Abstract
The catenin beta-1 (CTNNB1) gene, encoding a sub-unit of the cadherin/catenin protein complex that is involved in the Wnt signalling pathway important for proper interneuron development, is considered to be causative for the rare autosomal dominant mental retardation syndrome, formerly called MRD19 but later renamed neurodevelopmental disorder with spastic diplegia and visual defects (NEDSDV). Its main characteristics are moderate to severe intellectual disability (ID), disruptive autistic behaviours, microcephaly, absent or limited speech, facial dysmorphisms, peripheral hypertonia/spasticity, motor delay and visual defects. So far, 35 patients have been reported with a de novo loss-of-function variant in CTNNB1. In two other patients, a deletion comprising the full gene was found. Four out of the 37 patients were of adult age (range: 27–51 years), while the majority was infant or adolescent (range: 0–20 years). Here, a 32-year-old severely intellectually disabled female patient is described in whom exome sequencing disclosed a de novo heterozygous splice site variant in the CTNNB1 gene [Chr3(GRCh37): g.41267064G>T; NM_001904.3: 23. c.734+1G>T; r. spl?]. Somatic investigation disclosed significant microcephaly and minor facial dysmorphisms. Neurological examination demonstrated severe kyphoscoliosis, distal spastic tetraparesis, especially of the legs with increased tendon reflexes and bilateral Babinski sign, resulting in severely impaired walking capability with a broad-based gait. Apart from strabismus, no ophthalmological abnormalities were found. Here, the reported variant in the CTNNB1 gene was not published earlier nor is included in the international databases. This specific variant is considered to be causative for the severe ID, autism and the somato-neurological phenotype of the patient and corresponds with a diagnosis of NEDSDV.
Keywords: heterozygous splice site variant, CTNNB1, NEDSDV, autism, intellectual disability, distal spastic tetraparesis
Introduction
A small decade ago, diagnostic exome sequencing in a large group of patients with intellectual disability (ID) by De Ligt and coworkers yielded five candidate genes associated with ID including the β-catenin (CTNNB1) gene (OMIM: 116806) located on 3p22.1 in three patients.1 In all, main characteristics were severe ID, absent or limited speech, microcephaly, and spasticity with severely impaired walking ability. These observations led CTNNB1 to be postulated as a novel ID gene causing autosomal dominant mental retardation type 19 (MRD19). In a mouse model, it has been demonstrated that haploinsufficiency of this gene leads to disturbances in the regulation of dendritic branching and causes craniofacial abnormalities and cognitive dysfunction providing functional evidence for a causal relation between haploinsufficiency of CTNNB1 and the corresponding ID phenotype in humans.2
So far, 35 patients have been reported with a (de novo) loss-of-function variant of CTNNB1.1–6 In two other patients, a deletion comprising the full gene was found.3,7 Four out of this total of 37 patients were of adult age (27, 27, 29, and 51 years) while the majority were infant or adolescent (age range: 0–20 years). By systematic analysis of the earlier findings on clinical features, Kuechler and coworkers also identified multiple ocular abnormalities such as strabismus and hyperopia often to be present.3 Therefore, the MRD19 syndrome was renamed into neurodevelopmental disorder with spastic diplegia and visual defects (NEDSDV; OMIM: #615075). Findings on retinal detachment and proliferative vitreoretinopathy in a very limited number of patients seem to corroborate this.8,9
Summarizing, main clinical features in patients with CTNNB1-haploinsufficiency comprise moderate to severe ID, behavioural abnormalities including autistic traits, microcephaly, craniofacial dysmorphisms, truncal hypotonia, peripheral hypertonia/spasticity, motor delay, speech impairment and visual defects.3
We here report in detail the developmental trajectory of a 32-years-old intellectually disabled adult female, who was referred for the assessment and treatment of persistent severe challenging behaviours.
Patients and Methods
Ethical Aspects
Assessments were performed at the Raphael Institute Scorlewald, Centre for People with Intellectual Disabilities, in Schoorl, the Netherlands under supervision of consultants from the Dutch Centre for Consultation and Expertise, Utrecht, The Netherlands, that provided ethical approval for publication (CCE Directorate Approval letter #01082020). In addition, both parents gave written informed consent for publication including the picture of the patient (consent form dated April 2020). Both approval letters were provided to the Editorial Board.
Case Description
The patient is the second child of non-consanguineous healthy parents. Pregnancy and birth were unremarkable. Height, weight and head circumference at birth were 50cm, 3200gr and 33,5cm, respectively. She has one older brother and a younger sister and brother, all healthy.
