes can be of critical importance as it can lead to the development of new targeted therapies for diabetic foot ulceration, one of the most devastating complications of diabetes.
Keywords: Diabetes mellitus, Diabetic foot ulcer, Mast cells, Wound healing
Key Summary Points
| This is a review paper that focuses on the role of mast cells in diabetic wound healing. Skin mast cells are degranulated in diabetes. Stabilization of the mast cells reduces degranulation and promotes wound healing in animal models. This can be a new treatment for diabetic foot ulceration. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.12911561.
Introduction
More than 140 years have passed since Paul Ehrlich presented his doctoral thesis, “Contribution to The Theory and Practice of Histological Dyes,” in which mast cells (MCs) were first described [1, 2]. A wealth of knowledge has been generated since, and our perception of the role and functions of MCs in our bodies has been remodeled. From being known for their detrimental role in allergic diseases, such as food allergies, asthma, and anaphylaxis, for decades; to now recognized as crucial players in a diverse array of physiological and pathologic functions, including vasodilation, angiogenesis, lymphangiogenesis, pathogen elimination, innate and adaptive immune responses, wound healing, and homeostasis. Moreover, MCs play an important role in many diseases, such as gastrointestinal disorders, diabetes, malignancies, and cardiovascular diseases [3–6]. However, to this day, the pathophysiological roles of MCs are not well understood. In this review, we summarize the recent perception of MCs and mainly focus on MCs in diabetes and diabetic wound healing.
MCs Origin and Functions
MCs are granular, long-lived, tissue-residing immune cells derived from precursor cells in the bone marrow [7]. The progenitors release into the circulation and reach various organs to differentiate into multiple MC subtypes. These various subtypes have been characterized in both humans and animals on the basis of differences in cell morphology, histochemical properties, protease content in granules, anaphylactic types of degranulation, receptor expression, and functions [8–10]. MCs can change their phenotype depending on the tissue environment and culturing conditions [11, 12]. Recently, significant heterogeneity in gene expression of tissue-resident MCs has been described by using a comprehensive analysis of the transcriptome of individual anatomically distinct mouse MC populations [13]. MCs are distributed throughout nearly all tissues and are often found in close proximity to epithelia, fibroblasts, blood and lymphatic vessels, and nerves [6, 14]. They are commonly activated by (1) immunoglobulin E (IgE) antigen cross-linking, which results in the degranulation of the cells and the secretion of various pre-formed molecules including histamine, serotonin, tryptase, chymase, and lipid-derived mediators, as well as by (2) IgE-independent non-allergic responses, which results in the release of both pre-formed and newly synthesized MC mediators, including cytokines and chemokines [15–17].
MCs are key initiators and modulators of allergic disorders, such as bronchial asthma, allergic rhinitis, urticaria, food allergies, anaphylaxis, atopic dermatitis, and angioedema [4, 18]. As a result of their location, surface receptors, and a wide spectrum of inflammatory and immunomodulatory mediators, MCs are thought to act as the first sentinels in response to metabolic and immunological changes [19–22]. Additionally, MCs communicate and interact with many immune and non-immune cells, such as dendritic cells, macrophages, T cells, B cells, fibroblasts, eosinophils, endothelial cells, and epithelial cells. This facilitates their capacity to be involved in regulating the functions of many tissues and organs [23, 24]. There is increasing evidence that MCs play roles in organ development, skin barrier homeostasis, wound healing, angiogenesis, lymphangiogenesis, heart function, autoimmune diseases, and tumor initiation and progression [5, 6, 14, 25].
MCs in Diabetes
Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM), or insulin-dependent diabetes mellitus, is an autoimmune disease resulting from interactions of genetic, environmental, and immunologic factors that destroy insulin-producing pancreatic β cells in the islets of Langerhans and lead to insulin deficiency [26, 27]. While significant numbers of islet-infiltrating CD8+ cytotoxic T cells and macrophages have been observed in recent-onset T1DM, making them the most prominent infiltrating cell type in islets devoid of insulin-positive cells [28], several studies demonstrated the importance of other islet-infiltrating cell types such as leukocytes and MCs. Though the role of MCs in the pathogenesis of T1DM is not clear, it is generally accepted that MCs are able to present antigens to activate T cells in major histocompatibility complex class I and class II pathways in rodents and humans. It has also been suggested that MCs promote T cell migration into inflammatory sites by producing chemokines, or indirectly by increasing endothelial cell adhesion molecule expression [29]. In samples from donors with T1DM, a larger number of MCs were found to infiltrate pancreatic islets compared with those from donors without diabetes or with type 2 diabetes mellitus (T2DM). Furthermore, the extent of the infiltration correlated with β cell damage. Histamine, which is found at high levels in MCs, directly contributed to β cell death in isolated human islets and INS-1E cells, an insulin-secreting cell line, via a caspase-independent pathway [30]. The observation that patients with T1DM and their siblings have increased levels of circulating IgE compared to the general population also suggests that IgE-mediated activation of MCs could be involved in the pathogenesis of T1DM [31]. However, the exact role of MC, if any, in the activation and migration of autoreactive T cells to the islets has not been demonstrated, raising doubts regarding their possible role.
The DRlyp/lyp rats are a T1DM animal model that develops spontaneous T1DM with hyperglycemia, glycosuria, weight loss, decreased plasma insulin, and peripheral T cell lymphopenia. In contrast, the wild-type DR+/+ rats do not develop diabetes but have a genetic predisposition. Previous studies have shown that in pancreatic lymph nodes (PLN), MCs were more abundant in DRlyp/lyp rats than in DR+/+ rats and that their gene expression profile revealed an upregulation in specific MC genes. When treated with the MCs stabilizer disodium cromoglycate (DSCG or cromolyn), a membrane stabilizer that inhibits MC degranulation, DRlyp/lyp rats show delayed T1DM onset [32]. However, those results were not definitive since it is now clear that cromolyn is not a highly selective MC blocker and can affect other innate immune cells such as macrophages and basophils [33].
The role of MCs in the streptozocin-induced model of T1DM was evaluated by employing W/Wv or Wsh/Wsh MC-deficient mice. MC-deficient mice developed severe insulitis and accelerated hyperglycemia, with 100% of mice becoming diabetic compared to their littermates. They also had decreased numbers of T regulatory cells in the PLNs, and MCs deficiency caused a significant reduction in interleukins (IL), such as IL-10, IL-6, and transforming growth factor-beta (TGFβ) expression in the pancreatic tissue. These results indicate that MCs play a protective role in T1DM and suggest that a failure in MCs expression and function leads to the increased severity of this disease [34]. However, additional studies in two nonobese diabetic (NOD) mouse models with MC deficiency, namely the NOD.kitW-sh/W-sh and NOD.Cpa3Cre/+ mice, showed that the absence of MCs did not affect the progression or incidence of T1DM and had no discernible effects on the overall autoimmune response, which involves both innate and adaptive immune components [35]. Finally, in NOD mice, conditional MCs knockout that results in MCs selective depletion at an early stage, protects the mice from autoimmune T1DM. MCs of NOD mice were overly inflammatory, and they secrete a large amount of IL-6, which favors the differentiation of IL-17-secreting T cells but failed to acquire a tolerogenic IL-10+ phenotype [36]. The controversies of these outcomes partly result from the different models applied. Moreover, some of these models carry various immune abnormalities or have an increase or decrease in other cell types that can affect T1DM [37, 38].
Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM), or non-insulin-dependent diabetes mellitus, is the most common form of diabetes. It is characterized by increased plasma glucose levels due to insulin secretion deficiencies and insulin resistance, which can be a direct consequence of obesity. Obesity is associated with chronic, low-grade inflammation of adipose tissue (AT), characterized by recruitment of immune cells such as proinflammatory M1 macrophages, neutrophils, natural killer cells, T helper cells type 1, T helper cells type 17, B cells, and MCs [39–42]. AT is a reservoir for MCs, where their numbers are found to be particularly increased in obese individuals compared with lean subjects. Most MCs are degranulated as tryptase concentrations are significantly higher in serum obtained from patients with obesity [43, 44]. As potent inducers of inflammatory responses, MCs could potentially contribute to obesity-induced AT inflammation and metabolic dysregulation [45]. MC-deficient KitW-sh/W-sh mice and wild-type mice that are treated with cromolyn to stabilize MCs do not develop obesity in response to high-fat diet, nor do they show signs of increased levels of inflammatory cytokines, chemokines, and proteases in serum and white adipose tissue (WAT). Moreover, proinflammatory MCs play detrimental roles in obesity and diabetes. MCs in WAT obtained from lean humans and mice were leptin-deficient and polarized macrophages toward an anti-inflammatory M2 phenotype. An adoptive transfer of leptin-deficient MCs mitigates obesity and diabetes in obese mice [46].
MCs also play a role in regulating AT thermogenesis. Using the tryptophan hydroxylase 1 (Tph1) inhibitor and Tph1-deficient MCs, studies showed that MC-derived serotonin inhibits subcutaneous adipose tissue browning and systemic energy expenditure. Functional inactivation of MCs or inhibition of MC serotonin synthesis in subcutaneous adipose tissue promotes adipocyte browning and systemic energy metabolism in mice [47]. Furthermore, the exposure of mice to thermoneutrality promotes the infiltration of WAT with MCs that are highly enriched with Tph1. Engraftment of MC-deficient mice with Tph1−/− MCs or selective MCs deletion of Tph1 enhances uncoupling protein 1 expression in WAT and protects mice from developing obesity and insulin resistance [48].
However, conflicting findings have been reported on metabolic dysfunction that is related to obesity in MC-deficient mice, which are presented in detail in Table 1. More specifically, not only did some studies demonstrate that the absence of MCs does not affect AT inflammation and metabolic dysregulation but they also showed the protection of Kit mutant mice against the effects of hypercaloric diet resulted from effects of reduced Kit expression rather than MCs deficiency [49]. Studies on diet-induced obesity in Mcpt5-Cre R-DTA MC-deficient mice, in which the lack of MCs is caused by a principle different from MCs deficiency in Cpa3Cre/+ mice or Kit mutations, also observed no differences in terms of accumulation of M1 macrophages or upregulation of inflammatory cytokines including IL-1β, IL-6, IL-10, and tumor necrosis factor (TNF) between the MC-deficient and MC-proficient mice. Furthermore, MC deficiency had no marked changes in obesity and obesity-related dysregulation observed in weight gain, glucose tolerance, insulin resistance, and other metabolic parameters [50]. In another study, wild-type and MC-deficient mice were fed a high-fat or low-fat diet to study MC influence on inflammatory cell polarization in WAT and overall metabolic changes. The results demonstrated that MCs contributed to the local pro-inflammatory state within WAT in obesity but did not play a primary role in causing insulin resistance [51]. Thus, whether MCs play an essential role in obesity and related pathologies is still under debate. Generally, the MC population in AT is dynamic in nature, showing changes associated with tissue remodeling in obesity. On the basis of the anatomical positions of fat pads such as subcutaneous and epididymal fat, MCs may show different activity levels and distributions [52].
Table 1.
Studies examining whether metabolic dysfunction is related to obesity in MC-deficient mice
| Study year | Model | Main findings | Comments |
|---|---|---|---|
|
2006 Geoffrey et al. [32] |
DRlyp/lyp and DR+/+ rats | MCs were more abundant in DRlyp/lyp rats than in DR+/+ rats and that their gene expression profile revealed an upregulation in specific MC genes. When treated with the MCs stabilizer disodium cromoglycate (DSCG or cromolyn), a membrane stabilizer that inhibits MCs degranulation, DRlyp/lyp rats show delayed T1DM onset | Those results were not definitive since it is now clear that cromolyn is not a highly selective MC blocker and can have an effect on other innate immune cells such as macrophages and basophils [33] |
|
2015 Carlos et al. [34] |
W/Wv or Wsh/Wsh MC-deficient mice | W/Wv or Wsh/Wsh MC-deficient mice developed severe insulitis and accelerated hyperglycemia with 100% of mice becoming diabetic compared to their littermates. They also had decreased numbers of T regulatory cells in the PLNs and MCs deficiency caused a significant reduction in interleukins (IL), such as IL-10, IL-6, and transforming growth factor-beta (TGFβ) expression in the pancreatic tissue | Additional studies in two nonobese diabetic (NOD) mouse models with MC deficiency, namely the NOD.kitW-sh/W-sh and NOD.Cpa3Cre/+ mice, showed that the absence of MCs did not affect the progression or incidence of T1DM and had no discernible effects on the overall autoimmune response, which involves both innate and adaptive immune components [35] |
|
2015 Zhou et al. [46] |
MC-deficient KitW-sh/W-sh mice and wild-type mice | MC-deficient KitW-sh/W-sh mice and wild-type mice that are treated with cromolyn to stabilize MCs do not develop obesity in response to high-fat diet nor do they show signs of increased levels of inflammatory cytokines, chemokines, and proteases in serum and white adipose tissue (WAT). Moreover, proinflammatory MCs play detrimental roles in obesity and diabetes. MCs in WAT obtained from lean humans and mice were leptin-deficient and polarized macrophages toward an anti-inflammatory M2 phenotype. An adoptive transfer of leptin-deficient MCs mitigates obesity and diabetes in obese mice |
The protection of Kit mutant mice against the effects of hypercaloric diet resulted from effects of reduced Kit expression rather than MCs deficiency [49] Studies on diet-induced obesity in Mcpt5-Cre R-DTA MC-deficient mice, in which the lack of MCs is caused by a principle different from MCs deficiency in Cpa3Cre/+ mice or Kit mutations, also observed no differences in terms of accumulation of M1 macrophages or upregulation of inflammatory cytokines [50] |
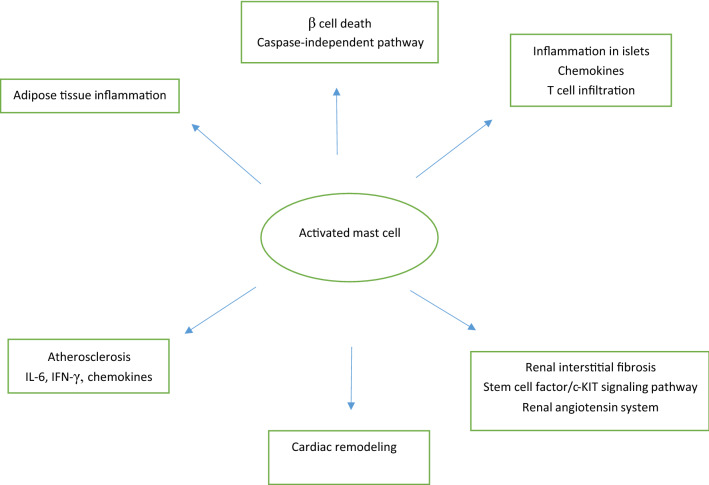
