Abstract
Background and Purpose
To compare cosmesis and local recurrence (LR) of definitive external beam radiation therapy (EBRT) vs brachytherapy (BT) for indolent basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the skin.
Materials and Methods
Studies including patients with T1-2N0 SCCs/BCCs treated with definitive EBRT/BT and ≥10 months follow-up were analyzed. The primary endpoint was post-treatment cosmesis, categorized as “good,” “fair,” or “poor.” The secondary endpoint was LR. Mixed effects regression models were used to estimate weighted linear relationships between biologically equivalent doses with α/β=3 (BED3) and cosmetic outcomes.
Results
A total of 9,965 patients received EBRT and 553 received BT across 24 studies. Mean age was 73 years, median follow-up was 36 months, and median dose was 45 Gy/10 fractions at 4.4 Gy/fraction. At BED3 of 100 Gy, “good” cosmesis was more frequently observed in patients receiving BT, 95% (95% CI: 88-100%) vs 79% (95% CI: 60-82%), p<0.05. Similar results were found for “good” cosmesis at BED3 >100 Gy. No difference in “poor” cosmesis was noted at any BED3. LR was <7% for both at one year.
Conclusion
BT has favorable cosmesis over EBRT for skin SCCs/BCCs at common fractionation regimens. Prospective studies comparing EBRT vs BT are warranted.
Keywords: external beam radiation therapy, brachytherapy, skin cancer, basal cell carcinoma, squamous cell carcinoma, meta-analysis
INTRODUCTION
Non-melanoma skin cancer is the most commonly diagnosed malignancy in the US [1], and its incidence increases with age [2]. Among these cancers, basal cell carcinoma (BCC) makes up 75-80% of diagnoses and squamous cell carcinoma (SCC) makes up the majority of the remaining cases [3,4]. Most localized (i.e. T1-2 N0) BCCs and SCCs are destroyed locally (excised, desiccated, frozen); however, several factors may preclude surgical extirpation, including patient comorbidities, anticoagulant use, and tumor location near a critical organ (e.g. orbit). Thus, radiation therapy (RT) is an efficacious alternative for localized tumors [5,6].
There are two categories of radiotherapy used for skin BCCs/SCCs: (1) external beam radiotherapy (EBRT) and (2) brachytherapy (BT), which is typically delivered using either radionuclide like Ir-192 (termed radionuclide BT in this work), or a miniature x-ray source (termed electronic brachytherapy, eBT, in this work). Subtypes of EBRT and BT are juxtaposed in Table 1. Generally, eBT is categorized as a type of BT due to its name and because it resembles surface applicator-based BT [7]. However, unlike radionuclide-based BT, eBT involves treatment with miniaturized x-ray sources in the 50-100 kV range, without a radionuclide [8]. Thus, eBT shares more similarities with superficial kV x-rays used in EBRT.
Table 1.
Comparison of treatment modalities in the management of skin cancer.
| Diagnostic x-rays | Superficial x-rays | Orthovoltage x-rays | MV x-rays (i.e. 6 MeV) | MV gamma ray | MeV electrons | Electronic BT (eBT) | BT with Ir-192 | Other BT radionuclide (e.g. I-125, Pd-103, Cs-137) | |
|---|---|---|---|---|---|---|---|---|---|
| Modality | N/A | EBRT | EBRT | EBRT | EBRT | EBRT | BT (per billing codes) | BT | BT |
| Used to treat skin cancer? | No | Yes | Yes | Yes | Yes, historically | Yes | Yes | Yes | No |
| Included in current analysis? | No | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Contemporary availability of technology | Available | Rare | Rare | Widely available. Robust data. | Depends | Widely available. Robust data. | Relatively new, as of ~2010 | Depends | Depends |
| Body of evidence for skin cancer (relative) | N/A | Robust historical data | Robust historical data | Robust historical, contemporary data | Minimal | Robust historical, contemporary data | Nearly non-existent, to meet inclusion criteria | Some historical and contemporary data | N/A |
| Particle | Photon | Photon | Photon | Photon | Gamma, Co-60 decay via beta- | Electron | Photon | Ir-192 decay mostly via beta- | Depends |
| Radionuclide replacement, radioactive waste | No | No | No | No | Yes, half-life = 5.3 y | No | No | Yes, half-life = 74 d | Yes |
| Energy | ~20-150 kV | ~40-200 kV | ~150-500 kV | 6 MV (max) | 1.25 MeV (average), similar to 4 MV x-ray | 6-15 MeV | 10-90 kV | 0.38 MeV, Max 1.09 MeV | Depends |
| Shielded room necessary | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Depends |
| Cone, cutout, block needed? | N/A | Cone | Cone | Blocks / collimator | Cone | Cutout | No | No, but mold or catheter needed | N/A |
| Bolus needed? | N/A | No | No | Yes | Yes | Yes | No | No | N/A |
| dmax | N/A | Skin surface | Skin surface | ~1.5 cm | 0.5 cm | Ē0 / 5 | Skin surface | Catheter surface | N/A |
| 90% isodose line | N/A | 5 mm | 2 cm | ~ 6 cm | 1.5 cm | Ē0 / 3.4-4, 1 cm inward from block edge | < 1cm | < 1cm | Depends |
Abbreviations: BT: brachytherapy; Ē0: initial energy of beam; EBRT: external beam radiation therapy; N/A: not applicable.
Note: Electronic brachytherapy (eBT) is regarded as a type of BT but actually involves x-ray treatment. BT uses a surface applicator, similar to that of superficial kV EBRT. Data for eBT are lacking; thus, it was excluded from this analysis.
