Abstract
Background/Aims:
Mucosal cutting biopsy (MCB) is useful for the histopathological diagnosis of gastric subepithelial tumors (SETs). However, there is little information on cases in which MCB did not establish a diagnosis. In the current study, we aimed to investigate the characteristics of cases in which MCB was unsuccessful.
Methods:
Cases in which MCB was used to histopathologically diagnose gastric SETs at Kobe University Hospital between August 2012 and October 2018 were retrospectively reviewed.
Results:
Forty-five cases in which MCB was used to diagnose 43 gastric SETs in 43 patients were analyzed. The median tumor size was 20 mm (range, 8–50 mm). Pathological examinations resulted in definitive and suspected diagnoses and no diagnosis in 29 (gastrointestinal stromal tumor: n=17, leiomyoma: n=7, aberrant pancreas: n=3, others: n=2), 6, and 10 cases, respectively. Failure to expose the tumor according to retrospective examinations of endoscopic images was significantly associated with no diagnosis. Other possible explanations included a less elevated tumor, biopsy of the surrounding field instead of the tumor due to the mobility, and poor endoscope maneuverability due to the tumor being close to the cardia.
Conclusions:
Clear exposure of gastric SETs during MCB may improve the diagnostic rate of such examinations.
Keywords: Gastric subepithelial tumor, Gastrointestinal stromal tumors, Mucosal cutting biopsy
INTRODUCTION
Gastric subepithelial tumors (SETs) arise in the muscularis mucosae, submucosa, or muscularis propria of the gastric wall [1]. They are sometimes incidentally found during screening examinations, such as upper gastrointestinal (GI) endoscopy, in patients without any symptoms [2,3]. They include gastrointestinal stromal tumors (GISTs), leiomyomas, schwannomas, aberrant pancreas, and non-neoplastic lesions such as inflammatory fibroid polyps [1,4]. Among them, GISTs are potentially malignant [5]; therefore, it is important to perform histological examinations to discriminate GIST from benign lesions. However, it is often difficult to obtain an accurate diagnosis using endoscopic ultrasonography (EUS) alone because the surfaces of gastric SETs are covered by normal mucosal tissue and many gastric SETs, including GIST, originate from the muscularis propria [4]. Hence, tissue sampling is required to definitively diagnose gastric SETs. The Japanese GIST therapeutic guidelines recommend that EUS-guided fine-needle aspiration biopsy (EUS-FNAB) examinations should be used to diagnose gastric SETs 2–5 cm in diameter, as well as those <2 cm in diameter that are growing or exhibit malignant findings, such as ulcer formation or an irregular surface [6]. However, EUS-FNAB can only be carried out at a limited number of medical institutions because it requires dedicated endoscopic equipment and experienced endoscopists, pathologists, and sometimes cytology technicians who are capable of performing rapid onsite evaluations [7-9].
As an alternative tissue sampling modality, mucosal cutting biopsy (MCB), a mucosal incision-assisted biopsy technique, has recently been employed, and some studies have already reported its usefulness [10-12]. However, there have been few reports of cases when MCB failed to establish a diagnosis, even though it is not possible to obtain a pathological diagnosis after MCB in some cases. Herein, we reviewed our consecutive experiences of MCB of gastric SETs and investigated the reasons for diagnostic failure in such cases.
PATIENTS AND METHODS
Patients
The cases of all patients who underwent MCB of gastric SETs at Kobe University Hospital between August 2012 and October 2018 were retrospectively reviewed.
Mucosal cutting biopsy
The appearance, origin, and size of each lesion were assessed by EUS prior to MCB. Most of the patients were hospitalized the day before the MCB procedure. Among the patients receiving antithrombotic therapy, those who were taking aspirin continued taking it, those who were taking thienopyridine derivative agents stopped taking them, and those who were taking warfarin received heparin-bridging therapy during the peri-MCB period.
