Abstract
Breast cancer is a common type of cancer in females. Our previous studies indicated that leucine aminopeptidase 3 (LAP3) promotes migration and invasion of breast cancer cells. Vimentin is a mesenchymal marker, and its upregulation represents the promotion of epithelial–mesenchymal transition. In this study, we found that LAP3 and vimentin were highly expressed in breast cancer tissues, and the overexpression of LAP3 in breast cancer cells promoted the expression of vimentin. Western blot analysis indicated that the overexpression of LAP3 upregulated the phosphorylation of Erk1/2. MEK inhibitor PD98059 downregulated the expression of vimentin, matrix metalloproteinase-2/9 (MMP-2/9), and fascin through the inhibition of Erk1/2 activity. We hypothesized that LAP3 promoted tumor migration and invasion by upregulating vimentin. The knockdown of vimentin resulted in the inhibited migration and invasion of MDA-MB-231 and MDA-MB-468 cells. The expression of MMP-2/9 and fascin could also be downregulated. In conclusion, vimentin might play an important role in the promotion of breast cancer metastasis by LAP3.
Keywords: Leucine aminopeptidase 3 (LAP3), Epithelial–mesenchymal transition (EMT), Vimentin, Metastasis
Introduction
Breast cancer is a common malignancy. Although great progress has been made in the treatment of breast cancer, the long-term survival rate of patients with breast cancer after operation or chemotherapy remains poor due to the high rate of recurrence and metastasis. Metastasis of breast cancer is the leading cause of death in patients (Lindsey et al. 2015; Castillo et al. 2016; Bhandari et al. 2018).
Leucine aminopeptidase 3 (LAP3) is an exopeptidase that catalyzes the hydrolysis of leucine residues from the amino terminus of a protein or peptide substrate (Tsujimoto et al. 2008). LAP3 is involved in the malignant development of breast cancer, endometrial cancer, ovarian cancer, esophageal squamous cell carcinoma, glioma cell carcinoma, and hepatocellular carcinoma; LAP3 is also associated with the proliferation, migration, invasion, and angiogenesis of tumor cells (Huang et al. 2009; Tian et al. 2014; Zhang et al. 2014; Wang et al. 2015; He et al. 2014; Fang et al. 2019). LAP3 is highly expressed in breast cancer tissues and promotes the migration and invasion of breast cancer cells by activating different signaling pathways (Fang et al. 2019).
Epithelial–mesenchymal transition (EMT) is a key process involved in cancer invasion and metastasis. Therefore, the prevention of EMT is a novel therapeutic strategy against tumor metastasis (Polyak and Weinberg 2009). EMT can induce various biochemical changes in polarized epithelial cells and cause them to express mesenchymal cell phenotypes, including high mobility, invasiveness, and enhanced anti-apoptotic properties (Thiery et al. 2009; Zeisberg and Neilson 2009). Loss of epithelial cell adhesion markers and acquisition of mesenchymal markers are important markers of EMT. Epithelial cell adhesion markers include E-cadherin, EpCAM, cytokeratin, and claudin-1. Mesenchymal markers include N-cadherin, vimentin, α-SMA, and fibronectin. Several transcription factors, including slug, SIP1, and twist, are involved in the regulation of EMT (Kang and Massagué 2004; Thiery et al. 2009). In addition, EMT can be regulated by various signaling networks, including mitogen-activated protein kinase (MAPK), PI3K/Akt, TGF-β, Wnt/β-catenin, and Notch pathways (Larue and Bellacosa 2005; Willis and Borok 2007; Turley et al. 2008; Craene and Berx 2013).