In 2010, eBT was introduced to for the treatment of skin SCCs and BCCs, and there has been a 20-fold increase in its use from 2011 to 2013, most likely due to its higher reimbursement [9]. However, Current Procedural Terminology codes for eBT were modified in 2016 and have made the criteria for reimbursement more stringent and also less profitable so it remains to be seen if eBT will continue to gain a foothold in skin BCC and SCC treatment [10]. As of 2017, the National Comprehensive Cancer Network (NCCN) and the American Society of Radiation Oncology (ASTRO) discourage the use of eBT given the lack of evidence regarding its efficacy or toxicity [5,11].
Although the proponents of skin BT (particularly those using eBT) report excellent cosmesis and local recurrence rates at short median follow-up times [12], a formal comparison of BT vs EBRT has not been performed. A 2017 meta-analysis reported favorable cosmesis with various fractionation regimens [13], 50Gy/15 fractions, 36.75Gy/7 fractions, or 35Gy/5 fractions; however, EBRT and BT were not juxtaposed. The purposes of the current study are to compare the cosmetic outcomes (i.e. normal tissue complication probability) and local recurrence (i.e. tumor control probability) of EBRT and BT. The results will provide patients, physicians, and payors evidence regarding the safety and effectiveness of the modalities and facilitate the treatment decision making process.
METHODS AND MATERIALS
Evidence acquisition
The inclusion criteria for the literature search was defined using the Population, Intervention, Control, Outcome, Study Design (PICOS; Supplementary Table 1) approach [14]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Supplementary Figure 1) literature selection protocol was used for article selection. Further, the meta-analysis of Observational Studies in Epidemiology (MOOSE; Supplementary Table 2) were used [15]. Clinical trials, prospective studies, retrospective studies, and case reports published in English at any time up to January 31, 2017 were searched in PubMed and MEDLINE. Details are reported in Supplementary Text 1. Minimum follow-up time was set at 10 months to capture recurrences [16]. Levels of evidence were assigned to each included study based on Centre of Evidence Based Medicine (CEBM) criteria. This meta-analysis shares similar methods for evidence acquisition and statistical analysis with the previously published study on hypofractionated RT for T1-2 non-melanoma skin cancer [17]; however, inclusion criteria were expanded to capture studies with shorter follow-up and BT. Studies in eBT were excluded systematically due to limited long-term data; in the future, eBT may be included as its own category as more data become available.
Outcomes Measures
Cosmesis
The primary endpoint was cosmesis because local control is generally >90%; thus, most clinicians are more concerned about cosmesis than control, and the proponents of BT systems tout them as having improved cosmesis over EBRT [9]. We characterized cosmesis discretely in three categories: “good,” “fair,” and “poor.” Most studies reported the presence of moderate-severe toxicities, and these were coded as “poor” for the purposes of our analysis. Two studies reported “excellent” cosmesis, and this was coded as “good” [18,19]. The Radiation Therapy Oncology Group (RTOG) grading system (or an analogous system) was used in coding.
Follow-up time when cosmesis was evaluated was not routinely reported in studies. Cosmesis grades were marked for individual fractionation regimens of each study at the latest time of follow-up available. Cosmesis is expected to worsen over time, and this is important in interpreting the findings of studies with relatively short follow-up times. All data from studies were reviewed and discussed by three of the authors to maintain reporting accuracy.
Local recurrence (LR)
The secondary endpoint, LR, was defined per authors of individual studies. We chose to analyze LR because this is a major outcome of interest for patients being treated with RT, particularly when comparing technologies. Other outcomes (e.g. lymph node metastases, distant metastases) are uncommon in BCCs and superficial SCCs, unless patients are immunocompromised and/or recurrent. Thus, cancer specific mortality is also uncommon [20].
Statistical analysis
Calculation of the biologically equivalent dose (BED) is described in Supplementary Text 1 [21–25]. Weighted mixed effects regression models were used to estimate weighted linear relationships between BED3s and the observed percentages of patients experiencing cosmetic outcomes, with 95% confidence intervals (CIs). In this analysis, BED3 and cosmesis were plotted as continuous variables. To characterize cosmesis at fractionation regimens with different BED3s that represented the gamut of the regimens used in the literature, we calculated the 95% confidence interval for cosmesis at each of three discrete values that represent this entire spectrum: BED3 of 80 Gy, 100 Gy, and 120 Gy.
RESULTS
Treatment characteristics, outcomes, and cosmesis of individual EBRT studies are listed in Table 2, and those of BT studies in Table 3. Table 4 lists cosmesis outcomes of BT vs EBRT at three dose levels that span the gamut of fractionations used.
Table 2.