In addition to the above, MCs contribute to the progression of complications in T2DM. An increased number of MCs were observed in the kidneys of diabetic animals where they have been found to release various mediators, such as TGFβ, chymase, tryptase, cathepsin G, rennin, and many others. The release of such mediators from MCs may contribute to renal diseases and renal interstitial fibrosis and cause extracellular matrix (ECM) accumulation [53, 54]. A recent study indicated that MC infiltration might promote renal interstitial fibrosis via the stem cell factor (SCF)/c-KIT signaling pathway, which can be inhibited by an antiallergic drug such as tranilast [55]. Another pathway in which MCs contribute to the pathogenesis of diabetic nephropathies might involve the renin–angiotensin system (RAS). Drugs that can inhibit renin directly prevented the increase of MCs in diabetic mice. This was observed when mice were treated with aliskiren [56]. Furthermore, MCs play a substantial role in the development of atherosclerosis in patients with diabetes as they secrete several cytokines including IL-6 and IFNγ, as well as chemokines such as eotaxin, monocyte chemoattractant protein 1 (MCP-1), and RANTES, which are involved in the recruitment of monocytes into the inflammation sites and promote differentiation in the arterial wall. Additionally, MCs participate in lipid retention, vascular cell remodeling, and atherosclerotic plaque progression by releasing vasoactive and angiogenic compounds, and pro-inflammatory mediators, such as arachidonic acid metabolites, histamine, cytokines, chemokines, platelet-activating factors, and proteolytic enzymes [57]. Studies also depicted the contribution of MCs to the pathogenesis of hyperglycemia-induced atrial fibrillation via the enhancement of inflammation and fibrosis with MC-deficient W/W(v) mice [58]. Cardiac MCs were supposed to be activated by metabolic byproducts resulted from hyperglycermia and then participate in the remodeling process of cardiomyopathy by releasing a multitude of cytokines and bioactive enzymes. Increased numbers of MCs and mediator release have been reported in explanted human hearts with dilated cardiomyopathy [59, 60]. Other studies have reported increased cardiac MC density and elevated MC secretions that correlated with gene expression and aberrant cytokine levels associated with cardiac remodeling. Nedocromil, a pharmacologic stabilizer of MC, halted contractile dysfunction in diabetic mice, reduced cardiac MC density, reduced the expression of MMP-2, MMP-9, TNFα, and IFNγ, and increased the expression of IL-4 and IL-10, resulting in attenuation of the diabetes-induced cardiomyopathy [61]. MC-deficiency protects mice from streptozotocin-induced diabetic cardiomyopathy [62]. The main MCs functions are summarized in Fig. 1.
Fig. 1.
Main mast cell functions in diabetes
MCs in Wound Healing
Wound healing is a complex and dynamic process that involves coordinated and overlapping phases, including coagulation, inflammation, proliferation, and remodeling aimed at ultimately restoring the barrier function and mechanical integrity of the skin. The vascular system and numerous cell types, cytokines, and mediators are involved in this process [63]. It is initiated with the vasoconstriction of blood vessels and platelet aggregation to stop the bleeding, followed by a flux of a variety of inflammatory cells, such as MCs, neutrophils, macrophages, and endothelial cells. These inflammatory cells then release various mediators and cytokines to promote fibroblast proliferation and migration to the wound area to secrete new ECM and granulation tissue, which then fills the wound prior to re-epithelialization. In the remodeling phase, collagen bundles increase in diameter and become organized to achieve greater tensile strength. Furthermore, fibroblasts undergo a transformation and differentiate into myofibroblasts to facilitate wound contraction [64].
MCs represent up to 8% of the total number of cells within the dermis and are localized adjacent to the epidermis and the subdermal vasculature and nerves. There is evidence supporting the participation of MCs in wound healing owing to their interaction with macrophages, endothelial cells, and fibroblasts [65–68]. At the onset of a cutaneous injury, MCs are recruited by anaphylatoxins C3a and C5a, produced under the influence of Hageman factor (XII) and SCF secreted by keratinocytes. Subsequently, recruited MCs release TNFα, histamine, vascular endothelial growth factor (VEGF), IL-6, IL-8, platelet-derived growth factor (PDGF), TGFβ, nerve growth factor, and other mediators. These MC-released mediators play significant roles in the wound healing process, where they (1) contribute to clot stabilization, neoangiogenesis, fibrinogenesis, and re-epithelialization, (2) increase endothelial permeability and vasodilation, (3) facilitate migration of monocytes and neutrophils to the site of injury, and (4) activate keratinocytes and fibroblasts. It has been observed that early skin wound healing is impaired in the absence of MCs, and after wound healing, vascular permeability and neutrophil recruitment is decreased. MCs degranulate in response to skin wounding and inhibition of histamine, but not in the absence of TNFα, which results in a delayed skin wound closure [67]. MCs were found contributing to scar formation during fetal wound healing. MCs enhance scar formation and mediate the transition from scarless to fibrotic healing during fetal wound repair. MC-deficient (KitW/W-v) was shown to produce less scar tissue when compared to wild-type (Kit+/+) fetuses [69]. The growth and differentiation factor activin A is a key regulator of tissue repair, inflammation, and fibrosis and can increase the number of mature MCs in mouse skin in vivo [69].
MCs were also involved in regulating the homeostatic expression of epidermal differentiation complex (EDC) genes, where decreased barrier functions were found in the absence of MCs. In MC-deficient mice, there is a diminished expression of multiple EDC genes and evidence of increased barrier permeability to protein antigens [70]. MCs have been reported as being more efficient in their ability to downregulate factor XIIIa than in contributing to its amounts and functions in homeostatic conditions. Activated MCs controlled thrombin-induced skin inflammation by MC protease 4, suggesting the complex function of MCs in wound healing, especially in chronic wound healing [71]. Further studies found that MC-deficient mice have a significantly delayed wound closure in infected skin wounds. The delay was associated with impaired bacterial clearance in the absence of MCs [22].
It should be emphasized that there is no universal consensus regarding the role of MCs in wound healing. Thus, studies using mated activin transgenic mice with CreMaster mice observed that loss of MCs did not affect the wound healing process. Furthermore, MC deficiency did not alter wounding-induced inflammation and new tissue formation or chemically induced angiogenesis in mice with normal activin levels. This led to the hypothesis that MCs are not major targets of activin during wound healing and do not play a major role in wound healing in general [72]. However, a drawback of these studies is that they were performed with different mice models, which have additional abnormalities in the immune system, and this can be the main reason for the observed contradictory results. A more specific and reliable model of MC deficiency is needed to fully comprehend the role of MC in normal wound healing.
Diabetic Foot Ulceration
Diabetic foot ulcers (DFUs) are a leading cause of hospital admission for patients with diabetes. DFUs are a major morbidity, often causing patients to suffer from severe pain and a poor quality of life. Approximately 10–15% of patients with diabetes develop foot ulcers, and 15% of patients with DFU require amputation, both of which are associated with high mortality rates of 16.7% at 12 months and over 50% at 5 years [73]. In contrast to non-diabetic wounds, diabetic wounds are characterized by prolonged inflammation, impaired angiogenesis, and delayed wound closure. The etiology of non-healing DFUs is multifactorial because of a combination of peripheral neuropathy, peripheral artery disease, and altered immune function. Additionally, the recruitment of endothelial progenitor cells is impaired in diabetes as a result of reduced nitric oxide production, which ultimately results in impaired angiogenesis. However, the most direct effects on wound healing come from the functional alterations in cells activated by the immune response, including platelets, macrophages, neutrophils, endothelial cells, fibroblasts, and keratinocytes, all contributing to a failure to progress through the normal phases of wound healing [74].