Already in her first months, there were marked feeding problems and her parents noticed lack of eye contact, a general refusal of communication and delay of development, reason why she was examined by a pediatrician at the age of seven months. Physical examination demonstrated striking microcephaly (head circumference: 38.5cm; <P0.6), short stature (63cm; P2), markedly low weight (5800gr; P0.6) without, however, any dysmorphisms or other abnormalities. Ultrasound and X-ray of the skull as well as EEG were all normal. No explanation for the developmental delay was found and physical therapy was advised to stimulate motor development.
About four months later, re-examination revealed strabismus convergens alternans, head and truncal instability as well as the tendency to overstretch. Height, weight and head circumference were now 69.5cm (<P10), 7570gr (P3) and 40.5cm (<P0.6), respectively. Ophthalmological examination refined the diagnosis of strabismus into left sided strabismus convergens. Again, no further explanation could be provided.
Aged 17 months, she was hospitalized for two weeks because of the suspicion of a metabolic disease that, however, was excluded. Examination disclosed absent sucking reflex, nearly lacking contact, suboptimal head balance, absent truncal balance, and inability to sit, stand or walk. Between her second and fifth years, she underwent several follow-up examinations. Chromosomal analysis at age two revealed a normal 46, XX karyotype. During that period general growth followed the same pattern with, eg, head circumference of 45cm (<P2) at age three. At neurological examination, tendon reflexes were normal. Concerning motor development, she did not show any balance while standing even with support. X-pelvis demonstrated marked right hip dysplasia for which a pavlik harness was prescribed. Now, facial dysmorphic features and autistic behaviours were noticed. All these years, she underwent intensive physical and logopedic therapy which led to gradual but only limited improvement of motor skills, speech and language.
The delayed milestones became more apparent after the birth of her younger sister and brother, reason why she, at about seven years of age, was hospitalized for six months to a pedagogic observation clinic. At that time, there were challenging and ritualistic behaviours with temper tantrums and mood instability. Speech was restricted to 2–4 words and the developmental level of receptive and expressive language was about 3–4 years. There were marked hypotonia with an uncoordinated global movement pattern, persisting mild overstretch, and valgus deformity. Because of the latter, aged 9, the patient successfully underwent bilateral extra-articular subtalar arthrodesis after which her walking pattern stabilized in that she was able to walk with support for a short distance. Nevertheless, she needed a wheeled walker and was partially wheelchair bound. Communication was hampered by frequent crying, involuntary gestures, and head banging. Behavioural observation revealed clear autistic traits. Special education and individual guidance within a structured environment were recommended.
At the age of 12, she was examined at a university clinic for child neurology. MRI scanning of the brain disclosed no abnormalities and a cause for her developmental delay and motor dysfunction could not be established. Shortly thereafter, a diagnosis of autism spectrum disorder was made at an outpatient department for child psychiatry.
During the following years, she lived at her parent’s home, followed specialized education for some years, stayed during daytime within an activity center of an institute for people with intellectual disabilities where she was employed with simple work at the pottery and remained several weekends at the institute. During adolescence, her significant right convex thoracolumbar scoliosis increased markedly (40º), leading to a right structural kyphosis. In addition, she developed a spastic atactic walking pattern.
Aged 21, she was institutionalized permanently because of progressive problems with walking (nearly completely wheelchair bound) and intensifying of challenging behaviours with temper tantrums, anger, and screaming especially in situations with less structure and predictability. Over the following years, the patient participated in various simple daycare activities at the institute for people with ID while staying at her parent’s home during the weekends. She was treated symptomatically with 0.1mg haloperidol twice daily and, at night, 40mg pipamperone in combination with 2mg melatonin. In addition, 800IE cholecalciferol once daily was prescribed.
Investigations
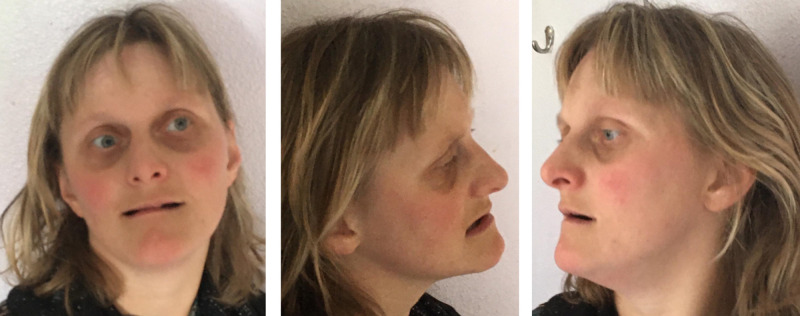
Somatic investigation at the age of 32 years disclosed significant microcephaly with a head circumference of 50cm (P<0.6). Height and weight were 150cm and 50.1kg, respectively. She was almost completely wheelchair bound and there was no active language apart from limited 3 to 4 words sentences. Her eye movements were not always well coordinated with intermittent strabismus. Her behaviour showed clear autistic traits and she was not fully toilet trained with respect to urine continence. There were mild facial dysmorphisms, including divergent strabismus, thin lips, small alae nasi, full nasal tip, short philtrum, pointed chin, and slightly upslanted palpebral fissures (right>left) (Figure 1). As assessed with the Vineland Adaptive Behaviour Scale (VABS10), developmental age scores on domains of communication, daily activities, socialization, and motor skills were 34, 44, 18 and 16 months, respectively. Social-emotional development as measured by the Dutch scale for emotional development in people with intellectual disability (SEO-R11) corresponded with a developmental age of 18 months. With the revised scale for autism and related disorders (AVZ-R12), a total score of 16 was established (scores between 10 and 19 are indicative for autism). In addition, the total score on the Autism Diagnostic Observation Schedule (ADOS-213) of 27 (cut off: 9) corroborated a diagnosis of autism.