EBRT outcomes and toxicity
| Study | CEBM Level of Evidence | n | Technique, energy | Total dose (Gy) | Gy/fraction | Mean FU (mo) | LR 1y (%) | LR 5y (%) | OS | Cosmesis rating (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Good | Fair | Poor | ||||||||||
| Abbatucci, 1988 | 2b | 675 | Muller RT 100, 1000R/min | 31 | 10.2 | 24 | 1 | 5.3 | NR | 47.5 | 50.1 | 2.4 |
| Avril, 1997 | 1b | 20 | 85-250 kV | 60-65 | 2-4 | 41 | 1.2 | 7.5 | 72.6 | 76 | 20 | 3 |
| Caccialanza, 2005 | 2b | 110 | 55-120 kV | 45-70 | 5 | 28.8 | NR | 10.4 | 98.2 | 74.8 | 13.5 | 1.8 |
| Caccialanza, 2009 | 2b | 671 | 55-120 kV | 30-75 | 5 | 38 | 2.5 | 5.7 | NR | 74.5 | 22.4 | 2.4 |
| 60+23 | 2+5 | 38 | ||||||||||
| Lovett, 1990 | 2b | 325 | kV, MV, and MeV | 40-60 | 1-4 | 24 | NR | 14 | NR | 88.3 | 3.0 | 6.8 |
| Mazeron, 1989 | 2b | 71 | MV, Mev, Co-60 | 30.6-70 | 2-10.2 | >24 | 19a | NR | NR | 52 | 37 | 11 |
| 639 | ~100kV | 4.8a | NR | NR | 61 | 22 | 17 | |||||
| Van Hezewijk, 2010 | 2b | 159 | 4-12 MeV | 54 | 3 | 42.8 | 1.5 | 2.5 | NR | 62 | 13 | 25 |
| 275 | 44 | 4 | 42.8 | 2 | 3.9 | NR | 67 | 33 | 0 | |||
| Ashby, 2001 | 2b | 360 | 5-7 MeV, MV | 24 | 6 | 12 | 0.7 | 4.3 | 98.9 | NR | NR | NR |
| Chan, 2007 | 2b | 464 | 45-100kV | 22.5 | 22.5 | 18 | 1 | 8 | NR | NR | NR | NR |
| 499 | 20 | 20 | 18 | 3 | 10 | NR | NR | NR | NR | |||
| Cognetta, 2012 | 2b | 1715 | 80 kV | 35 | 7 | 31.5 | 1.1 | 5 | NR | NR | NR | NR |
| Hernandez, 2007 | 2b | 710 | 14-50 kV | 45-56 | 4 | 12 | 1.9 | 5.9 | NR | NR | NR | NR |
| 36 | 9 | |||||||||||
| Hall, 1986 | 2c | 19 | 130 kV | 35 | 7 | 12 | 0 | NR | NR | NR | NR | NR |
| 30 | 38 | 3.8 | 24 | 6.7 | ||||||||
| Grossi Marconi, 2016 | 2b | 521 | 80-200 kV | 50 | 3 | 44 | NR | 0.8 | NR | NR | NR | NR |
| 55 | 3 | |||||||||||
| 500 | 60 | 2 | 44 | NR | 2.7 | NR | NR | NR | NR | |||
| Pampena, 2015 | 2b | 275 | 50-300 kV | 37 | 5 | 30.4 | 1.1 | 5.5 | 69.5 | NR | NR | NR |
| 161 | 45 | 3 | 34.3 | 1.2 | 3.7 | 83.9 | NR | NR | NR | |||
| Schulte, 2005 | 2b | 1019 | 10-100 kV | 45 | 5 | 77 | NR | 4.5 | 74.4 | NR | NR | NR |
| 245 | 60 | 5 | NR | 6.9 | 57.1 | NR | NR | NR | ||||
| Silva, 2000 | 2b | 47 | 100-250 kV, MV MeV, Co-60 | 18-20 | 18-20 | 40 | 6.2 | 21.8 | 63.8 | NR | NR | NR |
| 123 | 35 | 7 | ||||||||||
| 68 | 43-45 | 4-5 | ||||||||||
| 41 | 50-65 | 2-3 | ||||||||||
| Tsao, 2002 | 2b | 34 | 75-250 kV, 9-20 MeV | 35 | 7 | 35 | 5 | 15 | 58 | NR | NR | NR |
| 20 | 45 | 5 | ||||||||||
| 10 | 50 | 3 | ||||||||||
| 11 | 20 | 20 | ||||||||||
| Zagrodnik, 2003 | 2b | 148 | 20-50 kV | 40-48 | 8 | 48 | 6.4 | 15.8 | NR | NR | NR | NR |
| 40-52 | 4 | |||||||||||
| 52-60 | 2 | |||||||||||
Abbreviations: BED: biologically equivalent dose; CSS: cancer-specific survival; EBRT: external beam radiation therapy; FU: follow-up; Gy: Gray; n: number of patients per group; NR: not reported; LR: local recurrence; OS: overall survival
Note: Gy and BED rounded to whole numbers. LR rounded to nearest tenth. Combined cells apply to all doses as they were the only results given in their respective studies. Bold line separates studies with cosmesis, above, from studies without cosmesis, below. For technique/energy, kv implies orthovoltage photons, MV implies megavoltage photons, MeV implies electrons, Co-60 implies Cobalt units.
LR at 2 years
Table 3.
HDR-BT outcomes and toxicity
| Study | CEBM Level of Evidence | n | Source | Total dose (Gy) | Gy/fraction | Mean FU (mo) | LR 1y (%) | LR 5y (%) | OS | Cosmesis rating (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Good | Fair | Poor | ||||||||||
| Delishaj, 2015 | 2c | 48 | Ir-192 + applicator | 40 | 5 | 12 | 0 | NR | NR | 98 | 2.1 | 0 |
| 9 | 50 | 5 | 0 | NR | NR | 100 | 0 | 0 | ||||
| Gauden, 2013 | 2b | 236 | Ir-192 + applicator | 36 | 3 | 66 | 2a | NR | NR | 88 | 6.5 | 5.5 |
| Guix, 2000 | 2b | 136 | Ir-192 + mold | 60-65 | 1.8 | 12 | NR | 2.21 | NR | 98 | 0 | 2 |
| 75-80 | 1.8 | |||||||||||
| Somanchi, 2008 | 2c | 25 | Ir-192 + mold | 42.5 | 5.3 | 60 | 0 | 0 | NR | 100 | 0 | 0 |
| Svoboda, 1995 | 2b | 54 | Ir-192 + mold | 32 | 4.5 | 10 | 0 | NR | NR | NR | NR | NR |
| Tormo, 2014 | 2c | 45 | Ir-192 + applicator | 42 | 7 | 47 | NR | 2b | NR | NR | NR | NR |
Abbreviations: BED: biologically equivalent dose; BT: brachytherapy; CSS: cancer-specific survival; FU: follow-up; Gy: Gray; n: number of patients per group; NR: not reported; LR: local recurrence; OS: overall survival
Note: Gy and BED rounded to whole numbers. LR rounded to nearest tenth. Combined cells apply to all doses as they were the only results given in their respective studies. Bold line separates studies with cosmesis, above, from studies without cosmesis, below.
