ABSTRACT
This study aimed to identify the elderly generation with the worst prognoses for high-grade astrocytoma and find independent predictors of good outcomes. We conducted a retrospective analysis of 91 patients, ≥65 years old, with anaplastic astrocytoma or glioblastoma. Progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan–Meier method and compared using log-rank test or multivariate Cox regression analysis. We included 21 (23%) and 70 (77%) patients aged 65–69 years and ≥70 years. In the two generations, significant differences were found in the Charlson comorbidity index, extent of resection, chemoradiotherapy (CRT) as adjuvant therapy, and radiation dose (all P < 0.05). The median PFS was 9.9 and 6.9 months in patients aged 65–69 and ≥70 years (P = 0.10). The median OS was 22.8 and 11.6 months in patients aged 65–69 and ≥70 years (P = 0.009). In the multivariate analyzes in patients ≥70 years, only postoperative Karnofsky performance status (KPS) scores ≥70 were significantly related to prolonged PFS (hazard ratio [HR]: 0.48, P = 0.04), and postoperative KPS, CRT as adjuvant therapy, and salvage therapy were significantly related to prolonged OS (HR: 0.45, P = 0.03, HR: 0.38, P = 0.002, and HR: 0.43, P = 0.01, respectively). In conclusion, in patients ≥70 with high-grade astrocytoma, OS was significantly shorter compared to those aged 65–69. Postoperative KPS score was significantly related to prolonged PFS and OS. Postoperative CRT and salvage therapy at recurrence may be effective in the selected elderly.
Key Words: chemoradiotherapy, elderly, high-grade astrocytoma, postoperative KPS, salvage therapy
INTRODUCTION
The landmark EORTC-NCIC (the European Organization for Research and Treatment of Cancer/National Cancer Information Center) trial has established a standard therapy for patients under 70, which includes adjuvant chemoradiation with temozolomide (TMZ) after maximum safe resection.1 However, there is no consensus regarding the standard of care for elderly patients with high-grade astrocytoma because they are often excluded from randomized clinical trials.2,3 Prospective trials in elderly patients with high-grade gliomas, such as the NOA-084 and Nordic study,5 showed the efficacy of TMZ alone and short-course RT alone, and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation as a therapeutic marker for outcomes.6,7 In recent years, Perry et al showed that adding TMZ to short-course RT (40 Gy in 15 fractions) significantly improved OS, compared to short-course RT alone, in newly diagnosed patients with glioblastoma multiforme (GBM) over 65.8 However, the results may not be applicable in the real world because these studies were limited to elderly patients with relatively good preoperative performance status.
Because high-grade astrocytoma is the most common form of malignant primary brain tumor in adults, and the incidence of high-grade astrocytoma in the elderly has increased over the past decades,9,10 an appropriate treatment strategy must be established for the elderly population. In fact, several reports on the elderly with high-grade astrocytoma have been published in recent years, but the definition of elderly in terms of age is not constant.
In the present study, we compared patients aged 65–69 years to those aged ≥70 years and evaluated clinical characteristics that influence clinical outcomes, clarifying independent predictors for progression-free survival (PFS) and overall survival (OS). Specifically, we focused on the extent of resection (EOR) at the initial surgery, adjuvant and salvage therapy for the outcomes, and maintenance of Karnofsky performance status (KPS) after the initial surgery.
MATERIALS AND METHODS
Patient population
A retrospective, single-center analysis was conducted for patients ≥65 with histopathologically confirmed anaplastic astrocytoma or GBM. All patients underwent tumor biopsy or resection between April 2002 and June 2018 at Shizuoka Cancer Center, Japan. Postoperative KPS was measured within 1 week to 1 month postoperatively. All analyzes were approved in advance by the institutional review board.
Surgical intervention and image evaluation
Surgical indications and the surgical approach were discussed for each patient and decided by multiple, board-certified neurosurgeons beforehand. Gross total resection (GTR) was defined as removal of >98% of preoperative contrast-enhanced lesion.11 Partial removal (PR) was defined as any status other than biopsy and GTR. Radiological tumor progression was assessed using the RANO criteria.12 Progression disease (PD) was defined as ≥25% increase in lesion size.