We previously showed that increased skin inflammation and aberrant growth factor levels are the main factors associated with a failure to heal DFUs. Patients with DFUs had more severe neuropathy, higher white blood cell count, and lower endothelium-dependent and independent vasodilation in the macrocirculation. Patients whose ulcers failed to heal had higher TNFα, MCP-1, matrix metallopeptidase-9 (MMP-9), and fibroblast growth factor 2 serum levels when compared with those who healed. Skin biopsy analysis showed that compared with control subjects, patients with diabetes had increased immune cell infiltration, expression of MMP-9, and protein tyrosine phosphatase 1B, which negatively regulates the signaling of insulin, leptin, and various growth factors [75]. We also observed that increased chronic inflammation and blood vessel density in the skin of patients with diabetes and in experimental diabetes models [75, 76], as well as the defective inflammation resolution in diabetic mouse wounds, impair the healing process [77].
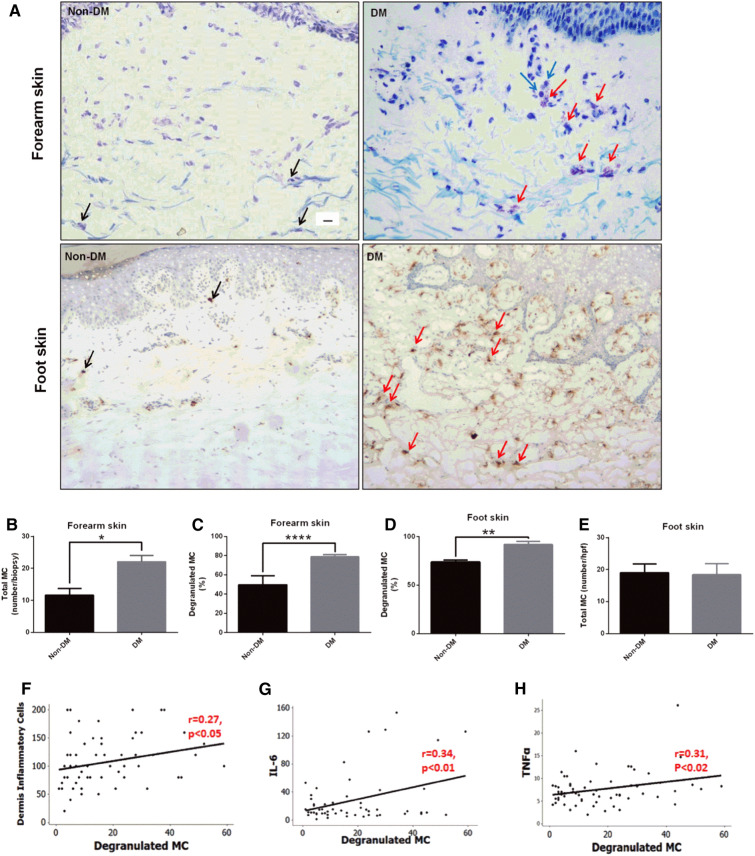
Subsequent studies in our unit showed that the number of degranulated MCs was increased in unwounded forearm and foot skin of patients with diabetes and in the unwounded dorsal skin of diabetic mice (Figs. 2 and 3). Conversely, post-wounding MCs degranulation increased in non-diabetic mice, but not in diabetic mice. Pretreatment with the MCs degranulation inhibitor DSCG rescued diabetes-associated wound-healing impairment in mice. Pretreatment with DSCG also shifted macrophages to the regenerative M2 phenotype. Macrophages are critical for the healing of DFUs and can be broadly characterized as “pro-inflammatory” M1 or “immunomodulatory/regenerative” M2 [78, 79]. M1 activation is required during the acute inflammatory phase, although it is also present in chronic wounds characterized by persistent inflammation [80]; whereas M2 activation during the proliferative phase promotes angiogenesis and collagen production [81]. Single-cell RNA sequencing analyses of healthy lower extremity skin and DFUs found that the DFU groups had significantly more M1 polarized as compared to M2 [82]. Furthermore, our studies showed that non-diabetic and diabetic mice deficient in MCs had delayed wound healing compared with their wild-type (WT) controls. These results indicate that intact, non-degranulated MCs are necessary for proper healing, and therapies inhibiting MC degranulation could improve wound healing in diabetes [83].
Fig. 2.
Skin MC degranulation, assessed by toluidine blue and/or tryptase immunostaining, is increased in patients with DM and is associated with inflammation. a Representative images of toluidine blue-stained MCs in forearm skin (top panel) and of tryptase-immunostained MCs in foot skin specimens (bottom panel) from subjects with and without DM (scale bar 10 µm). Black arrows show non-degranulated MCs and red arrows show degranulated MCs. Degranulated MCs were in proximity to inflammatory cells (blue arrows). b The total number and c percentage of degranulated MCs stained with toluidine blue were increased in forearm skin specimens from subjects with DM. d MC degranulation was also increased in foot skin specimens from subjects with DM stained with tryptase, while e the total number of MC was not different. *p < 0.05. f–h A positive correlation was observed between degranulated MCs and the f dermis inflammatory cells, the serum levels of g IL-6 and h TNFα. (Ref. [77])
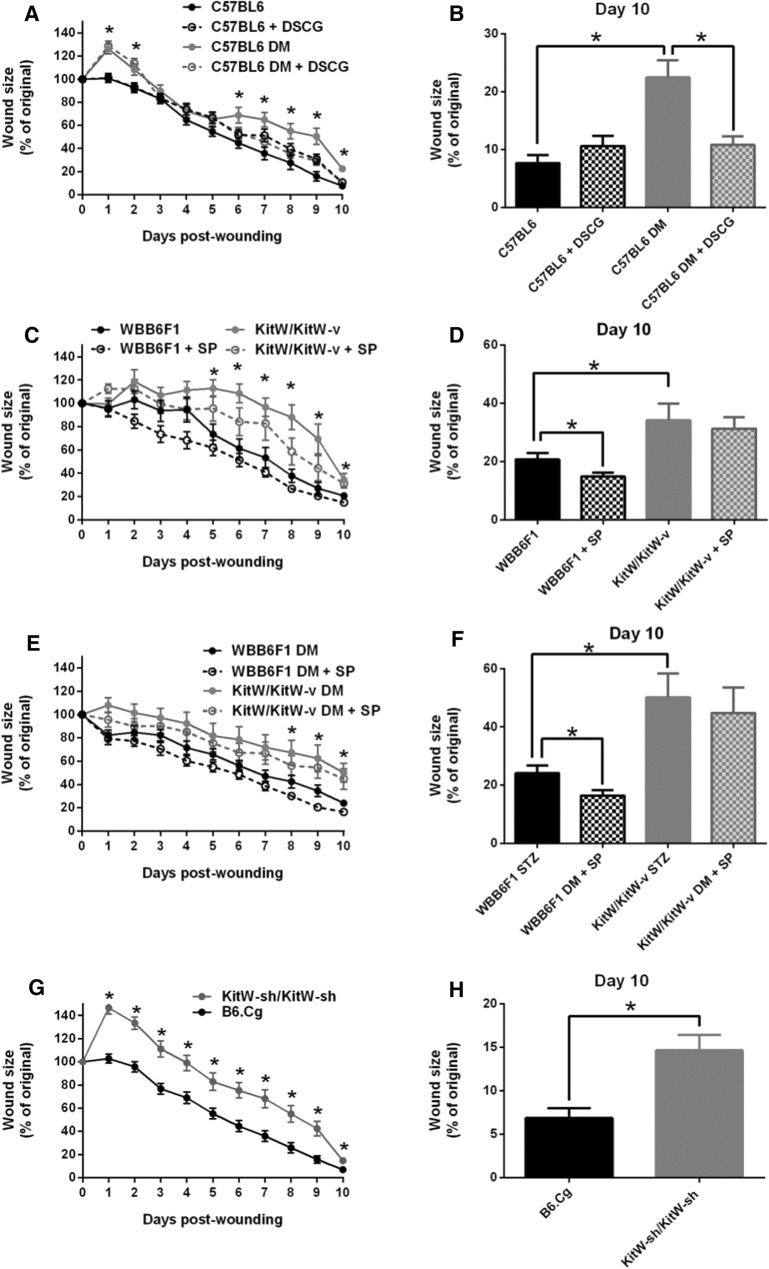
Fig. 3.