LR at 2 years
LR at 4 years
Table 4.
Cosmesis outcomes of BT vs. EBRT
| BED3=80 | BED3=100 | BED3=120 | Slope┼ | |
|---|---|---|---|---|
| Good cosmesis | ||||
| BT | 94% (82-100%) | 95% (88-100%)* | 99% (90-100%)* | 2.8% p=0.002 |
| EBRT | 79% (60-95%) | 79% (60-82%)* | 68% (60-74%)* | −2.5% p=0.121 |
| Fair cosmesis | ||||
| BT | 4% (0-10%) | 3% (0-9%) | 2% (0-9%)* | −1.7% p=0.010 |
| EBRT | 12% (0-31%) | 18% (8-31%) | 22% (18-28%)* | 3.2% p=0.068 |
| Poor cosmesis | ||||
| BT | 5% (0-9%) | 5% (0-7%) | 4% (0-8%) | −1.3% p=0.005 |
| EBRT | 10% (1-18%) | 8% (2-12%) | 5% (1-7%) | −1.2% p=0.130 |
Note:
denotes p-value < 0.05 for BT vs. EBRT for particular cosmetic outcome, 95% CI in parentheses,
denotes percent change vs 10 unit change in BED
Note that this analysis is performed on all patients in the dataset. The selected dose levels (80 Gy, 100 Gy, 120 Gy) represent the gamut of the doses, as shown in Figure 1; further, there are no “good”/”fair”/”poor” cosmesis all.
Study characteristics
The meta-analysis included 10,518 patients (n) from 24 studies (N) [12,16,18,19,26–45]. The patients were treated from year 1985 to 2016. There were 9,965 patients treated with definitive EBRT [16,26–42] and 553 treated with BT [12,18,19,43–45]. Only one study was prospective [16]. The studies were from the United States [31,33,34], United Kingdom [30,32,43,44], France [26,42], Germany [36], Italy [12,28,29,35], Australia [18,27], Spain [19,39,45], Canada [37,38], the Netherlands [40], and Switzerland [41]. Overall, patient follow up times ranged from 12-77, median 36 months. For EBRT, median follow-up was 36 months (range: 18-77); for BT, median follow-up was 30 months (range: 10-66). The median patient age range was 73 years (range: 62-84). For EBRT, median age was 74 years (range: 62-81), and for BT, median age was 74 years (range: 67-84). The vast majority of studies included patients with T1-2 BCCs/SCCs, only 4 studies included tumors >T2 (60 patients total) [16,29,36,44]. No study focused on very elderly (>80 years) or immunocompromised patients.
Median dose among all studies was 45 Gy/10 fractions (interquartile range [IQR]: 36 Gy/5 fractions-55 Gy/17 fractions) at 4.4 Gy/fraction (IQR: 3 – 7 Gy); the most hypofractionated was 22.5 Gy/1 fraction using EBRT [30]. For EBRT, median dose was 45 Gy/12 fractions (interquartile range [IQR]: 36 Gy/6 fractions-55 Gy/19 fractions) and mean BED3 was 112 Gy (range: 60-191 Gy). For BT, the median dose was 41 Gy/9 fractions (interquartile range [IQR]: 31.5 Gy/5 fractions-49 Gy/12 fractions) and mean BED3 was 109 Gy (range: 60-153 Gy).
Cosmesis
There were 3,399 patients whose long-term cosmesis was evaluated, 2,945 of whom received EBRT and 454 of whom received BT. The majority of patients in both EBRT and BT groups had “good” cosmetic outcome for any fractionation regimen included in the meta-analysis: the median % of patients with “good” cosmesis was 95% (IQR: 75% - 100%). The median % of patients with “fair” cosmesis was 1% (IQR: 0% - 15%). Notably, there were 675 patients treated to 30.6 Gy in 10.2 Gy/fraction in a single study; of these, 50% developed “fair” cosmesis with this very hypofractionated technique [26]. The median % of patients with “poor” cosmesis was 2% (IQR: 0% - 7%).
Cosmetic results from BEDs representative of the dose fractionation spectrum are shown in Table 4 and Figure 1. “Good” and “fair” cosmesis were similar at BED3 of 80 Gy for BT and EBRT, as evidenced by the overlapping 95% CI regions. At BED3 of 100 Gy, there was a slight benefit for BT over EBRT in terms of “good” cosmesis over “fair” cosmesis: 79% (95% CI: 60-82%) vs 95% (95% CI: 88-100%), p<0.05. At BED3 of 120 Gy, “good” cosmesis was more frequently observed in patients receiving BT, and this difference was more pronounced: 68% (95% CI: 60-74%) vs 99% (95% CI: 90-100%), p<0.05.