Adjuvant and salvage therapy
Adjuvant therapy includes RT, chemotherapy with TMZ or ACNU (1-(4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-cholroethyl)-3-nitrosourea hydrochloride), and combined chemoradiotherapy (CRT). RT was planned, with three-dimensional planning systems, for a total dose of 40.05 Gy, administered in 15 daily fractions for three weeks8 or for a total dose of 60 Gy, administered in 30 daily fractions for 6 weeks.1 ACNU 70 mg/m2, calculated based on body surface area, was administered intravenously every 4–6 weeks. Concomitant TMZ 75 mg/m2 was administered during RT, and consecutive TMZ 150 mg/m2 was administered for at least six cycles according to Stupp’s protocol.1 The best supportive care was selected for patients who could not receive adjuvant therapy.
Salvage therapy, which included RT, chemotherapy, bevacizumab treatment, and reoperation, was performed after identifying clinical or radiological PD. The indications of salvage therapy were discussed and decided by multiple, board-certified neurosurgeons and radiation oncologists.
Pathology
Pathological diagnoses were conducted in a routine, histological manner using hematoxylin–eosin stained sections according to the World Health Organization (WHO) criteria. IDH1 Arg132His mutations were examined by immunohistochemistry (IHC) with a mutation-specific antibody (internal clone H14, Dianova, Hamburg, Germany). MGMT expression was examined using immunohistochemistry with a specific antibody (Thermo Scientific, Fremont, CA, USA). As previously reported, a patient was defined as MGMT-immunonegative and MGMT-immunopositive if <10% and >10%, respectively, of all approvable, viable neoplastic cells in the specimen were MGMT-IHC positive.13
Statistical analysis
Statistical analyzes were performed using EZR statistical software.14 PFS was defined as the time from surgery to the first clinical or radiological evidence of tumor progression. OS was defined as the time from surgery to death or the last follow-up. PFS and OS were calculated using the Kaplan–Meier method and compared using log-rank test or multivariate Cox regression analysis. P<0.05 was considered statistically significant.
RESULTS
Patient characteristics
Table 1 summarizes the characteristics of 91 patients ≥65 with histopathologically confirmed anaplastic astrocytoma or GBM. Of the 91 patients, 21 (23%) and 70 (77%) were aged 65–69 and ≥70, respectively. The median preoperative KPS score was 70 (range, 40–100), and the preoperative KPS scores were high (≥70) in 12 patients (57%) aged 65–69. The median postoperative KPS score was 70 (range, 50–100), and postoperative KPS scores were high in 11 patients (52%) aged 65–69. The median preoperative KPS score was 60 (range, 30–100), and preoperative KPS scores were high in 29 patients (41%) aged ≥70. The median postoperative KPS score was 60 (range, 40–90), and postoperative KPS scores were high in 22 patients (31%) aged ≥70. With regard to the change between pre- and postoperative KPS, the KPS scores of 38 patients (42%) decreased postoperatively. No statistically significant differences were found in the change in perioperative KPS between these two age groups. Thirty-one patients (44%) aged ≥70 and three patients (14%) aged 65–69 scored ≥1 on the Charlson comorbidity index (CCI). A statistically significant difference was found in the percentages of patients with higher CCI between the two age groups (P = 0.04). GTR, PR, and biopsies were performed in 7 (33%), 10 (48%), and 4 patients (19%) aged 65–69, respectively. Of 70 patients, biopsy was mainly adopted in 36 patients (51%) aged ≥70 years. A statistically significant difference was found in the EOR between the two age groups (P=0.01). GBM was diagnosed in 20/21 patients (95%) aged 65–69 and 42/70 patients (74%) aged ≥70. All patients, except for one patient aged 65–69 years, exhibited wild-type IDH1. Chemotherapy with TMZ was administered in 19/21 patients (90%) aged 65–69, and chemotherapy with TMZ (45 patients) or ACNU (2 patients) were administered in 47 patients (67%) aged ≥70. Radiotherapy was performed in 84/91 patients (92%). In patients aged ≥70, approximately half received 40.05 Gy, whereas most patients aged 65–69 received 60 Gy. A statistically significant difference was found in the radiation dose between the two age groups (P<0.001). CRT was performed as an adjuvant therapy in 19 (90%) and 41 patients (60%) aged 65–69 and ≥70, respectively (P = 0.015). Salvage treatment was administered to 29 patients at the time of recurrence (Table 2). Among them, 12 (41.4%) were treated with bevacizumab.
Table 1.