Functional MCs are required for proper wound healing. Wound healing progress was evaluated over a 10-day period in non-DM and DM mice DSCG treated or not treated and MC-deficient mice. a, b Wound healing was delayed in C57BL6 DM mice compared to non-DM mice and DSCG pre-treatment accelerated it from days 6 to 10 post wounding. DSCG had no effect on non-DM mice. c–f Wound healing was delayed in KitW/KitW-v mice with or without DM, when compared to their respective non-DM or DM WBB6F1 controls. Topical SP improved healing at day 10 post wounding in both non-DM and DM WBB6F1 mice, but failed to have an effect in either non-DM KitW/KitW-v or DM KitW/KitW-v mice. g, h Wound healing was delayed in MC-deficient KitW-sh/KitW-sh mice when compared to their respective B6.Cg controls. *p < 0.05. (Ref. [77])
MCs may also be involved in modulating VEGF, which acts on multiple components of the wound healing cascade, including angiogenesis, epithelization, and collagen deposition. MCs are a significant source of VEGF in mouse skin, and its release from human MCs is reduced in hyperglycemic conditions [84]. Local MC activation increased blood flow through the hind limb (46% at day 9) compared to that in non-activated control mice. Histological analysis of the muscle tissue revealed an increased number of CD31+ capillaries [85].
In addition to the above, additional modes of action for MCs have been proposed. MCs have been shown to promote mesenchymal stem cell (MSC) proliferation and migration by the activation of the PDGF pathway, downregulation of miR-145/143, and modulation of the myocardin–Klf4 axis [86]. Chymase released by MC increased proliferation of skin fibroblast and expression of TGFβ1 and interleukin IL-1β in a dose-dependent way [87]. MC protease mMCP-6, an MC-specific tryptase, has scar-suppressing properties after spinal cord injury via indirect cleavage of axon growth-inhibitory scar components and alteration of the gene expression profile of these factors [88]. MCs protect from the exacerbated allergic skin inflammation induced by repeated allergen challenges [89]. MCs were also utilized as an alternative target in the vasoplegic response in cardiac surgery [90], while MC protease 7 could promote angiogenesis by the degradation of integrin subunits [91].
MCs-Related Diabetic Wound Therapy
DFUs treatment requires fully identifying the etiology and assessing the co-morbidities. Adequate care for DFUs should include a focus on appropriate wound debridement, infection control, relieving pressure while standing or walking, and optimizing blood flow. With technological advancement, a series of advanced therapies have been implemented, such as bioengineered skin substitutes, negative pressure wound therapy, and the development of new wound dressings [92]. Though these treatments have provided encouraging results, most of them are expensive, and some even have significant side effects that often result in non-compliance. As MC and their secreted factors can regulate the process of wound healing at multiple levels, MCs stabilizers may be a new therapeutic modality to treat DFUs.
MCs Stabilizers
MC stabilizer ketotifen was used to treat both Yorkshire and red Duroc pigs. Studies showed that ketotifen treatment significantly reduced the first phase of contraction in red Duroc pig wounds to a level equivalent to Yorkshire wounds, but had no detectable effects on the post-epithelialization phase of contraction. Ketotifen treatment also reduced the deposition of collagen within the red Duroc wounds but did not affect Yorkshire pig wounds contraction or collagen deposition, suggesting that ketotifen was an effective treatment for the reduction of excessive wound contraction and fibrosis in human cutaneous injuries without affecting the normal healing process [93]. Another study investigated the effects of systemic usage of DSCG, the most widely used MC stabilizer, on wound healing in BALB/c mice. The authors reported that the application of DSCG could reduce the levels of inflammatory cytokines, such as IL-1α, IL-1β, and CXCL1, decrease scar width, and accelerate collagen reorganization [94].
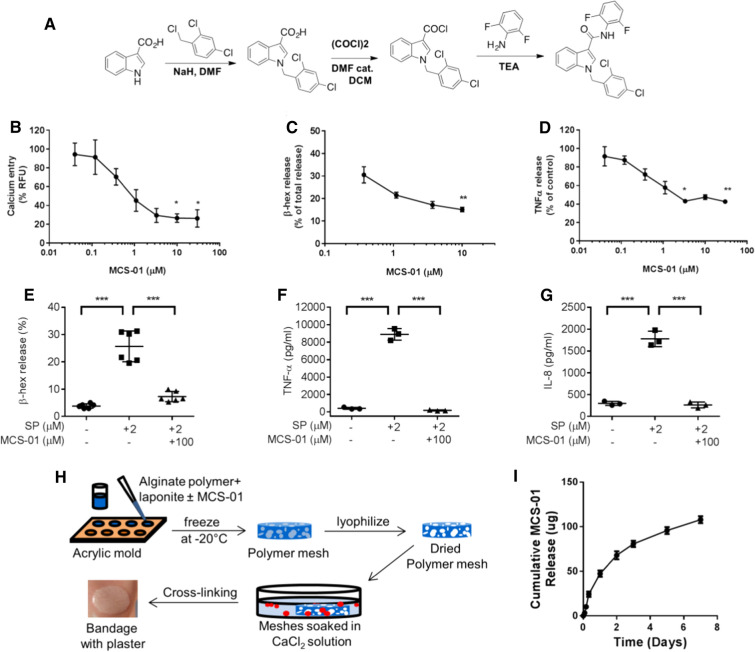
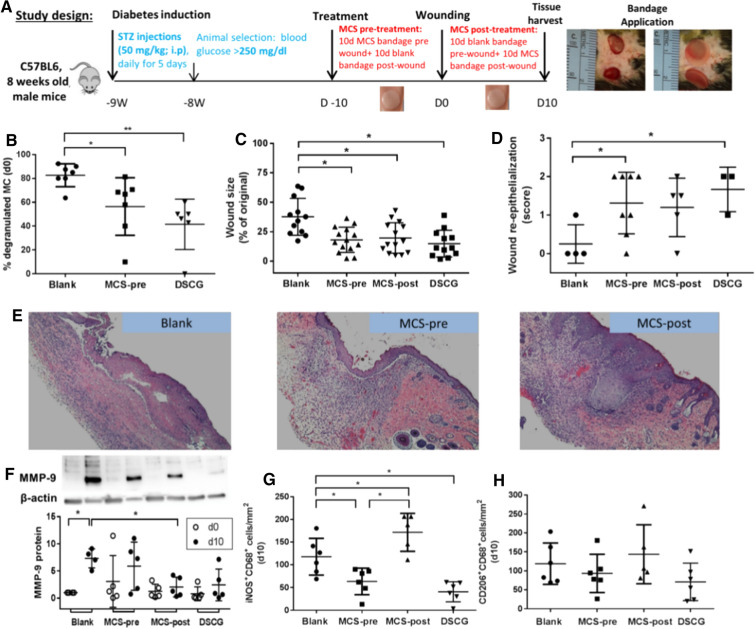
As our previous studies have shown an increase in degranulated skin MCs in patients with diabetes and animal models of diabetes, a study from our unit investigated the effect of systemic administration of DSCG. It showed that it increased angiogenesis and improved wound healing in diabetic mice [83]. As DSCG may not be a good candidate for topical use, we further developed a new indole carboxamide-type of MCs stabilizer, MCS-01, and proved it to be an effective MCs degranulation inhibitor in vitro by calcium channel blockade. MCS-01 can be delivered topically for prolonged periods through controlled release by specifically designed alginate bandages (Fig. 4). Analyses of MC supernatants showed that MCS-01 significantly inhibited the release of β-hex and TNFα in a concentration-dependent manner. Human LAD2 cells stimulated with substance P (SP, 2 mM), an established inducer of MCs degranulation, showed that MCS-01 inhibited β-hex release and reduced both TNFα and IL-8. This proved that MCS-01 could affect the degranulation and release of pro-inflammatory mediators