Figure 1. SCC and BCC post-radiation cosmesis as a function of BED for BT and EBRT.
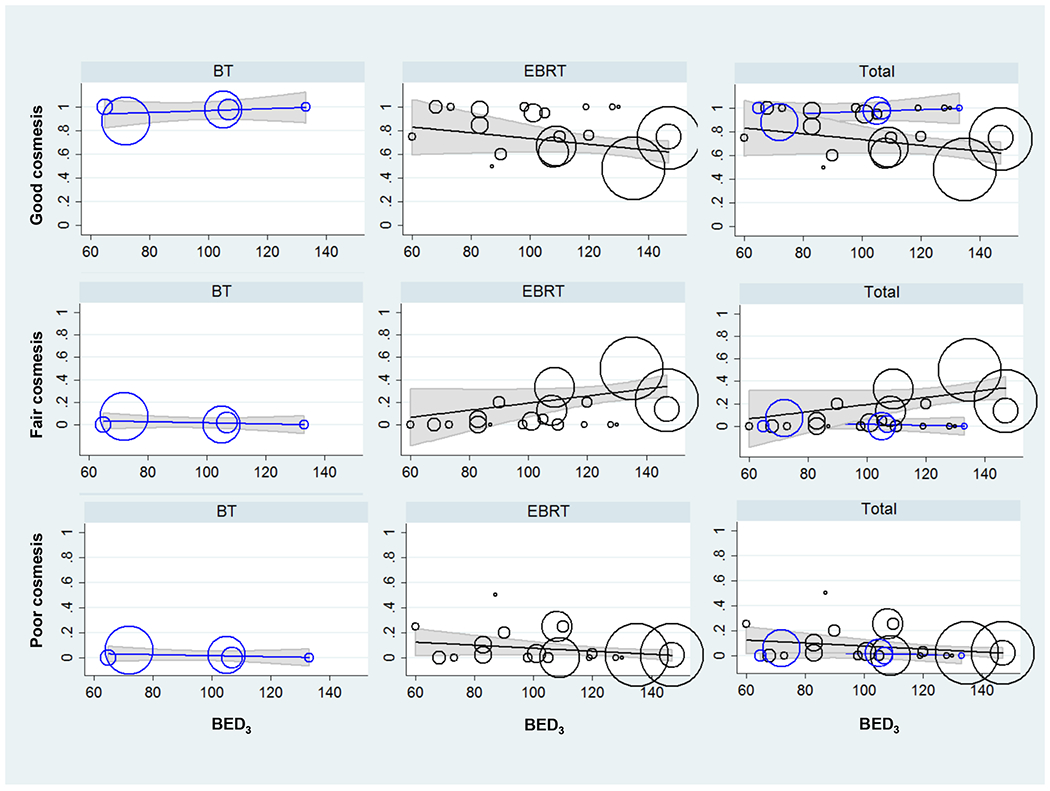
The rates of “good” (upper panel), “fair” (middle panel), and “poor” (lower panel) cosmesis are plotted vs. biologically equivalent doses with α/β=3 (BED3). The % good/fair/poor cosmesis vs BED3 is plotted for brachytherapy (BT, left column), external beam radiation therapy (EBRT, center column), and both techniques co-plotted (right column). The cosmetic outcomes for BT and EBRT were similar up to BED3 of ~100 Gy (as evidenced by overlapping 95% confidence intervals), which is equivalent to about 64 Gy/32 fractions, 55 Gy/20 fractions, 50 Gy/15 fractions. Neither technique was associated with poor cosmetic outcomes, independent of the dose or fractionation. At higher BED3s, e.g. 35 Gy/5 fractions, or BED3 of 120 Gy, BT was associated with higher likelihood of good cosmesis vs. fair cosmesis.
Overall, with increasing BED3, there was a decreasing frequency of “good” cosmesis for EBRT and increasing frequency of “fair” cosmesis. Percent change in cosmesis vs 10 Gy change in BED (slope) of the linear regression for “good” cosmesis in EBRT was -2.5%, p=0.121. Percent change in cosmesis vs 10 Gy change in BED of the linear regression for “good” cosmesis in BT was 2.8%, p=0.002. Slope of the linear regression for “fair” cosmesis in EBRT was 3.2%, p=0.068. Slope of the linear regression for “fair” cosmesis in BT was -1.7%, p=0.010. “Poor” cosmesis was noted in <10% of patients for EBRT and BT for any BED3 (p>0.05).
The most common late toxicities noted were hyperpigmentation and telangiectasias. There were no reports of ulceration or necrosis. There were no Grade 4-5 toxicities or any surgical intervention necessary to correct late toxicity. There was no evidence of increased toxicity for higher doses or more hypofractionated schedules (Figure 1, lower panel).
Outcomes
Overall, LR was <7% for both EBRT and BT at one year; there was too few events to evaluate BT at a longer time point. The 1-year and 5-year LR percentages of individual studies, as well as cosmesis and fractionation regimens, are listed in Tables 2 and 3. The 1- year LR rate was typically <10% for any fractionation regimen. The 5-year LR rate was <20% for any fractionation regimen, and only one study using BT reported these long-term outcomes. The median 1 year LR rate was 2% (IQR: 1-5%) and the 5-year LR rate was 14% (IQR: 7-14%) for all fractionation regimens. For EBRT, the median 1 year and 5 year LR rates were 3% (IQR: 1-6%) and 14% (IQR 6-15%), respectively. For BT, the median 1 year and 5 year LR rates were 0% (IQR: 0-0%) and 2% (IQR: 2-2%), respectively.