Vimentin is a 57 KD, type III intermediate filament protein, which plays an important role in the deformation and movement of tumor cells. It is found in the developmental stages of mesenchymal cells in various tissues and maintains the integrity of cells and tissues (Coulombe and Wong 2004). The MEK/Erk1/2 pathway is a part of the classic MAPK signaling pathway, which controls various cellular processes (such as proliferation, survival, differentiation, and movement) and is usually activated in human tumors (Sun et al. 2015). Fascin is an important component of the cytoskeleton and is closely related to the migration tumor cells (Adams 2004; Han et al. 2016). The increased expression of matrix metalloproteinase (MMP)-2 and MMP-9 is associated with aggressive forms of cancer, including colorectal cancer, breast cancer, ovarian cancer, and melanoma (Richard et al. 2001; Saito et al. 2004; Mendes et al. 2005; Zhang et al. 2005; Kessenbrock et al. 2010). In many tumor cells, the increased expression of vimentin, MMPs, and fascin is associated with increased metastatic potential (Li et al. 2010; Gutschalk et al. 2013). On the basis of our earlier results, the present study aimed to investigate the relation of EMT to LAP3 in the promotion of the migration and invasion of breast cancer cells and to elucidate the mechanism involved.
Materials and methods
Cell culture and Lentivirus transfection
Human breast cancer cells MDA-MB-231 and MDA-MB-468 were obtained from the Cell Bank of Shanghai (Shanghai, China). MDA-MB-231 was cultured in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% fetal calf serum (FCS) and MDA-MB-468 in RPMI-1640 supplemented with 15% FCS. All cell lines were cultured at 37 °C in a humidified atmosphere of 5% CO2.
MDA-MB-231 cell lines were infected with a recombinant Lentivirus vector to overexpress or knock down LAP3, and all lentiviruses were synthesized by Genechem (Shanghai, China). The target for human LAP3 shRNA was 5′-CCCAAGTCTTGGATTGAGGAA-3′. Cells were selected using puromycin for 2 weeks and subjected to analysis after showing stability (Fang et al. 2019).
Western blot analysis
Total protein was extracted from tissues and cells with RIPA buffer (Solarbio, China), and protein concentration was quantified using the Bradford method. Approximately 30 μg of protein extract from each sample was separated by SDS–polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore, USA) (Dou et al. 2017). The membranes were blocked with 5% skimmed milk and incubated separately with antibodies against LAP3 (1:1000; Santa Cruz Biotechnology, USA), fascin (1:1000; Santa Cruz Biotechnology, USA), MMP-2 (1:500; Santa Cruz Biotechnology, USA), MMP-9 (1:500; Santa Cruz Biotechnology, USA), vimentin (1:1000; Santa Cruz Biotechnology, USA), E-cadherin (1:500; Santa Cruz Biotechnology, USA), N-cadherin (1:500; Santa Cruz Biotechnology, USA), p-Erk1/2 (1:1000; Santa Cruz Biotechnology, USA), p-Erk1/2 (1:1000; Santa Cruz Biotechnology, USA), Erk1/2 (1:1000; Santa Cruz Biotechnology, USA), and β-actin (1:5000; Santa Cruz Biotechnology, USA) at 4 °C overnight. The following day, membranes were washed in TBST and were incubated with secondary antibodies (1:5000 dilution) for 1 h at room temperature. Then, the membranes were visualized by enhanced chemiluminescence (Millipore, USA) and analyzed using the Image J software.
Human tissue samples were obtained from the affiliated hospital of Weifang Medical University. The adjacent tissue of breast cancer at least 5 cm away from the edge of the tumor is normal breast tissue with pathological confirmation. For the use of these clinical materials, prior patients’ consent and approval from the Institutional Research Ethics Committee were obtained, the approval number: 2019SL061.
siRNA transfection
The RNA interference technique was used to downregulate vimentin in MDA-MB-231 and MDA-MB-468 cells. Control siRNA and vimentin-specific siRNA were synthesized in duplex (Sangon Biotech, China). The sequences were as follows: siVIM#1, 5′-GCAGGAUGAGAUUCAGAAUTT-3′; siVIM#2, 5′-GAGGGAAACUAAUCUGGAUTT-3′; negative control (NC) siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′.
siRNA (100 pmol) was transfected using Lipofectamine®2000 (5 µL) transfection reagent (Life Technologies, USA) and serum-free RPMI-1640 in a six-well plate. At 6 h after transfection, siRNA was removed by changing the medium with RPMI-1640 with 10% FBS. After 48 h of transfection, we performed Western blot to clarify whether the transfection of siRNA downregulated vimentin protein expression and to determine the knockdown efficiency.