from MCs. Similar to the effects of a systemic application of DSCG for MCs stabilization, bandages releasing MCS-01 reduced MC degranulation in diabetic mouse skin, and the topical application of MCS-01 accelerated wound healing in both pre- and post-wounding of diabetic mice (Fig. 5). We further investigated the global changes in gene expression patterns. Bulk transcriptome analysis from wounds treated with MCS-01 or placebo showed that MCS-01 (1) significantly modulated messenger RNA and microRNA profiles of diabetic wounds, (2) stimulated upregulation of pathways linked to acute inflammation and immune cell migration, and (3) activated NF-κB, IL-6, and TREM1 signaling pathways. Single-cell RNA sequencing analysis of 6154 cells from wounded and unwounded mouse skin revealed that MCS-01 primarily altered the gene expression of MCs, monocytes, and keratinocytes, while fibroblasts, adipocytes, and T cells had no significant changes. All the above suggests that topical MC stabilization can be a potentially successful treatment for DFUs [95].
Fig. 4.
Synthesis and validation of mast cell stabilization by MCS-01. a Chemical synthesis of MCS-01. In rat RBL-2H cells MCS-01 reduced b Ca2+ influx measured as % relative fluorescence of Fluo-4, c β-hex release measured as % of total β-hex in cell lyses at 30 min, and d TNFα release measured at 3 h of MCS-01 stimulation (0.033–30 µM) after thapsigargin (Tg, 1 µM) activation (n = 3). MCS-01 (100 µM) treatment inhibited SP (2 μM)-mediated e β-hex release (after 30 min pre-treatment), f TNFα or g IL-8 release (after 24 h pre-treatment) from human mast cells. h Development of MCS-0-releasing bandage for topical delivery: the schematic of alginate bandage fabrication. Mixture of alginate polymer, laponite, and MCS-01 drug is added to acrylic molds and is frozen at − 20° C. The molds are then placed in a lyophilizer to remove ice crystals, leaving polymer meshes with large voids. The dried polymer meshes are then placed in a large 100 mM CaCl2 bath for 15 min to form ionic cross-linking for gelation. Alginate bandages are formed. i In vitro release of MCS-01 from alginate bandages. Data shown as mean ± SD (n ≥ 3, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001) (Ref. [89])
Fig. 5.
Topical MCS-01 applied either pre or post wounding and DSCG (i.p. injected pre-wounding) accelerated wound healing in diabetic mice. a Experimental design of in vivo wound healing in STZ-induced diabetic mice. 6-mm full-thickness wounds were created on the dorsum. Effects after 10 days of topical treatment with MCS-01-loaded bandages (800 µg/bandage), applied either before or after wounding (MCS-pre or MCS-post, respectively), were compared to that of vehicle-only bandages (blank) or intraperitonially administered mast cell stabilizer disodium cromoglycate (DSCG). Wounds before and after bandage application are shown to the right. b DSCG or pre-MCS-01 treatment reduced skin mast cell degranulation assessed by toluidine blue staining compared to blank controls. c Wound size measurement, d wound re-epithelialization analysis, and e representative images on day 10 (D10) post wounding showed improvement in treatment groups compared to blank controls. Topical post-MCS-01 treatment and DSCG also f rescued the elevation in MMP-9 protein expression at D10 vs D0 observed in diabetic mice. g D10 counts of M1 macrophages were elevated upon post-MCS-01 treatment, but reduced with pre-MCS-01 and DSCG treatments. h M2 macrophages showed similar trends as M1. Data shown as mean ± SD (n ≥ 3, *p ≤ 0.05, **p ≤ 0.01. Results in Fig. 2d are based on two-sample t test comparisons between blank and pre-MCS-01 and DSCG) (Ref. [89])
Finally, photoimmodulation, or photoimmodulation combined with condition medium derived from human bone marrow MSCs, significantly decreases both the total numbers of MCs and their degranulation and improves wound healing in diabetes [96]. Omega-3 fatty acids significantly decreased the diabetic wound area by affecting the concentration of MCs [97]. Topical administration of 0.03% naltrexone, an opioid receptor antagonist, could accelerate diabetic wound closure by promoting deoxyribonucleic acid synthesis, increasing MCs, and enhancing the expression of PDGF and VEGF [98]. DSCG in subcutaneously mesh-implanted C57BL/6J mice decreased early inflammation and fibrotic responses [99].
Concluding Remarks
Recent work has significantly increased our knowledge of MC as multifaceted immune modulators and operators of health. MCs have also been implicated in the development of non-healing, chronic DFU. The development of new, potent mast cell stabilizers that are appropriate for topical use has the potential to improve impaired diabetic wound healing, one of the most severe complications of diabetes.
Acknowledgements
Funding
This work was supported by the National Institutes of Health Grant DP3DK108224 (A.V.). A.V. received funding from the National Rongxiang Xu Foundation. No Rapid Service or Open Access Fees were received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Aristidis Veves is a Section Editor for Advances in Therapy. The remaining authors Jie Dong, Lihong Chen, Ying Zhang, Navin Jayaswal, Ikram Mezghani and Weijie Zhang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12911561.