DISCUSSION
EBRT and BT (historically delivered with Ir-192) are treatment options for BCCs and SCCs of the skin. eBT has had a dramatic increase in use since 2010, and the NCCN guidelines state that there is insufficient evidence for its use [5]. Despite the stance of the NCCN, no studies comparing the EBRT vs BT (with Ir-192 or eBT) have been published. We performed the first meta-analysis to compare cosmesis and tumor control of EBRT and BT. We found that the rate of “good” cosmesis is improved with BT over EBRT when using common fractionation regimens of 64 Gy/32 fractions, 55 Gy/20 fractions, 50 Gy/15 fractions, which are also endorsed by the NCCN [5]. Among fractionation regimens with higher dose, cosmesis appears to also be superior with BT.
EBRT has been available as a treatment option for indolent skin cancers not amenable to extirpation for longer than BT; thus, data supporting its use for BCC and SCC are more robust. As such, only 6% of patients from studies that met the inclusion criteria were treated with Ir-192 BT [12,18,19,43–45]. Despite this discrepancy, fractionation regimens and median follow-up times were similar between EBRT and BT studies. Three of six BT studies included in our meta-analysis reported median follow-up times of ≤12 months [12,19,44]. Other eBT studies in the studies that did not meet inclusion criteria for this analysis similarly had shorter follow-up times of 10 and 12 months [46,47].
Cancer recurrence rates were similar between EBRT and Ir-192 BT, although most of the included Ir-192 BT studies lacked 5-year LR. Recurrence for skin tumors is best captured when follow-up extends to 4 years [16]. Several studies using EBRT also report a 6-fold increase in LR at 5 years [16,27]. We encourage investigators of eBT to similarly report long-term outcomes of this new technology.
Overall, both EBRT and BT demonstrate similar “good” and “fair” cosmesis at low BED3 of approximately 80 Gy. At higher BED3 of 100 Gy, which encompasses most common definitive fractionation regimens, there is a significantly higher proportion of patients with “good” cosmesis with BT compared to EBRT, 95% (95% CI 88-100%) vs 79% (95% CI: 60-82%), as per Figure 1 and Table 4. Even a higher BED3 of 120 Gy results in significant increase in “fair” cosmesis in patients treated with EBRT over BT.
This difference in “good” and “fair” cosmesis at common fractionation regimens may be attributable to a number of factors. First, there is patient selection that benefits BT. Given the higher reimbursement for BT [9,11], there may be a financial conflict of interest for studies mentioning name brands of the devices used. Further, it is expected that larger sized tumors would be preferentially treated with EBRT, specifically, electron therapy with bolus. Electron therapy would therefore require larger fields (e.g. 4x4 cm) to account for the beam penumbra and setup uncertainty. The idea of the small field is only relevant to an extremely small tumor, and is not applicable to larger tumors. Unfortunately, we are unable to differentiate T1 vs T2 tumors in this analysis. Further, the issues with penumbra and bolus do not apply to orthovoltage therapy and are a reason that it is favored as an EBRT modality to treat skin cancers. We are not able to differentiate electrons from orthovoltage therapy in the current analysis. BT does not have a large penumbra like electron therapy and would be expected to have less setup uncertainty. These differences likely contribute to some of the cosmesis benefits noted of BT over EBRT.
This work has other limitations. First, we based toxicity evaluations on clinician evaluations of patients, using a simple scoring system. Patient evaluation of their own cosmesis was taken into account in only one study [16]. In general, most acute reactions (e.g. erythema, edema, itch) resolve within 3 months; late reactions (e.g. fibrosis, ulceration, necrosis) start to develop after 3 months, with a rising incidence over time. Thus, cosmesis depends on the median follow-up time of each study. Intra- and inter-observer differences may be present. In some studies, assistants were able to help grade toxicity [16,40]. We also did not blindly evaluate photographs of the skin after treatment.
We did not evaluate ethnic subpopulations or patient-reported outcomes, which have been shown to be important in prostate and breast cancer [48–50]. We do not have comorbidity data of these patients; those with peripheral arterial disease and diabetes would be expected to have worse toxicity [51]. We strongly encourage future investigators to provide detailed reports of toxicities among their patients, and for basic scientists to identify biomarkers of RT toxicity [52].
Additionally, despite the cosmesis benefits of BT at higher BED3, few BT studies on non-melanoma skin cancer were found that met inclusion criteria (Table 1 and Table 3). The published experience with BT is lacking, and thus we performed the current meta-analysis. There are currently no guidelines on the required expertise to deliver BT; data from prostate cancer radionuclide BT suggest that practitioners need >20 cases to become proficient [53]. The existing radionuclide BT literature may be limited due to the special equipment necessary, which is not widely available. The use of eBT has increased 20-fold from 2011 to 2013, likely secondary to self-referral and increased reimbursement [9,11]. Cost of setup of eBT is also lower than traditional radionuclide BT due to the minimal shielding needed and eliminating the handling costs of radioactive sources [8].
Further, the BED equation may not adequately characterize extremely hypofractionated regimens (e.g. >8 Gy/fraction), cellular death due to different modes surrounding mitotic catastrophe (e.g. necroptosis) [54] or molecular pathways behind recurrence (e.g. vasculogenesis) [55]. The BED equation also does not take into account target volume, treatment field sizes, presence of hot spots, or prescription method (e.g. organ, margin around tumor, isodose line) [56]. We have a limited follow-up time of < 5 years because most elderly skin cancer patients are unable to come for extended follow-up. Many of the BT studies reported near 100% “good” cosmesis outcomes and 0% LR although mean follow-up times 10-12 months.