During the procedure, the patients were mainly sedated using midazolam, flunitrazepam, and pentazocine. The MCB procedure was performed with a single-channel endoscope equipped with a water jet function (GIF-Q260J, H260Z; Olympus Medical Systems, Tokyo, Japan). An ERBE VIO 300 D high-performance cautery device (Erbe Elektromedizin GmbH, Tubingen, Germany) was utilized in all cases.
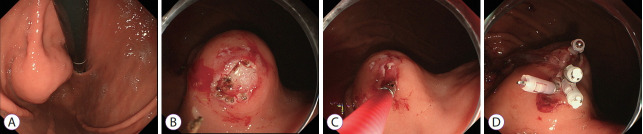
The MCB procedure was performed as follows (Fig. 1). In some cases, saline was injected into the submucosa at the endoscopist’s discretion, although it was typically not performed. Then, an incision measuring 10–15 mm in length was made in the mucosa covering the SET using a needle-knife (KD-1L; Olympus Medical Systems). When the endoscopist was convinced that the tumor had been reached, a tissue sample was collected using biopsy forceps. After obtaining the specimen, any resultant bleeding was coagulated with hemostatic forceps, and the incised wound was closed with endoclips to prevent postoperative bleeding and reduce the risk of tumor dissemination during any subsequent surgical resection.
Fig. 1.
(A) A 30-mm subepithelial tumor was found on the posterior wall of the fornix. (B) The mucosa was incised, and the tumor was exposed. (C) A biopsy of the tumor was performed. (D) The wound was closed with endoclips.
The procedures were conducted by either experienced endoscopists who had performed more than 100 endoscopic submucosal dissection (ESD) procedures or non-experienced endoscopists under the supervision of experienced endoscopists.
Oral intake was allowed on the day after the procedure, and a proton pump inhibitor was administered for 1–4 weeks after the procedure at the discretion of the attending doctor. The patients were discharged 2 or 3 days after the MCB procedure.
Definitions of pathological findings
The pathological findings were categorized as follows: (1) diagnostic, (2) diagnostically suspicious or suggestive, and (3) non-diagnostic. Non-diagnostic was considered to represent diagnostic failure.
Factors related to diagnostic failure
The following variables were selected as factors potentially related to diagnostic failure: tumor location, tumor size, tumor origin, the number of biopsies conducted, the endoscopist who performed MCB (experienced/inexperienced), whether the tumor was exposed during the biopsy procedure according to a retrospective examination of endoscopic images, and the degree of bleeding during the procedure. These variables were examined via statistical analyses. Furthermore, the factors responsible for each non-diagnostic finding were examined in each case.
Definition of tumor exposure according to retrospective examinations of endoscopic images
Tumor exposure was judged by retrospectively examining endoscopic images. Tumors were classified as clearly exposed when a whitish tumorous structure could be seen in the area where the mucosa had been incised, as not exposed when no tumorous structure could be seen because the mucosal incision was insufficient, and as unclear when the mucosa was incised sufficiently and the presence of a tumorous structure was suspected but could not be confirmed due to bleeding and/or scorching of the incised area after coagulation.
Definitions of procedure-related bleeding and its severity
Procedure-related bleeding was defined as bleeding that was caused by MCB.
The degree of bleeding was assessed retrospectively based on endoscopic images and the reports of the attending endoscopists. When there was excessive bleeding from the incised area that could not be easily stopped with hemostatic forceps, it was defined as massive bleeding. When the bleeding was very minor and there was no need to stop it, it was defined as slight bleeding. When the degree of bleeding was between massive and slight, it was defined as moderate bleeding.
The degree of bleeding was also investigated according to the histology of the tumor and the presence/absence of antithrombotic drug treatment.
Ethics
All patients were informed of the risks and benefits of MCB and provided written informed consent to undergo the procedure. The study protocol was approved by the ethics committee at Kobe University Hospital (No. 180181).