Cell migration assays
The cells were collected at logarithmic growth phase and inoculated with 1 × 105/mL cells per well in a six-well plate. The layer of cells was scraped with a 20–200 µL micropipette tip to create a wound of 1 mm width. The cells were washed twice with PBS and replaced with serum-free medium. At 0, 24, and 48 h, pictures of the migrated cells were captured under a microscope (IX81, Olympus, Japan). The images were processed using the Image-J software to determine the area of the gap. Migration rate = (initial area − area at a certain point in time)/initial area × 100%.
Invasion assays
Cell invasion was analyzed with the aid of a Transwell (8 µm pore size; Corning Incorporated, Corning, USA). Matrigel (BD Biosciences, USA) was diluted with serum-free RPMI-1640 in 1:10, and 50 µL of the solution was used to coat the upper invasion surface for 2 h. Cells (1 × 105) suspended in 200 µL of serum-free medium were seeded onto the upper compartment of the Transwell, and the lower chambers were filled with medium containing 10% FCS in a 24-well plate. After incubation for 24 h, the non-invaded cells and gel in the upper chamber were carefully removed with a cotton swab. The invaded cells on the lower surface were fixed with methanol and stained with 1 mg/mL crystal violet dye. Images of the invaded cells were captured, and the number of cells was counted under a microscope (IX81, Olympus, Japan).
Statistics
All experiments were repeated at least three times with similar results, and measurements from distinct samples were always taken. All data were presented as mean ± standard error of mean. Comparison was performed using two-tailed student’s t-test or one-way ANOVA. Differences with P < 0.05 were considered statistically significant.
Result
Overexpression of LAP3 in breast cancer tissues and cells promotes the expression of vimentin
Previous immunohistochemical results showed that LAP3 is strongly positively expressed in invasive ductal carcinoma of breast (Fang et al. 2019). In the current study, we found that LAP3 and vimentin were strongly overexpressed in breast cancer tissues. The expression of LAP3 and vimentin was relatively weakly positive in the adjacent tissues of breast cancer (Fig. 1a). Western blot data indicated that the overexpression or knockdown LAP3 could promote or inhibit the expression of vimentin in MDA-MB-231, respectively, but had minimal effect on the expression of E-cadherin and N-cadherin (Fig. 1b).
Fig. 1.
Expression of LAP3 and EMT markers in breast cancer tissues and cells. Western blot analysis showed the expression of LAP3 and vimentin in two groups: breast cancer adjacent tissues and breast cancer tissues (a). Western blot analysis was used to detect the expression of EMT markers (vimentin, E-cadherin, and N-cadherin) in MDA-MB-231 cells with LAP3 overexpression or knockdown (b)