Figure 1.
Clinical photographs of the patient at age 32 years showing mild facial dysmorphic features including divergent strabismus left eye, thin lips, small alae nasi, full nasal tip, short philtrum, pointed chin, and slightly upslanted palpebral fissures (right>left).
Neurological examination demonstrated truncal hypotonia with severe kyphoscoliosis, mild intention tremor of both arms and distal spastic tetraparesis of the arms and legs, most pronounced at the lower extremities with increased tendon reflexes and bilateral Babinski sign. This neurological condition resulted in severely impaired walking capability in that she was, with support, able to take a few steps with a broad-based gait, only. Apart from strabismus, ophthalmologic evaluation showed no irregularities, especially no vitreoretinopathy. Relevant hematological (eg, white blood count and thrombocytes) and biochemical parameters (eg, vitamin status, thyroid and liver parameters, glucose, and lipid spectrum) were all normal. MRI scanning of the brain disclosed no abnormalities.
Whole exome sequencing analysis was performed as described before.14 Essentially, DNA was sequenced on an Illumina HiSeq system after exome enrichment using the Agilent SureSelectXT Human All Exon 50Mb Kit. Reads were aligned to the Hg19 reference genome with BWA and variants were called using GATK and annotated using an in-house developed pipeline. Data analysis/filtering was performed essentially based on allele frequencies and mutation severity. This analysis demonstrated a heterozygous splice donor site variant in the CTNNB1 gene [Chr3(GRCh37): g.41267064G>T; NM_001904.3: c.734+1G>T; r. spl?]. Subsequent targeted Sanger sequencing analysis of the patient and both her parents showed that this concerns a de novo variant (see Supplementary figure for the Sanger sequencing electropherograms of the index and her parents). This specific variant in the CTNNB1 gene has not been described before in the literature, but another variant of the same splice donor site was reported in 2019 in a patient with both severe ID and visual defects.6 The de novo CTNNB1 splice variant is considered to be causative for the severe ID and the clinical phenotype of the patient and is corresponding with a diagnosis of NEDSDV without, however, the vitreoretinopathy.
Outcome and Follow-Up
After extensive multidisciplinary discussion of the etiological diagnosis and the potential neurological consequences, ie, slow progression of the spastic atactic paraparesis especially of the legs, it was decided to focus individualized daily guidance as much as possible to both the patients autistic behaviour repertoire and her slowly progressive neurological dysfunctions. This approach resulted in a significant reduction of her challenging behaviours, in particular aggression, temper tantrums and anxieties. Although the effectiveness of medication was doubtful, the symptomatic psychopharmacotherapy was kept unchanged.
Discussion
Here, an adult severely intellectually disabled female patient, aged 32, is described with a de novo heterozygous CTNNB1 variant that, to the best of our knowledge, has not been published earlier nor is included in the international DECIPHER database or present in our inhouse database. Her main phenotypical characteristics include ID, mild facial dysmorphic features, severe speech and language impairment, microcephaly, truncal hypotonia, progressive distal peripheral spasticity leading to broad-based ataxic gait, and strabismus. In addition, mild overstretch was present as well as severe thoracolumbar kyphoscoliosis. Whereas the first decades of the developmental trajectory of the patient were dominated by the above described somato/neurological abnormalities, from young adulthood and up, behavioural abnormalities and psychopathology within the autism spectrum became more prominent.
While this set of characteristics is fully compatible with those as described by both Kuechler and Kharbanda and their coworkers,3,5 in the present patient, for the first time, the results of extensive formal assessment of behaviour and psychopathology are reported confirming an AVZ-R and ADOS-compliant diagnosis of autism. This observation is in line with the report by Dong and associates who showed that loss-of-function of CTTNB1 in mice leads to behaviours showing similarity to autism, like significantly impaired object recognition and social interactions as well as elevated repetitive behaviours.15
Conclusion
As the case presented here demonstrates, medical professionals should at present consider whole-exome sequencing as the starting point in etiological investigation, especially in older patients with so far unexplained ID with or without neurological and/or somatic comorbidities. This may be of great importance to elucidate course and prognosis of newly discovered genetic disorders. In the here presented patient, haploinsufficiency of CTNNB1 is responsible for the craniofacial/ID/somato-neurological phenotypes and may be crucially involved in the gradual behavioural development from autistic traits to definite autism that necessitates permanent adjustment of daily guidance.