Statistical analysis
The Mann–Whitney U test was used to compare continuous variables, and the chi-squared test or Fisher’s exact probability test was used to compare categorical variables. P-values of <0.05 were considered statistically significant. All statistical analyses were performed using JMP version 10 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
A total of 45 MCB procedures were performed to examine 43 gastric SETs in 43 patients. Four patients were taking antithrombotic agents, including aspirin, thienopyridine derivative agents, and warfarin. The patients who were taking thienopyridine derivative agents stopped taking them 5–6 days before the procedure and resumed 1–2 days after the procedure.
The characteristics of the patients and lesions are shown in Table 1. There were 22 male and 21 female patients. Their ages ranged from 37 to 89 years (median age, 60 years). The tumors were located in the upper third of the stomach in 25 patients, the middle third in 17 patients, and the lower third in 1 patient. One tumor located in the upper third of the stomach and another tumor in the middle third of the stomach were subjected to MCB twice on separate days because the first MCB did not yield a definitive diagnosis. All the tumors exhibited intraluminal growth. EUS indicated that 31 tumors originated from the muscularis propria, 2 originated from the submucosa/muscularis propria, 2 originated from the submucosa, and 2 originated from the mucosa. The origins of 6 tumors were unclear. The median tumor size was 20 mm (range, 8–50 mm). Saline was injected into the submucosa in 6 cases at the discretion of the attending endoscopists. The median number of biopsies per case was 10 (range, 3–13). The median time from the saline injection or mucosal incision to the closure of the incised wound with endoclips was 20 minutes (range, 6–82 minutes).
Table 1.
Characteristics of the Patients and Lesions
| Sex, Male/Female | 22/21 |
| Age, median (range) | 60 (37–89) |
| Lesion location (Upper/Middle/Lower) | 25/17/1 |
| Growth pattern (Intraluminal/Extraluminal) | 43/0 |
| Tumor size (mm), median (range) | 20 (8–50) |
| Tumor origin (Muscularis propria/Submucosa and muscularis propria/Submucosa/Mucosa/Unknown) | 31/2/2/2/6 |
| Number of biopsies conducted (times), median (range) | 10 (3–13) |
| Submucosal injection (Yes/No) | 6/39 |
| Procedure time (min), median (range) | 20 (6–82) |
| Endoscopist (Experienced/Non-experienced) | 18/27 |
| Adverse events | Perforation: 1, postoperative bleeding: 1 |
| Diagnosis (Diagnostic/Suspected/Non-diagnostic) | 29/6/10 |
| Histology based on mucosal cutting biopsy | GIST: 17, leiomyoma: 11, aberrant pancreas: 3, adenocarcinoma: 2, mesenchymal myxoid tumor: 1, old hematoma: 1, non-diagnostic: 10 |
| Cases that underwent surgical resection and their histology | 21 (GIST: 15, leiomyoma: 2, adenocarcinoma: 1, mesenchymal myxoid tumor: 1, no diagnosis: 2) |
GIST, gastrointestinal stromal tumor.
Adverse events occurred in 2 cases: perforation occurred in 1 case during the biopsy of a lesion in the greater curvature of the fornix, which was closed with endoclips and managed conservatively, and postoperative bleeding occurred in another case, which was treated via additional endoclip application. The median duration of the patients’ hospital stays was 4 days (range, 0–8 days).
Among the 45 cases, definitive pathological diagnoses were obtained in 29 cases (GIST: n=17, leiomyoma: n=7, aberrant pancreas: n=3, adenocarcinoma: n=1, old hematoma: n=1), suspected diagnoses were obtained in 6 cases (leiomyoma: n=4, adenocarcinoma: n=1, mesenchymal myxoid tumor: n=1), and no diagnosis was obtained in 10 cases. In the cases with definitive diagnoses, a diagnosis was obtained during the first biopsy (median, 1; range, 1–4). The suspected diagnoses were made for the following reasons: (1) discriminating between a leiomyoma and the normal muscularis mucosa was difficult in 4 cases; (2) the tissue was damaged by heat denaturation in 1 case of adenocarcinoma, which was reexamined by an additional MCB leading to a definitive diagnosis; and (3) a soft tissue tumor was found and plexiform fibromyoma was considered, but a definitive diagnosis was difficult in 1 case. In the cases of suspected leiomyomas, the pathologist commented that they would have been able to confirm the diagnosis if they were sure that the tissue sample had definitely been obtained from the tumor itself.