LAP3 promotes the expression of vimentin, MMP-2/9, and fascin through the MEK/Erk1/2 signaling pathway
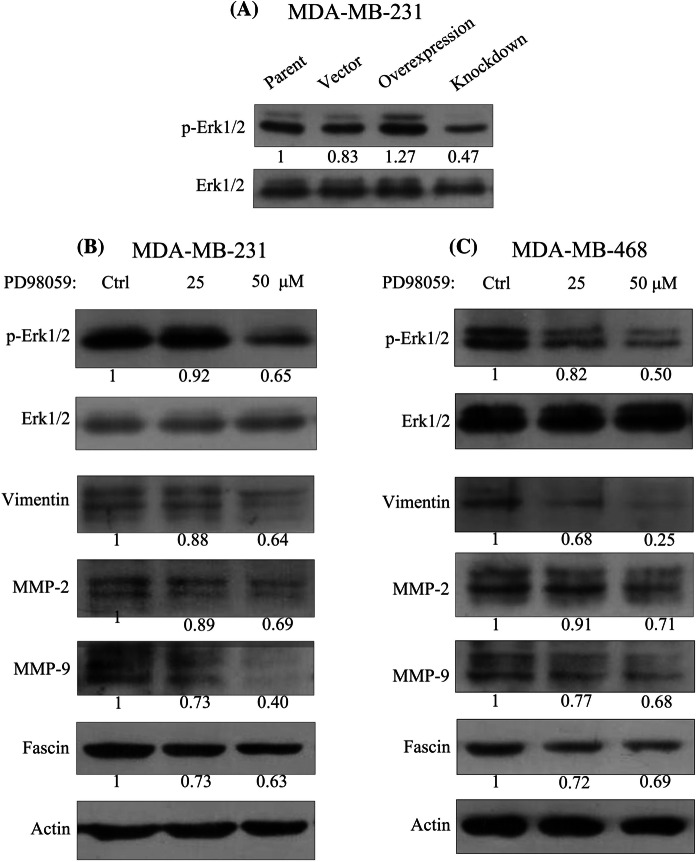
The overexpression of LAP3 could upregulate the phosphorylation of Erk1/2, whereas the knockdown of LAP3 could downregulate the phosphorylation of Erk1/2 in MDA-MB-231 cells (Fig. 2a). Our previous results indicated that LAP3 promotes the expression of fascin and MMP-2/9 and the migration and invasion of breast cancer cells (Fang et al. 2019). PD98059 (Beyotime, China) is a potent, selective, and cell-permeable inhibitor of MEK1 and MEK2. In this study, PD98059 could inhibit the phosphorylation of Erk1/2 and decrease the expression of MMP-2/9, fascin, and vimentin in MDA-MB-231 and MDA-MB-468 cells (Fig. 2b, c).
Fig. 2.
LAP3 promotes the expression of vimentin, MMP-2/9, and fascin through the MEK/Erk1/2 signaling pathway. The phosphorylation of p-Erk1/2 was detected by immunoblotting in MDA-MB-231 cells with LAP3 overexpression or knockdown (a). The expression of p-Erk1/2, vimentin, MMP-2/9, and fascin was detected by immunoblotting after the treatment of MEK/Erk1/2 inhibitor PD98059 (Ctrl, 25 μM, 50 μM) for 48 h in MDA-MB-231 (b) and MDA-MB-468 (c) cells
Knockdown of vimentin expression inhibits the expression of MMP-2/9 and fascin and the migration and invasion of breast cancer cells
The silencing efficiency of siRNA–vimentin in MDA-MB 231 (Fig. 3a) and MDA-MB 468 (Fig. 3b) cells was examined by Western blot. The results showed that siVIM#2 had a good knockdown effect in the two sequences we designed. Treatment of breast cancer cells with LAP3 inhibitors can reduce the ability of cell migration and invasion (Fang et al. 2019). We speculated that vimentin played an important role in promoting breast cancer cell migration and invasion by LAP3. In MDA-MB-231 and MDA-MB-468 cells with vimentin knockdown, the migration and invasion of cells were markedly inhibited (Fig. 4a–f). Western blot data indicated that the silencing of vimentin could inhibit the expression of MMP-2/9 and fascin in MDA-MB-231 (Fig. 3c) and MDA-MB-468 (Fig. 3d) cells. These results demonstrated that the knockdown of vimentin inhibited the expression of MMP-2/9 and fascin and the migration and invasion of breast cancer cells.
Fig. 3.
Knockdown of vimentin expression inhibits the expression of MMP-2/9 and fascin. The protein levels of vimentin were examined in siRNA (siVIM) expressing stable siVIM#1 and siVIM#2 clones for various siRNA sequences in MDA-MB-231 (a) and MDA-MB-468 (b) cells. NC served as a control. The protein levels of MMP-2/9 and fascin in control (NC) and vimentin knockdown stable clones in MDA-MB 231 (c) and MDA-MB 468 (d) cells were examined via Western blot. Actin served as an internal control
Fig. 4.