CONCLUSION
In this landmark meta-analysis, we found that BT has more favorable cosmesis over EBRT for skin BCCs and SCCs at common fractionation regimens of 64 Gy/32 fractions, 55 Gy/20 fractions, and 50 Gy/15 fractions. BT demonstrates an extraordinarily high rate of clinician reported good cosmesis after treatment. Whether this is secondary to the superiority of the technique, patient selection, or another unidentified factor is unknown. Prospective studies comparing the techniques are warranted.
Supplementary Material
Acknowledgements:
This article has no funding source. We have no acknowledgements to make. This work was presented at the American Society for Radiation Oncology 2017 Annual Meeting.
ABBREVIATIONS
- BCC
basal cell carcinoma
- BED
biologically equivalent dose
- CI
confidence interval
- CPT
current procedural terminology
- EBRT
external beam radiation therapy
- HDR-BT
high dose rate brachytherapy
- IQR
interquartile range
- PICOS
Population, Intervention, Control, Outcome, Study Design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RT
radiation therapy
- RTOG
Radiation Therapy Oncology Group
- SCC
squamous cell carcinoma
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med 2005;353:2262–9. [DOI] [PubMed] [Google Scholar]
- [4].Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg 2012;5:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bichakjian CK, Olencki T, Aasi SZ, et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:574–97. [DOI] [PubMed] [Google Scholar]
- [6].Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol 2011;25 Suppl 5:1–51. [DOI] [PubMed] [Google Scholar]
- [7].Skowronek J Brachytherapy in the treatment of skin cancer: an overview. Postepy Dermatol Alergol 2015;32:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramachandran P New era of electronic brachytherapy. World J Radiol 2017;9:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Linos E, VanBeek M, Resneck JS Jr. A Sudden and Concerning Increase in the Use of Electronic Brachytherapy for Skin Cancer. JAMA Dermatol 2015;151:699–700. [DOI] [PubMed] [Google Scholar]
- [10].Zachary CB. Electronic brachytherapy: overused and overpriced? Cutis 2015;96:153–4. [PubMed] [Google Scholar]
- [11].Haffty BG, Beyer DC, Kavanagh BD. Radiation Oncologist Concerns About Increased Electronic Brachytherapy Use for Skin Cancer. JAMA Dermatol 2015;151:1036. [DOI] [PubMed] [Google Scholar]
- [12].Delishaj D, Laliscia C, Manfredi B, et al. Non-melanoma skin cancer treated with high-dose-rate brachytherapy and Valencia applicator in elderly patients: a retrospective case series. J Contemp Brachytherapy 2015;7:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zaorsky NG, Lee CT, Zhang E, Keith SW, Galloway TJ. Hypofractionated radiation therapy for basal and squamous cell skin cancer: A meta-analysis. Radiother Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- [15].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [16].Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer 1997;76:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zaorsky NG, Lee CT, Zhang E, Keith SW, Galloway TJ. Hypofractionated radiation therapy for basal and squamous cell skin cancer: A meta-analysis. Radiother Oncol 2017;125:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gauden R, Pracy M, Avery AM, Hodgetts I, Gauden S. HDR brachytherapy for superficial non-melanoma skin cancers. J Med Imaging Radiat Oncol 2013;57:212–7. [DOI] [PubMed] [Google Scholar]
- [19].Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47:95–102. [DOI] [PubMed] [Google Scholar]
- [20].Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Avkshtol V, Dong Y, Hayes SB, et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Res Rep Urol 2016;8:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khan L, Breen D, Zhang L, et al. Predictors of recurrence after radiotherapy for non-melanoma skin cancer. Curr Oncol 2014;21:e326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Trott KR, Maciejewski B, Preuss-Bayer G, Skolyszewski J. Dose-response curve and split-dose recovery in human skin cancer. Radiother Oncol 1984;2:123–9. [DOI] [PubMed] [Google Scholar]
- [25].Douglas BG. Superfractionation: its rationale and anticipated benefits. Int J Radiat Oncol Biol Phys 1982;8:1143–53. [PubMed] [Google Scholar]
- [26].Abbatucci JS, Boulier N, Laforge T, Lozier JC. Radiation therapy of skin carcinomas: results of a hypofractionated irradiation schedule in 675 cases followed more than 2 years. Radiother Oncol 1989;14:113–9. [DOI] [PubMed] [Google Scholar]
- [27].Ashby MA, Smith J, Ainslie J, McEwan L. Treatment of nonmelanoma skin cancer at a large Australian center. Cancer 1989;63:1863–71. [DOI] [PubMed] [Google Scholar]
- [28].Caccialanza M, Piccinno R, Kolesnikova L, Gnecchi L. Radiotherapy of skin carcinomas of the pinna: a study of 115 lesions in 108 patients. Int J Dermatol 2005;44:513–7. [DOI] [PubMed] [Google Scholar]
- [29].Caccialanza M, Piccinno R, Percivalle S, Rozza M. Radiotherapy of carcinomas of the skin overlying the cartilage of the nose: our experience in 671 lesions. J Eur Acad Dermatol Venereol 2009;23:1044–9. [DOI] [PubMed] [Google Scholar]