Regarding the factors related to diagnostic failure, a failure to expose the tumor during the biopsy procedure according to a retrospective examination of endoscopic images was found to be significantly related to diagnostic failure (Table 2). The other examined variables, such as tumor size, tumor origin, number of biopsies conducted, endoscopist, and degree of bleeding during the procedure, were not found to be related to diagnostic failure.
Table 2.
Factors that Might be Related to the Failure of Tissue Sampling
| Variables | Category | Diagnostic rate (including suspected diagnoses) | p-value |
|---|---|---|---|
| Tumor location | Upper | 73% (19/26) | 0.077 |
| Middle | 89% (16/18) | ||
| Lower | 0% (0/1) | ||
| Cross-sectional tumor location | Lesser curvature | 67% (2/3) | 0.58 |
| Posterior wall | 69% (11/16) | ||
| Greater curvature | 83% (20/24) | ||
| Anterior wall | 100% (2/2) | ||
| Tumor size | <20 mm | 72% (13/18) | 0.48 |
| ≥20 mm | 81% (22/27) | ||
| Tumor origin | Muscularis propria | 73% (24/33) | 0.32 |
| Submucosa and muscularis propria/Submucosa/Mucosa | 100% (6/6) | ||
| Unknown | 83% (5/6) | ||
| Number of biopsies conducted (times) | ≥10 | 75% (18/24) | 0.73 |
| <10 | 81% (17/21) | ||
| Endoscopist | Experienced | 83% (15/18) | 0.72 |
| Non-experienced | 74% (20/27) | ||
| Tumor exposed | Yes | 100% (21/21) | <0.0001 |
| Unclear | 76% (13/17) | ||
| No | 14% (1/7) | ||
| Degree of bleeding | Massive | 79% (11/14) | 0.96 |
| Moderate | 75% (9/12) | ||
| Slight | 79% (15/19) |
The characteristics of the cases that ended in failure are shown in Table 3. In these cases, the tumors were mainly located in the upper third of the stomach, especially in the fornix or cardia. Tumor size, the number of biopsies, and the degree of bleeding varied among the cases. The factors that were suspected to be related to diagnostic failure included a failure to clearly expose the tumor in 7 cases, a small tumor or a tumor that lacked elevation in 3 cases, biopsy of the surrounding field instead of the actual tumor due to its mobility during the procedure in 2 cases, and poor maneuverability of the endoscope due to the proximity of the tumor to the gastric cardia in 1 case.
Table 3.
Characteristics of the Cases Which Did Not Reach Diagnosis
| No. | Age | Size (mm) | Number of biopsies | Tumor exposed | Tumor location | Cross-sectional tumor location | Degree of bleeding | Factors that might be related to failure besides tumor exposure |
|---|---|---|---|---|---|---|---|---|
| 1 | 72 | 20 | 12 | No | Middle | P | Slight | - |
| 2 | 56 | 10 | 6 | Unclear | Upper | P | Slight | Small tumor, surrounding field biopsies due to the tumor’s mobility |
| 3 | 65 | 23 | 6 | Unclear | Upper | G | Moderate | - |
| 4 | 72 | 16 | 5 | No | Upper | P | Massive | - |
| 5 | 57 | 25 | 10 | No | Upper | G | Moderate | - |
| 6a) | 59 | 15 | 10 | No | Upper | G | Moderate | Small tumor |
| 7a) | 59 | 15 | 5 | No | Upper | G | Massive | Tumor less elevated than before |
| 8 | 65 | 25 | 10 | No | Middle | P | Slight | - |
| 9 | 41 | 12 | 10 | Unclear | Upper | P | Massive | Poor endoscope maneuverability, surrounding field biopsies due to the tumor’s mobility |
| 10 | 46 | 30 | 10 | Unclear | Lower | L | Slight | - |
G, greater curvature; L, lesser curvature; P, posterior wall.