Knockdown of vimentin expression inhibits the migration and invasion of breast cancer cells. Representative images of a wound healing assay. The monolayer was scraped using pipette tips. The images of MDA-MB 231 (a) and MDA-MB 468 (c) cells were captured 0, 24, and 48 h after scraping. Quantitative data of the migration rate of MDA-MB 231 (b) and MDA-MB 468 (d) cells are shown in the right panel. The bars indicate mean ± SEM. *P < 0.05 and **P < 0.01. Transwell assays were used to investigate the invasion of MDA-MB 231 (e) and MDA-MB 468 (f) cells with altered expression of vimentin. The data represent the mean number of invaded cells from five different fields. The bars indicate mean ± SEM. *P < 0.05 and **P < 0.01
Discussion
LAP3 expression is positively correlated with lymph node metastasis and tumor grade (Fang et al. 2019). We found that breast cancer tissues with high expression of LAP3 also had high expression of vimentin. LAP3 can increase the expression of fascin through the p38-Hsp27 pathway to promote the migration of breast cancer cells, and can also affect the PI3K-Akt pathway to increase the expression of MMP-2/9 to further increase the invasion of breast cancer cells and promote tumor metastasis (Fang et al. 2019). The key to understanding malignant tumor metastasis is that EMT transformation is inextricably linked to invasion and migration of tumor cells (Thiery et al. 2009; Zeisberg and Neilson 2009). As previously reported, the expression levels of epithelial and mesenchymal markers are down- and upregulated in the overexpressed LAP3 tumor cells (Tian et al. 2014; Zhang et al. 2014; He et al. 2014). We speculated that LAP3 might be closely related to the promotion of breast cancer metastasis by regulating EMT transformation. Breast tumor cells were selected in the current study as the cell model to observe the effects of the overexpression or silencing of LAP3 on EMT. LAP3 could promote the expression of mesenchymal marker vimentin in breast cancer cells but had minimal effect on epithelial marker E-cadherin and mesenchymal marker N-cadherin. Therefore, LAP3 might affect the EMT transformation of breast cancer through the mediation of vimentin and further affect the metastasis of malignant tumors.
The Erk1/2 pathway mediates tumor metastasis via regulating the expression and activity of MMPs. This pathway plays a critical role in degrading ECM, which is an important step in tumor metastasis (Cao et al. 1998; Luo et al. 2009). The expression of MMP-2/9 and fascin was upregulated in MDA-MB-231 and MCF7 cells with the overexpression of LAP3 (Fang et al. 2019). As previously confirmed, the activation of Erk1/2 and the expression of MMP2 and vimentin can be inhibited by PD98059 (a specific inhibitor of MEK activation) in MCF-7 breast cancer cells (Li et al. 2017). In the current study, PD98059 could cause a dose-dependent decrease in the level of p-Erk1/2. By contrast, the level of p-Erk1/2 increased in MDA-MB-231 LAP3 overexpression cells. The knockdown of LAP3 could reduce the level of p-Erk1/2 in MDA-MB-231 cells. In addition, PD98059 could reduce the expression of vimentin, MMP-2/9, and fascin in MDA-MB-231 and MDA-MB-468 cells. Therefore, LAP3 might promote the expression of vimentin, MMP-2/9, and fascin through the MEK/Erk1/2 signaling pathway.
Vimentin is a major component of the intermediate filament cytoskeleton and is known as a marker of EMT in cancer; vimentin is also associated with the invasion and metastasis of cancer cells (Coulombe and Wong 2004; Mendez et al. 2010). The positive expression of vimentin is highly correlated with poor disease-free survival and overall survival in patients with breast cancer. Vimentin expression is also highly correlated with primary tumors and metastatic lymph nodes (Yamashita et al. 2015). The knockdown of vimentin also shows impaired wound healing ability in MDA-MB-231 cells and impaired invasive ability in renal cancer cells (Enokida et al. 2012; Liu et al. 2015). Vimentin also regulates the expression of slug (an EMT-associated transcription factor) to further enhance the EMT phenotype and the malignant degree of cancer (Divya et al. 2015; Virtakoivu et al. 2015). Vimentin, slug, and Erk2 are coexpressed in the majority of triple-negative breast cancers and are important regulators of the migration, invasion, and metastasis of breast cancer cells (Liu et al. 2015). In the current study, the knockdown of vimentin reduced the migration and invasion of breast cancer cells. The results suggested that vimentin affected the migration and invasion by regulating MMP-2/9 and fascin.