References
- 1.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crivellato E, Beltrami CA, Mallardi F, Ribatti D. Paul Ehrlich’s doctoral thesis: a milestone in the study of mast cells. Br J Haematol. 2003;123:19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 3.Lyons DO, Pullen NA. Beyond IgE: alternative mast cell activation across different disease states. Int J Mol Sci. 2020;21:1498. doi: 10.3390/ijms21041498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer M, Taube C, et al. Mast cells drive IgE-mediated disease but might be bystanders in many other inflammatory and neoplastic conditions. J Allergy Clin Immunol. 2019;144:S19–S30. doi: 10.1016/j.jaci.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Varricchi G, Marone G. Mast cells: fascinating but still elusive after 140 years from their discovery. Int J Mol Sci. 2020;21:464. [DOI] [PMC free article] [PubMed]
- 6.Varricchi G, Rossi FW, Galdiero MR, et al. Physiological roles of mast cells: Collegium Internationale Allergologicum update 2019. Int Arch Allergy Immunol. 2019;179:247–261. doi: 10.1159/000500088. [DOI] [PubMed] [Google Scholar]
- 7.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood J Am Soc Hematol. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 8.Dahlin JS, Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol Immunol. 2015;63:9–17. doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak AM, Morgan ES. Diamine oxidase-gold enzyme-affinity ultrastructural demonstration that human gut mucosal mast cells secrete histamine by piecemeal degranulation in vivo. J Allergy Clin Immunol. 1997;99:812–820. doi: 10.1016/s0091-6749(97)80016-4. [DOI] [PubMed] [Google Scholar]
- 10.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff S, Sellge G, Lorentz A, Sebald W, Raab R, Manns M. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci. 1999;96:8080–8085. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber M, Cato AC, Ainooson GK, et al. Regulation of the pleiotropic effects of tissue-resident mast cells. J Allergy Clin Immunol. 2019;144:S31–S45. doi: 10.1016/j.jaci.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer DF, Barrett NA, Austen KF. Immunological Genome Project C: expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 2016;17:878–887. doi: 10.1038/ni.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2016;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 19.Abraham SN, John ALS. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudeck A, Köberle M, Goldmann O, et al. Mast cells as protectors of health. J Allergy Clin Immunol. 2019;144:S4–S18. doi: 10.1016/j.jaci.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Piliponsky AM, Acharya M, Shubin NJ. Mast cells in viral, bacterial, and fungal infection immunity. Int J Mol Sci. 2019;20:2851. doi: 10.3390/ijms20122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann C, Troeltzsch D, Giménez-Rivera V, et al. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc Natl Acad Sci. 2019;116:20500–20504. doi: 10.1073/pnas.1908816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stassen M, Hartmann A-K, Delgado SJ, Dehmel S, Braun A. Mast cells within cellular networks. J Allergy Clin Immunol. 2019;144:S46–S54. doi: 10.1016/j.jaci.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Gri G, Frossi B, D’Inca F, et al. Mast cell: an emerging partner in immune interaction. Front Immunol. 2012;3:120. doi: 10.3389/fimmu.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elieh A, Komi D, Wohrl S, Bielory L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol. 2020;58:342–365. doi: 10.1007/s12016-019-08769-2. [DOI] [PubMed] [Google Scholar]
- 26.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nature Rev Endocrinol. 2009;5:219. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 28.Willcox A, Richardson S, Bone A, Foulis A, Morgan N. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi MA, Shi GP. Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front Immunol. 2012;3:7. doi: 10.3389/fimmu.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martino L, Masini M, Bugliani M, et al. Mast cells infiltrate pancreatic islets in human type 1 diabetes. Diabetologia. 2015;58:2554–2562. doi: 10.1007/s00125-015-3734-1. [DOI] [PubMed] [Google Scholar]
- 31.Svensson J, Eising S, Mortensen HB, et al. High levels of immunoglobulin E and a continuous increase in immunoglobulin G and immunoglobulin M by age in children with newly diagnosed type 1 diabetes. Hum Immunol. 2012;73:17–25. doi: 10.1016/j.humimm.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Geoffrey R, Jia S, Kwitek AE, Woodliff J, et al. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol. 2006;177:7275–7286. doi: 10.4049/jimmunol.177.10.7275. [DOI] [PubMed] [Google Scholar]
- 33.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Investig J Tech Methods Pathol. 2012;92:1472–1482. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlos D, Yaochite JN, Rocha FA, et al. Mast cells control insulitis and increase Treg cells to confer protection against STZ-induced type 1 diabetes in mice. Eur J Immunol. 2015;45:2873–2885. doi: 10.1002/eji.201545498. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez DA, Fu W, Schonefeldt S, et al. Type 1 diabetes in NOD mice unaffected by mast cell deficiency. Diabetes. 2014;63:3827–3834. doi: 10.2337/db14-0372. [DOI] [PubMed] [Google Scholar]
- 36.Betto E, Usuelli V, Mandelli A, et al. Mast cells contribute to autoimmune diabetes by releasing interleukin-6 and failing to acquire a tolerogenic IL-10+ phenotype. Clin Immunol. 2017;178:29–38. doi: 10.1016/j.clim.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Hübner MP, Larson D, Torrero MN, et al. Anti-FcεR1 antibody injections activate basophils and mast cells and delay type 1 diabetes onset in NOD mice. Clin Immunol. 2011;141:205–217. doi: 10.1016/j.clim.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valle A, Giamporcaro GM, Scavini M, et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–2077. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komi DEA, Shafaghat F, Christian M. Crosstalk between mast cells and adipocytes in physiologic and pathologic conditions. Clin Rev Allergy Immunol. 2020;2020:1–13. doi: 10.1007/s12016-020-08785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oishi Y, Manabe I. Integrated regulation of the cellular metabolism and function of immune cells in adipose tissue. Clin Exp Pharmacol Physiol. 2016;43:294–303. doi: 10.1111/1440-1681.12539. [DOI] [PubMed] [Google Scholar]
- 41.Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Żelechowska P, Wiktorska M, Różalska S, et al. Leptin receptor is expressed by tissue mast cells. Immunol Res. 2018;66:557–566. doi: 10.1007/s12026-018-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Divoux A, Moutel S, Poitou C. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metabol. 2012;97:E1677–E1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 44.Poglio S, De Toni-Costes F, Arnaud E, et al. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Yu X, Chen H, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22:1045–1058. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Wang X, Yin H, et al. Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep. 2019;28(792–803):e794. doi: 10.1016/j.celrep.2019.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yabut JM, Desjardins EM, Chan EJ, et al. Genetic deletion of mast cell serotonin synthesis prevents the development of obesity and insulin resistance. Nature Commun. 2020;2020:11. doi: 10.1038/s41467-019-14080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic kit deficiency, rather than lack of mast cells, protects mice from obesity and insulin resistance. Cell Metab. 2015;21:678–691. doi: 10.1016/j.cmet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Chmelař J, Chatzigeorgiou A, Chung K-J, et al. No role for mast cells in obesity-related metabolic dysregulation. Front Immunol. 2016;7:524. doi: 10.3389/fimmu.2016.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einwallner E, Kiefer FW, Di Caro G, et al. Mast cells are not associated with systemic insulin resistance. Eur J Clin Invest. 2016;46:911–919. doi: 10.1111/eci.12675. [DOI] [PubMed] [Google Scholar]