Cases No. 6 and No. 7 are the same lesions. The lesion was reexamined (No. 7) because no diagnosis was obtained during the original procedure (No. 6).
In terms of bleeding during the procedure, massive, moderate, and slight bleeding was seen in 14, 12, and 19 cases, respectively. The histology of the GIST was found to be significantly related to massive or moderate bleeding (p=0.0007) (Table 4). The use of antithrombotic medicines was not found to be related to the degree of bleeding.
Table 4.
Factors Related to the Degree of Bleeding during Mucosal Cutting Biopsy
| Variables | Category | Degree of bleeding |
Frequency of massive or moderate bleeding | p-value | ||
|---|---|---|---|---|---|---|
| Massive | Moderate | Slight | ||||
| Histology | GIST | 8 | 8 | 1 | 94% (16/17) | 0.0007 |
| Leiomyoma | 0 | 0 | 11 | 0% (0/11) | ||
| Aberrant pancreas | 0 | 1 | 2 | 33% (1/3) | ||
| Adenocarcinoma | 2 | 0 | 0 | 100% (2/2) | ||
| Mesenchymal myxoid tumor | 1 | 0 | 0 | 100% (1/1) | ||
| Old hematoma | 0 | 0 | 1 | 0% (0/1) | ||
| Non-diagnostic | 3 | 3 | 4 | 60% (6/10) | ||
| Use of antithrombotic medicines | Yes | 0 | 1 | 3 | 25% (1/4) | 0.29 |
| No | 14 | 11 | 16 | 61% (25/41) | ||
GIST, gastrointestinal stromal tumor.
Twenty-one patients underwent surgical tumor resection after a diagnosis was obtained using MCB (GIST: 15 cases, leiomyoma: 2 cases, adenocarcinoma: 1 case, mesenchymal myxoid tumor: 1 case, and no diagnosis: 2). In the 2 cases of leiomyoma, the reasons for surgery were that the tumor exhibited ulceration on its surface accompanied by bleeding and anemia in 1 case and that the tumor was slowly increasing in size in the other case. The histopathological and immunostaining findings of the surgically resected specimens were in agreement with those of MCB samples in 17 cases. One lesion that was diagnosed as a leiomyoma based on MCB was subsequently shown to be a schwannoma, 1 lesion that was suspected to be a mesenchymal myxoid tumor based on MCB was shown to be an inflammatory fibroid polyp, and 2 lesions that were classified as non-diagnostic based on MCB were diagnosed as a GIST and leiomyoma, respectively.
Among the 8 cases (7 patients) that were classified as non-diagnostic based on MCB, 4 were followed up via annual endoscopy and no tumor growth was seen. The other 4 cases (3 patients) were followed up at other institutions or lost to follow-up.
DISCUSSION
In the present study, we retrospectively reviewed 45 consecutive cases in which gastric SETs were subjected to MCB and investigated the factors related to the diagnostic failure of MCB. A definitive or suspected diagnosis was obtained in 35 of 45 (78%) cases, and the factors found to be related to the diagnostic failure of MCB included a failure to clearly expose the tumor, a less elevated tumor, the surrounding field being biopsied instead of the actual tumor due to its mobility during the procedure, and poor maneuverability of the endoscope due to the tumor’s location (close to the gastric cardia). The attending endoscopists had to be thoroughly convinced that the tumor was exposed before they performed the tissue sampling in all cases; however, diagnostic failure was more likely in the cases in which the tumor was not exposed according to retrospective examinations of endoscopic images. Therefore, we believe that exposing tumors to the extent that they are clearly recognizable in the endoscopic images is important for diagnosing gastric SETs via MCB.