In summary, vimentin may be an important part of LAP3 in promoting breast cancer metastasis. We find that LAP3 promotes migration and invasion of breast cancer cells through upregulation of vimentin expression through MEK/Erk1/2 signaling pathway. However, further studies on vimentin and transcription factors are needed to confirm the activity of this pathway and its potential effect on the progression, metastasis and maintenance of human breast cancer cells.
Funding
This work was supported by Project of Shandong Province Higher Educational Science and Technology Program (J17KA255), Foundation of Weifang Scientific Committee (2017YX064), and University Students Science and Technology Innovation Fund Project (201910438027X, KX2019025).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuejuan Wang—First author
Contributor Information
Xuejuan Wang, Email: 1532059394@qq.com.
Fang Yan, Email: yanfang303@163.com.
Chunyan Fang, Email: chunyanfang@163.com.
References
- Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590–596. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Xia E, Zhou Y, Guan Y, Xiang J, Kong L, Wang Y, Yang F, Wang O, Zhang X. ITGA7 functions as a tumor suppressor and regulates migration and invasion in breast cancer. Cancer Manag Res. 2018;10:969–976. doi: 10.2147/CMAR.S160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, et al. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273:11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- Castillo LF, Tascón R, Huvelle MAL, Novack G, Peters MG. Glypican-3 induces a mesenchymal to epithelial transition in human breast cancer cells. Oncotarget. 2016;7:60133–60154. doi: 10.18632/oncotarget.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. doi: 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- Craene BD, Berx G. Regulatory networks defining emt during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Divya S, Srivastava SK, Chaudhuri TK, Ghanshyam U. Multifaceted role of matrix metalloproteinases (mmps) Front Mol Biosci. 2015;2:19. doi: 10.3389/fmolb.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C, Fang C, Zhao Y, Fu X, Zhang Y, Zhu D, et al. Bc-02 eradicates liver cancer stem cells by upregulating the ros-dependent dna damage. Int J Oncol. 2017;51:1775–1784. doi: 10.3892/ijo.2017.4159. [DOI] [PubMed] [Google Scholar]
- Enokida H, et al. Tumor suppressive microrna?138 contributes to cell migration and invasion through its targeting of vimentin in renal cell carcinoma. Int J Oncol. 2012;41:805–817. doi: 10.3892/ijo.2012.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Zhang J, Yang H, Peng L, Wang K, Wang Y, Zhao X, Liu H, Dou C, Shi L, Zhao C, Liang S, Li D, Wang X. Leucine aminopeptidase 3 promotes migration and invasion of breast cancer cells through upregulation of fascin and matrix metalloproteinases-2/9 expression. J Cell Biochem. 2019;120:3611–3620. doi: 10.1002/jcb.27638. [DOI] [PubMed] [Google Scholar]
- Gutschalk CM, Yanamandra AK, Linde N, Meides A, Depner S, Mueller MM. Gm-csf enhances tumor invasion by elevated mmp-2, -9, and -26 expression. Cancer Med. 2013;2:117–129. doi: 10.1002/cam4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Huang J, Liu B, Xing B, Bordeleau F, Reinhart-King CA, Li W, Zhang JJ, Huang XY. Improving fascin inhibitors to block tumor cell migration and metastasis. Mol Oncol. 2016;10:966–980. doi: 10.1016/j.molonc.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Huang Q, Qiu X, Liu X, Wang D. Lap3 promotes glioma progression by regulating proliferation, migration and invasion of glioma cells. Int J Biol Macromol. 2014;72C:1081–1089. doi: 10.1016/j.ijbiomac.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Huang H, Tanaka H, Hammock BD, Morisseau C. Novel and highly sensitive fluorescent assay for leucine aminopeptidases. Anal Biochem. 2009;391:11–16. doi: 10.1016/j.ab.