Characteristics of elderly patients with high-grade astrocytoma
| Total | 65–69 years | 70–91 years | P value | ||
| Sex | Male | 51 | 10 | 41 | 0.46 |
| Female | 40 | 11 | 29 | ||
| Preop KPS | Median (range) | 60 (30-100) | 70 (40-100) | 60 (30-100) | |
| ≥70 | 41 | 12 | 29 | 0.22 | |
| ≤60 | 50 | 9 | 41 | ||
| Postop KPS | Median (range) | 60 (40-100) | 70 (50-100) | 60 (40-90) | |
| ≥70 | 33 | 11 | 22 | 0.12 | |
| ≤60 | 58 | 10 | 48 | ||
| Change in
perioperative KPS |
Increase or no
change |
53 | 12 | 41 | 1.00 |
| Decrease | 38 | 9 | 29 | ||
| CCI | 0 | 57 | 18 | 39 | 0.04 |
| 1 | 23 | 2 | 21 | ||
| 2 | 8 | 0 | 8 | ||
| 3 | 3 | 1 | 2 | ||
| ≥4 | 0 | 0 | 0 | ||
| EOR | Gross total removal | 16 | 7 | 9 | 0.01 |
| Partial removal | 35 | 10 | 25 | ||
| Biopsy | 40 | 4 | 36 | ||
| Pathology | Glioblastoma | 72 | 20 | 52 | 0.06 |
| Anaplastic
astrocytoma |
19 | 1 | 18 | ||
| IDH1 | Wild type | 55 | 14 | 41 | 0.16 |
| Mutation | 1 | 1 | 0 | ||
| No test | 35 | 6 | 29 | ||
| MGMT | Immunopositive
(≥10%) |
47 | 12 | 35 | 0.94 |
| Immunonegative
(<10%) |
30 | 6 | 24 | ||
| No test | 14 | 3 | 11 | ||
| Chemotherapy | Temozolomide | 64 | 19 | 45 | 0.10 |
| ACNU | 2 | 0 | 2 | ||
| None | 25 | 2 | 23 | ||
| Radiation dose | 60Gy | 52 | 19 | 33 | <0.001 |
| 40.05Gy | 32 | 1 | 31 | ||
| None | 7 | 1 | 6 | ||
| Adjuvant therapy | Chemoradiation | 62 | 19 | 41 | 0.015 |
| Others | 29 | 2 | 27 | ||
| Salvage therapy | Yes | 29 | 8 | 21 | 0.59 |
| No | 62 | 13 | 49 |
Abbreviations: KPS, Karnofsky Performance Status; CCI, Charlson comorbidity index; EOR, extent of resection; IDH1, isocitrate dehydrogenase 1; MGMT, O6-methylguanine-DNA methyltransferase; ACNU, 1-(4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-cholroethyl)-3-nitrosourea hydrochloride
Table 2.
Salvage treatment for elderly patients with high-grade astrocytoma
| Salvage treatment | Total | 65–69 years | 70–91 years |
| Bevacizumab | 12 (41.4%) | 4 | 8 |
| ACNU or TMZ | 11 (37.9%) | 2 | 9 |
| RT (including STI) | 6 (20.7%) | 0 | 6 |
| Resection | 3 (10.3%) | 1 | 2 |
Abbreviations: ACNU, 1-(4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-cholroethyl)-3-nitrosourea hydrochloride; TMZ, temozolomide; RT, radiation therapy; STI, stereotactic irradiation
PFS and OS
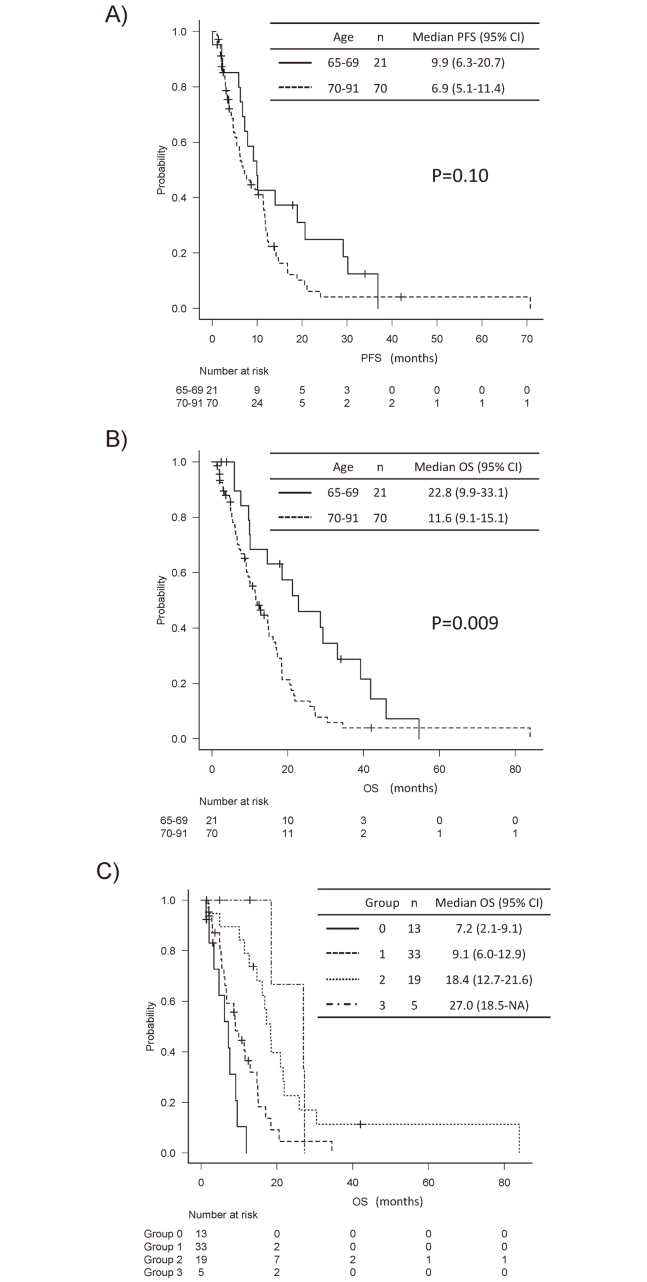