Furthermore, among the cases with suspected diagnoses, most of the lesions were diagnosed as suspected leiomyomas, but it was difficult to discriminate between the tumors and the normal muscularis mucosae. Leiomyomas are benign tumors composed of spindle cells, but they do not express any specific immunostaining markers, such as c-kit or CD34, which are found in GIST. Desmin and α-smooth muscle actin are expressed by leiomyomas, but they are also found in the normal muscularis mucosae, which makes those markers difficult to use. To differentiate between them, obtaining pathological findings of fascicular hyperplasia of the muscular fibers or confirming that the biopsy specimen was definitely taken from the tumor is essential. This also emphasizes the importance of clearly exposing tumors during MCB.
Certain technical measures, such as seeking the best approach for ensuring stable endoscopic maneuverability, stopping bleeding first and securing the endoscopic field to allow the tumor to be identified again in cases of bleeding, and performing wider and deeper incisions when tumors are not visible, might be effective in ensuring that SETs are sufficiently exposed during MCB.
As for adverse events, intraoperative perforation and postoperative bleeding occurred in 1 case each. In the case in which perforation occurred, the lesion was located in the greater curvature of the fornix and was classified as non-diagnostic. It is suspected that the biopsy samples were taken from the normal gastric mucosa, which led to the perforation. The gastric wall is thin in the greater curvature of the fornix; therefore, attention should be paid to the risk of perforation when MCB is performed at such locations. In the current study, postoperative bleeding occurred in a case of GIST. In addition, it was shown that bleeding easily occurred during MCB of GIST, which implies that GIST tissue may be hypervascular and carry a higher risk of bleeding.
Previous studies of MCB of gastric SETs reported diagnostic yields of 60% in 20 cases, 85% in 27 cases, and 100% in 18 cases [10-12].
Other procedures that are similar to MCB have also been reported. Liu et al. showed that the EUS-guided cutting of holes was useful and resulted in successful diagnoses in all 10 of their cases [13]. Lee et al. described the combined use of endoscopic partial resection with an unroofing technique, in which the mucosa overlying the SET was resected using a snare and the tumor was also grasped by the snare, which provided a diagnostic yield of 93.7% (15 out of 16 cases) [14]. Kobara et al. presented a novel method in which bloc biopsy was performed under direct endoscopic visualization using a combination of submucosal endoscopy and a mucosal flap method, which enabled immunohistological diagnoses to be obtained in all 8 of their cases [15]. The methods of Lee et al. and Kobara et al. allow the mucosa and submucosa to be opened more widely and expose the tumor to a greater degree than the standard MCB method, which could result in a higher diagnostic rate [14,15]. However, in the unroofing technique, exposing the tumor surface and leaving it open may cause postoperative bleeding and carry a risk of intraoperative tumor dissemination during subsequent surgical procedures. In addition, performing a bloc biopsy using ESD requires appropriate expertise and takes time. Therefore, each procedure has its advantages and disadvantages.
On the other hand, EUS-FNAB is considered to be a reliable diagnostic modality for obtaining preoperative histological diagnoses in gastric SETs. It has been reported that EUS-FNAB results in adequate tissue sampling in 81%–91% of cases, and the associated procedure-related adverse event rate is low [4,7,8,16]. However, the diagnostic yield for tumors <2 cm in size is reported to decrease to around 67%–71% [4,17]. In the present study, the diagnostic yield of MCB was 72% for lesions <20 mm in diameter and 81% for those ≥20 mm in diameter (p=0.48), which suggests that the success of MCB-based tissue sampling is less influenced by tumor size than that of EUS-FNAB. Furthermore, as mentioned above, the number of medical institutions where EUS-FNAB can be carried out is limited because it requires dedicated endoscopic equipment and skilled endoscopists. However, MCB does not require any dedicated endoscopic equipment and therefore can be carried out at any institution.