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Massagué Joan. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/akt pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guo Y, Duan L, Hu X, Zhang X, Hu J, et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of erk signaling. Oncotarget. 2017;8:33694–33703. doi: 10.18632/oncotarget.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey AT, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Liu CY, Lin HH, Tang MJ, Wang YK. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6:15966–15983. doi: 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Liang F, Zhang ZY. Prl1 promotes cell migration and invasion by increasing mmp2 and mmp9 expression through src and erk1/2 pathways\r, ? Biochemistry. 2009;48:1838–1846. doi: 10.1021/bi8020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes O, Kim HT, Stoica G. Expression of mmp2, mmp9 and mmp3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22:237–246. doi: 10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- Mendez MG, Kojima SI, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Richard EBS, Seftor Elisabeth A, Koshikawa Naohiko, Meltzer Paul S, Hendrix Mary J C. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- Saito T, Mizumoto H, Tanaka R, Satohisa S, Adachi K, Horie M, Kudo R. Overexpressed progesterone receptor form B inhibit invasive activity suppressing matrix metalloproteinases in endometrial carcinoma cells. Cancer Lett. 2004;209:237–243. doi: 10.1016/j.canlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu WZ, Liu T, Feng X, Zhou HF. Signaling pathway of mapk/erk in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:1–5. doi: 10.3109/10799893.2014.922576. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tian SY, Chen SH, Shao BF, Cai HY, Zhou Y, Zhou YL, Xu AB. Expression of leucine aminopeptidase 3 (LAP3) correlates with prognosis and malignant development of human hepatocellular carcinoma (HCC) Int J Clin Exp Pathol. 2014;7:3752–3762. [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M, Goto Y, Maruyama M, Hattori A. Biochemical and enzymatic properties of the m1 family of aminopeptidases involved in the regulation of blood pressure. Heart Fail Rev. 2008;13:285–291. doi: 10.1007/s10741-007-9064-8. [DOI] [PubMed] [Google Scholar]
- Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial–mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat Rev Clin Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtakoivu R, Mai A, Mattila E, De Franceschi N, Imanishi SY, Corthals G, et al. Vimentin-erk signaling uncouples slug gene regulatory function. Cancer Res. 2015;75:2349–2362. doi: 10.1158/0008-5472.CAN-14-2842. [DOI] [PubMed] [Google Scholar]
- Wang X, Shi L, Deng Y, Qu M, Mao S, Xu L, Xu W, Fang C. Inhibition of leucine aminopeptidase 3 suppresses invasion of ovarian cancer cells through down-regulation of fascin and MMP-2/9. Eur J Pharmacol. 2015;768:116–122. doi: 10.1016/j.ejphar.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. Tgf-beta-induced emt: mechanisms and implications for fibrotic lung disease. Am J Physiol. 2007;293:525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Tokunaga E, Inoue Y, Tanaka K, Nakashima Y, Ando K, et al. Clinical significance of co-expression of E-cadherin and vimentin in invasive breast cancer. Cancer Cytopathol. 2015;120:230–237. [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yan L, Tsang PCW, Moses MA. Matrix metalloproteinase-2 (mmp-2) expression and regulation by tumor necrosis factor alpha (tnfα) in the bovine corpus luteum. Mol Reprod Dev. 2005;70:122–132. doi: 10.1002/mrd.20196. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Shi H, Li M, Wang H. Overexpression of leucine aminopeptidase 3 contributes to malignant development of human esophageal squamous cell carcinoma. J Mol Histol. 2014;45:283–292. doi: 10.1007/s10735-014-9566-3. [DOI] [PubMed] [Google Scholar]