The median PFS with high-grade astrocytoma was 9.9 months (95% confidence interval [CI], 6.3–20.7 months) and 6.9 months (95% CI, 5.1–11.4 months) in patients aged 65–69 and ≥70, respectively (Figure A). The median OS was 22.8 months (95% CI, 9.9–33.1 months) and 11.6 months (95% CI, 9.1–15.1 months) in patients aged 65–69 and ≥70, respectively (Figure B). A statistically significant difference was found in the OS between the two age groups (P = 0.009).
Fig.
Kaplan–Meier analysis of relapse or survival
Fig. A: Progression free survival (PFS). Solid and dotted lines indicates the group aged 65–69 and ≥70, respectively. The median PFS with high-grade astrocytoma was 9.9 months and 6.9 months in patients aged 65–69 and ≥70, respectively (P = 0.10). Abbreviations; 95% CI, 95% confidence interval.
Fig. B: Overall survival (OS). Solid and dotted lines indicates the group aged 65–69 and ≥70, respectively. The median OS was 22.8 months and 11.6 months in patients aged 65–69 and ≥70, respectively (P = 0.009). Abbreviations; 95% CI, 95% confidence interval.
Fig. C: OS based on the number of independent predictive factors. Patients ≥70 were subdivided into four groups based on the number of the three independent prognostic factors (postoperative Karnofsky performance status, chemoradiotherapy as adjuvant therapy, and salvage therapy). Group 0: no prognostic factors, Group 1: one prognostic factor, Group 2: two prognostic factors, Group 3: three prognostic factors. Median OS of groups 0, 1, 2, and 3 was 7.2, 9.1, 18.4, and 27.0 months, respectively (P<0.001). Abbreviations; 95% CI, 95% confidence interval.
Univariate analysis of PFS and OS
A univariate analysis of PFS and OS was performed (Table 3). In patients aged 65–69, EOR and postoperative KPS scores ≥70 were significantly related to longer PFS (P = 0.005 and 0.003, respectively). In patients ≥70 years, pre- and postoperative KPS scores ≥70 were significantly related to longer PFS (P = 0.01 and 0.002, respectively). The median PFS of patients with pre- or postoperative KPS scores ≥70 was >1 year. The use of CRT as adjuvant therapy strongly correlated with prolonged PFS (P = 0.06). WHO grade, EOR, and MGMT-immunonegativity were not associated with prolonged PFS. Pre- and postoperative KPS scores ≥70, CRT as adjuvant therapy, and salvage therapy were significantly related to prolonged OS (P < 0.05, for all) in patients aged ≥70. WHO grade, EOR, and MGMT-immunonegativity were not related to prolonged OS.