Based on the results of the present study and previous studies of other types of tissue sampling procedures for gastric SETs, we contend that MCB should be explored at institutions where performing EUS-FNAB is difficult. Even at institutions where EUS-FNAB is feasible, MCB could be employed as a first-line option in cases where the tumor measures <20 mm in diameter, although it is important to ensure that the tumor is clearly exposed during the biopsy procedure. In cases involving larger tumors, EUS-FNAB may yield a higher definitive diagnosis rate. In addition, since rapidly growing SETs are considered likely to be GIST [18], EUS-FNAB may be used to avoid MCB-related intraoperative or postoperative bleeding in such cases. At institutions where ESD can be performed, it may also be possible to use submucosal dissection to obtain a direct endoscopic view of the tumor.
The present study had some limitations, which include its retrospective design and small population, as the data were collected from a single institution. Second, the assessments of tumor exposure and the degree of bleeding during the procedure were based on retrospective examinations of endoscopic images and were inevitably subjective, although all these examinations were performed to the same standard. Third, in 2 cases in which suspected diagnoses were obtained using MCB, the histopathological results derived from MCB samples were not in accordance with those obtained from the surgical specimens. Likewise, the accuracy of the histopathological results obtained with MCB could not be definitively determined because surgery was only performed in some of the cases in which MCB was used.
In conclusion, MCB can be a promising diagnostic modality for gastric SETs at institutions where performing EUS-FNAB is difficult, although it is important to ensure that the tumor is clearly exposed during the procedure to obtain a definitive diagnosis. Further studies are warranted to confirm our results and further assess the utility of MCB.
Footnotes
Conflicts of Interest:The authors have no financial conflicts of interest.
REFERENCES
- 1.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–307. doi: 10.1053/j.gastro.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 3.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–645. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 4.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–919. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–1301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 6.Japan Society of Clinical Oncology . Japanese Gastric Cancer Association, Japanese Study Group on GIST. GIST therapeutic guidelines. Tokyo: Kanehara & Co; 2008. [Google Scholar]
- 7.Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int. 2009;59:712–719. doi: 10.1111/j.1440-1827.2009.02433.x. [DOI] [PubMed] [Google Scholar]
- 8.Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 9.Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol. 2013;41:1075–1080. doi: 10.1002/dc.23047. [DOI] [PubMed] [Google Scholar]
- 10.Ihara E, Matsuzaka H, Honda K, et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013;5:191–196. doi: 10.4253/wjge.v5.i4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka M, Kawai T, Yagi K, et al. Mucosal cutting biopsy technique for histological diagnosis of suspected gastrointestinal stromal tumors of the stomach. Dig Endosc. 2013;25:274–280. doi: 10.1111/j.1443-1661.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- 12.Ikehara H, Li Z, Watari J, et al. Histological diagnosis of gastric submucosal tumors: a pilot study of endoscopic ultrasonography-guided fine-needle aspiration biopsy vs mucosal cutting biopsy. World J Gastrointest Endosc. 2015;7:1142–1149. doi: 10.4253/wjge.v7.i14.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YM, Yang XJ. Endoscopic ultrasound-guided cutting of holes and deep biopsy for diagnosis of gastric infiltrative tumors and gastrointestinal submucosal tumors using a novel vertical diathermic loop. World J Gastroenterol. 2017;23:2795–2801. doi: 10.3748/wjg.v23.i15.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CK, Chung IK, Lee SH, et al. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video) Gastrointest Endosc. 2010;71:188–194. doi: 10.1016/j.gie.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Kobara H, Mori H, Fujihara S, et al. Bloc biopsy by using submucosal endoscopy with a mucosal flap method for gastric subepithelial tumor tissue sampling (with video) Gastrointest Endosc. 2013;77:141–145. doi: 10.1016/j.gie.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Inoue H, Ryozawa S, et al. Fine-needle aspiration biopsy and endoscopic ultrasound for pretreatment pathological diagnosis of gastric gastrointestinal stromal tumors. Gastroenterol Res Pract. 2012;2012:139083. doi: 10.1155/2012/139083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada K, Maruyama K, Nagase H, et al. [A case of gastrointestinal stromal tumor of the stomach with rapid growth in a short term] Gan To Kagaku Ryoho. 2008;35:2080–2082. [PubMed] [Google Scholar]