- [30].Chan S, Dhadda AS, Swindell R. Single fraction radiotherapy for small superficial carcinoma of the skin. Clin Oncol (R Coll Radiol) 2007;19:256–9. [DOI] [PubMed] [Google Scholar]
- [31].Cognetta AB, Howard BM, Heaton HP, Stoddard ER, Hong HG, Green WH. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol 2012;67:1235–41. [DOI] [PubMed] [Google Scholar]
- [32].Hall VL, Leppard BJ, McGill J, Kesseler ME, White JE, Goodwin P. Treatment of basal-cell carcinoma: comparison of radiotherapy and cryotherapy. Clin Radiol 1986;37:33–4. [DOI] [PubMed] [Google Scholar]
- [33].Lovett RD, Perez CA, Shapiro SJ, Garcia DM. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys 1990;19:235–42. [DOI] [PubMed] [Google Scholar]
- [34].Grossi Marconi D, da Costa Resende B, Rauber E, et al. Head and Neck Non-Melanoma Skin Cancer Treated By Superficial X-Ray Therapy: An Analysis of 1021 Cases. PLoS One 2016;11:e0156544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pampena R, Palmieri T, Kyrgidis A, et al. Orthovoltage radiotherapy for nonmelanoma skin cancer (NMSC): Comparison between 2 different schedules. J Am Acad Dermatol 2016;74:341–7. [DOI] [PubMed] [Google Scholar]
- [36].Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol 2005;53:993–1001. [DOI] [PubMed] [Google Scholar]
- [37].Silva JJ, Tsang RW, Panzarella T, Levin W, Wells W. Results of radiotherapy for epithelial skin cancer of the pinna: the Princess Margaret Hospital experience, 1982–1993. Int J Radiat Oncol Biol Phys 2000;47:451–9. [DOI] [PubMed] [Google Scholar]
- [38].Tsao MN, Tsang RW, Liu FF, Panzarella T, Rotstein L. Radiotherapy management for squamous cell carcinoma of the nasal skin: the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys 2002;52:973–9. [DOI] [PubMed] [Google Scholar]
- [39].Hernandez-Machin B, Borrego L, Gil-Garcia M, Hernandez BH. Office-based radiation therapy for cutaneous carcinoma: evaluation of 710 treatments. Int J Dermatol 2007;46:453–9. [DOI] [PubMed] [Google Scholar]
- [40].van Hezewijk M, Creutzberg CL, Putter H, et al. Efficacy of a hypofractionated schedule in electron beam radiotherapy for epithelial skin cancer: Analysis of 434 cases. Radiother Oncol 2010;95:245–9. [DOI] [PubMed] [Google Scholar]
- [41].Zagrodnik B, Kempf W, Seifert B, et al. Superficial radiotherapy for patients with basal cell carcinoma: recurrence rates, histologic subtypes, and expression of p53 and Bcl-2. Cancer 2003;98:2708–14. [DOI] [PubMed] [Google Scholar]
- [42].Mazeron JJ, Chassagne D, Crook J, et al. Radiation therapy of carcinomas of the skin of nose and nasal vestibule: a report of 1676 cases by the Groupe Europeen de Curietherapie. Radiother Oncol 1988;13:165–73. [DOI] [PubMed] [Google Scholar]
- [43].Somanchi BV, Stanton A, Webb M, Loncaster J, Allan E, Muir LT. Hand function after high dose rate brachytherapy for squamous cell carcinoma of the skin of the hand. Clin Oncol (R Coll Radiol) 2008;20:691–7. [DOI] [PubMed] [Google Scholar]
- [44].Svoboda VH, Kovarik J, Morris F. High dose-rate microselectron molds in the treatment of skin tumors. Int J Radiat Oncol Biol Phys 1995;31:967–72. [DOI] [PubMed] [Google Scholar]
- [45].Tormo A, Celada F, Rodriguez S, et al. Non-melanoma skin cancer treated with HDR Valencia applicator: clinical outcomes. J Contemp Brachytherapy 2014;6:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Goyal U, Kim Y, Tiwari HA, Witte R, Stea B. A pilot study of ultrasound-guided electronic brachytherapy for skin cancer. J Contemp Brachytherapy 2015;7:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bhatnagar A Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy 2013;12:134–40. [DOI] [PubMed] [Google Scholar]
- [48].Kleinmann N, Zaorsky NG, Showalter TN, Gomella LG, Lallas CD, Trabulsi EJ. The effect of ethnicity and sexual preference on prostate-cancer-related quality of life. Nat Rev Urol 2012;9:258–65. [DOI] [PubMed] [Google Scholar]
- [49].Johnson ME, Zaorsky NG, Martin JM, et al. Patient reported outcomes among treatment modalities for prostate cancer. Can J Urol 2016;23:8535–45. [PubMed] [Google Scholar]
- [50].Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513–20. [DOI] [PubMed] [Google Scholar]
- [51].Zaorsky NG, Shaikh T, Ruth K, et al. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin Genitourin Cancer 2017;15:326–35 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Palmer JD, Zaorsky NG, Witek M, Lu B. Molecular markers to predict clinical outcome and radiation induced toxicity in lung cancer. J Thorac Dis 2014;6:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zaorsky NG, Davis BJ, Nguyen PL, et al. The evolution of brachytherapy for prostate cancer. Nat Rev Urol 2017;14:415–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Meng MB, Wang HH, Cui YL, et al. Necroptosis in tumorigenesis, activation of anti-tumor immunity, and cancer therapy. Oncotarget 2016;7:57391–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang HH, Cui YL, Zaorsky NG, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett 2016;375:349–59. [DOI] [PubMed] [Google Scholar]
- [56].Hawkey NM, Zaorsky NG, Galloway TJ. The role of radiation therapy in the management of sialorrhea: A systematic review. Laryngoscope 2016;126:80–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