Table 3.
Univariate analysis of PFS and OS
| 65–69 years | 70–91 years | ||||||||||
| n | PFS | OS | n | PFS | OS | ||||||
| mPFS | P | mOS | P | mPFS | P | mOS | P | ||||
| Sex | Male | 10 | 9.9 | 0.91 | 10 | 0.46 | 41 | 6.9 | 0.58 | 9.5 | 0.20 |
| Female | 11 | 10.1 | 28.6 | 29 | 7.6 | 16.1 | |||||
| CCI | Low (0 point) | 18 | 9.9 | 0.88 | 22.8 | 0.66 | 39 | 10.2 | 0.40 | 12.8 | 0.52 |
| Medium-high
(≥1point) |
3 | 30.2 | 28 | 31 | 4.2 | 9.9 | |||||
| WHO Grade | III | 1 | 7.9 | 0.49 | 39.2 | 0.7 | 18 | 8.5 | 0.81 | 11.4 | 0.84 |
| IV | 20 | 10.1 | 21.3 | 52 | 6.3 | 12.7 | |||||
| EOR | Gross total removal | 7 | 14 | 0.005 | 22.1 | 0.17 | 9 | 11.3 | 0.83 | 11.3 | 0.82 |
| Partial removal | 10 | 14 | 33.1 | 25 | 11.5 | 14.9 | |||||
| Biopsy | 4 | 4.3 | 9.9 | 36 | 6.3 | 10.1 | |||||
| MGMT | Immunopositive
(≥10%) |
12 | 9.9 | 0.65 | 22.8 | 0.69 | 35 | 6.6 | 0.91 | 12.9 | 0.65 |
| Immunonegative
(<10%) |
6 | 10.1 | 18.5 | 24 | 6.9 | 11.6 | |||||
| No test | 3 | 7.3 | 29.3 | 11 | 8.5 | 9.5 | |||||
| Preop. KPS | ≥70 | 12 | 9.2 | 0.38 | 29.3 | 0.82 | 29 | 12.3 | 0.01 | 16.8 | 0.01 |
| ≤60 | 9 | 10.1 | 18.5 | 41 | 5.5 | 9.1 | |||||
| Postop. KPS | ≥70 | 11 | 20.7 | 0.003 | 29.2 | 0.33 | 22 | 12.3 | 0.002 | 18.4 | 0.002 |
| ≤60 | 10 | 7.9 | 10.1 | 48 | 5.5 | 9.1 | |||||
| Adjuvant therapy | Chemoradiation | 19 | 11.5 | 0.48 | 16.1 | 0.44 | 41 | 11.4 | 0.06 | 16.1 | 0.02 |
| Others | 2 | 7.2 | 11.4 | 29 | 6.2 | 9.1 | |||||
| Salvage therapy | Yes | 8 | 20.7 | 0.3 | 28.9 | 0.5 | 21 | 8.5 | 0.39 | 17.3 | 0.04 |
| No | 13 | 7.9 | 12.4 | 49 | 6.2 | 9.5 | |||||
Abbreviations: mPFS, median progression free survival; mOS, median overall survival; CCI, Charlson comorbidity index; WHO, World Health Organization; EOR, extent of resection; MGMT, O6-methylguanine-DNA methyltransferase; KPS, Karnofsky Performance Status
Multivariate analysis of PFS and OS
In the multivariate analyzes of PFS and OS in relation to pre- and postoperative KPS, CRT as adjuvant therapy, and salvage therapy in patients ≥70, the only postoperative KPS score ≥70 was significantly related to prolonged PFS (hazard ratio [HR], 0.48; 95% CI, 0.24–0.98, P = 0.04) (Table 4). Postoperative KPS, CRT as adjuvant therapy, and salvage therapy were significantly related to prolonged OS (HR, 0.45; 95% CI, 0.23–0.90; P = 0.03; HR, 0.38; 95% CI, 0.20–0.70; P = 0.002, and HR, 0.43; 95% CI, 0.22–0.81; P = 0.01, respectively). Preoperative KPS score ≥70 was not significantly related to PFS or OS in the multivariate analysis. No clinical factors significantly associated with postoperative KPS were found except for preoperative KPS. Patients ≥70 were subdivided into four groups based on the three independent prognostic factors (postoperative KPS, CRT as adjuvant therapy, and salvage therapy). Thirteen patients had no prognostic factors (group 0), 33 had a prognostic factor (group 1), 19 had two prognostic factors (group 2), and five had all prognostic factors (group 3). The Kaplan–Meier curve of the four groups showed that the median OS of groups 0, 1, 2, and 3 was 7.2, 9.1, 18.4, and 27.0 months, respectively (Figure C). A statistically significant difference was found in the OS between the groups (P < 0.001).