Acknowledgments
Written informed consent was obtained from the parents for publication of the case history of the patient, including a photograph of her face. The patient was referred by the Centre of Consultation and Expertise, region West. The authors are indebted to the staff members of the Raphael Institute for Intellectual Disabilities, location Scorlewald Schoorl, for their careful observations of the behaviour status of the patient. Thanks are extended to Mrs. M. T Flohil, neurologist at the Northwest Hospital Group location Alkmaar and to Mrs. C. A. T. van den Berg, ophthalmologist at the Eye Centre North-Holland location Heerhugowaard, for their detailed neurological and ophthalmological examination of the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.De Ligt J, Willemsen MH, van Bon BWM, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524 [DOI] [PubMed] [Google Scholar]
- 2.Tucci V, Kleefstra T, Hardy A, et al. Dominant β-catenin mutations cause intellectual disability with recognizable syndromic features. J Clin Invest. 2014;124(4):1468–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuechler A, Willemsen MH, Albrecht B, et al. De novo mutations in beta-catenin (CTNNB1) appear to be a frequent cause of intellectual disability: expanding the mutational and clinical spectrum. Hum Genet. 2015;134(1):97–109. doi: 10.1007/s00439-014-1498-1 [DOI] [PubMed] [Google Scholar]
- 4.Winnczewka-Wiktor A, Badura-Stronka M, Monies-Nowicka A, et al. A de novo CTNNBI nonsense mutation associated with syndromic atypical hyperekplexia, microcephaly and intellectual disability: a case report. BMC Neurol. 2016;16(1):35. doi: 10.1186/s12883-016-0554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharbanda M, Piltz DT, Tomkins S, et al. Clinical features associated with CTNNB1 de novo loss of function mutations in ten individuals. Eur J Med Genet. 2017;60(2):130–135. doi: 10.1016/j.ejmg.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Zhao Y, Yang L, Han S, Qi M. Identification of a novel splice mutation in CTNNB1 gene in a Chinese family with both severe intellectual disability and serious visual defects. Neurol Sci. 2019;40(8):1701–1704. doi: 10.1007/s10072-019-03823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubruc E, Putoux A, Labalme A, Rougeot C, Sanlaville D, Edery P. A new intellectual disability syndrome caused by CTNNB1 haploinsufficiency. Am J Med Genet. 2014;164(6):1571–1575. doi: 10.1002/ajmg.a.36484 [DOI] [PubMed] [Google Scholar]
- 8.Li N, Xu Y, Li G, et al. Exome sequencing identifies a de novo mutation of CTNNB1 gene in a patient mainly presented with retinal detachment, lens and vitreous opacities, microcephaly, and developmental delay. Case report and literature review. Medicine. 2017;96(20):e6914. doi: 10.1097/MD.0000000000006914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panagiotou ES, Doriano CS, Poulter JA, et al. Defects in the cell signaling mediator β-catenin cause the retinal vascular condition FEVR. Am J Hum Genet. 2017;100(6):960–968. doi: 10.1016/j.ajhg.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bildt A, Kraijer D. Dutch Adaptation of the Vineland Adaptive Behavior Scale of Sparrow, Balla, and Cicchetti. Leiden, The Netherlands: PITS; 2003. [Google Scholar]
- 11.Claes L, Verduyn A. Scale for Emotional Development in People with Intellectual Disability [SEO-R: Schaal Voor Emotionele Ontwikkeling Bij Mensen Met Een Verstandelijke Beperking – Revised]. Apeldoorn: Garant; 2012. [Google Scholar]
- 12.Kraaijer DW. Scale for Autism and Related Disorders [AVZ-R: Autisme- En Verwante Stoornissenschaal-Z – Revised]. Amsterdam: Swets & Zeitlinger; 1999. [Google Scholar]
- 13.De Bildt A, De Jonge M, Greaves-Lord K. ADOS-2 Autism Diagnostic Observation Schedule (Dutch Version). Amsterdam: Hogrefe; 2013. [Google Scholar]
- 14.Haer-Wigman L, van Zelst-stams WAG, Ofundt R, et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment. Eur J Hum Genet. 2017;25(5):591–599. doi: 10.1038/ejhg.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong F, Jiang J, McSweeney C, Zou D, Liu L, Mao Y. Deletion of CTNNB1 in inhibitory circuitry contributes to autism-associated behavioral defects. Hum Mol Genet. 2016;25(13):2738–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]