- 52.Altintas MM, Nayer B, Walford EC, et al. Leptin deficiency-induced obesity affects the density of mast cells in abdominal fat depots and lymph nodes in mice. Lipids Health Dis. 2012;11:21. doi: 10.1186/1476-511X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickey FB, Martin F. Role of the immune system in diabetic kidney disease. Curr Diab Rep. 2018;18:20. doi: 10.1007/s11892-018-0984-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochim Biophys Acta. 2012;1822:14–20. doi: 10.1016/j.bbadis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin DD, Luo JH, Zhao ZY, Liao YJ, Li Y. Tranilast prevents renal interstitial fibrosis by blocking mast cell infiltration in a rat model of diabetic kidney disease. Mol Med Rep. 2018;17:7356–7364. doi: 10.3892/mmr.2018.8776. [DOI] [PubMed] [Google Scholar]
- 56.de Morais RB, do Couto-Muniz VP, Costa EN, et al. Mast cell population in the development of diabetic nephropathy: effects of renin angiotensin system inhibition. Biomed Pharmacother. 2018;107:1115–1118. doi: 10.1016/j.biopha.2018.08.066. [DOI] [PubMed] [Google Scholar]
- 57.Spinas E, Kritas S, Saggini A, et al. Role of mast cells in atherosclerosis: a classical inflammatory disease. London: SAGE; 2014. [DOI] [PubMed] [Google Scholar]
- 58.Uemura K, Kondo H, Ishii Y, et al. Mast cells play an important role in the pathogenesis of hyperglycemia-induced atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:981–989. doi: 10.1111/jce.12995. [DOI] [PubMed] [Google Scholar]
- 59.Patella V, de Crescenzo G, Lamparter-Schummert B, De Rosa G, Adt M, Marone G. Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res. 1997;46:31–32. doi: 10.1007/s000110050041. [DOI] [PubMed] [Google Scholar]
- 60.Patella V, Marino I, Arbustini E, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 61.Huang ZG, Jin Q, Fan M, et al. Myocardial remodeling in diabetic cardiomyopathy associated with cardiac mast cell activation. PLoS One. 2013;8:e60827. doi: 10.1371/journal.pone.0060827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.He A, Fang W, Zhao K, et al. Mast cell-deficiency protects mice from streptozotocin-induced diabetic cardiomyopathy. Transl Res. 2019;208:1–14. doi: 10.1016/j.trsl.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 64.Wallace HA, Basehore BM, Zito PM. Wound healing phases. In: StatPearls Treasure Island (FL). 2020. [PubMed]
- 65.Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regenerat. 2003;11:46–54. [DOI] [PubMed]
- 66.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 68.Noli C, Miolo A. The mast cell in wound healing. Vet Dermatol. 2001;12:303–313. doi: 10.1046/j.0959-4493.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 69.Antsiferova M, Martin C, Huber M, et al. Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J Immunol. 2013;191:6147–6155. doi: 10.4049/jimmunol.1301350. [DOI] [PubMed] [Google Scholar]
- 70.Sehra S, Serezani AP, Ocaña JA, Travers JB, Kaplan MH. Mast cells regulate epidermal barrier function and the development of allergic skin inflammation. J Investig Dermatol. 2016;136:1429–1437. doi: 10.1016/j.jid.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shubin NJ, Glukhova VA, Clauson M. Proteome analysis of mast cell releasates reveals a role for chymase in the regulation of coagulation factor XIIIA levels via proteolytic degradation. J Allergy Clin Immunol. 2017;139:323–334. doi: 10.1016/j.jaci.2016.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol. 2012;132:458–465. doi: 10.1038/jid.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamont P, Franklyn K, Rayman G, Boulton AJ. Update on the diabetic foot 2012: the 14th biennial Malvern Diabetic Foot Conference, May 9–11, 2012. Int J Lower Extremity Wounds. 2013;12:71–75. doi: 10.1177/1534734613476519. [DOI] [PubMed] [Google Scholar]
- 74.Den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019;204:39–50. doi: 10.1016/j.trsl.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tellechea A, Kafanas A, Leal EC, et al. Increased skin inflammation and blood vessel density in human and experimental diabetes. Int J Low Extrem Wounds. 2013;12:4–11. doi: 10.1177/1534734612474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leal EC, Carvalho E, Tellechea A, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185:1638–1648. doi: 10.1016/j.ajpath.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 79.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Investig. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Theocharidis G, Bhasin S, Kounas K, Bhasin M, Veves A. Single cell RNA-seq analyses of healthy lower extremity skin and diabetic foot ulcers uncover distinct immune landscape of diabetic wound healing. Diabetes. 2018;67:647-P. [Google Scholar]
- 83.Tellechea A, Leal EC, Kafanas A, et al. Mast cells regulate wound healing in diabetes. Diabetes. 2016;65:2006–2019. doi: 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strang H, Kaul A, Parikh U, et al. Chapter 11—role of cytokines and chemokines in wound healing. In: Bagchi D, Das A, Roy S, editors. Wound healing, tissue repair, and regeneration in diabetes. San Diego: Academic; 2020, p. 197–235.
- 85.Bot I, Velden DV, Bouwman M, et al. Local mast cell activation promotes neovascularization. Cells. 2020;2020:9. doi: 10.3390/cells9030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nazari M, Ni NC, Lüdke A, et al. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J Mol Cell Cardiol. 2016;94:32–42. doi: 10.1016/j.yjmcc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Dong X, Chen J, Zhang Y, Cen Y. Mast cell chymase promotes cell proliferation and expression of certain cytokines in a dose-dependent manner. Mol Med Rep. 2012;5:1487–1490. doi: 10.3892/mmr.2012.851. [DOI] [PubMed] [Google Scholar]
- 88.Vangansewinkel T, Geurts N, Quanten K, et al. Mast cells promote scar remodeling and functional recovery after spinal cord injury via mouse mast cell protease 6. FASEB J. 2016;30:2040–2057. doi: 10.1096/fj.201500114R. [DOI] [PubMed] [Google Scholar]
- 89.Gimenez-Rivera V-A, Siebenhaar F, Zimmermann C, Siiskonen H, Metz M, Maurer M. Mast cells limit the exacerbation of chronic allergic contact dermatitis in response to repeated allergen exposure. J Immunol. 2016;197:4240–4246. doi: 10.4049/jimmunol.1600236. [DOI] [PubMed] [Google Scholar]
- 90.Balsam LB, DeAnda A., Jr The mast cell demystified: a novel target for anti-inflammatory strategies after circulatory arrest? J Thorac Cardiovasc Surg. 2017;153:77. doi: 10.1016/j.jtcvs.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 91.de Souza Junior DA, Santana C, Vieira GV, Oliver C, Jamur MC. Mast cell protease 7 promotes angiogenesis by degradation of integrin subunits. cells. 2019;8:349. doi: 10.3390/cells8040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, et al. Current therapeutic strategies in diabetic foot ulcers. Medicina. 2019;55:714. doi: 10.3390/medicina55110714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regenerat. 2008;16:226–233. doi: 10.1111/j.1524-475X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 94.Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One. 2014;9:e85226. doi: 10.1371/journal.pone.0085226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tellechea A, Bai S, Dangwal S, et al. Topical application of a mast cell stabilizer improves impaired diabetic wound healing. J Invest Dermatol. 2020;140(901–911):e911. doi: 10.1016/j.jid.2019.08.449. [DOI] [PubMed] [Google Scholar]
- 96.Kouhkheil R, Fridoni M, Abdollhifar M-A, et al. Impact of photobiomodulation and condition medium on mast cell counts, degranulation, and wound strength in infected skin wound healing of diabetic rats. Photobiomodul Photomed Laser Surg. 2019;37:706–714. doi: 10.1089/photob.2019.4691. [DOI] [PubMed] [Google Scholar]
- 97.Babaei S, Ansarihadipour H, Nakhaei M, et al. Effect of Omegaven on mast cell concentration in diabetic wound healing. J Tissue Viabil. 2017;26:125–130. doi: 10.1016/j.jtv.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin PJ, Cain JD, Titunick MB, Sassani JW, Zagon IS. Topical naltrexone is a safe and effective alternative to standard treatment of diabetic wounds. Adv Wound Care. 2017;6:279–288. doi: 10.1089/wound.2016.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orenstein S, Saberski E, Klueh U, Kreutzer D, Novitsky Y. Effects of mast cell modulation on early host response to implanted synthetic meshes. Hernia. 2010;14:511–516. doi: 10.1007/s10029-010-0680-1. [DOI] [PubMed] [Google Scholar]