Table 4.
Multivariate analysis of PFS and OS in 70–91-year-old patients
| PFS | OS | |||||
| Hazard
ratio |
95% CI | P | Hazard
ratio |
95% CI | P | |
| Preop. KPS ≥ 70 | 0.71 | 0.37–1.37 | 0.31 | 0.73 | 0.38–1.40 | 0.34 |
| Postop. KPS ≥ 70 | 0.48 | 0.24–0.98 | 0.04 | 0.45 | 0.23–0.90 | 0.03 |
| Chemoradiation | 0.56 | 0.31–1.04 | 0.07 | 0.38 | 0.20–0.70 | 0.002 |
| Salvage therapy (+) | 0.77 | 0.41–1.45 | 0.42 | 0.43 | 0.22–0.81 | 0.01 |
Abbreviations: PFS, progression free survival; OS, median overall survival; 95% CI, 95% confidence interval; KPS, Karnofsky Performance Status
DISCUSSION
We showed a significant difference of OS between patients aged 65–69 and those ≥70. The median OS of patients aged 65–69 was >20 months, which was similar to previous studies including younger patients.1,15 However, the median OS of patients ≥70 was <1 year. We need to pay particular attention to the elderly ≥70. In this study, we found clearly different prognostic factors between these two age groups. Our multivariate analysis showed that higher postoperative KPS, CRT as adjuvant therapy, and salvage therapy were significantly related to good outcomes in patients ≥70.
Even in the elderly, EOR is a strong predictor of good outcome,2,16-20 provided that surgery does not change patients’ postoperative KPS scores.21 However, the overall rate of complications after resection in the elderly is 21.9%–31.9%.21,22 In addition, 13%–20% of elderly patients cannot receive complete adjuvant treatment due to neurological deterioration postoperatively.23 In this study, the perioperative KPS scores decreased in approximately 40% of patients. Although a maximally safe resection was essential for better prognosis, even in this age group, a surgical strategy to obtain tissue diagnosis while minimizing complications and maintaining postoperative KPS should be considered.
In this study, multivariate analysis of OS indicated that CRT, as adjuvant therapy was significantly related to prolonged OS. Some reports correlate adjuvant therapy with CRT with good outcomes.2,17-21,24-26 CRT as adjuvant therapy, rather than age and EOR, reportedly improves prognosis in the elderly.21 However, assessment tools to define which elderly patients can withstand aggressive therapy, such as CRT, are limited.3 Di Cristofori et al found that age, postoperative KPS, postoperative MMSE, and tumor volume, but not EOR, were significantly associated with CRT viability.23 We suggest that a higher postoperative KPS score should be an essential qualifier for CRT postoperatively. In this study, patients who underwent CRT postoperatively showed significantly prolonged OS. Our data support CRT’s efficacy as an adjuvant therapy in elderly patients with high postoperative KPS scores.
In this study, multivariate analyzes showed that salvage therapy was also significantly associated with prolonged OS, but not PFS, suggesting that aggressive treatment at recurrence is effective, even in selected elderly patients. Gorlia et al showed that performance status, not age, is a major prognostic factor for PFS and OS in recurrent GBM.27 Zanello et al found that elderly patients with KPS scores ≥70 at recurrence share similar OS from recurrence compared to younger patients. They reported that oncological treatment from recurrence, particularly surgical resection, TMZ, and bevacizumab therapy, were significant, independent predictors of longer OS from recurrence, and concluded that treatment options from recurrence for elderly patients should include repeat surgery, second-line chemotherapy, and anti-angiogenic agents.28
This study has some limitations. It is a retrospective study from a single institute, including relatively few patients and selection bias. In addition, our study lacks sufficient molecular information. Mutations in IDH1 were not investigated in approximately 40% of patients.
In conclusion, in patients ≥70 with high-grade astrocytoma, OS was significantly shorter compared to those aged 65–69. Our results suggest that postoperative KPS score ≥70 is significantly related to prolonged PFS and OS in elderly patients ≥70 years. Furthermore, CRT postoperatively and salvage therapy after recurrence may be effective methods in selected elderly population. A therapeutic strategy that prioritizes KPS maintenance may benefit survival in elderly patients ≥70 years.
ACKNOWLEDGEMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Abbreviations
- CCI
Charlson comorbidity index
- CRT
chemoradiotherapy
- EOR
extent of resection
- GBM
glioblastoma
- GTR
gross total resection
- KPS
Karnofsky performance status
- OS
overall survival
- PD
progression disease
- PFS
progression-free survival
- PR
partial removal
- TMZ
temozolomide
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed]
- 2.Heiland DH, Haaker G, Watzlawick R, et al. One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neurooncol. 2018;140(2):385–391. [DOI] [PubMed]
- 3.Palmer JD, Bhamidipati D, Mehta M, et al. Treatment recommendations for elderly patients with newly diagnosed glioblastoma lack worldwide consensus. J Neurooncol. 2018;140(2):421–426. [DOI] [PubMed]
- 4.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed]
- 5.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed]
- 6.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9(1):29–38. [DOI] [PubMed]
- 7.Yin AA, Zhang LH, Cheng JX, et al. The predictive but not prognostic value of MGMT promoter methylation status in elderly glioblastoma patients: a meta-analysis. PLoS One. 2014;9(1):e85102. [DOI] [PMC free article] [PubMed]
- 8.Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed]
- 9.Gallego PL, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed]
- 10.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed]
- 11.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed]
- 12.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed]
- 13.Watanabe R, Nakasu Y, Tashiro H, et al. O6-methylguanine DNA methyltransferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol. 2011;28(2):127–135. [DOI] [PubMed]
- 14.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed]
- 15.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed]
- 16.Babu R, Komisarow JM, Agarwal VJ, et al. Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg. 2016;124(4):998–1007. [DOI] [PubMed]
- 17.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007). Cancer. 2009;115(16):3758–3766. [DOI] [PMC free article] [PubMed]
- 18.Morgan ER, Norman A, Laing K, Seal MD. Treatment and outcomes for glioblastoma in elderly compared with non-elderly patients: a population-based study. Curr Oncol. 2017;24(2):e92–e98. [DOI] [PMC free article] [PubMed]
- 19.Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118(4):786–798. [DOI] [PubMed]
- 20.Tsang DS, Khan L, Perry JR, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol). 2015;27(3):176–183. [DOI] [PubMed]
- 21.Karsy M, Yoon N, Boettcher L, et al. Surgical treatment of glioblastoma in the elderly: the impact of complications. J Neurooncol. 2018;138(1):123–132. [DOI] [PubMed]
- 22.D’Amico RS, Cloney MB, Sonabend AM, et al. The safety of surgery in elderly patients with primary and recurrent glioblastoma. World Neurosurg. 2015;84(4):913–919. [DOI] [PubMed]
- 23.Di Cristofori A, Zarino B, Fanizzi C, et al. Analysis of factors influencing the access to concomitant chemo-radiotherapy in elderly patients with high grade gliomas: role of MMSE, age and tumor volume. J Neurooncol. 2017;134(2):377–385. [DOI] [PubMed]
- 24.Amsbaugh MJ, Yusuf MB, Gaskins J, Burton EC, Woo SY. Patterns of care and predictors of adjuvant therapies in elderly patients with glioblastoma: an analysis of the National Cancer Data Base. Cancer. 2017;123(17):3277–3284. [DOI] [PubMed]
- 25.Gately L, Collins A, Murphy M, Dowling A. Age alone is not a predictor for survival in glioblastoma. J Neurooncol. 2016;129(3):479–485. [DOI] [PubMed]
- 26.Huang J, Samson P, Perkins SM, et al. Impact of concurrent chemotherapy with radiation therapy for elderly patients with newly diagnosed glioblastoma: a review of the National Cancer Data Base. J Neurooncol. 2017;131(3):593–601. [DOI] [PubMed]
- 27.Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. [DOI] [PubMed]
- 28.Zanello M, Roux A, Ursu R, et al. Recurrent glioblastomas in the elderly after maximal first-line treatment: does preserved overall condition warrant a maximal second-line treatment? J Neurooncol. 2017;135(2):285–297. [DOI] [PubMed]